Preliminary evaluation of Roche Cobas Elecsys Anti-SARS-CoV-2 chemiluminescence immunoassay
-
Giuseppe Lippi
, Gian Luca Salvagno
To the Editor,
Coronavirus disease 2019 (COVID-19), the latest pandemic that has emerged during the past 20 years, is still massively spreading all around the world, causing several thousand hundreds deaths and contributing to the collapse of many healthcare systems [1]. Although the etiological diagnosis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is still based on direct detection of viral RNA in upper and/or lower respiratory tract specimens, serological testing may provide an important contribution for complementing molecular biology in certain, almost undetermined, cases, as well as for providing evidence of a humoral response against the virus for immuno-surveillance and epidemiological purposes [2].
Two technical approaches have currently been proposed for assessing the serologic immune response against SARS-CoV-2, the former encompassing the assessment of specific immunoglobulin classes (i.e., IgM, IgA, or IgG), whilst the later entails the evaluation of the “total” antibody immune response, thus encompassing the concomitant measurement of all antibodies subpopulations. A definitive recommendation on the most informative approach has not been currently endorsed by any national or international organization [3], [4], [5], so that additional studies would be needed to assess and interpret these two different measures, along with their possible strengths and limitations. For this purpose, we designed an original protocol aimed at analyzing the performance of the recently developed Roche Cobas Elecsys Anti-SARS-CoV-2 test (Roche Diagnostics GmbH, Mannheim, Germany), and comparing the clinical significance of its results with those of well-validated, commercially available, enzyme-linked immunosorbent assays (ELISA).
The new Roche Cobas Elecsys Anti-SARS-CoV-2 is an electrochemiluminescence immunoassay (ECLIA) for qualitative detection of total antibodies developed against SARS-CoV-2 in human plasma or serum specimens. The assay is based on a recombinant protein which represents the nucleocapsid (N) antigen of SARS-CoV-2. Briefly, this technique is based on a sandwich reaction where test sample, biotinylated SARS-CoV-2-specific recombinant antigen and SARS-CoV-2-specific recombinant antigen labeled with ruthenium are initially incubated altogether. After adding streptavidin-coated microparticles, the complex is bound to the solid phase through a biotin-streptavidin reaction and then aspirated into a measuring cell, where microparticles are magnetically captured. Unbound material is removed and chemiluminescent emission is finally assayed with a photomultiplier. Test results are generated by interpolating the electrochemiluminescence signal with that of a threshold previously generated during calibration. A cut-off index ≥1.0 is classified as “reactive”, and hence positive for SARS-CoV-2. The total procedure requires 12 μL of test sample and the total duration of the assay is 18 min.
The results generated by Elecsys Anti-SARS-CoV-2 were compared with those obtained with commercial SARS-CoV-2 IgA and IgG enzyme-linked immunosorbent assays (ELISAs; Euroimmun AG, Luebeck, Germany), whose clinical performance has been earlier validated elsewhere [6]. In particular, the diagnostic sensitivity and specificity of anti-SARS-CoV-2 antibodies 15 or more days after symptoms onset were found to be 90% and 99% for IgG, and 94% and 86% for IgA, respectively, whilst sensitivity and specificity of both antibody classes combined were found to be as high as 94% and 88% [6]. A test result ≥1.1 (absorbance of patient sample/absorbance of calibrator), is considered reactive, whilst the declared reproducibility ranges between 2 and 16%.
The final study population consisted of 150 consecutive patients (mean age, 52 ± 17 years; 79 women and 71 men), who underwent serology testing at the University Hospital of Verona for screening SARS-CoV-2 infection. The statistical analysis was carried out using Analyze-it (Analyze-it Software Ltd, Leeds, UK). The study was cleared by the local Ethical Board (University Hospital of Verona; SOPAV-2; protocol no. 35747).
A preliminary evaluation of intra-assay imprecision of Elecsys Anti-SARS-CoV-2, carried out to validate the repeatability of the method, yielded a coefficient of variation (CV%) of 2.5% for a low value plasma pool (12 repeats; mean value, 0.087 ± 0.002) and 1.0% for a very high value plasma pool (12 repeats; mean value, 96.125 ± 0.940), respectively.
Overall, the rate of positive test results was 16/150 (10.7%) with total Euroimmun IgG antibodies, 9/150 (6.0%) with Euroimmun IgG, 16/150 (10.7%) with Euroimmun IgA and 13/150 (8.7%) with Elecsys Anti-SARS-CoV-2. The Pearson’s correlation coefficient of raw values generated by Elecsys Anti-SARS-CoV-2 was 0.77 (95% CI, 0.70–0.83; p<0.001) vs. Euroimmun IgG antibodies values, and 0.70 (95% CI, 0.61–0.78; p<0.001) vs. Euroimmun IgA antibodies values, respectively. Notably, the correlation between Euroimmun IgG and IgA antibodies values was 0.81 (95% CI, 0.75–0.86; p<0.001).
The comparison of tests results obtained with Elecsys Anti-SARS-CoV-2 and Euroimmun Anti-SARS-CoV-2 IgG and IgA is shown in Figure 1. The area under the curve (AUC) of Elecsys Anti-SARS-CoV-2 results, expressed as raw values or as positive/negative (i.e., qualitative) results, was 0.89 (95% CI, 0.78–1.00; p<0.001) and 0.84 (0.72–0.95; p<0.001) vs. total Euroimmun antibodies positivity (Figure 1A), 0.91 (95% CI, 0.78–1.00; p<0.001) and 0.93 (95% CI, 0.82–1.00; p<0.001) vs. Euroimmun IgG antibodies positivity (Figure 1B), and 0.91 (95% CI, 0.80–1.00; p<0.001) and 0.83 (95% CI, 0.70–0.96; p<0.001) vs. Euroimmun IgA antibodies positivity (Figure 1C). The agreement of Elecsys Anti-SARS-CoV-2 positivity was 95% (kappa statistics, 0.71; 95% CI, 0.49–0.93) vs. total Euroimmun antibodies positivity, 96% (kappa statistics, 0.71; 95% CI, 0.49 vs. 0.93) vs. Euroimmun IgG antibodies positivity, and 94% (kappa statistics, 0.66; 95% CI, 0.45–0.88) vs. Euroimmun IgA antibodies positivity, respectively.
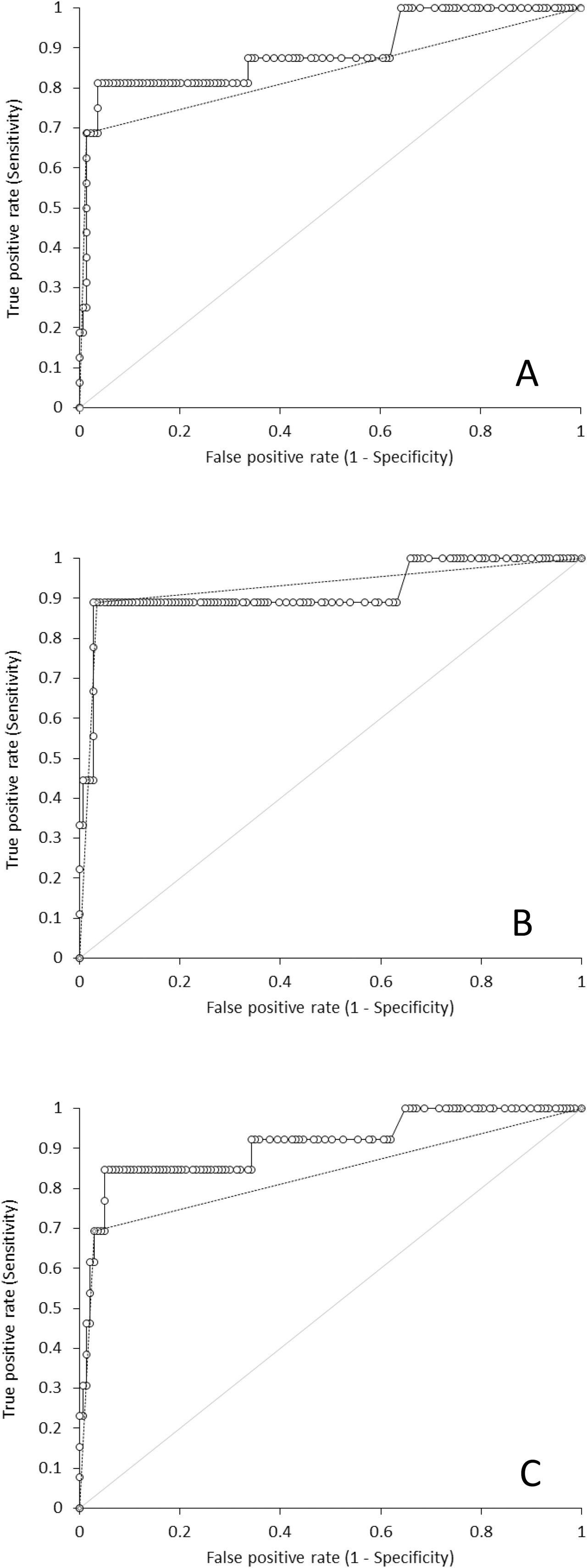
Receiver operating characteristics curve analysis of Roche Cobas Elecsys Anti-SARS-CoV-2 total antibodies.
O, raw values; - - , positive/negative vs. Euroimmun Anti-SARS-CoV-2 total antibodies either or both positive; (panel A), IgG (panel B) and IgA (panel C).
The results of this preliminary investigation on Elecsys Anti-SARS-CoV-2, based on its comparison with the Euroimmun Anti-SARS-CoV-2 IgG and IgA immunoassays, paves the way to some conclusions. First, a good correlation was found between the raw values of the new Roche total antibodies immunoassay and those of both Euroimmun IgGs and IgAs. Then, a good agreement was also found between the total antibodies measure and that of IgGs and IgAs combined or alone, with AUCs always >0.83 and agreement >94%. Therefore, although we could not evaluate IgM immune response in this study, it can be inferred that minor differences have emerged from using Elecsys Anti-SARS-CoV-2 total antibodies or Euroimmun Anti-SARS-CoV-2 IgGs and IgAs in our population screening, thus complementing previous evidence published by Egger et al. using another anti-SARS-CoV-2 IgM and IgG ELISA [7]. It is also noteworthy that an especially high concordance was noted when comparing data obtained with Euroimmun IgG antibodies positivity and Elecsys Anti-SARS-CoV-2 total antibodies. In particular, four more samples were found to be positive with Elecsys Anti-SARS-CoV-2 than with Euroimmun IgG, which may reflect perhaps the presence of IgMs, which could not be evaluated in this study. A further investigation of these cases showed that one had undermined results of nucleic acid amplification test (NAAT) on nasopharyngeal swabs, one was found to be negative and the remaining two were instead positive. This would actually suggest that Elecsys Anti-SARS-CoV-2 total antibodies may perhaps be characterized by higher diagnostic sensitivity in detecting acute SARS-CoV-2 infection, though further studies will be needed to verify this assumption.
Acknowledgments
The manufacturers provided in-kind support in the form of equipment and consumables for the evaluation, but had no role in directing the study or influencing the study outcomes.
-
Research funding: None declared.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: Authors state no conflict of interest.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Ethical approval: The study was cleared by the local Ethical Board (University Hospital of Verona; SOPAV-2; protocol no. 35747).
References
1. Lippi, G, Sanchis-Gomar, F, Henry, BM. COVID-19: unravelling the clinical progression of nature’s virtually perfect biological weapon. Ann Transl Med 2020;8:693. https://doi.org/10.21037/atm-20-3989.Suche in Google Scholar PubMed PubMed Central
2. Lippi, G, Mattiuzzi, C, Bovo, C, Plebani, M. Current laboratory diagnostics of coronavirus disease 2019 (COVID-19). Acta Biomed 2020;91:137–45.Suche in Google Scholar
3. Bohn, MK, Lippi, G, Horvath, A, Sethi, S, Koch, D, Ferrari, M, et al. Molecular, serological, and biochemical diagnosis and monitoring of COVID-19: IFCC taskforce evaluation of the latest evidence. Clin Chem Lab Med 2020;58:1037–52. https://doi.org/10.1515/cclm-2020-0722.Suche in Google Scholar PubMed
4. Plebani, M, Padoan, A, Sciacovelli, L, Basso, D. Towards the rational utilization of SARS-CoV-2 serological tests in clinical practice. Clin Chem Lab Med 2020;58:e189-91.10.1515/cclm-2020-0880Suche in Google Scholar PubMed
5. Plebani, M, Padoan, A, Negrini, D, Carpinteri, B, Sciacovelli, L. Diagnostic performances and thresholds: the key to harmonization in serological SARS-CoV-2 assays? Clin Chim Acta 2020;509:1–7. https://doi.org/10.1016/j.cca.2020.05.050.Suche in Google Scholar PubMed PubMed Central
6. Montesinos, I, Gruson, D, Kabamba, B, Dahma, H, Van den Wijngaert, S, Reza, S, et al. Evaluation of two automated and three rapid lateral flow immunoassays for the detection of anti-SARS-CoV-2 antibodies. J Clin Virol. 2020 Jul;128:104413. https://doi.org/10.1016/j.jcv.2020.104413. [Epub 2020 May 5].Suche in Google Scholar PubMed PubMed Central
7. Egger, M, Bundschuh, C, Wiesinger, K, Gabriel, C, Clodi, M, Mueller, T, et al. Comparison of the Elecsys® Anti-SARS-CoV-2 immunoassay with the EDI™ enzyme linked immunosorbent assays for the detection of SARS-CoV-2 antibodies in human plasma. Clin Chim Acta 2020;509:18–21. https://doi.org/10.1016/j.cca.2020.05.049.Suche in Google Scholar PubMed PubMed Central
© 2020 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Editorial
- Biotin interference in cardiac troponin immunoassay – where the wild things are?
- Review
- Laboratory-related issues in the measurement of cardiac troponins with highly sensitive assays
- Mini Review
- Chromatographic methods development for clinical practice: requirements and limitations
- Opinion Paper
- Harmonising EQA schemes the next frontier: challenging the status quo
- Genetics and Molecular Diagnostics
- Direct comparison study between droplet digital PCR and a combination of allele-specific PCR, asymmetric rapid PCR and melting curve analysis for the detection of BRAF V600E mutation in plasma from melanoma patients
- A novel mitochondrial m.14430A>G (MT-ND6, p.W82R) variant causes complex I deficiency and mitochondrial Leigh syndrome
- Obesity status modifies the association between rs7556897T>C in the intergenic region SLC19A3-CCL20 and blood pressure in French children
- General Clinical Chemistry and Laboratory Medicine
- Influence of reagent lots and multiple measuring systems on estimating the coefficient of variation from quality control data; implications for uncertainty estimation and interpretation of QC results
- Electrophoretic α1-globulin for screening of α1-antitrypsin deficient variants
- A continued method performance monitoring approach for the determination of pediatric renin samples – application within a European clinical trial
- Pilot study for cystic fibrosis neonatal screening: the Cuban experience
- Validation of the analytical performance of the NOVEOS™ System, a system which improves upon the third-generation in vitro allergy testing technology
- IgE cross-reactivity measurement of cashew nut, hazelnut and peanut using a novel IMMULITE inhibition method
- Sexual dimorphism in the cerebrospinal fluid total protein content
- Current state of the morphological assessment of urinary erythrocytes in The Netherlands: a nation-wide questionnaire
- Reference Values and Biological Variations
- Within-subject and between-subject biological variation of first morning void urine amino acids in 12 healthy subjects
- Proenkephalin as a new biomarker for pediatric acute kidney injury – reference values and performance in children under one year of age
- Hematology and Coagulation
- Quality performance for indirect Xa inhibitor monitoring in patients using international external quality data
- Cardiovascular Diseases
- Clinical risk assessment of biotin interference with a high-sensitivity cardiac troponin T assay
- Short- and long-term biological variation of cardiac troponin I in healthy individuals, and patients with end-stage renal failure requiring haemodialysis or cardiomyopathy
- Infectious Diseases
- Monocyte distribution width (MDW) as a screening tool for sepsis in the Emergency Department
- Performance of a Toxo IgM prototype assay for the diagnosis of maternal and congenital Toxoplasma infections
- Letters to the Editors
- Evaluation of an ELISA for SARS-CoV-2 antibody testing: clinical performances and correlation with plaque reduction neutralization titer
- Preliminary evaluation of Roche Cobas Elecsys Anti-SARS-CoV-2 chemiluminescence immunoassay
- Hypoalbuminemia and elevated D-dimer in COVID-19 patients: a call for result harmonization
- Total pathway to method validation
- Derivation of performance specifications for uncertainty of serum C-reactive protein measurement according to the Milan model 3 (state of the art)
- FGF23 measurement in burosumab-treated patients: an emerging treatment may induce a new analytical interference
- Use of a modified IDS-ISYS intact PTH assay for intraoperative PTH measurements
- Agreement of dried blood spot lyso-Gb3 concentrations obtained from different laboratories in patients with Fabry disease
- Influence of delayed separation of plasma from whole blood and centrifugation protocol on Zn plasma concentration
- A survey of order of draw on inpatient wards and adherence to EFLM-COLABIOCLI recommendations
- Successful implementations of automated minimum re-test intervals to overcome ferritin over-requesting in a Spanish hospital laboratory
- Remarkable pseudoleucocytosis induced by mild cryoglobulinemia
- Massive hemolysis due to Clostridium perfringens: a laboratory’s perspective
Artikel in diesem Heft
- Frontmatter
- Editorial
- Biotin interference in cardiac troponin immunoassay – where the wild things are?
- Review
- Laboratory-related issues in the measurement of cardiac troponins with highly sensitive assays
- Mini Review
- Chromatographic methods development for clinical practice: requirements and limitations
- Opinion Paper
- Harmonising EQA schemes the next frontier: challenging the status quo
- Genetics and Molecular Diagnostics
- Direct comparison study between droplet digital PCR and a combination of allele-specific PCR, asymmetric rapid PCR and melting curve analysis for the detection of BRAF V600E mutation in plasma from melanoma patients
- A novel mitochondrial m.14430A>G (MT-ND6, p.W82R) variant causes complex I deficiency and mitochondrial Leigh syndrome
- Obesity status modifies the association between rs7556897T>C in the intergenic region SLC19A3-CCL20 and blood pressure in French children
- General Clinical Chemistry and Laboratory Medicine
- Influence of reagent lots and multiple measuring systems on estimating the coefficient of variation from quality control data; implications for uncertainty estimation and interpretation of QC results
- Electrophoretic α1-globulin for screening of α1-antitrypsin deficient variants
- A continued method performance monitoring approach for the determination of pediatric renin samples – application within a European clinical trial
- Pilot study for cystic fibrosis neonatal screening: the Cuban experience
- Validation of the analytical performance of the NOVEOS™ System, a system which improves upon the third-generation in vitro allergy testing technology
- IgE cross-reactivity measurement of cashew nut, hazelnut and peanut using a novel IMMULITE inhibition method
- Sexual dimorphism in the cerebrospinal fluid total protein content
- Current state of the morphological assessment of urinary erythrocytes in The Netherlands: a nation-wide questionnaire
- Reference Values and Biological Variations
- Within-subject and between-subject biological variation of first morning void urine amino acids in 12 healthy subjects
- Proenkephalin as a new biomarker for pediatric acute kidney injury – reference values and performance in children under one year of age
- Hematology and Coagulation
- Quality performance for indirect Xa inhibitor monitoring in patients using international external quality data
- Cardiovascular Diseases
- Clinical risk assessment of biotin interference with a high-sensitivity cardiac troponin T assay
- Short- and long-term biological variation of cardiac troponin I in healthy individuals, and patients with end-stage renal failure requiring haemodialysis or cardiomyopathy
- Infectious Diseases
- Monocyte distribution width (MDW) as a screening tool for sepsis in the Emergency Department
- Performance of a Toxo IgM prototype assay for the diagnosis of maternal and congenital Toxoplasma infections
- Letters to the Editors
- Evaluation of an ELISA for SARS-CoV-2 antibody testing: clinical performances and correlation with plaque reduction neutralization titer
- Preliminary evaluation of Roche Cobas Elecsys Anti-SARS-CoV-2 chemiluminescence immunoassay
- Hypoalbuminemia and elevated D-dimer in COVID-19 patients: a call for result harmonization
- Total pathway to method validation
- Derivation of performance specifications for uncertainty of serum C-reactive protein measurement according to the Milan model 3 (state of the art)
- FGF23 measurement in burosumab-treated patients: an emerging treatment may induce a new analytical interference
- Use of a modified IDS-ISYS intact PTH assay for intraoperative PTH measurements
- Agreement of dried blood spot lyso-Gb3 concentrations obtained from different laboratories in patients with Fabry disease
- Influence of delayed separation of plasma from whole blood and centrifugation protocol on Zn plasma concentration
- A survey of order of draw on inpatient wards and adherence to EFLM-COLABIOCLI recommendations
- Successful implementations of automated minimum re-test intervals to overcome ferritin over-requesting in a Spanish hospital laboratory
- Remarkable pseudoleucocytosis induced by mild cryoglobulinemia
- Massive hemolysis due to Clostridium perfringens: a laboratory’s perspective


