Abstract
Common traumas to the skeletal system are bone fractures and injury-related articular cartilage damage. The healing process can be impaired resulting in non-unions in 5–10% of the bone fractures and in post-traumatic osteoarthritis (PTOA) in up to 75% of the cases of cartilage damage. Despite the amount of research performed in the areas of fracture healing and cartilage repair as well as non-unions and PTOA, still, the outcome of a bone fracture or articular cartilage damage cannot be predicted. Here, we discuss known risk factors and key molecules involved in the repair process, together with the main challenges associated with the prediction of outcome of these injuries. Furthermore, we review and discuss the opportunities for mass spectrometry (MS) – an analytical tool capable of detecting a wide variety of molecules in tissues – to contribute to extending molecular understanding of impaired healing and the discovery of predictive biomarkers. Therefore, the current knowledge and challenges concerning MS imaging of bone and cartilage tissue as well as in vivo MS are discussed. Finally, we explore the possibilities of in situ, real-time MS for the prediction of outcome during surgery of bone fractures and injury-related articular cartilage damage.
Introduction
Bone fractures and injury-related articular cartilage damage are common traumas to the skeletal system. A bone fracture is a complete or incomplete break of the anatomical continuation of the bone, resulting in its mechanical instability [1]. Normal fracture healing is a highly complex process involving a well-defined series of biological phases and mechanical requirements (see Figure 1) [1], [2], [3], [4], [5], [6], [7]. Although these phases are successive, there is great overlap between them, resulting in a continuously changing environment in terms of cell populations and signaling processes [2]. Bone fracture healing normally results in completely functional and structural restoration of the involved bone [2], [8]. Due to its complexity, fracture healing is not fully understood in detail, as the exact contribution of different molecules is largely unknown [2], [4], [7].
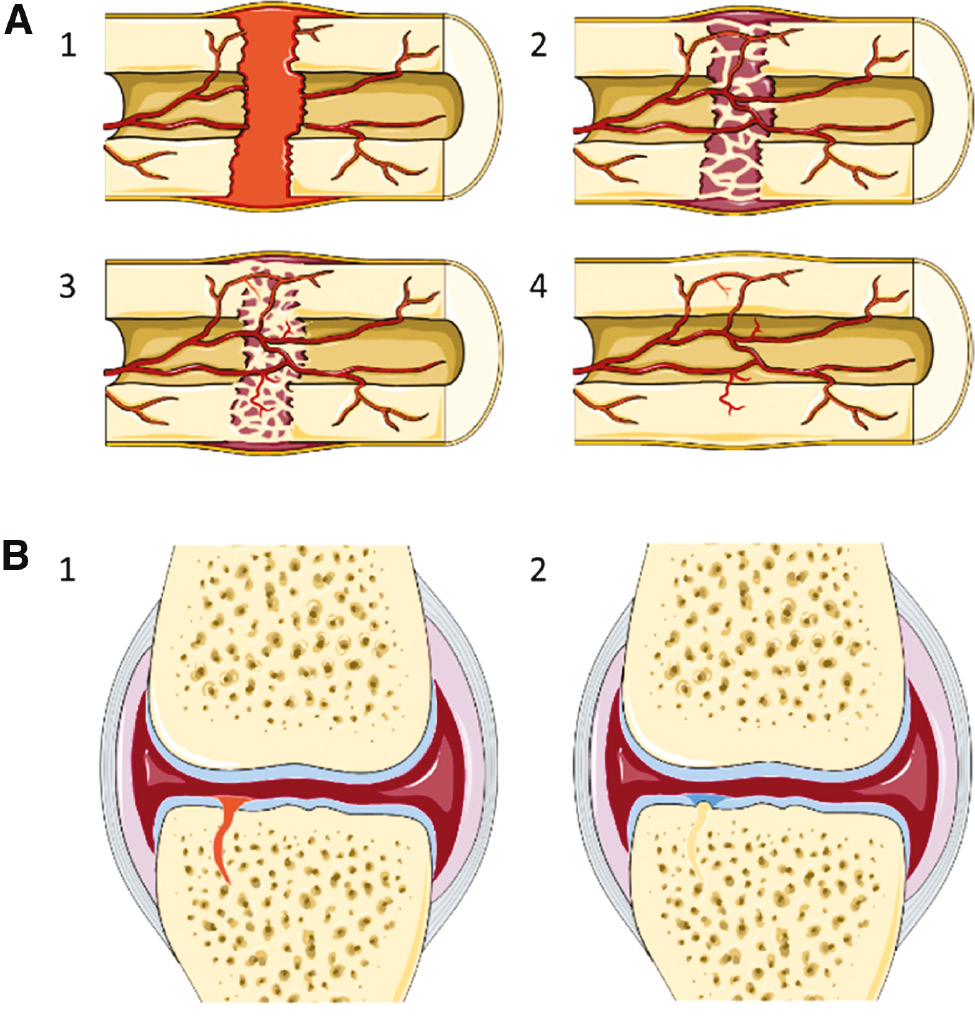
The phases in bone fracture healing and intra-articular fracture healing.
(A) The four phases of bone fracture healing are (1) hematoma (fibrin clot) formation, (2) soft callus formation, (3) hard callus formation, and (4) bone remodeling. This healing process results in the complete restoration of the structure and long-term function of the bone. (B) The two main phases of the healing response of the bone in intra-articular fractures are (1) fibrin clot formation to fill the defect and (2) bone formation in the osseous part and fibrocartilage formation in the chondral part. The structure of the fibrocartilage is significantly different from the original articular cartilage and will often fail. The healing response from the bone in intra-articular fractures does not result in long-term restoration of joint structure and function.
With cartilage injuries, its structures and functions are damaged, which can result in reduced mobility [9], [10], [11]. Cartilage damage can have different causes, but for post-traumatic osteoarthritis (PTOA) a previous joint injury is the cause [10], [11], [12], [13]. The repair capacity of articular cartilage is extremely limited and, therefore, small surface disruptions do not heal and even worsen over time [9], [14], [15]. Despite the extracellular matrix (ECM) deposition by chondrocytes, an injury to cartilage will often result in the loss of tissue and the formation of a defect [9], [14]. If an increasing defect reaches the underlying bone, scar tissue will be formed in the defect from the bone (see Figure 1) [9], [12], [16]. It is unknown when cartilage damage becomes irreversible and results in progressive damage [9], [14]. This is because of the complexity of cartilage interaction with surrounding joint tissues and response to external factors [12], [15], [16].
Abnormal healing of the bone and/or articular cartilage can result in long-lasting problems for the patient, such as non-unions and PTOA. The bone fracture healing process is abnormal in 5–10% of fractures, resulting in delayed healing or non-union [1], [2], [4], [17], [18]. Nevertheless, the non-union rate differs per anatomical location and per treatment method [1], [19], [20]. Non-union rates have been published in the range of 3–48% of the bone fractures [19], [21], [22]. Injury-related cartilage damage can result in PTOA in 10–75% of the cases, depending on the involved joint [13], [23], [24]. Furthermore, approximately 12% of the osteoarthritis (OA) cases can be assigned to PTOA [10], [11], [13], [16]. Non-unions and PTOA are difficult to predict, diagnose in an early phase, and treat, making them current challenges for surgeons and researchers [1], [2], [10], [12], [15], [16], [19]. Although some key molecules and risk factors associated with abnormal healing are known, these cannot predict the outcome of bone fractures or injury-related cartilage damages [15], [16], [25], [26]. Establishing predictive markers could contribute to early detection (e.g. at the time of the initial surgery) and/or prediction of abnormal healing, which can contribute to earlier and/or new treatment options.
Despite the knowledge of some key molecules in the healing processes, the exact molecular contribution to and interactions with these processes and how these molecules are involved in non-union and PTOA development are still unknown. This lack of knowledge contributes to the clinical challenges related to abnormal fracture healing. Extending the knowledge of molecules involved in abnormal fracture healing can support the development of new prediction, diagnosis, and treatment options. Mass spectrometry (MS) is an analytical technique that is used for an increasing number of applications in clinical research, including cancer research and prediction of cancer treatment outcome [27], [28], [29]. This technique allows for unlabeled analysis of a wide range of molecules without the need for prior knowledge or labeling; these include metabolites, lipids, peptides, and proteins [27], [29], [30], [31], [32]. Mass spectrometry imaging (MSI) is particularly used in clinical applications to study the distribution of different molecules in tissue sections for, among others, biomarker discovery, diagnosis, and studying the intra-tumor heterogeneity, which has been used to a lesser extent for imaging molecular distributions in bone and cartilage [29], [31], [33]. Furthermore, intra-operative techniques using MS are being developed mainly for diagnostic purposes in oncology, with one of the most important advantages being the real-time monitoring of the molecular profile of native tissues [34], [35], [36], [37]. The main focus of studies using MSI or intra-operative MS has been on tumor research, showing the potential of MS. The work performed on hard tissues (bone and cartilage) and abnormal fracture healing using MS is limited. Extending this work will provide new molecular insights, which can contribute to a better understanding and prediction of abnormal healing.
This article provides an overview of the current knowledge of delayed fracture healing and non-union development as well as the development of PTOA with a focus on hard tissues (bone and cartilage). Herein, important molecules in the healing processes and diagnostic opportunities will be discussed, which show the current challenges in the prediction of outcome and resulting complications. Lastly, we will discuss the potential for MS to overcome these current clinical challenges, as this technique can contribute to discovery of novel biomarkers and involved processes for development of delayed healing/non-union and PTOA, while it can also be used as a prediction tool for these conditions and make a major leap in patient management.
Delayed healing and non-union
A fracture non-union can be defined as the termination of the fracture healing process without reaching a bone union, while in delayed healing the fracture healing time is longer than expected [18], [21], [38], [39], [40]. Impaired healing can be caused by disturbance of any of the phases in the fracture healing process [4], [38]. A lack of consensus between surgeons exists about the definition of a non-union [1], [2], [19]. Non-union can be defined as no healing in 9 months and no progressive signs of healing in the last 3 months according to the United States Food and Drug Administration [1], [2], [18], [21], [39], [40]. Nevertheless, there is a great variation in healing time based on different skeletal locations and amount of soft tissue damage [1], [2], [40], [41].
An often-used classification for non-unions is the differentiation between hypertrophic and atrophic non-unions [2], [18], [21], [40], [42]. In a hypertrophic non-union, there is formation of a large callus, but no bony bridging due to instability [2], [18], [21], [39], [42]. Hypertrophic non-unions are characterized by their biological potential and can further be divided into elephant foot, horsefoot, or oligotrophic non-union [1], [18], [21], [39], [42]. An atrophic non-union shows no to very little callus formation, because of a lack of biological regeneration [2], [18], [39], [40], [42]. Damage to the vascularization at the fracture site is often a contributing factor in the development of an atrophic non-union [2], [21].
The general goal of treatment is to achieve a union and return the affected bone to complete functioning [2], [18], [21], [41]. Nevertheless, treatment of non-unions is complex and related to high costs [18], [20], [40], [41]. The type and location of non-union in combination with the alignment will dictate the best treatment option [18], [39], [40], [41]. The parameters necessary to achieve are based on the diamond concept: mechanical stability, osteogenic cells, osteoconductive scaffolds, and growth factors, which result in an adequate biological environment when adequate vascularity is present [1], [5], [17], [18], [21], [40]. These requirements can be reached using different treatment methods or a combination thereof, which can be classified into biological, mechanical, and biophysical treatments [2], [18]. Each patient will need an individualized treatment plan to achieve union, due to the unique nature of each non-union [18], [21], [41].
Despite the large amount of research performed on fracture healing as well as impaired healing and improvement of treatment, understanding of the causes of non-unions is limited and there is still no accurate way to predict the outcome [8], [19], [20], [41], [43]. Nevertheless, there are some known risk factors and essential molecules for the bone fracture healing process. Furthermore, the current diagnostic options will be discussed.
Risk factors for non-union development
Although there is still no way to predict the outcome of the fracture healing process, there are some risk factors that can be related to non-union development [25]. One of the most important factors is inadequate vascularization, as disturbance of the blood supply can increase the risk of non-union development [2], [18], [25], [38], [41], [42], [44]. This can be caused by high-energy trauma resulting in extensive soft tissue damage, excessive displacement, and operative treatment at the fracture site [22], [25], [38], [40], [41], [44]. In addition, the amount of vascularization can be related to the location of the fracture, which can influence its healing process, as delayed unions and non-unions occur mainly in diaphyseal parts of the bone [2], [18], [22], [25], [44]. Bones with lower blood supply (e.g. tibia and femoral neck) show higher non-union rates compared to, for example, humeral fractures, which receive higher blood supply [19], [40], [44]. High-energy trauma and open fractures can result in disruption of the biological environment and/or comminuted bone, which both are risk factors [1], [25], [44]. Another important factor is instability and/or movement between fracture fragments, as the natural fracture healing process is disturbed [2], [18], [22], [38], [40], [41], [42]. Other mechanical causes can be displacement and a fracture gap, in which the main fracture fragments are not in contact and the fracture gap is too large to bridge [2], [18], [21], [22], [25], [38], [41], [42], [44]. A contributing risk factor is the quality of the surgical treatment in terms of stabilization and reduction of the fracture gap [18], [20], [22], [41]. In addition, the type of fracture will also influence the fracture healing process, as unstable complex, comminuted, or segmental fractures have a higher risk of non-union development [8], [20], [25], [44]. A (deep) infection resulting from contamination of an open fracture or as a complication of operation is a risk factor for non-union development [2], [8], [18], [21], [22], [25], [40], [41], [44]. Furthermore, there are some factors that increase the risk of non-union development, but are not the main reason for impaired healing [4], [18], [39]. These contributing factors include old age, osteoporosis, malnutrition, anemia, alcohol consumption, smoking, genetics, systematic disorders (like diabetes mellitus), multiple trauma or fractures, and certain medications (such as corticosteroids, anticoagulants, chemotherapeutic agents, nonsteroidal anti-inflammatory drugs (NSAIDs), and some antibiotics) [2], [4], [8], [18], [20], [21], [22], 25], [40], [41], [44]. Because of the complexity and interaction between these risks factors, early detection and accurate prediction of the outcome of the healing process remain a challenge [8], [20], [25], [45]. Nevertheless, risk factors and prediction models could allow for early identification and treatment initiation of non-unions [25].
Key molecules involved in the bone fracture healing
Multiple classes of molecules have been reported to play a role in the normal bone fracture healing process, and can be divided into three main classes: pro-inflammatory cytokines, growth factors, and metalloproteinases and angiogenic factors [1], [3], [6]. Several cell types interact in a coordinated way with these molecules in the fracture healing process, including immune cells, bone and cartilage forming primary cells, and muscle mesenchymal stem cells (MSCs) [2], [3], [6], [7], [17]. Any alteration in local and systemic regulatory molecules can have an effect on the cellular response, which may result in impaired healing [1], [5], [7], [17]. Table 1 gives an overview of the molecules that have been shown to be involved in the bone fracture healing process.
Different molecules have been shown to contribute to the repair processes in bone and cartilage.
| Molecules | Bone healing | Cartilage loss | Intra-articular fracture |
|---|---|---|---|
| ADAM metallopeptidase with thrombospondin type 1 motifs 4 and 5 (ADAMTS-4 and -5) | – | [14], [26], [46], [47], [48] | – |
| Angiopoietins 1 and 2 | [3], [7], [17] | – | – |
| Bone morphogenetic protein (BMP) antagonists | [3], [17], [25] | – | – |
| Noggin | [1], [3], [8], [17], [25] | ||
| Bone morphogenetic protein and activin membrane-bound inhibitor (BAMBI) | [1], [8], [17] | ||
| sclerostin | [8], [17] | ||
| Cartilage oligomeric matrix protein | – | [14], [47], [49] | – |
| C-C chemokine receptors (CCRs) and ligands (CCLs), including | – | – | |
| CCR-2 | [46] | ||
| CCR-5 | [46] | ||
| CCL-2 | [46], [49] | ||
| CCL-4 | [46], [49] | ||
| Extracellular matrix molecules and their fragments, including | [8] | [14], [16], [26], [47] | – |
| Fibronectin | [7], [8] | [13], [14], [47] | |
| Fibromodulin | [8] | – | |
| Collagens | [7], [38] | [14], [47], [48], [49] | |
| Proteoglycans | – | [9], [13], [14], [48], [49] | |
| Fibroblast growth factors (FGFs), including | [1], [2], [3], [5], [7], [8] | [9], [14], [47] | – |
| FGF-1 | [3], [17] | – | |
| FGF-2 | [3], [7], [17], [20] | [16] | |
| Insulin-like growth factors (IGFs) | [1], [3], [5], [7], [8], [17] | [9], [14], [47] | [9], [12] |
| Interferon-γ (IFN-γ) | [7] | [46], [49] | – |
| Interleukins (ILs), including | [5], [7] | [13], [14], [26], [46], [48], [49] | – |
| IL-1 | [1], [3], [5], [7], [17], [20] | [13], [15], [16], [26], [46], [47] | |
| IL-6 | [1], [3], [5], [7], [17], [20] | [16], [46], [47], [48] | |
| Matrix metalloproteinases (MMPs) | [3], [7], [8], [17] | [13], [14], [15], [16], [26], [46], [47], [48], [49] | – |
| Nitric oxide | [7], [8] | [16], [49] | – |
| Osteocalcin (OC) | [8] | – | – |
| Osteopontin (OPN) | – | [14], [49] | – |
| Platelet-derived growth factor (PDGF) | [1], [2], [3], [5], [7], [8], [17], [20], [25] | – | [9], [12] |
| Prostaglandin E2 (PGE2) and cyclooxygenase-2 (COX-2) | [1], [7], [25], [38] | – | – |
| Tenascin-C (TnC) | [7] | [14], [46], [49] | – |
| Tissue inhibitors of matrix metalloproteinases (TIMPs) | [7], [17] | [14], [26], [48], [49] | – |
| Toll-like receptors (TLRs) | – | [46] | – |
| Transforming growth factor-β (TGF-β) superfamily, including | [5] | ||
| Activins | [2], [3], [7], [17] | – | – |
| BMPs | [1], [2], [3], [7], [8], [17], [20], [38] | [14] | [9], [12] |
| Growth differentiation factors (GDFs) | [1], [3], [8], [17] | [13], [16], [47] | – |
| TGF-β | [1], [2], [3], [7], [17], [20] | [9], [14] | [9], [12] |
| Tumor necrosis factor-α (TNF-α) | [1], [3], [5], [7], [8], [17], [20] | [13], [14], [15], [16], [26], [46], [47], [48], [49] | – |
| Vascular endothelial growth factor (VEGF) | [1], [3], [5], [7], [8], [17], [20] | – | – |
| Wnt signaling pathway | [8] | – | – |
These molecules have been reported in animal models of bone healing, cartilage damage, or intra-articular fracture as well as knock-out animal models. In addition, some molecules have been shown to differ between healing and non-healing patient groups. For bone healing, the molecules are given that have been shown to have an important role in bone healing. For cartilage damage, the molecules are given that have been shown to contribute to cartilage loss and changing chondrocyte metabolism. Lastly, for intra-articular fractures, some molecules are given that have been shown to be involved in the healing response from the subchondral bone. Although these molecules have been reported to have an important role in these processes, they are not used in the clinic for prediction outcome. In addition, some molecules, especially for bone healing, are investigated for therapeutic applications.
Despite the numerous molecules that have been discovered to contribute to the bone fracture healing process, there is still no complete understanding of which of these molecules are essential for the process [2], [6], [8]. No biomarkers for impaired healing have been used yet in a clinical setting for outcome prediction, as the consequences of the imbalance in the expression of different molecules are largely unknown [2], [8], [22], [45]. Thus, these molecules fail to serve as predictors of non-union [8], [45].
Diagnosis and prediction of non-unions
For the diagnosis of non-unions and evaluation of fracture healing, clinicians use mainly clinical, radiographic, and laboratory examinations [2], [8], [40], [41], [45]. However, the diagnosis of non-unions is often difficult [1], [22], [45]. Non-unions are diagnosed when they have already established [18], [20], [45]. Therefore, prediction of outcome and/or detection at the beginning of the fracture healing process would allow for earlier diagnosis, but this is lacking.
The clinical assessment contains documentation of the patient’s history and the bone fracture history, as well as a physical examination [18], [21], [22], [40], [41]. The physiological assessment of a non-union can show, among others, pain and tenderness at the fracture site, persistent motion, the inability of complete weight-bearing, and reduced motion in adjacent joints [18], [21], [22], [40], [41], [43].
The radiographic appearance of the fracture is still the gold standard used to confirm the non-union, to determine its classification, and to check the integrity of implants [18], [40], [41], [45]. General radiological signs of non-unions are the absence of bone bridging, persistent fracture lines, the presence of loose or broken implants, lack of progressive healing on serial radiographs, and hypertrophic or absent callus [20], [21], [22], [43]. Furthermore, multiple additional imaging modalities can be helpful, for example, computed tomography (CT), magnetic resonance imaging (MRI), and bone scans [18], [21], [22], [40], [43], [45].
Laboratory evaluations are used to define whether an infection is present as well as to discover metabolic or endocrine abnormalities that can explain the non-union [18], [22], [41], [43]. The laboratory evaluation should include a complete blood count (CBC), erythrocyte sedimentation rate (ESR), and C-reactive protein for the detection of infections as well as tests for the nutritional status when necessary [18], [21], [22], [40], [43]. Nevertheless, these tests have no direct linkage with the bone healing process. A few studies have been performed to analyze biological markers of fracture healing as predictors of impaired healing, for example, transforming growth factor-β (TGF-β) and bone morphogenetic proteins (BMPs), but they have not been applied in general clinical practice and sufficient evidence is lacking [1], [17], [45].
Despite the diagnostic options available, non-unions can only be diagnosed when they have already been established. Currently, it is not possible to predict the outcome of a bone fracture in the early phase, e.g. during the initial surgery. Early prediction would allow for adaption of treatment, if necessary, preventing non-union development and, thereby, avoiding additional surgeries and long burden for the patient.
Post-traumatic osteoarthritis
PTOA can be defined as the development of OA after a known joint injury causing cartilage degeneration, dysfunction, and pain [10], [11], [12], [13]. In general, PTOA has an earlier onset than OA and, therefore, affects a younger and more active population [15], [16], [23]. PTOA-causing injuries can be an intra-articular fracture, acute ligament sprain, or instability of ligaments [11], [13], [23], [26]. These injuries can be classified into three groups: (1) joint injury without visible mechanical disruption, but with articular cartilage and possibly subchondral bone damage; (2) joint injury with visible mechanical damage limited to the articular cartilage; and (3) joint injury with visible mechanical damage to the articular cartilage and the subchondral bone [9], [12], [16].
In PTOA development, the subchondral bone is of great importance and influence, as its properties affect the articular cartilage and vice versa [26], [50], [51], [52], [53], [54]. Nevertheless, the relation between bone changes and cartilage degeneration is not well understood [50], [51], [52], [53]. It has been shown that cartilage degeneration is associated with increased bone resorption and trabecular bone thinning in the early phase [52], [53], [54]. On the contrary, in the later phase of OA development, increased bone formation and trabecular bone thickening have been shown [50], [51], [53], [54]. Nevertheless, the mineralization of the formed bone reduces with the progression of OA [50], [51], [54].
Treatment of cartilage damage should be focused on slowing or preventing the progressive tissue damage in the development of PTOA [9], [12], [15], [16]. Orthopedic surgeons try to achieve this by restoring the alignment, stability, and congruity of the joint, which is currently the best possible treatment [13], [23], [24], [26]. Nevertheless, joint restabilization does not reduce the risk of PTOA development [12], [24], [46], [47], [55]. The treatment should focus on facilitating cartilage repair and remodeling, which is currently clinically unavailable [9], [12]. Surgeons can penetrate the subchondral bone to initiate a healing response resulting in fibrocartilage, which fails with time, however [9]. Cartilage repair may also be induced by periosteal or perichondrial grafts, cell transplants, scaffolds, and growth factors, but these methods have not been proven in clinical studies [9], [47]. Furthermore, cartilage can be replaced by osteochondral auto- or allografts [9].
Despite the amount of research performed for PTOA and OA, the reason for PTOA development after a joint injury is still poorly understood [11], [13], [24], [55]. Some risk factors and molecules, which have been defined, will be discussed as well as the current diagnostic options.
Risk factors for PTOA development
Cartilage health depends on a combination of biological, mechanical, and structural factors [14], [15], [16]. Any disturbance in one of these factors, which cannot be compensated for by the other factors, will potentially result in the development of OA [15].
PTOA cannot be predicted, but some risk factors are known. Certain injuries often result in development of PTOA, including joint impact loading; anterior cruciate ligament (ACL); meniscal, ligament, or joint capsule tears and injuries; joint dislocations; and recurrent joint instability [10], [11], [12], [15], [26], [46]. One of the major risk factors for the development of PTOA is damage to the chondral surface during injury, which is related to the injury severity [11], [13], [23], [26]. In intra-articular fractures, the underlying subchondral bone is also affected, which increases the risk of PTOA development [11], [13], [15], [56]. The severity of the injury depends on the magnitude, orientation, and rate of the applied force and determines the absorbed energy and mechanical damage to cartilage and the surrounding structures [9], [12], [16], [24], [47]. The injury results in chondrocyte death and ECM damage, which contributes to cartilage loss [13], [16], [26], [47]. Nevertheless, Zhang et al. suggested that chondrocyte catabolism instead of death results in cartilage degeneration, although the chondrocytes eventually will die [48]. Although mechanical trauma is a major risk factor, it is insufficient to explain all the changes in the cartilage in the unaffected areas [15], [16]. Therefore, the severity of the injury is not the only factor that determines whether damaged cartilage will repair or undergo progressive degeneration [12]. After injury and/or surgery, the joint kinematics change, which results in abnormal mechanical loading and contact pressures at the articular cartilage [12], [23], [24], [56]. Thus, chronic joint instability, incongruity, and malalignment contribute to the development of PTOA via abnormal distribution of joint loadings, which is worsened by surface gaps and step-offs [10], [11], [12], [13], [15], [16], [24], [26], [56]. It has been shown that these increased contact stresses can be related to cartilage thinning [24]. In addition, the biological response to the joint injury, for example, bleeding and inflammation, can lead to the development of PTOA [13], [46], [47]. Other risk factors of PTOA development are the involved joint, certain bone fracture types and related dislocation, time since injury, genetics, obesity and other co-morbidities, age, gender, muscle weakening, and joint disuse or overuse [9], [11], [13], [15], [16], [26], [46], [47], [57]. The relative contribution of each factor is poorly understood as PTOA is considered a multifactorial condition, which makes optimal treatment planning challenging [13], [24], [46], [55].
Key molecules relevant for cartilage health
The molecular pathways involved in the development of PTOA are still mostly unknown, which is mainly due to the complexity of cartilage interaction with surrounding joint tissues and response to external factors [12], [15], [16]. Inflammatory cytokines and chemokines are two major molecular classes involved in the development of PTOA and interact with chondrocytes and other joint tissue cell types [13], [15], [46], [49]. PTOA development is affected by cytokines and chemokines in both positive and negative ways [46], [49]. Cartilage loss is caused by progressive cartilage damage when the catabolic pathway is more active than the anabolic pathway and, therefore, the chondrocytes are unable to restore the damage [12], [14], [15], [16], 56]. Table 1 gives an overview of the molecules that have been shown to contribute to cartilage loss and to be involved in intra-articular fracture repair.
A number of metabolic changes after a joint injury have been shown, but relating these changes to healthy or pathological joint adaptions is challenging and using them as predictors of clinical outcome is extremely difficult [11], [14], [15], [46], [47], [49]. Therefore, at the moment, no predictive biomarkers for PTOA are available [15], [16], [49].
Diagnosis and prediction of PTOA
Early detection of OA-related changes to the cartilage would allow for early intervention, which might prevent the development of end-stage OA [15]. The detection of PTOA (or OA) is very difficult and limited by the lack of symptoms in the early phase [12], [14], [15]. The variability in the time between injury and the development of PTOA symptoms depending on the severity of the injury and differences between patients is adding to this challenge [15], [16], [26]. In addition, joint injuries that can cause PTOA are difficult to diagnose and often overlooked in cases of other joint tissue injuries [9]. Therefore, PTOA is often diagnosed after the onset of symptoms based on clinical and radiographic findings, when irreversible damage to the articular cartilage has already occurred [15], [55]. This clearly illustrates the need for early detection and prediction before irreversible changes occur.
Radiography and CT scans are often used for the assessment of articular fracture reduction and diagnosis of OA [13], [58]. With radiography, OA is diagnosed based on the bone (sclerosis and osteophytes) and cartilage changes as well as reduction of the joint space [15], [49], [51], [52]. CT scans have a higher sensitivity than radiography for articular cartilage assessment, with improved detection of surface gaps, step-offs, and other surface disruptions allowing for estimation of the absorbed energy during injury [13], [16], [24], [58]. Double-contrast CT can be used for the measurement of cartilage thickness to determine areas of cartilage thinning [24]. MRI can be used for the measurement of, among others, cartilage thickness and the detection of cartilage and bone marrow lesions [11], [24], [26]. Nevertheless, there is currently no method to measure the extent of cell and matrix damage [9], [12]. MRI has been proposed for studying the amount of damage for injuries without visible mechanical disruption [9].
Biomarkers that can be measured in, for example, blood, urine, or synovial fluid could contribute to early detection of OA, but they are currently not used for diagnosis because of lack of specificity [15], [49], [55]. Different molecules have been investigated, for example, ECM molecules and inflammation markers, but they have not been applied in general clinical practice, as there is only limited consensus about their predictive value [15], [16], [49], [55].
PTOA can only be diagnosed when irreversible changes to the articular cartilage have occurred, despite the current diagnostic options. Currently, it is not possible to predict the outcome of injury-related cartilage damage in the early phase, e.g. during the initial surgery. Early prediction would allow for adaption of treatment, if necessary, delaying or even preventing PTOA development and, thereby, avoiding additional surgeries and long burden for the patient.
Mass spectrometry imaging
A method is required that provides detailed molecular and spatial information to understand the molecular and structural changes in bone and cartilage during impaired healing. The spatial information also provides insight into the interaction of these affected areas with surrounding tissues. MSI is a widely used technique for the analysis of a broad variety of molecules in tissue slices, including metabolites, drugs, lipids, peptides, and proteins while maintaining their spatial integrity [27], [29], [30], [31], [32]. The advantages over traditional histopathological methods, such as immunohistochemistry (IHC), are rapid simultaneous detection and identification of a wide variety of molecules in complex biological tissues without the use of targeted labels [27], [29], [31]. In clinical research, MSI has been employed for biomarker discovery, tissue classification, and prediction of outcome [29], [31], [33].
The general workflow for MSI consists of sample preparation, MSI data acquisition, and data processing and visualization (see Figure 2) [27], [31]. Sample preparation depends partly on the ionization technique used, but for all of these techniques, tissue sections need to be cut and mounted on a flat support, such as glass slides for analysis [31]. The glass slide is placed in a mass spectrometer and the molecules from the tissue are desorbed and ionized with an appropriate ionization technique during MSI data acquisition [27], [29], [31]. The ionized molecules are subsequently analyzed based on their mass-to-charge ratio (m/z), which results in a complete mass spectrum per pixel [29]. Distribution images can be created for specific molecules based on the intensities of corresponding m/z values per pixel, often after data reduction and/or the removal of artifacts [29], [30], [31].
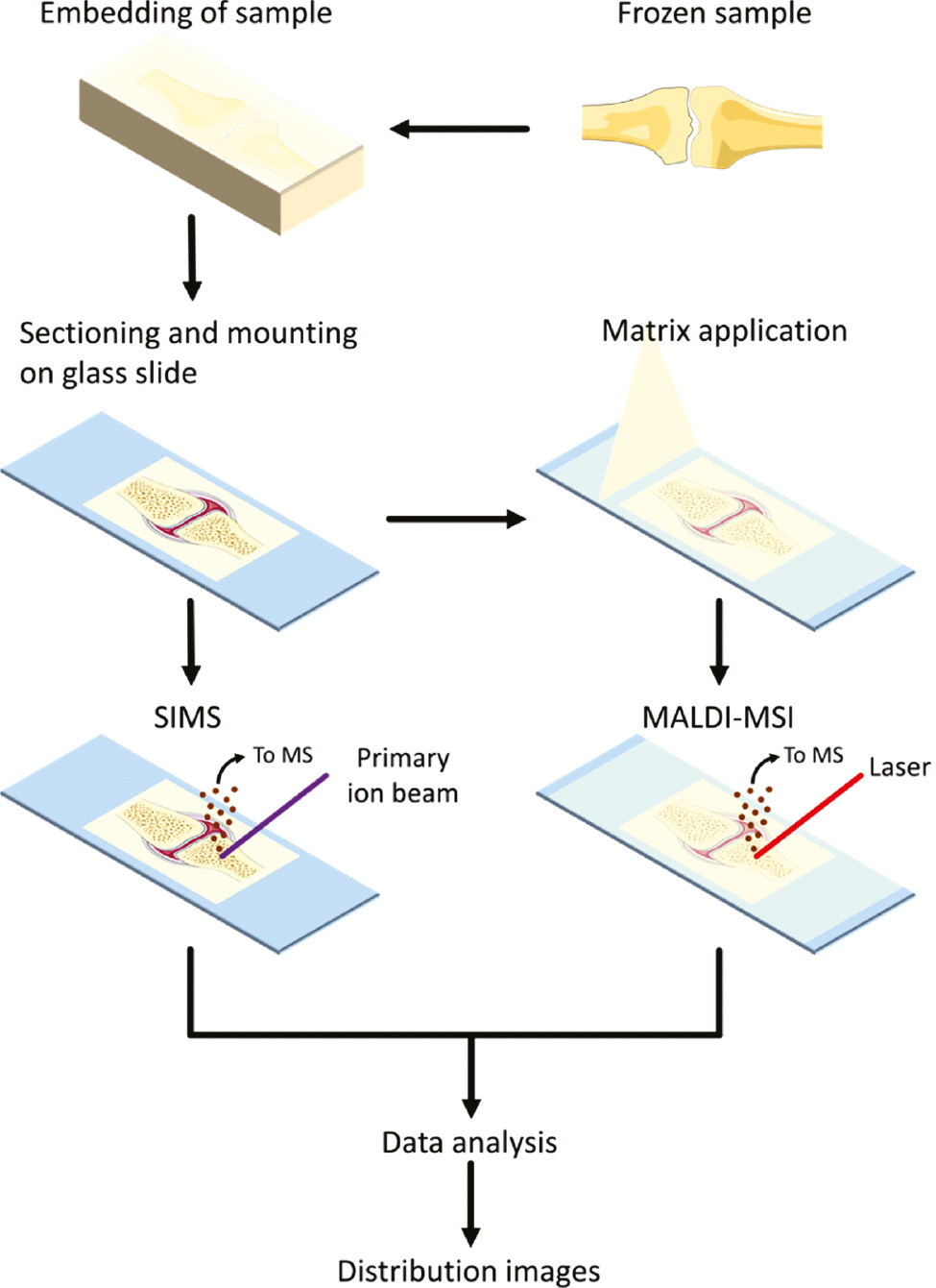
General mass spectrometry imaging workflow.
The sample is collected and stored frozen until use. For hard tissue samples, the tissue is embedded in an embedding material, which supports sectioning. After embedding, the sample is sectioned and mounted on a glass slide. For secondary ion mass spectrometry (SIMS), a primary ion beam shoots at the sample and secondary ions are created from the sample, which are analyzed by the mass spectrometer (MS). For matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI-MSI), first, a matrix is applied on the sample to support ion generation. Afterward, a laser shoots at the sample and generates ions, which are analyzed by the MS. Per pixel in the image, a mass spectrum is created of the generated ions. After data processing and analysis, distribution images of mass-to-charge values (m/z) can be created.
Numerous surface sampling and ionization techniques exist, which have been reviewed elsewhere [27], [29]. The techniques that have been specifically employed for MSI of hard tissue are matrix-assisted laser desorption/ionization (MALDI) and secondary ion mass spectrometry (SIMS) (see Table 2) [59]. MALDI requires a matrix to be deposited on the tissue section during sample preparation to support the desorption and ionization of the molecules from the tissue after exposure to laser radiation [27], [29], [30], [31]. SIMS utilizes the generation of secondary ions created and ejected from the tissue by sputtering its surface with a focused primary ion beam [27], [29]. SIMS can be used for the analysis of the elemental and mineral distribution in tissue sections, as well as small (<m/z 1000) inorganic and organic molecules, like lipids and peptides [29], [60], [61], [62], [63], [64], [65]. The sensitivity of this technique for the mass range above m/z 1000 is reduced as most larger organic ions will fragment as a result of the energetic impact of the primary ion beam [27], [63]. These fragments of larger molecules can, in selected cases, be informative on the surface composition nevertheless [27], [63]. The analytical possibilities of MALDI are broader and range from the analysis of small molecules such as metabolites to larger (intact) proteins, including also lipids, peptides, and glycans [27], [29], [31]. The spatial resolution of SIMS is higher (down to 50 nm) than the current achievable spatial resolution with MALDI (commonly down to 10 μm) [27], [29], [59], [64], [65].
Comparison of different mass spectrometry techniques useable on hard tissue, namely secondary ion mass spectrometry (SIMS), matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI-MSI), and rapid evaporative ionization mass spectrometry (REIMS).
| Mass spectrometry technique | Useable for analysis of hard tissues (bone, cartilage) | Spatial resolution | Molecular classes | Strengths | Limitations | |||
|---|---|---|---|---|---|---|---|---|
| Ex vivo | SIMS | Yes | Down to 50 nm | Elementals, minerals, metabolites, lipids | – | High spatial resolution | – | Limited molecular range |
| – | Detection of minerals | – | Long measurement times | |||||
| MALDI-MSI | Yes, only recently applied | Down to 10 μm | Metabolites, lipids, peptides, proteins, glycans | – | Broad molecular range | – | Not widely applied on hard tissues | |
| – | Simultaneous detection of a wide variety of molecules | – | Possible interference of salts and minerals | |||||
| In vivo | REIMS | Only for a laser coupled to REIMS | Depending on sampling tool, typically in mm range | Mainly lipids and lipid fragments | – | Near real-time feedback to the surgeon | – | Invasive technique |
| – | No sample preparation | – | Limited molecular range | |||||
The application of MALDI-MSI to the analysis of hard tissues (e.g. bone and cartilage) is very limited so far, due to the complex sample preparation and the lack of appropriate protocols [33], [66]. As bone consists of both hard and soft tissue parts (i.e. the calcified matrix and bone marrow, respectively), bone sectioning is very challenging [59]. Therefore, bone is often decalcified and/or dehydrated during the sample preparation to simplify the sectioning and to remove the interference of hydroxyapatite crystals in the determination of the molecular distributions in MALDI-MSI [59], [66]. During sample preparation for SIMS and sometimes for MALDI, the necessary fixation and dehydration causes substantial chemical changes to the tissue, such as the removal of phospholipids [59], [60]. However, decalcification and removal of interfering molecules are highly undesirable when the molecular distribution needs to be related to the in vivo situation, for example, for intra-operative applications. The importance of embedding a hard tissue sample to keep the structural composition has been shown, especially when soft tissue surrounds the bone or cartilage, or for diseased bone [27], [31], [59], [63], [67]. While sectioning, applying adhesive tape to the sample can contribute to maintaining the structural composition and spatial integrity of the hard tissue [27], [59], [67]. Despite the challenges in sample preparation of native hard tissue, analysis of these tissue types is essential for the understanding of impaired healing.
MSI of bone
Most of the research performed with MSI on bone has been performed with time-of-flight (ToF) SIMS, but more recent literature reports the application of MALDI-MSI. The limited number of studies applying MS proteomics methods on bone did not use MSI, as they were either applied to cell cultures and ECM, or blood products [68]. One of these studies was focused on bone fracture healing, in which Grgurevic et al. showed that the detected proteins were involved in cell growth and proliferation, transport, and coagulation using liquid chromatography-MS [69].
ToF-SIMS imaging of bone
One of the application fields of ToF-SIMS is to study bone mineralization in relation to, among others, fracture healing, bone-implant interaction, and disturbances of minerals (see Figure 3A) [71]. This is mainly due to the high sensitivity of SIMS for elements and elemental and molecular distributions of, for example, Ca, P, PO2, and PO3. In addition, different fragments of hydroxyapatite and collagen have been reported [61], [62], [65], [71], [72].
![Figure 3: Examples of application of mass spectrometry for the direct analysis of hard tissues, namely ToF-SIMS on bone, MALDI-MSI on cartilage, and CO2 laser coupled to REIMS on bone and cartilage.
(A) ToF-SIMS image of healthy sheep vertebra showing Ca+ (inorganic compound), C4H8N+ (organic compound), and overlay of the selected m/z values. ToF-SIMS measurement was performed with a Bi3+-primary ion beam and a lateral resolution of 10 μm. Reprinted with permission from Müller et al. [65]. (B) MALDI-MSI image of osteoarthritic cartilage showing an overlay image of SM (d18:1_16:0) in the damaged superficial part of the tissue, PC (18:0_18:1) specific for chondrocytes, and DMPE (34:1) in the matrix of the tissue. The arrows point at chondrocyte pellets. MALDI-MSI image was taken at a spatial resolution of 15 μm. Reprinted with permission from Barré et al. [70]. (C) Mass spectra for the mass range m/z 500–1200 obtained from a human femoral head with a CO2 laser (continuous wave with a laser power of 20 W with a pulse duration of 0.20 s) coupled to REIMS from cartilage (top) and cortical bone (bottom).](/document/doi/10.1515/cclm-2019-0857/asset/graphic/j_cclm-2019-0857_fig_003.jpg)
Examples of application of mass spectrometry for the direct analysis of hard tissues, namely ToF-SIMS on bone, MALDI-MSI on cartilage, and CO2 laser coupled to REIMS on bone and cartilage.
(A) ToF-SIMS image of healthy sheep vertebra showing Ca+ (inorganic compound), C4H8N+ (organic compound), and overlay of the selected m/z values. ToF-SIMS measurement was performed with a Bi3+-primary ion beam and a lateral resolution of 10 μm. Reprinted with permission from Müller et al. [65]. (B) MALDI-MSI image of osteoarthritic cartilage showing an overlay image of SM (d18:1_16:0) in the damaged superficial part of the tissue, PC (18:0_18:1) specific for chondrocytes, and DMPE (34:1) in the matrix of the tissue. The arrows point at chondrocyte pellets. MALDI-MSI image was taken at a spatial resolution of 15 μm. Reprinted with permission from Barré et al. [70]. (C) Mass spectra for the mass range m/z 500–1200 obtained from a human femoral head with a CO2 laser (continuous wave with a laser power of 20 W with a pulse duration of 0.20 s) coupled to REIMS from cartilage (top) and cortical bone (bottom).
Different studies have used ToF-SIMS on bone tissue to study its elemental and molecular composition. In osteoporotic animal models, ToF-SIMS was used to show decreasing trabecular number and thickness, and declining cortical bone based on calcium and collagen distribution [61], [65]. For quantification of the calcium content, calcium hydroxyapatite scaffolds have been used in a rat model with encouraging results, although quantification was affected by the surface properties of the standards [62]. Furthermore, the bone-titanium implant interaction has been studied with ToF-SIMS, showing higher mineralization around the implant after a longer time period and specific molecules for bone tissue, the implant, and the interface [60], [72]. Multiple studies have researched the effect of a strontium-enriched calcium phosphate cement on fracture healing on healthy and osteoporotic bone [64], [73], [74], [75]. It was shown that this implant improved bone formation and strontium could be found in the newly formed bone as well as pre-existing tissue as strontium diffused up to 6 mm from the implant [64], [73], [74], [75].
MALDI-MSI of bone
A few studies have shown the opportunity of using MALDI-MSI on bone tissue. Chughtai et al. studied lipids, peptides, proteins in bone, muscle, and skin from a limb of a mouse model with rheumatoid arthritis (RA) using both ToF-SIMS and MALDI-MSI [33]. With this multimodal approach, they showed the localization of proteins involved in inflammation in different regions related to the effect of RA on the tissues [33]. Fujino et al. compared different fixation and decalcification methods for the preparation of bone samples for MALDI and concluded that sections from trichloroacetic acid-treated samples were the most suitable [66]. Nevertheless, no significant differences between the obtained mass spectra were observed between bones that were decalcified, fixed, or both [66]. Svirkova et al. showed the combination of MALDI-MSI and micro X-ray fluorescence (μXRF) to study the lipid (focus on sphingomyelins and phosphatidylcholines) as well as the elemental composition of bone and surrounding soft tissue without tissue decalcification [67]. They were able to combine the elemental distribution obtained from the undecalcified tissue (μXRF) with the lipid distribution of the surrounding soft tissue (MALDI-MSI) [67]. Atmospheric-pressure scanning microprobe MALDI (AP-SMALDI) and ToF-SIMS have been applied on undecalcified human bone sections by Schaepe et al. [59]. They showed the lateral localization of different major lipid classes, including glycerolipids, glycerolphospholipids, and sphingolipids [59]. Signals specific for the hard part of the bone as well as the bone marrow were found with ToF-SIMS [59]. With AP-SMALDI, a hydroxyapatite fragment could be found in the mineralized tissues as well as different triacylglycerols in the bone marrow [59].
The recent developments in the usage of MALDI-MSI on bone tissue show the opportunities of this technique for the application in the impaired healing research on bone, allowing for the discovery of a wide range of molecules that could be linked to impaired molecular pathways.
MSI of cartilage
Several MSI studies have been performed on cartilage, but most studies use electrospray ionization or liquid chromatogram MS/MS. These methods have been used to study mainly proteins, peptides, and lipids in cartilage, other joint tissues, serum, and synovial fluid. The MSI studies mainly employed ToF-SIMS and MALDI for the analysis of cartilage and other joint tissues [31].
ToF-SIMS imaging of cartilage
With ToF-SIMS, osteoarthritic and healthy human cartilage were studied and showed that these two types of cartilage could be distinguished by specific peaks [76]. Furthermore, colocalization of cholesterol and other lipids in lipid droplets was seen in the superficial area of the cartilage, with a similar localization of fatty acids in osteoarthritic cartilage [76]. Calcium and phosphate ions were observed in the areas surrounding chondrocytes in osteoarthritic cartilage [76].
MALDI-MSI of cartilage
In a study on peptides and proteins in human healthy and osteoarthritic cartilage using MALDI-MSI, osteoarthritic-specific peptides and proteins were found with a higher intensity in the deep cartilage [30]. Distinctive peptides for OA included fibronectin, cartilage oligomeric matrix protein, and fibromodulin [30]. The peptides of young, old, and osteoarthritic equine cartilage showed different profiles between the groups using MALDI-MSI [77]. Increased intensities of fibronectin peptides and decreased intensities of fibromodulin peptides were seen in osteoarthritic cartilage, while collectin-43 and cartilage oligomeric matrix protein were age-specific [77]. Recently, in a MALDI-MSI study about the lipid distribution in human osteoarthritic cartilage, the superficial layer and the cartilage layer could be distinguished by specific molecules, but more interestingly a phosphatidylcholine was found to be specific for chondrocyte cluster cells (see Figure 3B) [70]. Furthermore, N-glycans have been studied in cartilage and bone marrow with MALDI-MSI, as the subchondral bone is affected by OA by bone marrow lesions [78]. Different N-glycans showed different distributions over the cartilage and bone marrow, with a high mannose N-glycan being prominent in osteoarthritic cartilage and a disialylated biantennary complex glycan was prominent in bone marrow of the patient with bone marrow lesion stage 1 [78]. The distribution of triamcinolone acetonide (TAA) in cartilage was shown for sample submerged in TAA with MALDI-MSI using an essential derivatization step [79].
MALDI-MSI has already been applied to cartilage to study a wide range of molecules, including lipids and peptides, although most research is applied on late osteoarthritic tissue. Applying this technique to impaired healing research on cartilage could contribute to a better understanding of, among others, the early phase of PTOA.
Ambient ionization mass spectrometry and the potential toward in vivo tissue evaluation
The development of ambient ionization techniques opened up the possibility to employ MS for in vivo tissue analysis, evaluating metabolic and lipid composition and changes [34], [35], [37], [80]. An advantage of the in vivo application of MS is the near real-time evaluation of the tissue with minimal sample preparation [34], [35], [37], [80], [81]. The instant availability of complex molecular information allows for improved decision-making by surgeons [35], [81]. The ideal in vivo technology should have a high clinical sensitivity and specificity and low invasiveness. Therefore, these in vivo techniques have the potential to be employed for the early prediction of non-unions and PTOA during the initial surgery. Existing or new surgical handpieces are connected to a mass spectrometer for the evaluation of the molecular profile of the tissue for future in vivo intra-operative MS-based diagnostic techniques. The development of these in vivo techniques focuses on the ablation and ionization of minimal amounts of molecules from the tissue that are analyzed by a mass spectrometer [35]. This ionization method allows for the analysis of living tissue in its native environment while creating tissue-specific mass spectra, which is considered its major advantage [82]. These techniques currently result in mass spectra that predominantly contain lipids and their fragments [37], [80], [81]. Different techniques have been coupled to MS systems, including the electrocautery, different laser-based techniques, and others. Supplementary Table 1 gives an overview of the studies performed with these techniques, which are reviewed below.
Electrocautery
Electrocautery combined with rapid evaporative ionization MS (REIMS) for rapid, in situ profiling has been mainly used for cancer research and tumor margin assessment (see Supplementary Table 1) [32], [35], [80], [83]. The coupling of electrocautery with REIMS is often referred to as the intelligent Knife (iKnife) [32], [35], [80], [83]. With this approach, electrocautery is used for cauterizing and cutting of the tissue resulting in aerosol-rich smoke containing aerosolized molecules, which is transported to the mass spectrometer via a tube using a Venturi pump [28], [32], [83], [84], [85], [86]. The basis for this technique is rapid thermal evaporation of biological tissue by, for example, electrocautery resulting in the formation of gaseous ions [29], [32], [80], [82], [83], [84], [85]. Several research groups have shown that different tissues show different and characteristic mass spectra of specific lipid patterns allowing for differentiation between healthy and cancer tissue, different tumor grades, and tumor necrosis [32], [82], [83], [84], [85], [86]. In general, these studies show high accuracy (range 74.7–97%), sensitivity (range 78.6–97.7%), and specificity (range 89.7–100%) (see Supplementary Table 1) [32], [83], [84], [85], [86], [87]. Although most of the research is currently performed ex vivo, the usage of the electrocautery-REIMS has also been tested in vivo [32], [84], [85], [86].
In a similar manner, Balog et al. used electrosurgical dissection via an endoscopic polypectomy snare connected to REIMS for the classification of gastrointestinal tract tissue [87]. This technique was able to differentiate between healthy intestinal wall tissue, colorectal cancer, and adenomatous polyps [87].
Laser-based methods
Laser ablation of tissues has shown to result in characteristic ion patterns and combined with MS could be used for the near real-time in vivo identification of tissues [81], [88]. For this, both ultraviolet and infrared lasers have been used, whereby the CO2 laser is the most common employed infrared laser in surgical procedures [35], [80], [81], [88].
Schäfer et al. used laser desorption ionization mass spectrometry (LDI-MS) for the ex vivo analyses of biological tissues comparing different ultraviolet and infrared lasers [88]. The reproducibility of the spectra obtained with the CO2 laser was higher than of those obtained with the ultraviolet (Nd:YAG) laser, resulting in high accuracies for tissue identification with the CO2 laser (see Supplementary Table 1) [88]. The mass spectra generated with the CO2 laser differed considerably between the two modes of the laser (continuous wave and pulsed mode) and was associated with the different laser powers [88]. This indicates that laser-tissue interaction differs per mode and, therefore, should be optimized per tissue type and clinical application. The obtained spectra showed high similarity with spectra obtained with electrocautery, suggesting similar ion formation mechanisms, which was confirmed by Balog et al. [84], [88]. Genangeli et al. showed that the quality and reproducibility of the mass spectra obtained with the CO2 laser coupled to REIMS were higher compared to electrocautery [89]. In addition, the CO2 laser enabled to generate a lipid-rich signal from hard tissues, i.e. bone, bone marrow, and cartilage [89]. An example of the mass spectra obtained from hard tissues is provided in Figure 3C. The use of the CO2 laser has been tested in vivo [88].
Fatou et al. developed an in vivo near real-time analysis technique, named the SpiderMass, which uses resonant infrared laser ablation (RIR-LA) for the production of mainly lipid ions [28], [29], [90]. Different signals could be obtained for normal, cancer, and necrotic tissue and high correct classification rates have been shown (see Supplementary Table 1) [28], [90]. The minimal invasiveness of this technique was shown in vivo on human skin and canine tissue, although the technique can result in local dehydration of the tissue [28], [90].
Woolman et al. developed a handheld sampling probe using picosecond infrared laser (PIRL) ablation MS for the sampling of tissue with minimal thermal damage and tissue removal [91]. In different studies, m/z values in the signal were identified, which belonged to fatty acids, ceramides, and (fragments of) phospholipids, including phosphatidic acids, phosphatidylethanolamines, and phosphatidylcholines [91], [92], [93]. Ex vivo studies using PIRL showed good separation of tissue types and medulloblastoma subgroups with high classification rates (see Supplementary Table 1) [91], [92], [93]. PIRL combined to MS has not been used in vivo yet.
Other techniques
Schäfer et al. combined a cavitron ultrasonic surgical aspirator (CUSA), which uses ultrasound for the disintegration of tissue, to sonic-spray ionization MS [81], [94]. A clear differentiation could be made between different tissues and tumor types (see Supplementary Table 1) [94]. With this technique, besides lipid-related ions, the spectra also contained ions of metabolic constituents, carbohydrates, and peptides [94]. The combination of CUSA with MS has not been used in vivo yet, but is expected to give similar results [94].
The MasSpec Pen extracts molecules (metabolites, lipids, and proteins) from the tissue via a water droplet, which is analyzed in a mass spectrometer [95], [96]. This device allows for the rapid and nondestructive analysis of tissue, independently of the tissue shape and rigidity [95]. The MasSpec Pen has been shown to be able to differentiate between different tissue types and cancer and normal tissue with high accuracy, sensitivity, and specificity (see Supplementary Table 1) [95], [96]. No visible tissue damage was observed in the in vivo testing of the MasSpec Pen [95].
Implementation and limitations
Although each potential in vivo MS technique has its own application field, only a CO2 laser coupled to REIMS has recently been used on bone and cartilage (see Table 2) [89]. The implementation and limitations of the different techniques will be discussed to explore which technique could be used for the prediction of non-unions and PTOA.
The main advantages of these ambient techniques are the possibility to analyze intact samples in vivo in their native environment with the near real-time feedback [37], [80], [84], [91], [97]. Furthermore, multiple techniques, including electrocautery and the CO2 laser, use surgically approved handpieces for the ablation and ionization of tissue, which allows for easier implementation in the operating room [80], [81], [84], [89]. In contrast, other techniques, like the SpiderMass and the MasSpec Pen, are not medically approved, which will extend the implementation process in the operating room in the near future.
The amount of unwanted tissue damage generated by these in vivo techniques varies depending on the approach used. The main disadvantage of the electrocautery is the thermal and mechanical damage to healthy surrounding tissue, which is unavoidable during surgery but additional damage is an unwanted side effect [28], [35], [37], [90], [95]. Consequently, the spatial resolution of this method is low and defined by the size of the blade used [37], [98]. Furthermore, this tissue damage complicates the use on healthy tissues and the histopathological validation of tissues sampled with electrocautery [32], [37], [83], [92]. Other methods prevent extensive tissue damage, with limited damage for different ablation-based methods (infrared and ultraviolet lasers, CUSA, and PIRL) and almost no damage for other techniques (SpiderMass and MasSpec Pen) [28], [37], [89], [90], [91], [92], [93], [94], [95], [96], [97]. For CO2 laser and PIRL-based techniques, another advantage compared to electrocautery is the minimal thermal spread as well as the fact that these probes interact mostly with the top tissue surface [80], [81], [91], [92], [93]. Compared to the electrocautery, CO2 laser-based techniques allow for the more precise sampling of the tissue with less tissue damage [88], [89]. The higher reproducibility of the CO2 laser compared to other lasers allows for more frequent sampling during surgical interventions to improve the efficiency of analysis and decrease the invasiveness of intentional sampling [88].
In addition, different techniques have their own disadvantages. As already mentioned, electrocautery results in extensive tissue damage [28], [35], [37], [90], [95]. The possible contamination with tissue debris limits the usage of the SpiderMass, which is the result of the close contact of the device and the tissue [90]. In the PIRL-based technique, a separate plume collection tube is used for the transfer of the molecules to the mass spectrometer [91], [92], [93]. The need for this additional tube might limit the translation to the operating room because of practical reasons. For the MasSpec Pen, the disposable tip can be cleaned by automated flushing of the system or needs to be replaced [95]. Due to the contact of the tip with the tissue, contamination can occur when the tip is only flushed while replacing the tip every time is clinically unpractical [37].
Until now, implementation of in vivo methods in the clinic has been limited, which can partly be explained by the lack of multicenter and validation studies performed in the operating room [81]. In addition, the implementation is constrained by the need for the development of large data sets (which can take years) to improve the accuracy of the built tissue classification models as well as the need for validated and quick data processing workflows [36], [80], [81]. These databases are generated based on ex vivo measurements of patient material and associated clinical information. The potential for intra-operative MS techniques in the improvement of patient care is foreseen as revolutionary, especially for those techniques using existing surgical handpieces. For the prediction of non-unions and PTOA, we propose the usage of a CO2 laser coupled to REIMS, as it can be used on hard tissues, employs an existing surgical handpiece, and has low invasiveness. This in vivo MS technique can be used intra-operatively in patients for the prediction of outcome of fracture healing by sampling the bone and/or cartilage without tissue removal.
Conclusions and outlook
Non-unions and PTOA can only be diagnosed in a later phase and, therefore, early prediction of outcome of the healing process is required. Nevertheless, currently, outcome prediction is not possible, despite the known risk factors and limited knowledge of involved molecules. More detailed molecular information about the bone fracture healing and cartilage repair processes is necessary, which can be provided by MS, as this technique can be used for the analysis of bone, cartilage, and surrounding tissue. MSI has the potential to analyze the involved molecules in the repair processes in more detail, while in vivo MS can be used for the outcome prediction during the initial surgery.
MSI analysis of bone, cartilage, and surrounding tissue can contribute to the improvement of our knowledge and understanding of the key molecular processes involved in bone fracture healing and cartilage repair. As a broad range of molecules can be studied, in the upcoming years, this can result in defining key molecules and pathways, e.g. lipids and proteins, as well as their distribution. This information can be used for the development of new treatment options for earlier intervention.
Only recently a potential in vivo MS technique using CO2 laser coupled to REIMS has been shown to allow for the analysis of bone and cartilage. Therefore, this technique has to be tested further for optimization of analysis of these tissues and to assess the predictive value for outcome of bone fractures and injury-related cartilage damage. Nevertheless, we expect that the CO2 laser coupled to REIMS can already be used as an in vivo MS technique in the operating room in the upcoming years for the first analyses and, later on, for prediction of outcome.
Improved patient management will be possible by earlier intervention and more treatment options based on both the improvement of the understanding of key molecular pathways in the repair processes as well as the outcome prediction of bone fracture and injury-related cartilage damage.
Acknowledgments
S.P.N., T.P.S., and R.M.A.H. acknowledge the M4I institute and financial support of the Dutch Province of Limburg through the LINK program.
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: The LINK program of the Dutch province of Limburg and MUMC+ (funder name: Maastricht Universitair Medisch Centrum, funder id: http://dx.doi.org/10.13039/501100004528, Grant number: 15.0869).
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
1. Harwood PJ, Newman JB, Michael AL. An update on fracture healing and non-union. Orthop Trauma 2010;24:9–23.10.1016/j.mporth.2009.12.004Suche in Google Scholar
2. den Boer FC, Patka P, Bakker FC, Haarman HJ. Current concepts of fracture healing, delayed unions, and nonunions. Osteo Trauma Care 2002;10:1–7.10.1055/s-2002-30627Suche in Google Scholar
3. Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury 2005;36:1392–404.10.1016/j.injury.2005.07.019Suche in Google Scholar
4. Gaston MS, Simpson AH. Inhibition of fracture healing. J Bone Joint Surg Br 2007;89:1553–60.10.1302/0301-620X.89B12.19671Suche in Google Scholar
5. Giannoudis PV, Einhorn TA, Marsh D. Fracture healing: the diamond concept. Injury 2007;38(Suppl 4):S3–6.10.1016/S0020-1383(08)70003-2Suche in Google Scholar
6. Tsiridis E, Upadhyay N, Giannoudis P. Molecular aspects of fracture healing: which are the important molecules? Injury 2007;38(Suppl 1):S11–25.10.1016/j.injury.2007.02.006Suche in Google Scholar PubMed
7. Oryan A, Monazzah S, Bigham-Sadegh A. Bone injury and fracture healing biology. Biomed Environ Sci 2015;28:57–71.Suche in Google Scholar
8. Pountos I, Georgouli T, Pneumaticos S, Giannoudis PV. Fracture non-union: can biomarkers predict outcome? Injury 2013;44:1725–32.10.1016/j.injury.2013.09.009Suche in Google Scholar PubMed
9. Buckwalter JA. Articular cartilage injuries. Clin Orthop Relat Res 2002;402:21–37.10.1097/00003086-200209000-00004Suche in Google Scholar PubMed
10. Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma 2006;20:739–44.10.1097/01.bot.0000246468.80635.efSuche in Google Scholar PubMed
11. Thomas AC, Hubbard-Turner T, Wikstrom EA, Palmieri-Smith RM. Epidemiology of posttraumatic osteoarthritis. J Athl Train 2017;52:491–6.10.4085/1062-6050-51.5.08Suche in Google Scholar PubMed PubMed Central
12. Buckwalter JA, Brown TD. Joint injury, repair, and remodeling: roles in post-traumatic osteoarthritis. Clin Orthop Relat Res 2004;423:7–16.10.1097/01.blo.0000131638.81519.deSuche in Google Scholar
13. Schenker ML, Mauck RL, Ahn J, Mehta S. Pathogenesis and prevention of posttraumatic osteoarthritis after intra-articular fracture. J Am Acad Orthop Surg 2014;22:20–8.10.5435/JAAOS-22-01-20Suche in Google Scholar
14. Lorenz H, Richter W. Osteoarthritis: cellular and molecular changes in degenerating cartilage. Prog Histochem Cytochem 2006;40:135–63.10.1016/j.proghi.2006.02.003Suche in Google Scholar
15. Carbone A, Rodeo S. Review of current understanding of post-traumatic osteoarthritis resulting from sports injuries. J Orthop Res 2017;35:397–405.10.1002/jor.23341Suche in Google Scholar
16. Anderson DD, Chubinskaya S, Guilak F, Martin JA, Oegema TR, Olson SA, et al. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res 2011;29:802–9.10.1002/jor.21359Suche in Google Scholar
17. Tosounidis T, Kontakis G, Nikolaou V, Papathanassopoulos A, Giannoudis PV. Fracture healing and bone repair: an update. Trauma 2009;11:145–56.10.1177/1460408609335922Suche in Google Scholar
18. Brinker MR, O’Connor DP. Nonunions: evaluation and treatment. In: Browner BD, Jupiter JB, Krettek C, Anderson PA, editors. Skeletal trauma: basic science, management, and reconstruction. Philadelphia: Elsevier, 2015:637–718.e8.Suche in Google Scholar
19. Tzioupis C, Giannoudis PV. Prevalence of long-bone non-unions. Injury 2007;38(Suppl 2):S3–9.10.1016/S0020-1383(07)80003-9Suche in Google Scholar
20. Hak DJ, Fitzpatrick D, Bishop JA, Marsh JL, Tilp S, Schnettler R, et al. Delayed union and nonunions: epidemiology, clinical issues, and financial aspects. Injury 2014; 45(Suppl 2):S3–7.10.1016/j.injury.2014.04.002Suche in Google Scholar PubMed
21. Miranda MA, Moon MS. Treatment strategy for nonunions and malunions. In: Stannard JP, Schmidt AH, Kregor PJ, editors. Surgical treatment of orthopaedic trauma. New York: Thieme, 2007:77–100.Suche in Google Scholar
22. Bishop JA, Palanca AA, Bellino MJ, Lowenberg DW. Assessment of compromised fracture healing. J Am Acad Orthop Surg 2012;20:273–82.10.5435/JAAOS-20-05-273Suche in Google Scholar PubMed
23. McKinley TO, Borrelli Jr J, D’Lima DD, Furman BD, Giannoudis PV. Basic science of intra-articular fractures and posttraumatic osteoarthritis. J Orthop Trauma 2010;24:567–70.10.1097/BOT.0b013e3181ed298dSuche in Google Scholar PubMed PubMed Central
24. Anderson DD, Marsh JL, Brown TD. The pathomechanical etiology of post-traumatic osteoarthritis following intraarticular fractures. Iowa Orthop J 2011;31:1–20.Suche in Google Scholar
25. Copuroglu C, Calori GM, Giannoudis PV. Fracture non-union: who is at risk? Injury 2013;44:1379–82.10.1016/j.injury.2013.08.003Suche in Google Scholar PubMed
26. Riordan EA, Little C, Hunter D. Pathogenesis of post-traumatic OA with a view to intervention. Best Pract Res Clin Rheumatol 2014;28:17–30.10.1016/j.berh.2014.02.001Suche in Google Scholar PubMed
27. Chughtai K, Heeren RM. Mass spectrometric imaging for biomedical tissue analysis. Chem Rev 2010;110:3237–77.10.1021/cr100012cSuche in Google Scholar PubMed PubMed Central
28. Fatou B, Saudemont P, Leblanc E, Vinatier D, Mesdag V, Wisztorski M, et al. In vivo real-time mass spectrometry for guided surgery application. Sci Rep 2016;6:25919.10.1038/srep25919Suche in Google Scholar PubMed PubMed Central
29. Vaysse PM, Heeren RM, Porta T, Balluff B. Mass spectrometry imaging for clinical research – latest developments, applications, and current limitations. Analyst 2017;142:2690–712.10.1039/C7AN00565BSuche in Google Scholar
30. Cillero-Pastor B, Eijkel GB, Kiss A, Blanco FJ, Heeren RM. Matrix-assisted laser desorption ionization-imaging mass spectrometry: a new methodology to study human osteoarthritic cartilage. Arthritis Rheum 2013;65:710–20.10.1002/art.37799Suche in Google Scholar PubMed
31. Rocha B, Cillero-Pastor B, Blanco FJ, Ruiz-Romero C. MALDI mass spectrometry imaging in rheumatic diseases. Biochim Biophys Acta Proteins Proteom 2017;1865:784–94.10.1016/j.bbapap.2016.10.004Suche in Google Scholar PubMed
32. St John ER, Balog J, McKenzie JS, Rossi M, Covington A, Muirhead L, et al. Rapid evaporative ionisation mass spectrometry of electrosurgical vapours for the identification of breast pathology: towards an intelligent knife for breast cancer surgery. Breast Cancer Res 2017;19:59.10.1186/s13058-017-0845-2Suche in Google Scholar PubMed PubMed Central
33. Chughtai S, Chughtai K, Cillero-Pastor B, Kiss A, Agrawal P, MacAleese L, et al. A multimodal mass spectrometry imaging approach for the study of musculoskeletal tissues. Int J Mass Spectrom 2012;325:150–60.10.1016/j.ijms.2012.07.008Suche in Google Scholar
34. Hsu CC, Dorrestein PC. Visualizing life with ambient mass spectrometry. Curr Opin Biotechnol 2015;31:24–34.10.1016/j.copbio.2014.07.005Suche in Google Scholar PubMed PubMed Central
35. Ifa DR, Eberlin LS. Ambient ionization mass spectrometry for cancer diagnosis and surgical margin Evaluation. Clin Chem 2016;62:111–23.10.1373/clinchem.2014.237172Suche in Google Scholar
36. Zhang J, Yu W, Suliburk J, Eberlin LS. Will Ambient ionization mass spectrometry become an integral technology in the operating room of the future? Clin Chem 2016;62:1172–4.10.1373/clinchem.2016.258723Suche in Google Scholar
37. Hanel L, Kwiatkowski M, Heikaus L, Schluter H. Mass spectrometry-based intraoperative tumor diagnostics. Future Sci OA 2019;5:FSO373.10.4155/fsoa-2018-0087Suche in Google Scholar
38. Hayda RA, Brighton CT, Esterhai Jr JL. Pathophysiology of delayed healing. Clin Orthop Relat Res 1998;355(Suppl):S31–40.10.1097/00003086-199810001-00005Suche in Google Scholar
39. Megas P. Classification of non-union. Injury 2005;36(Suppl 4):S30–7.10.1016/j.injury.2005.10.008Suche in Google Scholar
40. Weinlein JC. Delayed union and nonunion of fractures. In: Azar FM, Beaty JH, Canale ST, editors. Campbell’s operative orthopaedics. Philadelphia: Elsevier, 2017:3081–116.e8.Suche in Google Scholar
41. Lynch JR, Taitsman LA, Barei DP, Nork SE. Femoral nonunion: risk factors and treatment options. J Am Acad Orthop Surg 2008;16:88–97.10.5435/00124635-200802000-00006Suche in Google Scholar
42. Naimark A, Miller K, Segal D, Kossoff J. Nonunion. Skeletal Radiol 1981;6:21–5.10.1007/BF00347342Suche in Google Scholar
43. Gelalis ID, Politis AN, Arnaoutoglou CM, Korompilias AV, Pakos EE, Vekris MD, et al. Diagnostic and treatment modalities in nonunions of the femoral shaft: a review. Injury 2012;43:980–8.10.1016/j.injury.2011.06.030Suche in Google Scholar
44. Calori GM, Albisetti W, Agus A, Iori S, Tagliabue L. Risk factors contributing to fracture non-unions. Injury 2007;38(Suppl 2):S11–8.10.1016/S0020-1383(07)80004-0Suche in Google Scholar
45. Zimmermann G, Muller U, Wentzensen A. The value of laboratory and imaging studies in the evaluation of long-bone non-unions. Injury 2007;38(Suppl 2):S33–7.10.1016/S0020-1383(07)80007-6Suche in Google Scholar
46. Lieberthal J, Sambamurthy N, Scanzello CR. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthritis Cartilage 2015;23:1825–34.10.1016/j.joca.2015.08.015Suche in Google Scholar PubMed PubMed Central
47. Lotz MK, Kraus VB. New developments in osteoarthritis. Posttraumatic osteoarthritis: pathogenesis and pharmacological treatment options. Arthritis Res Ther 2010;12:211.10.1186/ar3046Suche in Google Scholar PubMed PubMed Central
48. Zhang M, Mani SB, He Y, Hall AM, Xu L, Li Y, et al. Induced superficial chondrocyte death reduces catabolic cartilage damage in murine posttraumatic osteoarthritis. J Clin Invest 2016;126:2893–902.10.1172/JCI83676Suche in Google Scholar PubMed PubMed Central
49. Harkey MS, Luc BA, Golightly YM, Thomas AC, Driban JB, Hackney AC, et al. Osteoarthritis-related biomarkers following anterior cruciate ligament injury and reconstruction: a systematic review. Osteoarthritis Cartilage 2015;23:1–12.10.1016/j.joca.2014.09.004Suche in Google Scholar PubMed
50. Burr DB. The importance of subchondral bone in osteoarthrosis. Curr Opin Rheumatol 1998;10:256–62.10.1097/00002281-199805000-00017Suche in Google Scholar PubMed
51. Burr DB. The importance of subchondral bone in the progression of osteoarthritis. J Rheumatol Suppl 2004;70:77–80.Suche in Google Scholar
52. Botter SM, van Osch GJ, Waarsing JH, Day JS, Verhaar JA, Pols HA, et al. Quantification of subchondral bone changes in a murine osteoarthritis model using micro-CT. Biorheology 2006;43:379–88.Suche in Google Scholar
53. Hashimoto S, Rai MF, Janiszak KL, Cheverud JM, Sandell LJ. Cartilage and bone changes during development of post-traumatic osteoarthritis in selected LGXSM recombinant inbred mice. Osteoarthritis Cartilage 2012;20:562–71.10.1016/j.joca.2012.01.022Suche in Google Scholar PubMed PubMed Central
54. Yu DG, Nie SB, Liu FX, Wu CL, Tian B, Wang WG, et al. Dynamic alterations in microarchitecture, mineralization and mechanical property of subchondral bone in rat medial meniscal tear model of osteoarthritis. Chin Med J (Engl) 2015;128:2879–86.10.4103/0366-6999.168045Suche in Google Scholar PubMed PubMed Central
55. Olson SA, Guilak F. From articular fracture to posttraumatic arthritis: a black box that needs to be opened. J Orthop Trauma 2006;20:661–2.10.1097/01.bot.0000245683.89152.55Suche in Google Scholar PubMed
56. Dare D, Rodeo S. Mechanisms of post-traumatic osteoarthritis after ACL injury. Curr Rheumatol Rep 2014;16:448.10.1007/s11926-014-0448-1Suche in Google Scholar PubMed
57. Lubbeke A, Salvo D, Stern R, Hoffmeyer P, Holzer N, Assal M. Risk factors for post-traumatic osteoarthritis of the ankle: an eighteen year follow-up study. Int Orthop 2012;36:1403–10.10.1007/s00264-011-1472-7Suche in Google Scholar PubMed PubMed Central
58. Borrelli Jr J, Ricci WM, Steger-May K, Totty WG, Goldfarb C. Postoperative radiographic assessment of acetabular fractures: a comparison of plain radiographs and CT scans. J Orthop Trauma 2005;19:299–304.Suche in Google Scholar
59. Schaepe K, Bhandari DR, Werner J, Henss A, Pirkl A, Kleine-Boymann M, et al. Imaging of lipids in native human bone sections using TOF-secondary ion mass spectrometry, atmospheric pressure scanning microprobe matrix-assisted laser desorption/ionization Orbitrap mass spectrometry, and Orbitrap-secondary ion mass spectrometry. Anal Chem 2018;90:8856–64.10.1021/acs.analchem.8b00892Suche in Google Scholar PubMed
60. Palmquist A, Emanuelsson L, Sjovall P. Chemical and structural analysis of the bone-implant interface by TOF-SIMS, SEM, FIB and TEM: experimental study in animal. Appl Surf Sci 2012;258:6485–94.10.1016/j.apsusc.2012.03.065Suche in Google Scholar
61. Henss A, Rohnke M, El Khassawna T, Govindarajan P, Schlewitz G, Heiss C, et al. Applicability of ToF-SIMS for monitoring compositional changes in bone in a long-term animal model. J R Soc Interface 2013;10:20130332.10.1098/rsif.2013.0332Suche in Google Scholar PubMed PubMed Central
62. Henss A, Rohnke M, Knaack S, Kleine-Boymann M, Leichtweiss T, Schmitz P, et al. Quantification of calcium content in bone by using ToF-SIMS – a first approach. Biointerphases 2013;8:31.10.1186/1559-4106-8-31Suche in Google Scholar PubMed
63. Henss A, Hild A, Rohnke M, Wenisch S, Janek J. Time of flight secondary ion mass spectrometry of bone – impact of sample preparation and measurement conditions. Biointerphases 2015;11:02A302.10.1116/1.4928211Suche in Google Scholar PubMed PubMed Central
64. Kern C, Quade M, Ray S, Thomas J, Schumacher M, Gemming T, et al. Investigation of strontium transport and strontium quantification in cortical rat bone by time-of-flight secondary ion mass spectrometry. J R Soc Interface 2019;16:20180638.10.1098/rsif.2018.0638Suche in Google Scholar PubMed PubMed Central
65. Muller R, Henss A, Kampschulte M, Rohnke M, Langheinrich AC, Heiss C, et al. Analysis of microscopic bone properties in an osteoporotic sheep model: a combined biomechanics, FE and ToF-SIMS study. J R Soc Interface 2019;16:20180793.10.1098/rsif.2018.0793Suche in Google Scholar PubMed PubMed Central
66. Fujino Y, Minamizaki T, Yoshioka H, Okada M, Yoshiko Y. Imaging and mapping of mouse bone using MALDI-imaging mass spectrometry. Bone Rep 2016;5:280–5.10.1016/j.bonr.2016.09.004Suche in Google Scholar PubMed PubMed Central
67. Svirkova A, Turyanskaya A, Perneczky L, Streli C, Marchetti-Deschmann M. Multimodal imaging of undecalcified tissue sections by MALDI MS and uXRF. Analyst 2018;143:2587–95.10.1039/C8AN00313KSuche in Google Scholar PubMed
68. Nielson CM, Jacobs JM, Orwoll ES. Proteomic studies of bone and skeletal health outcomes. Bone 2019;126:18–26.10.1016/j.bone.2019.03.032Suche in Google Scholar PubMed PubMed Central
69. Grgurevic L, Macek B, Durdevic D, Vukicevic S. Detection of bone and cartilage-related proteins in plasma of patients with a bone fracture using liquid chromatography-mass spectrometry. Int Orthop 2007;31:743–51.10.1007/s00264-007-0404-zSuche in Google Scholar PubMed PubMed Central
70. Barre F, Rocha B, Dewez F, Towers M, Murray P, Claude E, et al. Faster raster matrix-assisted laser desorption/ionization mass spectrometry imaging of lipids at high lateral resolution. Int J Mass Spectrom 2019;437:38–48.10.1016/j.ijms.2018.09.015Suche in Google Scholar
71. Malmberg P, Nygren H. Methods for the analysis of the composition of bone tissue, with a focus on imaging mass spectrometry (TOF-SIMS). Proteomics 2008;8:3755–62.10.1002/pmic.200800198Suche in Google Scholar PubMed
72. Eriksson C, Malmberg P, Nygren H. Time-of-flight secondary ion mass spectrometric analysis of the interface between bone and titanium implants. Rapid Commun Mass Spectrom 2008;22:943–9.10.1002/rcm.3445Suche in Google Scholar PubMed
73. Rohnke M, Henss A, Kokesch-Himmelreich J, Schumacher M, Ray S, Alt V, et al. Mass spectrometric monitoring of Sr-enriched bone cements-from in vitro to in vivo. Anal Bioanal Chem 2013;405:8769–80.10.1007/s00216-013-7329-8Suche in Google Scholar PubMed
74. Thormann U, Ray S, Sommer U, Elkhassawna T, Rehling T, Hundgeburth M, et al. Bone formation induced by strontium modified calcium phosphate cement in critical-size metaphyseal fracture defects in ovariectomized rats. Biomaterials 2013;34:8589–98.10.1016/j.biomaterials.2013.07.036Suche in Google Scholar PubMed
75. Rohnke M, Pfitzenreuter S, Mogwitz B, Henss A, Thomas J, Bieberstein D, et al. Strontium release from Sr(2+)-loaded bone cements and dispersion in healthy and osteoporotic rat bone. J Control Release 2017;262:159–69.10.1016/j.jconrel.2017.07.036Suche in Google Scholar PubMed
76. Cillero-Pastor B, Eijkel G, Kiss A, Blanco FJ, Heeren RM. Time-of-flight secondary ion mass spectrometry-based molecular distribution distinguishing healthy and osteoarthritic human cartilage. Anal Chem 2012;84:8909–16.10.1021/ac301853qSuche in Google Scholar PubMed
77. Peffers MJ, Cillero-Pastor B, Eijkel GB, Clegg PD, Heeren RM. Matrix assisted laser desorption ionization mass spectrometry imaging identifies markers of ageing and osteoarthritic cartilage. Arthritis Res Ther 2014;16:R110.10.1186/ar4560Suche in Google Scholar PubMed PubMed Central
78. Briggs MT, Kuliwaba JS, Muratovic D, Everest-Dass AV, Packer NH, Findlay DM, et al. MALDI mass spectrometry imaging of N-glycans on tibial cartilage and subchondral bone proteins in knee osteoarthritis. Proteomics 2016;16:1736–41.10.1002/pmic.201500461Suche in Google Scholar PubMed
79. Barre FP, Flinders B, Garcia JP, Jansen I, Huizing LR, Porta T, et al. Derivatization strategies for the detection of triamcinolone acetonide in cartilage by using matrix-assisted laser desorption/ionization mass spectrometry imaging. Anal Chem 2016;88:12051–9.10.1021/acs.analchem.6b02491Suche in Google Scholar PubMed
80. Takats Z, Strittmatter N, McKenzie JS. Ambient mass spectrometry in cancer research. Adv Cancer Res 2017;134:231–56.10.1016/bs.acr.2016.11.011Suche in Google Scholar PubMed
81. St John ER, Rossi M, Pruski P, Darzi A, Takats Z. Intraoperative tissue identification by mass spectrometric technologies. Trac-Trend Anal Chem 2016;85:2–9.10.1016/j.trac.2016.05.003Suche in Google Scholar
82. Schafer KC, Denes J, Albrecht K, Szaniszlo T, Balog J, Skoumal R, et al. In vivo, in situ tissue analysis using rapid evaporative ionization mass spectrometry. Angew Chem Int Ed Engl 2009;48:8240–2.10.1002/anie.200902546Suche in Google Scholar PubMed
83. Phelps DL, Balog J, Gildea LF, Bodai Z, Savage A, El-Bahrawy MA, et al. The surgical intelligent knife distinguishes normal, borderline and malignant gynaecological tissues using rapid evaporative ionisation mass spectrometry (REIMS). Br J Cancer 2018;118:1349–58.10.1038/s41416-018-0048-3Suche in Google Scholar PubMed PubMed Central
84. Balog J, Szaniszlo T, Schaefer KC, Denes J, Lopata A, Godorhazy L, et al. Identification of biological tissues by rapid evaporative ionization mass spectrometry. Anal Chem 2010;82:7343–50.10.1021/ac101283xSuche in Google Scholar PubMed
85. Balog J, Sasi-Szabo L, Kinross J, Lewis MR, Muirhead LJ, Veselkov K, et al. Intraoperative tissue identification using rapid evaporative ionization mass spectrometry. Sci Transl Med 2013;5:194ra93.10.1126/scitranslmed.3005623Suche in Google Scholar PubMed
86. Alexander J, Gildea L, Balog J, Speller A, McKenzie J, Muirhead L, et al. A novel methodology for in vivo endoscopic phenotyping of colorectal cancer based on real-time analysis of the mucosal lipidome: a prospective observational study of the iKnife. Surg Endosc 2017;31:1361–70.10.1007/s00464-016-5121-5Suche in Google Scholar PubMed PubMed Central
87. Balog J, Kumar S, Alexander J, Golf O, Huang J, Wiggins T, et al. In vivo endoscopic tissue identification by rapid evaporative ionization mass spectrometry (REIMS). Angew Chem Int Ed Engl 2015;54:11059–62.10.1002/anie.201502770Suche in Google Scholar PubMed
88. Schäfer KC, Szaniszlo T, Gunther S, Balog J, Denes J, Keseru M, et al. In situ, real-time identification of biological tissues by ultraviolet and infrared laser desorption ionization mass spectrometry. Anal Chem 2011;83:1632–40.10.1021/ac102613mSuche in Google Scholar PubMed
89. Genangeli M, Heeren RM, Porta Siegel T. Tissue classification by rapid evaporative ionization mass spectrometry (REIMS): comparison between a diathermic knife and CO2 laser sampling on classification performance. Anal Bioanal Chem 2019;411:7943–55.10.1007/s00216-019-02148-8Suche in Google Scholar PubMed PubMed Central
90. Saudemont P, Quanico J, Robin YM, Baud A, Balog J, Fatou B, et al. Real-time molecular diagnosis of tumors using water-assisted laser desorption/ionization mass spectrometry technology. Cancer Cell 2018;34:840–51.e4.10.1016/j.ccell.2018.09.009Suche in Google Scholar PubMed
91. Woolman M, Gribble A, Bluemke E, Zou J, Ventura M, Bernards N, et al. Optimized mass spectrometry analysis workflow with polarimetric guidance for ex vivo and in situ sampling of biological tissues. Sci Rep 2017;7:468.10.1038/s41598-017-00272-ySuche in Google Scholar PubMed PubMed Central
92. Woolman M, Ferry I, Kuzan-Fischer CM, Wu M, Zou J, Kiyota T, et al. Rapid determination of medulloblastoma subgroup affiliation with mass spectrometry using a handheld picosecond infrared laser desorption probe. Chem Sci 2017;8:6508–19.10.1039/C7SC01974BSuche in Google Scholar
93. Woolman M, Kuzan-Fischer CM, Ferry I, Kiyota T, Luu B, Wu M, et al. Picosecond infrared laser desorption mass spectrometry identifies medulloblastoma subgroups on intrasurgical timescales. Cancer Res 2019;79:2426–34.10.1158/0008-5472.CAN-18-3411Suche in Google Scholar PubMed
94. Schäfer KC, Balog J, Szaniszlo T, Szalay D, Mezey G, Denes J, et al. Real time analysis of brain tissue by direct combination of ultrasonic surgical aspiration and sonic spray mass spectrometry. Anal Chem 2011;83:7729–35.10.1021/ac201251sSuche in Google Scholar PubMed
95. Zhang J, Rector J, Lin JQ, Young JH, Sans M, Katta N, et al. Nondestructive tissue analysis for ex vivo and in vivo cancer diagnosis using a handheld mass spectrometry system. Sci Transl Med 2017;9:eaan3968.10.1126/scitranslmed.aan3968Suche in Google Scholar PubMed PubMed Central
96. Sans M, Zhang J, Lin JQ, Feider CL, Giese N, Breen MT, et al. Performance of the MasSpec Pen for rapid diagnosis of ovarian cancer. Clin Chem 2019;65:674–83.10.1373/clinchem.2018.299289Suche in Google Scholar PubMed PubMed Central
97. Keung EZ, Roland CL. Accurate and reproducible diagnosis of canine soft tissue sarcoma using mass spectrometry: a step in the right direction. Cancer Cell 2018;34:697–9.10.1016/j.ccell.2018.10.013Suche in Google Scholar PubMed PubMed Central
98. Bodai Z, Cameron S, Bolt F, Simon D, Schaffer R, Karancsi T, et al. Effect of electrode geometry on the classification performance of rapid evaporative ionization mass spectrometric (REIMS) bacterial identification. J Am Soc Mass Spectrom 2018;29:26–33.10.1007/s13361-017-1818-5Suche in Google Scholar PubMed PubMed Central
Supplementary Material
The online version of this article offers supplementary material (https://doi.org/10.1515/cclm-2019-0857).
©2020 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Editorial
- Advancements in mass spectrometry as a tool for clinical analysis: part II
- Quantitative protein assessment
- Complexity, cost, and content – three important factors for translation of clinical protein mass spectrometry tests, and the case for apolipoprotein C-III proteoform testing
- Vedolizumab quantitation using high-resolution accurate mass-mass spectrometry middle-up protein subunit: method validation
- Development and evaluation of an element-tagged immunoassay coupled with inductively coupled plasma mass spectrometry detection: can we apply the new assay in the clinical laboratory?
- MALDI-MS for the clinic
- Matrix-assisted laser desorption ionisation (MALDI) mass spectrometry (MS): basics and clinical applications
- Clinical use of mass spectrometry (imaging) for hard tissue analysis in abnormal fracture healing
- Cellular resolution in clinical MALDI mass spectrometry imaging: the latest advancements and current challenges
- Bacterial identification by lipid profiling using liquid atmospheric pressure matrix-assisted laser desorption/ionization mass spectrometry
- Clinical application of ’omics technologies
- Individualized metabolomics: opportunities and challenges
- Diagnostic amyloid proteomics: experience of the UK National Amyloidosis Centre
- The “olfactory fingerprint”: can diagnostics be improved by combining canine and digital noses?
- Peptidomic and proteomic analysis of stool for diagnosing IBD and deciphering disease pathogenesis
- The influence of hypoxia on the prostate cancer proteome
- Laboratory automation and kit-based approaches
- Mass spectrometry and total laboratory automation: opportunities and drawbacks
- The pathway through LC-MS method development: in-house or ready-to-use kit-based methods?
- Evaluation of the 25-hydroxy vitamin D assay on a fully automated liquid chromatography mass spectrometry system, the Thermo Scientific Cascadion SM Clinical Analyzer with the Cascadion 25-hydroxy vitamin D assay in a routine clinical laboratory
Artikel in diesem Heft
- Frontmatter
- Editorial
- Advancements in mass spectrometry as a tool for clinical analysis: part II
- Quantitative protein assessment
- Complexity, cost, and content – three important factors for translation of clinical protein mass spectrometry tests, and the case for apolipoprotein C-III proteoform testing
- Vedolizumab quantitation using high-resolution accurate mass-mass spectrometry middle-up protein subunit: method validation
- Development and evaluation of an element-tagged immunoassay coupled with inductively coupled plasma mass spectrometry detection: can we apply the new assay in the clinical laboratory?
- MALDI-MS for the clinic
- Matrix-assisted laser desorption ionisation (MALDI) mass spectrometry (MS): basics and clinical applications
- Clinical use of mass spectrometry (imaging) for hard tissue analysis in abnormal fracture healing
- Cellular resolution in clinical MALDI mass spectrometry imaging: the latest advancements and current challenges
- Bacterial identification by lipid profiling using liquid atmospheric pressure matrix-assisted laser desorption/ionization mass spectrometry
- Clinical application of ’omics technologies
- Individualized metabolomics: opportunities and challenges
- Diagnostic amyloid proteomics: experience of the UK National Amyloidosis Centre
- The “olfactory fingerprint”: can diagnostics be improved by combining canine and digital noses?
- Peptidomic and proteomic analysis of stool for diagnosing IBD and deciphering disease pathogenesis
- The influence of hypoxia on the prostate cancer proteome
- Laboratory automation and kit-based approaches
- Mass spectrometry and total laboratory automation: opportunities and drawbacks
- The pathway through LC-MS method development: in-house or ready-to-use kit-based methods?
- Evaluation of the 25-hydroxy vitamin D assay on a fully automated liquid chromatography mass spectrometry system, the Thermo Scientific Cascadion SM Clinical Analyzer with the Cascadion 25-hydroxy vitamin D assay in a routine clinical laboratory


