To the Editor
Aplastic anemia (AA) is a kind of bone marrow failure syndrome. Hematopoietic stem cell transplantation (HSCT) and immunosuppressive therapy (IST) significantly improve symptoms and prolong life of AA.[1] However, about one third of AA patients are still refractory. Studies have shown that thrombopoietin receptor agonist (TPO-RA) eltrombopag was effective in the treatment of newly diagnosed and refractory AA.[2, 3, 4] Due to the potential hepatotoxicity of eltrombopag, it is still uncertain whether AA patients with liver disease can be treated with eltrombopag.
Avatrombopag, an oral TPO-RA, has been approved to treat chronic liver disease (CLD) related thrombocytopenia. Avatrombopag has achieved good Results in the patients with thrombocytopenia caused by CLD[5] and liver cirrhosis[6], with increased platelet levels, reduced bleeding events and platelet transfusion, without hepatotoxicity.
In this study, we used avatrombopag to treat AA patients with liver disease. Fourteen patients were enrolled between January 2021 and April 2022. Acquired severe AA (SAA) was diagnosed by the criteria[7]. All patients had liver disease. All patients were treated with IST combined with avatrombopag (Doptelet, Kawashima Plant, Eisai Co., Ltd. Japan). The initial dose of avatrombopag was 40 mg/d, and it was added to 60 mg/d after 2 weeks (the dose of avatrombopag was 20 mg/d in four children). Rabbit anti-human Thymocyte Immunoglobulin (ATG, Thymoglobuline, Sanofi, France) were administered intravenously with a dose of 3.0–3.5 mg/kg per day for 5 days. The initial dose of cyclosporine (Neocyspin, Huadong, China) was 3–5 mg/kg per day, and then the dose was adjusted according to the blood concentration to maintain the blood concentration between 150–250 ng/mL. This clinical trial was approved by the Ethics Committee of General Hospital of Tianjin Medical University (No. IRB2021-YX-045-01) and was registered on Chinese Clinical Trial Registry (No. ChiCTR2100050045). Informed written consents were obtained from all patients or their guardians according to the Helsinki Declaration.
Fourteen SAA patients with liver disease were included in this study, including 7 males and 7 females, with a median age of 35 (range 7–71) years. Eight cases were very SAA(VSAA), 5 cases of SAA and 1 case of SAA-PNH syndrome. All patients were red blood cell and platelet transfusion dependent. All patients had normal chromosome karyotype. One patient detected TET2 mutation, one patient detected DNMT3a mutation, and the others did not detect myeloid tumor related mutations. The median time from diagnosis to this study was 3 (range 1–60) months. The baseline blood cell levels of the patients were as follows, hemoglobin 57 (range 50–74) g/L, absolute neutrophil count 0.28 (range 0–3.24) × 109/L, platelet count 4 (range 1–19) × 109/L, absolute reticulocyte count 6.9 (range 0.4–29.8) × 109/L (Table 1).
The characteristics of the patients (n = 14)
| Male/Female, n | 7/7 |
| Age, median (range) , years | 35.5 (7-71) |
| Complete blood count at diagnosis, median (range) | |
| Absolute neutrophil count, ×109/L | 0.28 (0-3.24) |
| Hemoglobin, g/L | 57 (50-74) |
| Platelet count, ×109/L | 6 (1-19) |
| Reticulocyte count, ×109/L | 6.95 (0.4-29.8) |
| AA subtype, n | |
| VSAA | 8 |
| SAA | 5 |
| SAA-PNH | 1 |
| Transfusion | |
| RBC transfusion dependent | 14 |
| platelet transfusion dependent | 14 |
| Chromosome karyotype | |
| Normal karyotype | 14 |
| MDS-FISH*, n | |
| normal | 14 |
| MDS-related gene mutation#, n | |
| No mutation | 12 |
| TET2 | 1 |
| DNMT3A | 1 |
| Hepatic conditions | |
| Hepatitis associated aplastic anemia | 5 |
| Alanine transaminase (U/L) | 14 |
| Aspartate Transaminase (U/L) | 13 |
| Total bilirubin (μmol/L) | 6 |
| Hepatic cirrhosis | 2 |
| HBcAb-IgG positive | 1 |
| Hepatitis B virus positive | 1 |
| Time from diagnosis to therapy (months) | 3 (1-60) |
*MDS-FISH (myelodysplastic syndrome-fluorescence in situ hybridization) includes del(5q33), del(5q31), -5, -7, del(7q31), 20q-, +8, -Y and 17p13.1; #MDS-related gene mutation includes ASXL1, BCOR, BCORL1, CBL, CEBPA, CALR, CSF3R, DNMT3A, ETV6, EZH2, GATA2, IDH1, IDH2, JAK2, KIT, KRAS, MLL, MPL, NPM1, NRAS, PIGA, PHF6, RUNX1, SETBP1, SF3B1, SH2B3, SRSF2, TET2, TP53, U2AF1, ZRSR2, NF1, STAG2, STAT3, PPM1D. AA: aplastic anemia; SAA: severe AA; VSAA: very SAA; PNH: paroxysmal nocturnal hemoglobinuria.
All patients had abnormal liver function, including 14 cases with increased alanine transaminase (range 54–1801 U/L), 13 cases with increased aspartate transaminase (range 19–1183 U/L) and 6 cases with increased total bilirubin (range 8.3–226.3 μmol/L). Five patients were diagnosed as hepatitis associated AA (HAAA). Concomitant liver diseases included 2 cases of liver cirrhosis, one case of hepatitis C virus, one case of hepatitis B virus.
The median follow-up time was 6 (range 3–12) months. Four patients achieved complete remission (CR), 4 patients achieved partial remission (PR), and 6 patients were no remission (NR). The total response rate was 57.1% (8/14). The time of obtaining CR in 4 patients was 1, 2.5, 4 and 9 months, respectively (Table 2).
The therapeutic regimes and efficacy
| Therapeutic regimes | Number |
|---|---|
| ATG + cyclosporine + avatrombopag | 14 |
| TPO-RA used previously | |
| Eltrombapag | 6 |
| No | 8 |
| efficacy | |
| Complete response (CR) | 4 |
| Partial response (PR) | 4 |
| No response (NR) | 6 |
| RBC transfusion independent | 9 |
| Platelet transfusion independent | 8 |
| Survival | |
| Alive | 12 |
| Death | 2 |
ATG: antithymocyte globulin; TPO-RA: Thrombopoietin receptor agonist
The patients were divided into the younger group (< 50 years old, 8 cases) and the older group (> 50 years old, 6 cases). All the response patients were in the younger group, and the response rate was 100% (8/8). All elderly patients were no response (0/6).
All patients were treated with liver protecting drugs, including magnesium isoglycyrrhizinate, ademetionine and ursodeoxycholic acid. Except one patient died due to disease progression and multiple organ failure, the liver functions of the others recovered to normal level.
During the follow-up period, two patients died, including one patient who died of systemic multiple organ failure caused by lung infection and sepsis, and one patient who died of sudden acute heart event. The total survival rate of the patients at 12 months was 85.7% (Figure 1). No patients stopped treatment because of the toxicity of avatrombopag.
All patients were tested for chromosome and gene mutations 3 months after treatment, and no chromosomal abnormalities and progression of myeloid tumor gene mutations were found.
HAAA is bone marrow failure syndrome after acute hepatitis. Hepatocyte injury is usually considered as a poor prognosis.[8] In western countries, about 5% of cases of AA are related to hepatitis,[9] but in East Asia, the proportion is as high as 10%. China is one of the most prevalent areas of hepatitis B and has the highest incidence rate of hepatitis related AA, accounting for 23.9% of AA.[10] Our previous studies have shown that HAAA have higher early infection rate and infection related mortality in the first 2 years after diagnosis, and lower 2-year survival rate. Without HSCT or ATG, almost all patients die within 2 years.[10] With the progress of HSCT and ATG, the survival of HAAA has improved, especially in young patients. A Japanese study shows that children with HAAA have better response. Forty-four children with HAAA were treated with ATG and cyclosporin. Fourteen patients (31.8%) achieved CR and 17 patients (38.6%) achieved PR. After 6 months, the total response rate was 70.4%. Seven non responders received HSCT from HLA matched unrelated donors, and 6 of the 7 survived. The 10-year overall survival rate was 88.3%.[11]
Ma et al.[12] retrospectively analyzed 15 young HAAA patients (3–36 years old) who received haploid HSCT. The three-year overall survival rate (OS) was 100%, and the liver event free survival rate was 80.0%. The Results showed that haploid HSCT was a feasible HAAA treatment when no HLA matched donor was available, and the risk of transplant related mortality and complications was low.
At present, the TPO-RAs on the market mainly include eltrombopag, romiplostim, avatrombopag, lusutrombopag and hetrombopag. Eltrombopag, romiplostim and hetrombopag alone or in combination with ATG and cyclosporine have shown good efficacy in the treatment of primary and refractory AA, which can improve the response rate, shorten the response time, and promote bone marrow hematopoiesis.[2-4]
Patients with long-term use of eltrombopag can have abnormal liver function, including elevated alanine aminotransferase, aspartate aminotransferase, and blood bilirubin, which is especially obvious in AA.[3,4] AA patients had significantly higher TPO-RAs dose and longer time. In addition, they also used cyclosporine and other drugs that affect liver function.
The Results of our small sample study showed that the CR was 28.6%, the PR was 28.6%, and the response rate was 57.1% in the treatment of AA with abnormal liver function by avatrombopag combined with IST. Especially young patients have achieved better response. However, the effect of elderly patients was poor, of which 2 patients died during treatment. Previous studies have confirmed that age is an independent prognostic factor for the treatment of AA. The response of young patients with AA is better than that of elderly patients with AA, whether with IST or HSCT treatment.[1] Elderly patients have more complications. After IST treatment, the immune deficiency period is longer, which is easy to be complicated with serious infection and bleeding, affecting the recovery of the disease, and even leading to the death of patients.
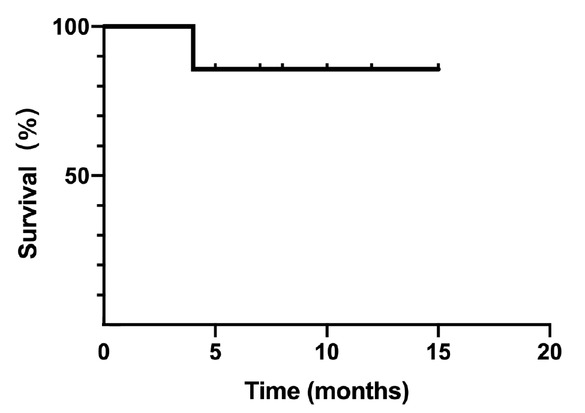
The survival curve of the patients (n = 14).
In conclusion, avatrombopag combined with IST is effective in the treatment of AA with abnormal liver function, especially in young patients, without obvious hepatotoxicity.
-
Author Contributions
Wang HW and Fu R conceived and designed the study, collected, assembled, analyzed, and interpreted data, and wrote the manuscript; He GS, Jia JS, Mengyuan Liu MY, Liu H, Wang T, Liu CY contributed patients or analyzed and interpreted data. All authors read and approved the manuscript.
-
Ethics Approval and Consent to Participate
The clinical trial was approved by the Ethics Committee of General Hospital of Tianjin Medical University (No. IRB2021-YX-045-01) and was registered on Chinese Clinical Trial Registry (No. ChiCTR2100050045)
-
Conflict of Interest
There is no conflict of interest among the authors.
References
1 Young NS. Aplastic anemia. N Engl J Med 2018;379:1643–5610.1016/j.diabet.2020.11.003Search in Google Scholar PubMed
2 Olnes MJ, Scheinberg P, Calvo KR, Desmond R, Tang Y, Dumitriu B, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med 2012;367:11–910.1055/a-1117-8446Search in Google Scholar PubMed
3 Townsley DM, Scheinberg P, Winkler T, Desmond R, Dumitriu B, Rios O, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med 2017;376:1540–5010.1016/j.ahj.2007.04.057Search in Google Scholar PubMed
4 Peffault de Latour R, Kulasekararaj A, Iacobelli S, Terwel SR, Cook R, Griffin M, et al. Eltrombopag added to immunosuppression in severe aplastic anemia. N Engl J Med 2022;386:11–2310.1155/2023/8818502Search in Google Scholar PubMed PubMed Central
5 Terrault N, Chen Y-C, Izumi N, Kayali Z, Mitrut P, Tak WY, et al. Avatrombopag before procedures reduces need for platelet transfusion in patients with chronic liver disease and thrombocytopenia. Gastroenterology 2018;155:705–1810.2147/IJGM.S418520Search in Google Scholar PubMed PubMed Central
6 Terrault NA, Hassanein T, Howell CD, Joshi S, Lake J, Sher L, et al. Phase II study of avatrombopag in thrombocytopenic patients with cirrhosis undergoing an elective procedure. J Hepatol 2014;61:1253–910.2169/internalmedicine.8424-21Search in Google Scholar PubMed PubMed Central
7 Killick SB, Bown N, Cavenagh J, Dokal I, Foukaneli T, Hill A, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol 2016;172:187–20710.1210/clinem/dgac034Search in Google Scholar PubMed PubMed Central
8 Brown KE, Tisdale J, Barrett AJ, Dunbar CE, Young NS. Hepatitisassociated aplastic anemia. N Engl J Med 1997;336:1059–6410.1055/a-1730-5029Search in Google Scholar PubMed
9 Locasciulli A, Bacigalupo A, Bruno B, Montante B, Marsh J, Tichelli A, et al. Hepatitis-associated aplastic anaemia: epidemiology and treatment results obtained in Europe. A report of The EBMT aplastic anaemia working party. Br J Haematol 2010;149:890–510.3390/ijms22168393Search in Google Scholar PubMed PubMed Central
10 Wang H, Tu M, Fu R, Wu Y, Liu H, Xing L, et al. The clinical and immune characteristics of patients with hepatitis-associated aplastic anemia in China. PloS One 2014;9:e9814210.1177/14791641221137736Search in Google Scholar PubMed PubMed Central
11 Osugi Y, Yagasaki H, Sako M, Kosaka Y, Taga T, Ito T, et al. Antithymocyte globulin and cyclosporine for treatment of 44 children with hepatitis associated aplastic anemia. Haematologica 2007;92:1687–9010.1136/bmjdrc-2020-002032Search in Google Scholar PubMed PubMed Central
12 Ma X, Zuo Y, Xu Z, Zhang Y, Cheng Y, Han T, et al. Comparable clinical outcomes of haploidentical hematopoietic stem cell transplantation in patients with hepatitis-associated aplastic anemia and non-hepatitis-associated aplastic anemia. Ann Hematol 2022;101:1815–2310.3390/medicina60010061Search in Google Scholar PubMed PubMed Central
© 2023 Huaquan Wang, Guangsheng He, Jinsong Jia, Mengyuan Liu, Hui Liu, Ting Wang, Chunyan Liu, Rong Fu, published by Sciendo
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Perspective
- Revising the hemodynamic criteria for pulmonary hypertension: A perspective from China
- Animal models: An essential tool to dissect the heterogeneity of chronic obstructive pulmonary disease
- Effective albumin – A novel paradigm in the management of decompensated liver cirrhosis
- Monkeypox: A real new warning or just a sign of times?
- Severe acute hepatitis of unknown origin in children: Clinical issues of concern
- Commentary
- Manipulating cell motility by Legionella: Speeding up or slowing down?
- Standardized inhalation capability assessment: A key to optimal inhaler selection for inhalation therapy
- Review Article
- Mesenchymal stem cells and connective tissue diseases: From bench to bedside
- Predictors of progression in idiopathic inflammatory myopathies with interstitial lung disease
- Original Article
- Moderate-intensity continuous training has time-specific effects on the lipid metabolism of adolescents
- Point-of-care ultrasound-guided submucosal paclitaxel injection in tracheal stenosis model
- Gas chromatography-mass spectrometry pilot study to identify volatile organic compound biomarkers of childhood obesity with dyslipidemia in exhaled breath
- Letter to Editor
- Efficacy and safety of avatrombopag in aplastic anemia patients with liver disease
- Retraction Note
- Retraction note: Hydrogel: A promising new technique for treating Alzheimer’s disease (in Volume 10 Issue 3)
Articles in the same Issue
- Perspective
- Revising the hemodynamic criteria for pulmonary hypertension: A perspective from China
- Animal models: An essential tool to dissect the heterogeneity of chronic obstructive pulmonary disease
- Effective albumin – A novel paradigm in the management of decompensated liver cirrhosis
- Monkeypox: A real new warning or just a sign of times?
- Severe acute hepatitis of unknown origin in children: Clinical issues of concern
- Commentary
- Manipulating cell motility by Legionella: Speeding up or slowing down?
- Standardized inhalation capability assessment: A key to optimal inhaler selection for inhalation therapy
- Review Article
- Mesenchymal stem cells and connective tissue diseases: From bench to bedside
- Predictors of progression in idiopathic inflammatory myopathies with interstitial lung disease
- Original Article
- Moderate-intensity continuous training has time-specific effects on the lipid metabolism of adolescents
- Point-of-care ultrasound-guided submucosal paclitaxel injection in tracheal stenosis model
- Gas chromatography-mass spectrometry pilot study to identify volatile organic compound biomarkers of childhood obesity with dyslipidemia in exhaled breath
- Letter to Editor
- Efficacy and safety of avatrombopag in aplastic anemia patients with liver disease
- Retraction Note
- Retraction note: Hydrogel: A promising new technique for treating Alzheimer’s disease (in Volume 10 Issue 3)

