Human albumin: A pleiotropic molecule
Synthesis
Human albumin, a 66.5-kDa globular protein synthesized by hepatocytes, is the most abundant serum protein whose concentration ranges from 3.5 to 5 g/dL. Under normal conditions, albumin production is stable (10–15 g daily), but can increase up to 3–4 fold. Hormonal factors, such as insulin, cortisol, and growth hormone, stimulate, while mediators of inflammation, such as proinflammatory cytokines, inhibit albumin synthesis.[1, 2, 3]
Structure and functional properties
A single chain of 585 amino acids developed through nine loops and organized into three domains constitutes the albumin structure, stabilized by 34 disulfide bonds involving cysteine residues. The circulatory half-life of albumin is approximately 16–18 h, while the total half-life varies from 13 to 18 days in healthy adult subjects.[1, 2, 3]
Albumin is the principal modulator of fluid distribution among the body’s compartments, accounting for 70%–75% of the plasma oncotic pressure due to its osmotic property. Furthermore, its net negative charge attracts and binds cations, such as sodium, ultimately leading fluids to move from the extra- to the intravascular compartment. These features and its prolonged half-life explain why albumin is employed as a plasma expander in several clinical contexts.
Albumin is the most abundant circulating antioxidative system in the human body due to the sulfhydryl group in the Cys-34 position, which can scavenge reactive oxygen species (ROS). In healthy adults, most (70%–80%) of these sulfhydryl groups are in the reduced form (human mercaptalbumin), about 20% are involved in reversible disulfide bonds with sulfhydryl compounds (non-mercaptalbumin 1), and a small amount is irreversibly oxidized to sulfonic or sulfinic acid (non-mercaptalbumin 2). The N-terminal and a metal-binding domain contribute to the antioxidative capacity of albumin by binding highly reactive, toxic metals such as copper, cobalt, nickel, zinc, and iron.
The negative charges and tertiary structure of albumin account for its binding, storage, transport, solubilization, and detoxification of several endogenous and exogenous substances, including drugs, bilirubin, bile acids, hormones, metals, anions, long-chain fatty acids, l-thyroxine, nitric oxide, endotoxin, and other pathogen molecular patterns (PAMPs). Another nononcotic property of albumin is immunomodulation. Not all mechanisms underlying this function are well defined. However, there is convincing evidence that albumin modulates the immune response to sepsis. Its binding with circulating molecules, such as PAMPs and danger-associated molecular patterns (DAMPs), avoids these molecules from reacting with recognition receptors, preventing immune cell activation. Internalization of albumin into the leukocyte cytoplasm interacts with toll-like receptor trafficking and signaling, attenuating immune cell responses to stimulating molecules such as proinflammatory cytokine. Albumin prevents or attenuates macrophage dysfunction due to prostaglandin E2 (PGE2) and protects tissues from tumor necrosis factor-α (TNF-α)-induced immunopathology. These immunomodulatory mechanisms exert relevant indirect effects. The attenuation of immune system activation also leads to a decreased generation of ROS. Furthermore, along with interactions with the extracellular matrix, immunomodulation is also relevant in protecting endothelia and preserving capillary integrity and permeability. Also, albumin influences hemostasis by reducing platelet aggregation and acid-base balance by acting as a weak acid and buffering nonvolatile acids.[2, 3, 4, 5, 6]
Pathophysiology of advanced cirrhosis
For decades, we considered many features of advanced cirrhosis as the expression of arterial vasodilation mainly occurring in the splanchnic circulation.[7] This abnormality, secondary to portal hypertension, ultimately endangers effective volemia. Compensatory responses include activation of vasoconstrictor and sodium-retaining systems and increase in cardiac output. Despite these responses, effective hypovolemia can persist, especially in the advanced stages of cirrhosis, where cardiac dysfunction does not allow the cardiac output to cope with the needs of the systemic circulation. Ascites formation and impaired renal perfusion up to renal failure are the principal consequences of these events. For these reasons, the current, well-established indications for the use of albumin in cirrhosis pertain to conditions characterized by an acute worsening of effective volemia.[8]
Our understanding of the pathophysiological background of decompensated cirrhosis has improved.[9,10] Cardiocirculatory dysfunction still plays a central role, but the pathophysiological network has become far more complex. The systemic spread of PAMPs, due to abnormal translocation from the gut, and DAMPs released from the liver, where inflammation and cell necrosis occur, after recognition by innate pattern recognition receptors, activates the immune cells to produce proinflammatory cytokines and chemokines, along with ROS and reactive nitrogen species. This cascade of events contributes to the development of cardiocirculatory dysfunction and favors multiorgan dysfunction and failure through direct effects called immunopathology. Besides immunopathology, other mechanisms promote multiorgan dysfunction in advanced cirrhosis, like immune dysmetabolism. Indeed, sustained activation of the immune cells enhances their energetic demand. Thus, body nutrient allocation prioritizes the immune tissue at the expense of nonimmune cells. The latter adapt to nutrient scarcity by reducing mitochondrial oxidative phosphorylation and, therefore, adenosine triphosphate (ATP) production. The improved knowledge of the pathophysiological background of decompensated cirrhosis offers a network where novel therapeutic approaches, including human albumin administration, could find their targets. Interestingly, human albumin administration, by reducing immune system activity, may also diminish the energy demand from the immune cells, indirectly attenuating immune metabolism abnormalities.
Albumin abnormalities in patients with cirrhosis
Low serum albumin concentration is a long-recognized feature in patients with cirrhosis and is associated with reduced survival.[11] Impaired synthesis due to extensive reduction of functional liver parenchyma and sustained inflammation, hemodilution secondary to renal sodium and water retention, and enhanced transvascular escape, especially in the most advanced stages of cirrhosis, cooperate in inducing hypoalbuminemia.
More recent studies have clearly shown that, as it occurs in other chronic diseases such as diabetes mellitus, chronic renal failure, and ischemic heart disease, changes in albumin molecule also develop in cirrhosis.[3] The systemic inflammatory and pro-oxidant milieu that characterize the disease and intensify in parallel with its progression represent the principal cause of albumin damage. These abnormalities are clinically relevant. Indeed, whereas the oncotic property of albumin is derived from highly conserved features, such as the high molecular weight and the negative net charge, the nononcotic properties are strictly related to the integrity of the molecular structure.[1] Thus, when structural damages occur, one or more functions of albumin fail. The posttranscriptional molecular abnormalities most frequently reported, alone or in combination, consist of the oxidation of the Cys-34 residue, leading to an increase in the proportion of non-mercaptalbumin 1 and non-mercaptalbumin 2, oxidation of N-terminus (ischemia-modified albumin [IMA]), truncation of N- and C-termini, and glycosylation.[12, 13, 14] These alterations generally parallel the severity of cirrhosis, from outpatients with a compensated disease to those affected by acute-on-chronic liver failure (ACLF). Some are also associated with specific complications of cirrhosis, such as ascites, renal failure, or bacterial infections.
Therefore, the alterations of serum albumin in patients with cirrhosis are not only quantitative but also qualitative, as posttranscriptional molecular abnormalities lead to impairments of nononcotic functions (Figure 1). The clinical consequences are relevant, especially in advanced cirrhosis, where the persistent systemic inflammatory and pro-oxidative state would require fully preserved albumin functions. Moreover, altered albumin isoforms may contribute to systemic inflammation. Indeed, the inflammatory response of peripheral blood mononuclear cells, assessed in vitro by measuring the production of interleukin-6 and TNF-α, is enhanced by a challenge with non-mercaptalbumin 1.[15]
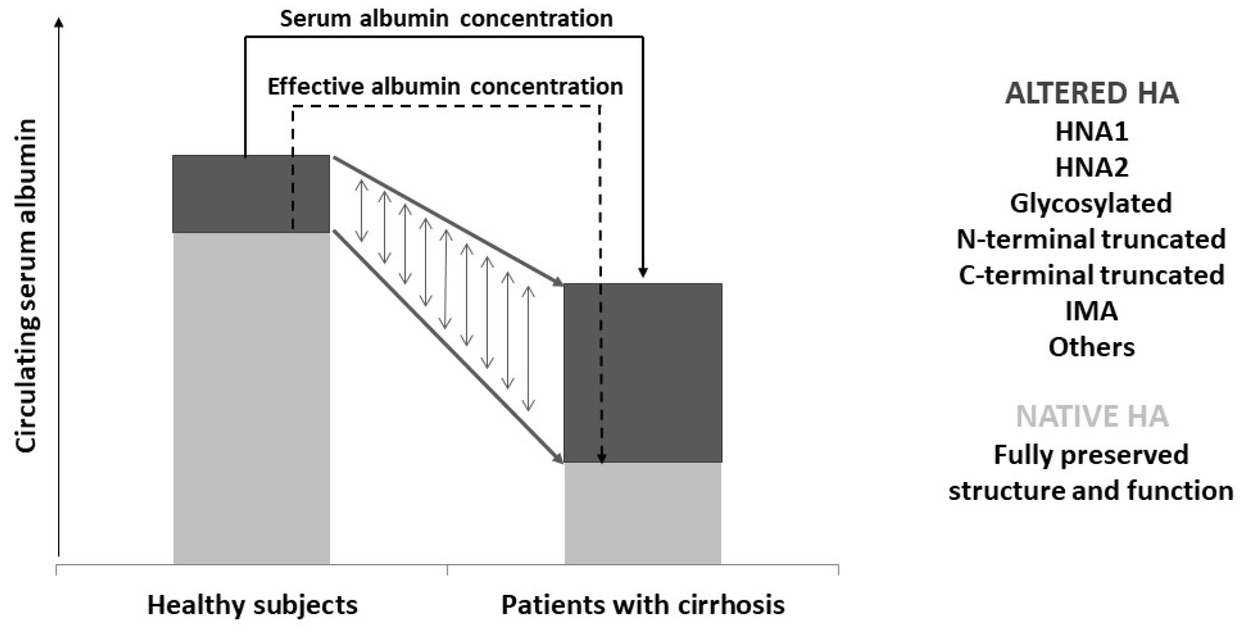
Graphic representation of the concept of effective albumin. In healthy subjects, the native isoform, with preserved molecular structure and functions, largely prevails among the circulating albumin isoforms. Patients with advanced cirrhosis show a reduction in total serum albumin concentration. However, the relative proportions of altered isoforms are increased, so that the amount of effective albumin can be drastically reduced. HA: human albumin; HNA1: non-mercaptalbumin 1; HNA2: non-mercaptalbumin 2; IMA: ischemia-modified albumin.
The traditional measurement of serum albumin concentration cannot provide information on the global albumin status. Thus, it would be clinically relevant to determine the proportion of circulating structurally and functionally intact albumin: the serum effective albumin concentration (EAc).[2]
Clinical relevance of albumin nononcotic properties
Human albumin administration to patients with advanced cirrhosis traditionally aims at improving effective volemia. However, there are several direct and indirect pieces of evidence that the beneficial effects obtained from this treatment are also, sometimes prevalently, due to the nononcotic properties of albumin. Albumin, but not hydroxyethyl starch, improved stroke work index and peripheral vascular resistance in patients with spontaneous bacterial peritonitis.[16] These effects were associated with a significant decrease in the plasma levels of factor VIII and Von Willebrand-related antigen, suggesting an attenuation of endothelial activation. In rats with carbon tetrachloride-induced cirrhosis, albumin administration, but not hydroxyethyl starch or saline, restored impaired cardiac contractility, counteracting the adverse effects of TNF-α and oxidative stress in the cardiac tissue.[17] Albumin administration in patients with cirrhosis improved immune competence by binding free PGE2, whose excess endangers macrophage function.[6] Long-term albumin administration to patients with cirrhosis and ascites reduced the occurrence of complications and improved survival.[18] Considering the relatively low dose administered (40 g/week), it is likely that these effects were mainly due to the nononcotic properties of albumin. Thus, restoring EAc may become a primary target in clinical practice.
Effective albumin in cirrhosis: What we know and perspectives
The measurement of EAc requires the assessment of the relative abundance of the native, intact albumin molecule by techniques like combination of liquid chromatography/ electrospray ionization/mass spectrometry. Then, EAc derives from the formula, serum albumin concentration (g/dL) × native albumin (%)/100.
The available information about the measurement of EAc in patients with cirrhosis is limited.[19] The principal results obtained are as follows: (1) EAc progressively declines with the worsening of cirrhosis and is superior to serum albumin concentration in stratifying patients among compensated cirrhosis, acute decompensation, and ACLF; (2) EAc better reflects the residual binding and detoxification activity of albumin than serum albumin concentration; (3) EAc at admission in hospitalized patients with acute decompensation of cirrhosis predicts the 30-day occurrence of ACLF and 90-day mortality better than serum albumin concentration.
These data suggest that EAc can help physicians as a biomarker for predicting prognosis and monitoring response to treatment. Moreover, it can provide information about the extent of albumin dysfunction and may guide treatments to a more personalized approach. In other words, the reach of a given level of EAc may represent the target for human albumin administration. Furthermore, the extent of EAc deficit may guide the dosages of drugs whose pharmacokinetics and pharmacodynamics are influenced by defects in albumin binding and detoxification activities. The assessment of the relative amount of albumin isoforms requires specific techniques, which are certainly more complex than the routine methods of assessing serum albumin concentration. However, they are high-throughput techniques and provide results in a relatively short time at a contained cost. Therefore, the measurement of EAc could well represent a second-level evaluation.
-
Conflict of Interest
The author declares the following potential conflicts of interest with respect to the publication of this article. The author received personal fees from CLS Behring GmbH (consultant, speaker), Grifols SA (consultant, speaker), Takeda (consultant, speaker), PPTA (speaker), Octapharma AG (speaker).
References
1 Quinlan GJ, Martin GS, Evans TW. Albumin: biochemical properties and therapeutic potential. Hepatology 2005;41:1211–9.10.1002/hep.20720Search in Google Scholar PubMed
2 Garcia-Martinez R, Caraceni P, Bernardi M, Gines P, Arroyo V, Jalan R. Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology 2013;58:1836–46.10.1002/hep.26338Search in Google Scholar PubMed
3 Bernardi M, Angeli P, Claria J, Moreau R, Gines P, Jalan R, et al. Albumin in decompensated cirrhosis: new concepts and perspectives. Gut 2020;69:1127–38.10.1136/gutjnl-2019-318843Search in Google Scholar PubMed PubMed Central
4 Casulleras M, Flores-Costa R, Duran-Güell M, Alcaraz-Quiles J, Sanz S, Titos E, et al. Albumin internalizes and inhibits endosomal TLR signaling in leukocytes from patients with decompensated cirrhosis. Sci Transl Med 2020;12:eaax5135.10.1126/scitranslmed.aax5135Search in Google Scholar PubMed
5 Duran-Güell M, Flores-Costa R, Casulleras M, López-Vicario C, Titos E, Díaz A, et al. Albumin protects the liver from tumor necrosis factor α-induced immunopathology. FASEB J 2021;35:e21365.10.1096/fj.202001615RRRSearch in Google Scholar PubMed
6 O’Brien AJ, Fullerton JN, Massey KA, Auld G, Sewell G, James S, et al. Immunosuppression in acutely decompensated cirrhosis is mediated by prostaglandin E2. Nat Med 2014;20:518.10.1038/nm.3516Search in Google Scholar PubMed PubMed Central
7 Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodés J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology 1988;8:1151–7.10.1002/hep.1840080532Search in Google Scholar PubMed
8 EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69:406–60.10.1016/j.jhep.2018.03.024Search in Google Scholar PubMed
9 Bernardi M, Moreau R, Angeli P, Schnabl B, Arroyo V. Mechanisms of decompensation and organ failure in cirrhosis: From peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol 2015;63:1272–84.10.1016/j.jhep.2015.07.004Search in Google Scholar PubMed
10 Engelmann C, Clària J, Szabo G, Bosch J, Bernardi M. Pathophysiology of decompensated cirrhosis: Portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J Hepatol 2021;75(Suppl 1):S49–S66.10.1016/j.jhep.2021.01.002Search in Google Scholar PubMed PubMed Central
11 Salerno F, Borroni G, Moser P, Badalamenti S, Cassarà L, Maggi A, et al. Survival and prognostic factors of cirrhotic patients with ascites: a study of 134 outpatients. Am J Gastroenterol 1993;88:514–9.Search in Google Scholar
12 Oettl K, Birner-Gruenberger R, Spindelboeck W, Stueger HP, Dorn L, Stadlbauer V, et al. Oxidative albumin damage in chronic liver failure: relation to albumin binding capacity, liver dysfunction and survival. J Hepatol 2013;59:978–83.10.1016/j.jhep.2013.06.013Search in Google Scholar PubMed
13 Jalan R, Schnurr K, Mookerjee RP, Sen S, Cheshire L, Hodges S, et al. Alterations in the functional capacity of albumin in patients with decompensated cirrhosis is associated with increased mortality. Hepatology 2009;50:555–64.10.1002/hep.22913Search in Google Scholar PubMed
14 Domenicali M, Baldassarre M, Giannone FA, Naldi M, Mastroroberto M, Biselli M, et al. Posttranscriptional changes of serum albumin: clinical and prognostic significance in hospitalized patients with cirrhosis. Hepatology 2014;60:1851–60.10.1002/hep.27322Search in Google Scholar PubMed
15 Alcaraz-Quiles J, Casulleras M, Oettl K, Titos E, Flores-Costa R, Duran-Güell M, et al. Oxidized albumin triggers a cytokine storm in leukocytes through P38 mitogen-activated protein kinase: Role in systemic inflammation in decompensated cirrhosis. Hepatology 2018;68:1937–52.10.1002/hep.30135Search in Google Scholar PubMed
16 Fernández J, Monteagudo J, Bargallo X, Jiménez W, Bosch J, Arroyo V, et al. A randomized unblinded pilot study comparing albumin versus hydroxyethyl starch in spontaneous bacterial peritonitis. Hepatology 2005;42:627–34.10.1002/hep.20829Search in Google Scholar PubMed
17 Bortoluzzi A, Ceolotto G, Gola E, Sticca A, Bova S, Morando F, et al. Positive cardiac inotropic effect of albumin infusion in rodents with cirrhosis and ascites: molecular mechanisms. Hepatology 2013;57:266– 76.10.1002/hep.26021Search in Google Scholar PubMed
18 Caraceni P, Riggio O, Angeli P, Alessandria C, Neri S, Foschi FG, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet 2018;391:2417–29.10.1016/S0140-6736(18)30840-7Search in Google Scholar PubMed
19 Baldassarre M, Naldi M, Zaccherini G, Bartoletti M, Antognoli A, Laggetta M, et al. Determination of effective albumin in patients with decompensated cirrhosis: Clinical and prognostic implications. Hepatology 2021;74:2058–73.10.1002/hep.31798Search in Google Scholar PubMed PubMed Central
© 2023 Mauro Bernardi, published by Sciendo
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Perspective
- Revising the hemodynamic criteria for pulmonary hypertension: A perspective from China
- Animal models: An essential tool to dissect the heterogeneity of chronic obstructive pulmonary disease
- Effective albumin – A novel paradigm in the management of decompensated liver cirrhosis
- Monkeypox: A real new warning or just a sign of times?
- Severe acute hepatitis of unknown origin in children: Clinical issues of concern
- Commentary
- Manipulating cell motility by Legionella: Speeding up or slowing down?
- Standardized inhalation capability assessment: A key to optimal inhaler selection for inhalation therapy
- Review Article
- Mesenchymal stem cells and connective tissue diseases: From bench to bedside
- Predictors of progression in idiopathic inflammatory myopathies with interstitial lung disease
- Original Article
- Moderate-intensity continuous training has time-specific effects on the lipid metabolism of adolescents
- Point-of-care ultrasound-guided submucosal paclitaxel injection in tracheal stenosis model
- Gas chromatography-mass spectrometry pilot study to identify volatile organic compound biomarkers of childhood obesity with dyslipidemia in exhaled breath
- Letter to Editor
- Efficacy and safety of avatrombopag in aplastic anemia patients with liver disease
- Retraction Note
- Retraction note: Hydrogel: A promising new technique for treating Alzheimer’s disease (in Volume 10 Issue 3)
Articles in the same Issue
- Perspective
- Revising the hemodynamic criteria for pulmonary hypertension: A perspective from China
- Animal models: An essential tool to dissect the heterogeneity of chronic obstructive pulmonary disease
- Effective albumin – A novel paradigm in the management of decompensated liver cirrhosis
- Monkeypox: A real new warning or just a sign of times?
- Severe acute hepatitis of unknown origin in children: Clinical issues of concern
- Commentary
- Manipulating cell motility by Legionella: Speeding up or slowing down?
- Standardized inhalation capability assessment: A key to optimal inhaler selection for inhalation therapy
- Review Article
- Mesenchymal stem cells and connective tissue diseases: From bench to bedside
- Predictors of progression in idiopathic inflammatory myopathies with interstitial lung disease
- Original Article
- Moderate-intensity continuous training has time-specific effects on the lipid metabolism of adolescents
- Point-of-care ultrasound-guided submucosal paclitaxel injection in tracheal stenosis model
- Gas chromatography-mass spectrometry pilot study to identify volatile organic compound biomarkers of childhood obesity with dyslipidemia in exhaled breath
- Letter to Editor
- Efficacy and safety of avatrombopag in aplastic anemia patients with liver disease
- Retraction Note
- Retraction note: Hydrogel: A promising new technique for treating Alzheimer’s disease (in Volume 10 Issue 3)

