Abstract
The pathogenesis of connective tissue diseases (CTDs), represented by systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), systemic sclerosis (SSc), primary Sjögren’s syndrome (pSS), and idiopathic inflammatory myopathies (IIM), includes various immune cells involved in both innate and adaptive immunity. The mesenchymal stem cells (MSCs) are unique due to their regulatory effect on immunity. This makes them a promising therapeutic approach for patients with immune-mediated disorders such as CTD. The safety and clinical efficacy of MSC treatment in CTD have been tested in a growing number of preclinical and clinical studies. Administration of MSCs has consistently shown benefits with both symptomatic and histologic improvement in CTD animal models. MSC therapies in severe and drug-resistant CTD patients have shown promise in a number of the pilot studies, cohort studies, and randomized controlled trials in SLE, RA, and SSc, but some problems still need to be resolved in the transition from the bench to the bedside. The relevant studies in pSS and IIM are still in their infancy, but have displayed encouraging outcomes. Considerable efficacy variations have been observed in terms of the route of delivery, time of MSC injection, origin of the MSCs and dosage. Furthermore, the optimization of conventional drugs combined with MSC therapies and the applications of novel cell engineering approaches requires additional research. In this review, we summarize the current evidence about the immunoregulatory mechanism of MSCs, as well as the preclinical and clinical studies of MSC-based therapy for the treatment of CTDs.
Introduction
Mesenchymal stem cells (MSCs), also referred to as multipotent stromal cells or mesenchymal stromal cells, have profound immunomodulatory functions and have been shown to have promising therapeutic effects for autoimmune diseases. They are found to be capable of interacting with immune cells via direct cell contact or through the secretion of various cytokines in vitro and in vivo.[1] MSCs can be isolated from almost all adult tissues, in particular bone marrow, umbilical cord, adipose tissue, and placenta. Bone marrow-derived MSCs (BM-MSCs) are the most extensively used MSCs and are characterized by remarkable osteogenic and chondrogenic differentiation potential.[2] However, the effectiveness of BM-MSCs is dependent on the donor’s condition, and the risk of infection during extraction cannot be ignored. Human umbilical cord-derived MSCs (UC-MSCs) can be obtained more easily with painless extraction procedures. In terms of proliferation and immunosuppressive potential, UC-MSCs have the most rapid growth rate and they secret multiple growth factors that can reduce inflammation and reverse tissue damage.[3] Adipose tissue-derived MSCs (AD-MSCs) have gained increasing attention since they can be extracted in large quantities from various sites of human body. Hence, the current studies have focused on exploring the cell surface markers and secretome of AD-MSCs from different body sites because this knowledge is critical to improve the clinical efficacy of AD-MSCs. Placenta-derived stem cells (PDSCs) have a longer culture period and higher proliferative capacity, which is related to their short population doubling time. The therapeutic efficacy of PDSCs varies depending on the anatomical site, and they have been reported to have a better effect for vascular disease.[4]
Autoimmune connective tissue diseases (CTDs) are a group of chronic multisystem disorders characterized by immune-mediated inflammation of connective tissues. Conventional treatments of CTD include corticosteroids, nonsteroidal anti-inflammatory drugs (NSAIDs), immunosuppressants, and biologic agents. This treatment approach has had a significant impact on improving the prognosis of CTD patients. However, disease control remains unsatisfactory in a subset of these patients, and conventional immunotherapy often results in serious adverse effects including infection, haemocytopenia, impairment of liver and kidney function, and metabolic disorders. Therefore, the development of novel treatments for CTD patients is an important unmet need. A growing understanding of the immune system has identified a group of cells that could be the potential therapeutic targets for CTDs, such as B cells, regulatory T (Treg) cells, natural killer (NK) cells, and MSCs.[5] Furthermore, accumulating evidence has suggested that the differentiation and function of stem cells are dysregulated in CTD patients, and thus MSC-based therapies have emerged as a potential therapeutic modality to restore immune tolerance in autoimmune diseases. MSCs have been approved for the treatment of some immune-mediated disorders, such as Crohn’s disease and graft versus host disease (GvHD), by the European Medicines Agency (EMA) and the Japanese Ministry of Health, Labour, and Welfare (MHLW). Currently, hundreds of clinical trials focusing on the reparative capabilities of MSC therapy in CTD patients have either been completed or are in progress.[6] In this review, we summarize the studies on the immunoregulatory mechanism of MSCs, as well as the preclinical and clinical evidence for MSC-based therapy for CTD.
Immunoregulatory mechanism of MSCs in CTD
MSCs can interact with various immune cells and secrete soluble factors and extracellular vesicles (EVs), thus representing a powerful tool to modulate the immune system in CTD. The immunoregulatory activity of MSCs could be promoted by the participation of some types of immune cells, including T-lymphocytes, B-lymphocytes, macrophages, NK cells, and monocytes, which are discussed in detail below. In terms of soluble inflammatory factors, MSCs can secrete cytokines, growth factors, and chemokines, that are crucial to their autocrine or paracrine activities. The autocrine effect of MSCs is characterized by the secretion of these factors, which then act on themselves. The autocrine signalling activities of interleukin (IL)-6, IL-8, prostaglandin E2 (PGE2), and vascular endothelial growth factor (VEGF) was reported to enhance the stemness of MSCs.[7] The main beneficial effects of MSC-based therapy are attributed to these secreted factors or the packaging of these factors in EVs that act on nearby cells via paracrine signalling. Key factors have been identified, such as IL-1 receptor antagonists, VEGF, transforming growth factor-β (TGF-β), stromal cell-derived factor-1 (SDF-1),[8] tumour necrosis factor-α (TNF-α)–stimulated TNF-inducible gene 6 (TSG6),[9] PGE2,[10] and others. Through these paracrine pathways and intercellular contact, MSCs have been shown to significantly downregulate T helper (Th) 1 cytokines (TNF-α), IL-1, interferon-γ (IFN-γ), inducible nitric oxide synthase (iNOS), matrix metalloprotein-9 (MMP-9), TGF-β1, and granulocyte– macrophage colony-stimulating factor (GM-CSF), as well as upregulate Th2 cytokine (IL-10).[11,12] These findings provide a basis for promoting cell-based treatment through combination therapies and bioengineering.[13,14] Therefore, we summarize the immunoregulatory mechanisms of MSCs in CTD (Figure 1) and address the importance of understanding the properties of MSCs before their further application in clinical trials.
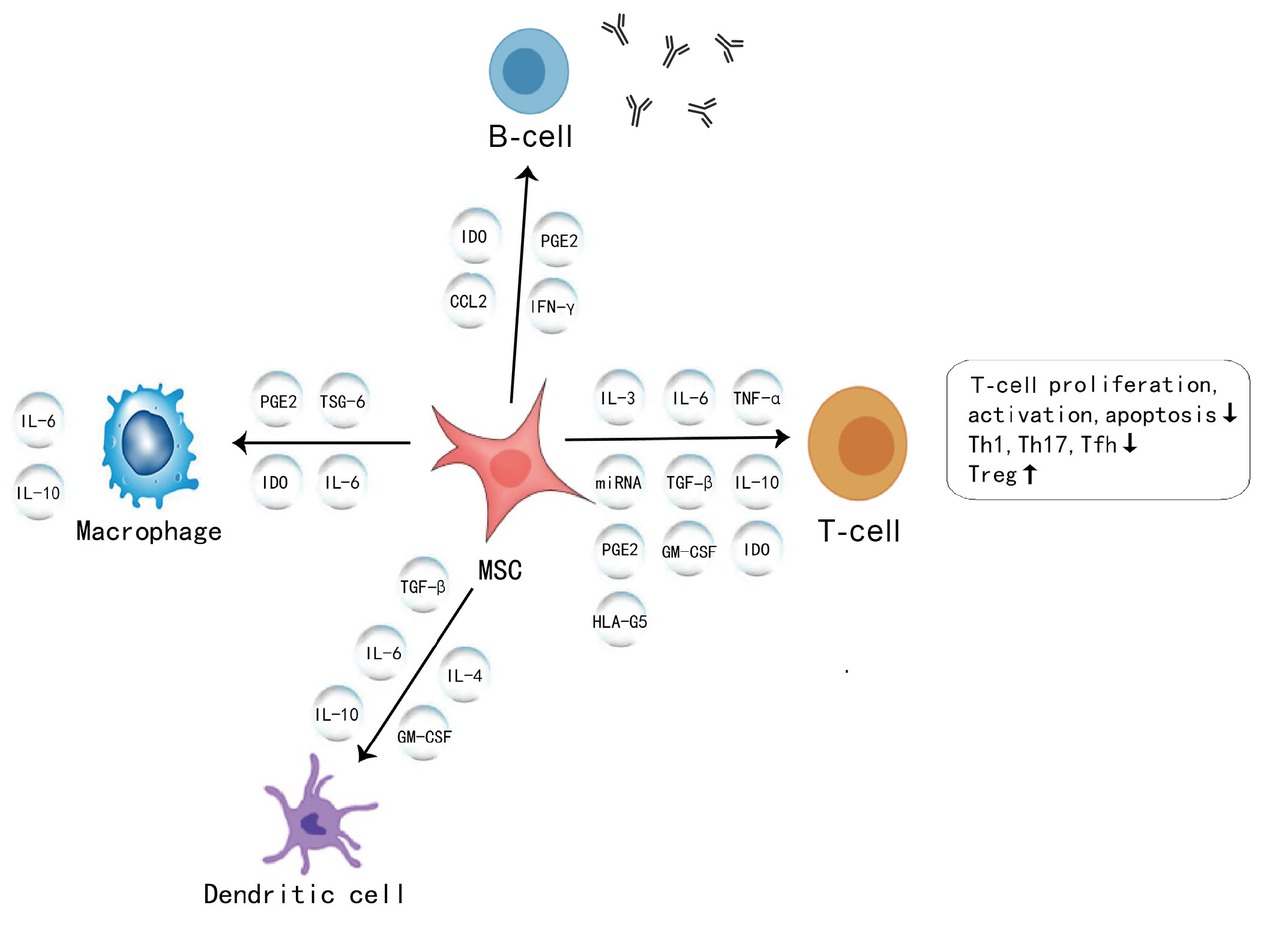
A diagrammatic representation of immunomodulatory properties of MSCs in connective tissue diseases. MSC: mesenchymal stromal cell; IL-16: interleukin-16; IFN-γ: interferon-γ; TNF: tumour necrosis factor; IDO: indoleamine 2,3-dioxygenase; PGE2: prostaglandin E2; CCL2: CC chemokine ligand 2; HLA-G5: human leukocyte antigen-G5; TSG-6: TNF-α–stimulated gene 6 protein; miRNA: microRNA; TGF-β: transforming growth factor β; GM-CSF: granulocyte– macrophage colony-stimulating growth factor; Th1: helper T-cell 1; Th 17: helper T-cell 17; Tfh: T follicular helper cells; Treg cells: regulatory T cells.
T-lymphocytes
Various types of MSCs have been proven to have the capacity to inhibit T cell responses and T-cell–mediated diseases.[15] Increased apoptosis of peripheral T cells has been reported in systemic lupus erythematosus (SLE) patients. MSCs could rescue T-cell by decreasing apoptosis, which is mediated by mitochondrial transfer.[16] However, the immunosuppressive effect of MSCs on T-cell proliferation could be reversed in the presence of TNF-α, and this is associated with an increase in the level of IL-6.[17] IL-3, a cytokine secreted by activated T-lymphocytes, was proven to enhance the migration of MSCs through the C–X–C motif chemokine receptor 4 (CXCR4)/SDF-1α axis.[18] Secretion of human leukocyte antigen-G5 (HLA-G5) by MSCs contributes to the direct inhibition of T-cell responses.[19] Accumulating evidence supports the crucial role of microRNAs in the therapeutic effects of MSCs. MSCs may upregulate microRNA (miR)-181a in T-lymphocytes to improve the efficacy of MSC-based therapies.[20] Th1 and Th17 cells that mediate responses to cartilage antigens and joint components are the important causes of joint inflammation in CTD. MSCs have been proven to ameliorate autoimmune arthritis by increasing the number of different subsets of T cells in the draining lymph nodes, including Th17 and Th1 cells.[21,22] The inhibition of Th1/Th17 responses has also been shown in the treatment of experimental arthritis by MSCs.[23] Epigenetic modification of MSCs can regulate the Th17-related immune responses and enhance the immunomodulatory potential of MSCs.[24]
It is well established that Treg cells are critical for the maintenance of immunological self-tolerance and immune homeostasis via TGF-β, IL-10, and the newly described IL-12 family member, IL-35. Darlan et al.[25] suggested that MSCs could upregulate the functional Treg cells by releasing TGF-β1 to control SLE disease activity. In rheumatoid arthritis (RA), the role of MSCs in controlling the development of the disease also depends on the induction of particular functional Treg cells.[15,22] IL-10 was found to be critical in the generation of Treg cells. MSCs increased the level of IL-10 in lymph nodes and joints and then induced de novo generation of Treg cells, thus restoring the regulatory/inflammatory balance.[22] Moreover, miR-663 impaired MSC-mediated regulation of Treg cells by inhibiting TGF-β1 production.[26] Meanwhile, miR-663 overexpression impaired the therapeutic effect of MSCs in vivo. Breaking the Th17/Treg balance could be responsible for the development of SLE. It is clear that MSCs can reset the immune balance by upregulating Tregs and downregulating Th17 cells, Th1 cells, and follicular helper T (Tfh) cells.[10] MSCs secrete TGF-β and PGE2 to mediate this process. Tregs/Th17 cells also influence the gut microbiota in MSC-treated RA via the aryl hydrocarbon receptor, which is a cytoplasmic receptor that modulates the response to environmental stimuli.[27] Cell-to-cell contact may hamper the immunoregulatory function of MSCs in CTD, thus, the use of a microencapsulation technology could provide additional benefits in MSC-based therapies.[28] MSC-derived exosomes and microparticles also have the capacity to decrease the percentage of T-cell subsets and increase the number of Treg cells in inflammatory arthritis.[29] T cells are a key source of GM-CSF cytokines, which are pivotal during the induction phase of CTD.[30] MSCs modify the early adaptive T-cell responses by reducing the total number of pathogenic GM-CSF+CD4+ (cluster of differentiation 4 receptors) T cells in RA.[22]
B-lymphocytes
B cells are of great importance in the pathogenesis of CTD through autoantibody-dependent and autoantibody-independent mechanisms. MSCs can inhibit B-lymphocyte proliferation, differentiation, and antibody secretion via many cytokines and chemokines. Che et al.[31] showed that CC chemokine ligand 2 (CCL2), a chemokine that induces the migration of monocytes and macrophages to inflammatory loci, played an important role in MSC-mediated B-cell immunoregulation. Schena et al.[32] noted that MSCs affected both follicular and marginal zone B-cell activation after stimulation with B-cell receptor (BCR) and Toll-like receptor 9 (TLR-9) agonists, which was dependent on IFN-γ and cell-to-cell contact. In the absence of BCR triggering, MSCs induced both polyclonal expansion and differentiation of B cells into plasma cells after being stimulated with a TLR-9 agonist.[33] Tfh cells promote the development of CTD by assisting B cells in producing autoantibodies. Alunno et al.[34] suggested an inhibitory effect of MSCs on circulating Tfh cells through the secretion of indoleamine 2,3-dioxygenase (IDO).
Macrophages and dendritic cells
Macrophages are a key component of innate immunity. MSC–macrophage interaction interactions occur during anti-inflammatory processes. Németh et al.[35] showed that MSCs could reprogram the IL-10 secretion signalling pathway of macrophages by releasing PGE2, and macrophages produce more IL-10 when cultured with MSCs. In addition, macrophages are re-educated to attenuate inflammation and facilitate repair due to the effects of MSCs, which manipulate their metabolic programs.[36] The anti-inflammatory protein TSG-6 secreted by the MSCs suppresses nuclear factor-κB (NF-κB) signalling in resident macrophages through the CD44 receptor.[9]
Dendritic cells (DCs) are professional antigen-presenting cells characterized by a unique primary immune response initiation ability. Regulation of DCs differentiation is relevant to the immunoregulatory function of MSCs. MSCs inhibit the differentiation and function of monocyte-derived DCs via either cell-to-cell contact or soluble factors.[37] MSCs induce peripheral blood CD1c+DCs through a recombinant FMS-like tyrosine kinase-3 ligand (FLT3 L)–FLT3 interaction, which is a key regulator of DCs haematopoiesis.[38] MSCs can also drive mature DCs to differentiate into regulatory DCs and rescue them from apoptosis.[39] MSCs suppress IL-12 production by DCs and inhibit the initial differentiation of monocytes to DCs via GM-CSF and IL-4. These results further support the immunomodulatory function of MSCs in the treatment of CTD.
Preclinical studies of MSC therapies in CTD
Systemic lupus erythematosus
Studies have shown that BM-MSCs from patients with SLE are impaired in proliferation, cytokine production, and immune modulation, which might contribute to the pathogenesis of lupus.[40] BM-MSCs from active SLE patients were reported to have defective IDO production, which resulted in less responsiveness to IFN-γ and CD8+ T-cell proliferation.[41] Abnormal expression of cytokines such as IL-10 and TGF-β was also found in lupus BM-MSCs.[42] Moreover, lupus BM-MSCs exhibited increased apoptosis, which was reflected by higher intracellular reactive oxygen species and increased levels of senescence-related genes in MSCs from SLE patients.[43] Thus, SLE was speculated by some experts to be a stem cell disease.
Current therapies for SLE are mainly aimed at mitigating disease activity, and the drugs have considerable side effects. Thus, MSCs have attracted much attention as a novel therapeutic cell type for lupus. The efficacy of MSC therapies in SLE animal models is summarized in Table 1. A total of 28 preclinical studies evaluated MSC treatment in animal models of lupus nephritis (LN).[44] These studies showed that MSC therapies resulted in a reduction of anti-double-stranded DNA (anti-dsDNA) antibody, antinuclear antibody (ANA), serum creatinine, blood urea nitrogen, and proteinuria levels. Other studies using murine SLE models demonstrated that allogeneic MSC transplantation could also decrease the levels of cytokines, autoantibody, and complement 3 (C3) deposition in glomeruli and prolong the lifespan.[45, 46, 47] Transplantation of human MSCs has also been found to ameliorate severe bone reduction through IL-17 suppression in an MRL/lpr SLE model.[48] The wide variation in the origin of MSCs, including UC-MSCs, BM-MSCs, AD-MSCs, and gingiva-derived MSCs, is one of the variables commonly encountered in preclinical studies. UC-MSCs were reported to be more potent immunosuppressors and less immunogenic than BM-MSCs.[49] However, other in vitro and in vivo studies showed that there was very little difference in the effect of different MSCs.[50] In addition, some new subsets of BM-MSCs, including those lacking the ability to adhere to plastic culture dishes, were identified and they showed increased immunomodulatory capacity in treating SLE mice compared to regular BM-MSCs. The efficacy of MSCs based on their source of origin remains controversial. The interaction between MSCs and other drugs has also been investigated. Rapamycin, an inhibitor of the mammalian target of rapamycin (mTOR) signalling pathway, was proven to reverse the senescent phenotype and improve the immunoregulatory capacity in MRL/ lpr mice.[51] Moreover, metformin-treated AD-MSCs had stronger anti-inflammatory effects mediated through signal transducer and activator of transcription 1 (STAT1).[52] Mutually reinforcing the interaction between IL-37 and MSCs enhanced immunosuppression in vivo in terms of improved survival and reduced signs of SLE.[53] These studies may help to improve the therapeutic potential of MSCs in the future.
Preclinical studies investigating the efficacy of MSCs in CTD animal models
| Author (year) | Disease | Species, model | Groups | MSC origin | MSC dose | Infusion | Efficacy | Follow-up (weeks) |
|---|---|---|---|---|---|---|---|---|
| Akiyama et al. (2012)[72] | SLE | Mouse, MRL/ lpr | PBS group (n = 5) MSC group (n = 24) | BM | 1 × 105 cells/10 g body weight | Intravenous | Proteinuria↓, mesangial proliferation↓, anti-dsDNA↓, ANA↓, serum albumin↑ | 2 |
| Chang et al. (2011)[45] | SLE | Mouse, NZB/W F1 | Control group (n = 8) MSC group (n = 15) | UC | 1 × 106 cells | Intravenous | Proteinuria↓, creatinine↓, anti-dsDNA↓, mesangial proliferation and sclerosis↓, survival rates↑ | 8 |
| Cheng et al. (2021)[73] | SLE | Mouse, MRL/ lpr | Control group (n = 5) PBS group (n = 5) MSC group (n = 5) | UC | 5 × 105 cells | Intravenous | Proteinuria↓, kidney lesion↓ | 4 |
| Choi et al. (2016)[74] | SLE | Mouse, MRL/ lpr | Control group (n = 20) C3H/HeJ normal group (n = 15) CTX group (n = 20) MSC group (n = 20) | AD | 1 × 106 cells/2 weeks (18 times) | Intravenous | anti-dsDNA↓, survival rates↑ | 35 |
| Choi et al. (2012)[75] | SLE | Mouse, NZB/W F1 | Control group (n = 41) MSC group (n = 56) CTX group (n = 14) | AD | 5 × 105 cells (28 times) or 2 × 106 cells (11 times) | Intravenous | Proteinuria↓, BUN↓, anti-dsDNA↓, survival rates↑ | 54 |
| Dang et al. (2020)[76] | SLE | Mouse, NZM2328 | PDF control group MSC group | GMSCs | 2 × 106 cells | Intravenous | Proteinuria↓, anti- dsDNA↓, survival rates↑ | 23 |
| Gu et al. (2016)[51] | SLE | Mouse, MRL/ lpr | Control group (n = 12) RAPA group (n = 12) MSC group (n = 24) | BM | 1 × 106 cells | Intravenous | 24 h proteinuria↓, anti-dsDNA↓, survival rates↑, glomerular sclerosis↓, interstitial fibrosis↓ | 12 |
| Jang et al. (2020)[52] | SLE | Mouse, MRL/ lpr | Control group (n = 6) MSC group (n = 10) | AD | 1 × 106 cells (8 times) | Intravenous | Proteinuria↓, anti-dsDNA↓, glomerulonephritis↓ | 7 |
| Ma et al. (2015)[48] | SLE | Mouse, MRL/ lpr | PBS group MSC group | BM | 1 × 105 cells/10 g body weight | Intravenous | Secondary bone loss↓, osteoblast and osteoclast dysregulation↓ | 4 |
| Xu et al. (2020)[53] | SLE | Mouse, MRL/ lpr | PBS group MSC group | BM | 1 × 106 cells (4 times) | Intravenous | Proteinuria↓, renal pathologic score↓, survival rates↑, total antibody↓, anti-dsDNA↓, ANA↓ | 7 |
| Ahmed et al. (2021)[12] | RA | Rat, CFA subcutaneous | Normal group CFA group CFA + MSC group CFA + IMC group CFA + IMC + MSC group | BM | 1 × 106 cells (4 times) | Intravenous | Right hind leg paw diameter and circumference↓, serum anti-CCP↓ | 3 |
| Papadopoulou et al. (2012)[77] | RA | Rat, rAIA and SSEA | PBS group Bortezomib group MSC group Bortezomib + MSC group | BM | rAIA: 20 × 106 cells (2 times) SSEA: 12 × 106 cells (2 times) | Intraperitoneal | Histologic improvement | NA |
| Park et al. (2011)[78] | RA | Mouse, CIA | PBS group MSC group | BM | 1 × 106 cells | Intraperitoneal | Bone erosion↓, cartilage destruction↓ | NA |
| Rui et al. (2016)[79] | RA | Mouse, CIA | PBS group BM-MSC group OE-MSC group | OE or BM | 1 × 106 cells (2 times) | Intravenous | Arthritis onset↓, disease severity↓, anti-CII↓ | 3 |
| Shin et al. (2016)[80] | RA | Mouse, CIA | Negative control (n = 5) Positive control (n = 10) Etanercept group (n = 7) Fibroblast-injected group (n = 7) MSC group (n = 10) | UC | 1 × 106 cells (1 or 5 times) | Intraperitoneal | Clinical severity of CIA↓, histologic damages↓ | 3 |
| Wei et al. (2021)[81] | RA | Rat, CIA | PBS group MSC group | BM | 2 × 106 cells | Intraarticular | Ankle swelling↓, joint destruction↓, articular index score↓, ankle circumference↓ | 7 |
| Wu et al. (2020)[82] | RA | Mouse, CIA | PBS group (n = 5) MSC group (n = 5) | GMSCs | 2 × 106 cells | Intravenous | Anti-dsDNA↓, anti-CII↓, cartilage damage↓, histologic damages↓ | 7 |
| Yu et al. (2019)[83] | RA | Mouse, CIA | PBS group MSC group (n = 5 in each group) | UC | 1 or 3 or 5× 106 cells (3 times) | Intravenous | Clinical arthritis score↓, cartilage damage↓ | 3 |
| Jiang et al. (2017)[84] | SSc | Mouse, bleomycin subcutaneous | PBS group (n = 6) MSC group (n = 12) | BM | 1 × 106 cells | Subcutaneous | Skin fibrosis and apoptosis↓ | 2 |
| Jin et al. (2021)[68] | SSc | Mouse, bleomycin subcutaneous | PBS group (n = 6) MSC group (n = 12) | BM | 1 × 106 cells | Subcutaneous | Skin fibrosis↓, collagen content↓ | 2 |
| Maria et al. (2016)[85] | SSc | Mouse, HOCI intradermal | Control group (n = 4) MSC group (n = 4) | AD | 2.5 × 105 cells | Intravenous | Skin and lung fibrosis↓, collagen content↓ | 3 |
| Maria et al. (2018)[69] | SSc | Mouse, HOCI intradermal | HOCI Control (n = 7–10) PBS group (n = 7–10) MSC group (n = 7–10) | BM | 2.5 × 105 cells | Intravenous | Skin inflammation↓, Skin thickness↓, collagen content↓ | 3 |
| Yang et al. (2020)[86] | SSc | Mouse, bleomycin subcutaneous | Control group (n = 10) SSc group (n = 10) MSC group (n = 10) | UC | 1 × 106 cells (2 times) | Intravenous | Skin fibrosis↓, collagen synthesis↓, local inflammation↓ | 2 |
| Okamura et al. (2020)[67] | SSc | Mouse, bleomycin intradermal | PBS group (n = 5) MSC group (n = 5) | AD | 2 × 105 cells | Intravenous | Skin and lung fibrosis↓, immune cell infiltration into the skin↓ | 4 |
| Aluri et al. (2012)[70] | pSS | Mouse, NOD | PBS group (n = 10) MSC group (n = 10) | BM | 1 × 106 cells | Intraperitoneal | Tear production↑ | 4 |
| Xu et al. (2012)[87] | pSS | Mouse, NOD | Control group (n = 6) MSC group (n = 12) | BM | 1 × 105 cells | Intravenous | Saliva flow rate↑, inflammatory area in SG↓ | 8 or 18 |
| Tian et al. (2020)[71] | pSS | Mouse, SG protein subcutaneous | Control group (n = 6) MSC group (n = 6) | BM | 5 × 105 cells (2 times) | Intravenous | Saliva flow rates↑, serum autoantibodies↓, ANA↓ | 2 |
ANA: antinuclear antibody; CTD: connective tissue disease; MSC: mesenchymal stem cells; SLE: systemic lupus erythematosus; RA: rheumatoid arthritis; SSc: systemic sclerosis; pSS: primary Sjögren’s syndrome; PDF: primary dermal fibroblast; RAPA: rapamycin; HOCI: hypochlorite; SG: salivary gland; CFA: complete Freund’s adjuvant; IMC: indomethacin; rAIA: adjuvant-induced arthritis; SSEA: severe arthritis spontaneously; CIA: collagen-induced arthritis; PBS: phosphate-buffered saline; CTX: cyclophosphamide; BM: bone marrow-derived MSCs; UC: human umbilical cord-derived MSCs; AD: human adipose tissue-derived MSCs; OE: olfactory ecto-MSCs; GMSCs: gingiva-derived MSCs; BUN: blood urea nitrogen; NA: not available. The arrow pointing up indicates an increase in a numerical value, and the arrow pointing down indicates a decrease.
An important advantage of MSC therapies using MSCs is that they exert therapeutic effects in various ways. B cells play an important role in the pathogenesis of SLE through autoantibody-dependent mechanisms. Ma et al.[54] reported that MSCs could inhibit B cell activation and immunoglobulin production via suppression of the B cell activating factor (BAFF). Lee et al.[55] recently reported that MSCs inhibited mouse T cells in the early stage after injection, whereas priming of MSCs with IFN-γ improved their ability to inhibit B cells in SLE.
Meanwhile, epigenetic modification, such as DNA methylation, is also crucial in the mechanisms underlying MSC treatment. MSCs could rescue the global hypomethylation pattern of the recipient BM-MSCs and improve osteopenia in mice.[56] Furthermore, epigenetic modulation appears to constitute a promising experimental target in MSC research. Kim et al.[57,58] reported that a combination of hypomethylating agents and histone deacetylase inhibitors could increase the gene expression of IL-10 and IDO in MSCs, which enhanced the Th17-related immune responses of MSC-based therapy in RA. Accumulating evidence has shown that priming of MSCs with epigenetic modifiers may enhance cell proliferation, cell survival, and cell differentiation potential, but more studies are needed in CTD. MSC transplantation in animal models helps us to better understand the function of MSCs in vivo, which might be useful to generate more responsive MSCs in different SLE host microenvironments.
Rheumatoid arthritis
Reports have shown that the function of MSCs is altered in RA patients. Anti-citrullinated protein antibodies (ACPAs) have been identified as an important biomarkers for the aetiology of RA. It has been proven that ACPAs can reduce the efficacy of BM-MSCs by increasing IL-6, IL-8, and CCL2 expression and decreasing the production of IDO.[59] Another defect of BM-MSCs from RA patients is the loss of A20, also called TNF-α–induced protein 3, which leads to increased IL-6 secretion and further affects the pathogenesis of RA.[60] It is important to explore the functional features of MSCs in RA patients, as they may be a potential targets for the cell-based therapies.
There are more than 100 articles that have reported the use of MSCs in experimental models of RA. Among these, the most widely used animal model was collagen-induced arthritis (CIA) mice. The most commonly used routes of delivery were intravenous (IV), intraperitoneal, intra-articular, intramuscular, and subcutaneous delivery. The effective dosage of MSCs reported was in the range of 2 × 106–3 × 106 cells per injection, as noted in a meta-analysis preclinical studies.[61] In some studies, a single MSC injection could prevent the occurrence of bone damage and lead to disease remission, without the need for multiple administrations.[15,62] In most of the studies, the efficacy of MSC treatment was evaluated by joint swelling, histologic assessment, serum antibody, and joint imaging (Table 1). Most of the studies concluded that MSC therapies were effective in reducing inflammation, joint swelling, and cartilage destruction in RA without adverse effects. However, some studies have demonstrated that allogeneic and syngeneic transplantation do not influence the disease course of RA.[63,64] These conflicting results suggest that further studies investigating the effect of different sources, quantities, and administration regimens of MSCs are warranted. MSCs can interact with many immune cells via both direct contact and secreted anti-inflammatory factors. In preclinical studies of RA, the therapeutic effect of MSCs was mediated by downregulating Th1 and Th17 cells and upregulating Treg cells.[21,23] Furthermore, an increase in anti-inflammatory factors such as TGF-β and IL-10,[65] together with a reduction in TNF-α, IL-6, and monocyte chemoattractant protein-1 have been reported.[65]
Recently, some researchers have developed novel approaches to improve the efficacy and long-lasting beneficial effects of MSCs in arthritis. Cosenza et al.[29] suggested that MSC-derived exosomes, one of the main types of EVs containing a large variety of proteins, messenger RNAs, and miRNAs, were efficient in suppressing inflammation in inflammatory arthritis. In addition, a three-dimensional (3D) priming strategy to improve the efficacy of the resulting UC-MSC secretomes induced a faster remission of local and systemic RA manifestations, compared to secretomes produced under conventional two-dimensional (2D) monolayer conditions.[66] The therapeutic potential of exosomes has attracted increasing attention because they reduce the risks of triggering immune reactions against MSCs. TNF-α plays a critical role in the pathogenesis of RA. Liu et al.[14] genetically modified MSCs to deliver human soluble TNF receptor II (hsTNFR). They reported that the sTNFR-transduced rat MSCs have the same effect of inhibiting joint inflammation as etanercept, which is a recombinant fusion protein of hsTNFR used to treat RA. Moreover, combined MSC and IL-4 therapy showed decreased rheumatoid factor (RF) and C-reactive protein (CRP) levels, compared to MSCs alone.[13] All of these favorable results achieved in preclinical studies demonstrated that modified MSCs might have improved safety and efficacy in the clinic.
Other CTDs
MSC therapies have been used in several animal models of systemic sclerosis (SSc) including bleomycin-induced models and hypochlorite (HOCI)-injected models (Table 1). In the bleomycin-induced SSc model, a single infusion of 2 × 105 AD-MSCs attenuated the skin and lung fibrosis by inhibiting the infiltration of T cells and macrophages into the dermis.[67] The effect of UC-MSCs on skin fibrosis and collagen formation was confirmed in this model. At present, BM-MSC-derived EVs have also been proven to be effective in treating skin dysfunction and fibrosis of skin.[68] The injection time point after disease induction is an important factor affecting the impact of MSCs during the fibrogenesis process. Maria et al.[69] identified a three-phase process leading to skin fibrosis in the HOCI-induced model, and MSC-based therapies exerted different benefits in all these three phases through immunosuppressive, trophic, or regenerative properties. This team also indicated that iNOS is required for the antifibrotic function of MSCs in HOCI-SSc mice, which highlighted the importance of the antioxidant activity of MSCs in future applications. Taken together, MSCs have immunosuppressive, antifibrotic, and antioxidative properties, all providing a promising approach for the treatment of SSc.
Preclinical studies in primary Sjögren’s syndrome (pSS) have revealed the expected capacity of MSCs in controlling the inflammation and preserving salivary function. A single intraperitoneal injection of BM-MSCs into a NOD mouse model was able to increase the tear production, although the number of lymphocytic foci in the lacrimal glands did not decrease.[70] In another study, BM-MSCs alleviated the disease progression in an experimental Sjögren’s syndrome model induced by salivary gland (SG) protein by upregulating the immunosuppressive effect of myeloid-derived suppressor cells.[71] Further experiments are needed to explore in detail the underlying mechanisms of improved lacrimal gland function after MSC transplantation.
Clinical evidence of MSC therapies in CTD
Systemic lupus erythematosus
Some SLE patients do not respond to conventional immunosuppressive and immunomodulatory therapies and are in urgent need of newer therapeutic approaches. Over the last few decades, increasing evidence has suggested that MSCs are a potential therapeutic tool for refractory SLE. The first case series study using allogenic MSCs in SLE patients was published in 2009.[88] Four LN patients enrolled in this study were drug resistant, and all achieved stable remission after MSC therapy for 12–18 months. Subsequently, several initial pilot studies that involving patients with severe LN or multiorgan involvement reported that systemic administration of BM-MSCs or UC-MSCs decreased disease activity and improved renal function (Table 2). Meanwhile, few serious adverse effects attributed to the MSCs were observed. In these studies, the popular treatment regimens were transfusions for one to three times, each dosed at 1 × 106 cells/kg body weight. The transplanted stem cells were derived from either the bone marrow of healthy donors or umbilical cords from healthy mothers after normal deliveries. A multicentre clinical study including 40 active SLE patients showed that MSCs resulted in a significant decline of the SLE disease activity index (SLEDAI) and British Isles Lupus Assessment Group (BILAG) scores and ameliorated the hematological and cutaneous manifestations.[89] In their investigation of the long-term efficacy and safety, Wang et al. reported the long-term follow-up results of MSC-treated patients in their series of cohort studies.[90, 91, 92] The 5-year disease remission rate was 34%, and the 5-year overall rate of relapse was 24%.[90] To date, there have been no reports of MSC-associated tumour formation or changes in serum tumour biomarkers after infusion in SLE patients. A double-blind placebo-controlled trial was conducted in 18 newly diagnosed severe LN patients from a single centre. However, this study showed that UC-MSCs did not achieve a better therapeutic effect than standard immunosuppressive therapies.[93] This controversial result indicated that there still remain some challenges in designing appropriate study protocols for randomized controlled trial (RCT) to determine the efficacy of MSCs for SLE.
Clinical studies of MSCs in connective tissue diseases
| Author (year) | Disease | Study design | Patients (n) | MSC type | MSC dose | Infusion | AE | Efficacy | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| Barbado et al. (2018) [112] | SLE | Case series | 3 | BM | 9 × 107 cells | Intravenous | None | 24 h proteinuria↓, SLEDAI↓, drug dosage↓ | 9 |
| Wang et al. (2017)[92] | SLE | Observational | 9 | UC | 1 × 106 cells/kg (2 times) | Intravenous | Mild dizzy and warm sensation (n = 1) | Normal liver function, no newly onset abnormality on electrocardiogram and chest radiography, no rise in serum tumour markers | 72 |
| Wang et al. (2013)[91] | SLE | Observational | 87 | UC, BM | 1 × 106 cells/kg | Intravenous | Nontreatment- related events (n = 5) | Overall rate of survival (94%), complete clinical remission (28%, 31%, 42%, and 50% at 1, 2, 3, and 4 years, respectively) | 27 |
| Wen et al. (2019)[113] | SLE | Observational | 69 | UC, BM | 1 × 106 cells/ kg (1–2 times) | Intravenous | NA | Low disease activity (58%), clinical remission (23%) | 12 |
| Wang et al. (2018)[90] | SLE | Observational | 81 | UC, BM | 1 × 106 cells/ kg (1–3 times) | Intravenous | Death (n = 15), diarrhoea (n = 2), herpesvirus infection (n = 3), tuberculosis infection (n = 2), Klebsiella pneumoniae pneumonia (n = 2), cryptococcal meningitis (n = 1) | Five-year overall survival rate (84%), complete remission (27%), partial clinical remission (7%) | 60 |
| Wang et al. (2014)[89] | SLE | Single arm | 40 | UC | 1 × 106 cells/kg | Intravenous | Herpesvirus infection (n = 4), death (n = 3) | SLEDAI↓, BILAG↓, 24 h proteinuria↑, anti-dsDNA↓, ANA↓ | 12 |
| Sun et al. (2010)[114] | SLE | Single arm | 16 | UC | 1 × 106 cells/kg | Intravenous | Severe nausea (n = 1) | SLEDAI↓, ANA↓, anti-dsDNA↓, serum albumin↑, C3↑, renal function↑ | 8.25 |
| Liang et al. (2018)[115] | SLE, pSS, SSc | Observational | 404 | UC, BM | 1 × 106 cells/kg | Intravenous | Malignancies (n = 5), death (n = 45), transplantation- related mortality (n = 1), infection (n = 119), serious infection (n = 52) | The 5- and 8-year survival rates were 90.4% and 88.9%, respectively | 43.4 ± 25.9 |
| Liang et al. (2010)[116] | SLE | Single arm | 15 | BM | 1 × 106 cells/kg | Intravenous | None | SLEDAI↓, 24 h proteinuria↓, anti-dsDNA↓, GFR↓ | 12 |
| Li et al. (2013)[117] | SLE | Single arm | 35 | UC, BM | 1 × 106 cells/ kg (2–3 times) | Intravenous | Uncontrolled disease recurrence after infection (n = 2) | Blood cell count↑↓, disease activity↓ | 21 |
| Deng et al. (2017)[93] | SLE | RCT | 18 | UC | 2 × 108 cells | Intravenous | Leukopenia, pneumonia, and subcutaneous abscess (n = 1), severe pneumonia (n = 1) | Remission occurred in 75% versus 83% (placebo) | 12 |
| Wang et al. (2013)[100] | RA | RCT | 172 | UC | 4 × 104 cells (2 times) | Intravenous | Chills or fever (≤38.5°C) (n = 6) | DAS28↓, HAQ↓ | 8 |
| Yang et al. (2018)[118] | RA | RCT | 105 | UC | 1 × 106 cells/kg | Intravenous | Chills or fever (≤39°C) (n = 3) | DAS28↓, drug dosage↓ | 12 |
| Wang et al. (2019)[99] | RA | Single arm | 64 | UC | 4 × 107 cells | Intravenous | None | ESR↓, CRP↓, RF↓, anti- CCP↓, health index↓, DAS28↓ | 36 |
| Shadmanfar et al. (2018) [102] | RA | RCT | 30 | BM | 42 ± 4 × 106 Cells | Intraarticular | Minor AEs: postimplantation pain and/ or articular swelling | WOMAC score↑, VAS score↓, standing time↑ | 12 |
| Park et al. (2018)[105] | RA | Single arm | 9 | UC | 2.5 × 107, 5 × 107, or 1 × 108 cells | Intravenous | None | DAS28↓ | 1 |
| He et al. (2020)[107] | RA | RCT | 63 | UC | 1 × 106 cells/kg | Intramuscular | None | ACR20 response rates were 53.3% in patients with MSCT monotherapy and 93.3% in patients with MSCT combined with IFN-γ treatment | 12 |
| Ghoryani et al. (2020) [103] | RA | Single arm | 13 | BM | 1 × 106 cells/kg | Intravenous | None | DAS28-ESR↓ | 12 |
| Ghoryani et al. (2019) [104] | RA | RCT | 9 | BM | 1 × 106 cells/kg | Intravenous | None | DAS28-ESR↓, VAS score↓, no significant difference was found in serum CRP and anti-CCP | 12 |
| Alvaro- Gracia et al. (2017)[101] | RA | RCT | 53 | AD | 1, 2, and 4 × 106 cells/kg (3 times) | Intravenous | Severe AEs (n = 8): lacunar infarction, diarrhoea, tendon rupture, rheumatoid nodule and arthritis, sciatica, asthenia | ACR20 responses were 20%–45% versus 29% (placebo) at month 1 and 15%–25% versus 0% at month 3 | 6 |
| Del Papa et al. (2015) [119] | SSc | Single arm | 15 | AD | 0.5–1 mL | NA | None | Blood flow without improvement, pain↓, improvement in ulcers without the appearance of new ones, number of capillaries↑ | 6 |
| Takagi et al. (2014)[120] | SSc | Single arm | 11 | BM | 0.4–5.1 × 1010 cells | Intramuscular | Amputation (n = 1, osteomyelitis prior to treatment) | Complete resolution of ulcer size (n = 9), recurrence (n = 1), VAS score↓, TcPO2↑ | 1–24 |
| Kamata et al. (2007) [121] | SSc | Case series | 4 | BM | 3.5 × 108 cells | Intramuscular | NA | VAS score↓, arterial flow↓ (n = 1) | 12 |
| Keyszer et al. (2011) [108] | SSc | Case series | 5 | BM | 1 × 106 cells/kg | Intravenous | Mild respiratory tract infections (n = 4) | Temporary MRSS↓, improved acral necrosis, oxygen saturation over the involved tissue↑ | 6 |
| Park et al. (2020)[109] | SSc | Single arm | 20 | AD | 3.61 × 106 cells/ finger | Subcutaneous in hand | None | Skin fibrosis↓, hand oedema↓, 31.6% of active ulcers were healed at 24 weeks after injections | 6 |
| Zhang et al. (2017)[110] | SSc | Single arm | 14 | UC | 1 × 106 cells/kg | Intravenous | Minor respiratory tract infection (n = 5), diarrhoea (n = 1) | MRSS↓, anti-Scl70↓, improvement of lung function and CT images in three ILD patients | 12 |
| Xu et al. (2012)[87] | pSS | Single arm | 24 | UC | 1 × 106 cells/kg | Intravenous | None | SSDAI score↓, anti-SSA/ Ro production↓, salivary flow rate↑, improved refractory haemolytic anaemia | 12 |
| Wang et al. (2011)[111] | IIM | Single arm | 10 | BM or UC | 1 × 106 cells/kg | Intravenous | Disease recurrence (n = 2) | Improvements in CK, CK- MB, and muscle strength | 6 |
MSC: mesenchymal stem cell; SLE: systemic lupus erythematosus; RA: rheumatoid arthritis; SSc: systemic sclerosis; pSS: primary Sjögren’s syndrome; IIM: idiopathic inflammatory myopathies; RCT: randomized controlled trial; DM: dermatomyositis; PM: polymyositis; BM: bone marrow-derived MSCs; UC: human umbilical cord-derived MSCs; AD: human adipose tissue-derived MSCs; AEs: adverse events; SLEDAI: the SLE disease activity index; BILAG: British Isles Lupus Assessment Group; GFR: glomerular filtration rate; DAS28-ESR: disease activity score 28-ESR; HAQ: Health assessment questionnaire; WOMAC: Western Ontario and McMaster Universities Arthritis Index; VAS: visual analog scale; ACR: American College of Rheumatology; TcPO2: transcutaneous (partial) pressure of oxygen; MRSS: modified Rodnan skin score; CT: computed tomography; ILD: interstitial lung disease; CK: serum creatine kinase; NA: not available. The arrow pointing up indicates an increase in a numerical value, and the arrow pointing down indicates a decrease.
Although MSCs have powerful anti-inflammatory properties in SLE, a challenge for improving their therapeutic efficacy is that their mechanism of action is still unclear. In LN patients, MSC transplantation inhibited the common complement terminal pathways, which may contribute to controlling excessive complement activation in glomerular diseases.[94] In addition, MSCs can engulf accumulated apoptotic cells and subsequently exhibit enhanced immunosuppression via PGE2 activation. In MSC-treated SLE patients, the plasma PGE2 levels increased significantly, which revealed another mechanism active in MSC-based therapy in SLE.[95] By analysing the baseline cytokine levels and the treatment effect of MSCs, serum IFN-γ was proven to be a potential predictor of a clinical response to MSC therapy.[96] The pathogenesis of SLE is very complicated, and a certain subgroup of SLE patients would benefit from MSC treatment. Further clinical and molecular studies are needed to explore the underlying mechanisms.
Rheumatoid arthritis
RA is an autoimmune disease that results in progressive joint damage and multiorgan comorbidities. The conventional therapies for RA are corticosteroids, nonbiologic disease-modifying antirheumatic drugs (DMARDs), and biologic agents. Recently, dozens of clinical trials on MSCs for RA have been conducted (Table 2) based on the evidence that they can regulate the immune response, stimulate injury repair, and reduce the inflammatory response, as shown in preclinical studies.[97]
The first pilot study of MSCs in RA was reported in 2011.[98] Three patients were infused IV with autologous in one, two, or four doses.[98] This study proved that multiple infusions of up to 8 × 108 of AD-MSCs were safe and potentially effective. In another pilot study of a single IV infusion of autologous BM-MSCs in nine RA patients, significant decreases in the 28-joint disease activity score using erythrocyte sedimentation rate (ESR) (DAS28-ESR) and the visual analog scale (VAS) score were observed at 1 and 12 months after MSC transplantation. Serum anti-CCP showed no significant difference after the intervention. To assess the long-term safety and efficacy of MSC-based therapies in RA, Wang et al. conducted a 3-year prospective Phase I/II study in 64 RA patients.[99] They found improvements in ESR, CRP, RF, and DAS28 after 1 year and 3 years of UC-MSC treatment. Recently, several randomized placebo-controlled trials of autologous or allogeneic MSCs for RA were conducted. In a single-centre RCT with 172 patients, the MSC-treated group (n = 136) received DMARDs plus 4 × 107 UC-MSCs, while the control group received DMARDs plus medium.[100] The therapeutic effects of the MSCs were maintained for 3–6 months according to the American College of Rheumatology (ACR) criteria and DAS28 without serious adverse events. In a multicentre Phase Ib/IIa trial, 53 patients were treated with three IV infusions of AD-MSCs: 1 × 106/kg, 2 × 106/kg, 4 × 106/ kg or placebo. There was one dose-limiting toxicity event, and a lacunar infarction occurred. Signs of clinical efficacy were observed in the ACR20 responses in all 4 groups.[101] In 2018, Shadmanfar et al.[102] conducted an RCT of intra-articular knee implantation of BM-MSCs in RA.[102] The MSC group had a superior effect according to the VAS, the Western Ontario and McMaster Universities Arthritis Index (WOMAC), and the standing time. In summary, although the safety and efficacy of MSC therapies were reported in RA, there was a great heterogeneity in terms of the sources of MSCs, cell dosing, and routes of delivery. Further studies are needed to determine the optimal regimen and identify the subgroup of RA patients who are most likely to respond to MSC treatment.
Numerous immune responses and mechanisms of action have been explored in MSC-treated RA patients. Substantial changes in the serum levels of cytokines and T-cell subtypes, such as TGF-β, TNF-α, IL-10, IL-17, IFN-γ, Th17, and Treg cells, were detected after transplantation of MSCs into patients with refractory RA.[103, 104, 105] Recent studies also showed that the functions of MSCs could be affected by the inflammatory milieu, and some novel strategies have been employed to improve the ability of MSCs to modulate anti-inflammatory actions and promote tissue repair.[106,107] He et al.[107] demonstrated that the combination of IFN-γ and MSCs was safe and could synergistically improve the clinical efficacy. However, the safety profile of recombinant therapies still needs to be considered.
Other CTDs
There is evidence of efficacy of MSC therapies in other CTDs. The proangiogenic and antifibrotic properties of MSCs provide a strong rationale for their use in SSc. In 2011, a German team reported five cases of severe progressive SSc treated with BM-MSCs.[108] No immediate toxicity or severe infection was noted. The BM-MSCs exerted a marked effect on the healing of the skin ulcers. The stromal vascular fraction (SVF) consisting of AD-MSCs, as well as growth factors and cytokines, has been reported as a potential therapeutic option in SSc. A single-centre pilot study investigated the clinical efficacy of autologous SVF injection into each finger of 20 SSc patients with hand disability.[109] The amelioration of skin fibrosis was prominent, and 31.6% of active ulcers were healed at the 24-week follow-up. Additionally, the combined treatment effects of MSCs with other therapies have also been reported. Zhang et al.[110] investigated a combination of plasmapheresis and single MSC transplantation in 14 SSc patients. The modified Rodnan skin scores (MRSS) were significantly improved, and anti-topoisomerase I antibodies (anti-Scl70), serum TGF-β, and VEGF levels were also significantly decreased at 12 months of follow-up. In this trial, all three patients with interstitial lung disease had better pulmonary functions and improved computed tomography (CT) images. A relevant RCT has not yet been conducted for SSc.
One Chinese study investigated the responses to BM-MSC treatment in 24 pSS patients to treatment with BM-MSCs.[87] All patients had an improvement in the unstimulated salivary flow rate and oral dryness and a decrease in anti-SSA/anti-SSB without serious adverse events. Interestingly, this study also showed that the infused allogeneic MSCs migrated towards the inflammatory regions in an SDF-1–dependent manner, which indicated the key role of the SDF-1/CXCR4 signalling pathway in the immunoregulatory functions of MSCs. For idiopathic inflammatory myopathies (IIMs), a single-arm trial involving 10 drug-resistant polymyositis/ dermatomyositis patients was conducted by Wang et al.[111] in 2011. Improvements were seen in serum creatine kinase (CK) and CK-MB in eight patients, and amelioration of muscle strength was seen in all patients. Two patients died due to disease recurrence and infection. The numbers of enrolled pSS and IIM patients in the published papers are low, so more evidence is required to draw a conclusion.
Perspectives on the use of MSCs as a treatment for CTD
In this review, we summarized the current status of the immunological mechanisms, preclinical studies, and clinical trials of MSC therapies for CTDs. MSC-based therapies modulate inflammation by affecting different immune cells and maintaining a balance of highly complex biochemical and cellular interactions. Preclinical results showed a reduction in disease activity and the degree of organ involvement in various CTD models. These experimental models also help us to better understand the pathogenesis of stem cell dysfunction in CTDs and improve the efficacy and increase the duration of the effects of MSCs via cell engineering approaches or combination therapies. To date, the safety and efficacy of MSC-based therapies for CTD have been investigated in pilot studies, long-term follow-up studies, and small randomized controlled clinical trials in CTDs, especially for SLE and RA. These clinical trials showed a low rate of adverse events, and most of them demonstrated positive clinical outcomes. Clinical studies of MSC therapies in other CTDs, such as SSc, pSS, and IIM, are still in their early stages and are worthy of further study in well-designed controlled trials for future evaluation. Cell therapy with MSCs is a very attractive new approach to address unresolved treatment difficulties for patients with CTD. Current reports indicate that MSCs have favourable prospects for the treatment of CTD, but the evidence is insufficient to come to a definite conclusion. RCTs with large sample sizes and studies on specific organ involvement need to be conducted. A better understanding of the mechanisms of action underlying MSC therapies would contribute to the development of an optimized regimen and allow for exploring serum biomarkers of CTD patients to predict treatment outcomes.
-
Source of Funding
This study was supported by the Chinese National Key Technology R&D Program, Ministry of Science and Technology (2021YFC2501301-5, 2019YFC0840603, 2017YFC0907601,2017YFC0907604, 2017YFC0907605), CAMS Innovation Fund for Medical Sciences (CIFMS) (2021-I2M-1-005), The Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2019PT330004), Beijing Municipal Science & Technology Commission (No.Z201100005520022, 23, 25–27).
-
Conflict of Interest
The authors have no conflicts of interest to disclose.
-
Author Contributions
Li M, Zeng X, and Tian X conceived and designed this study. Shi Y and Jiang N searched for and extracted data from the included articles and drafted the manuscript. Tian X critically revised the manuscript. All authors have read and approved the final manuscript.
References
1 El-Jawhari JJ, El-Sherbiny Y, McGonagle D, Jones E. Multipotent Mesenchymal Stromal Cells in Rheumatoid Arthritis and Systemic Lupus Erythematosus; From a Leading Role in Pathogenesis to Potential Therapeutic Saviors? Front Immunol 2021;12:643170.10.3389/fimmu.2021.643170Search in Google Scholar PubMed PubMed Central
2 Mohamed-Ahmed S, Fristad I, Lie SA, Suliman S, Mustafa K, Vindenes H, et al. Adipose-derived and bone marrow mesenchymal stem cells: a donor-matched comparison. Stem Cell Res Ther 2018;9:168.10.1186/s13287-018-0914-1Search in Google Scholar PubMed PubMed Central
3 Li X, Bai J, Ji X, Li R, Xuan Y, Wang Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int J Mol Med 2014;34:695–704.10.3892/ijmm.2014.1821Search in Google Scholar PubMed PubMed Central
4 Du WJ, Chi Y, Yang ZX, Li ZJ, Cui JJ, Song BQ. et al. Heterogeneity of proangiogenic features in mesenchymal stem cells derived from bone marrow, adipose tissue, umbilical cord, and placenta. Stem Cell Res Ther 2016;7:163.10.1186/s13287-016-0418-9Search in Google Scholar PubMed PubMed Central
5 Liao J, Chang C, Wu H, Lu Q. Cell-based therapies for systemic lupus erythematosus. Autoimmun Rev 2015;14:43–8.10.1016/j.autrev.2014.10.001Search in Google Scholar PubMed
6 Munir H and McGettrick HM. Mesenchymal Stem Cell Therapy for Autoimmune Disease: Risks and Rewards. Stem Cells Dev 2015;24:2091–100.10.1089/scd.2015.0008Search in Google Scholar PubMed
7 Sid-Otmane C, Perrault LP, Ly HQ. Mesenchymal stem cell mediates cardiac repair through autocrine, paracrine and endocrine axes. J Transl Med 2020;18:336.10.1186/s12967-020-02504-8Search in Google Scholar PubMed PubMed Central
8 Guiducci S, Manetti M, Romano E, Mazzanti B, Ceccarelli C, Dal Pozzo S, et al. Bone marrow-derived mesenchymal stem cells from early diffuse systemic sclerosis exhibit a paracrine machinery and stimulate angiogenesis in vitro. Ann Rheum Dis 2011;70:2011–21.10.1136/ard.2011.150607Search in Google Scholar PubMed
9 Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood 2011;118:330–8.10.1182/blood-2010-12-327353Search in Google Scholar PubMed PubMed Central
10 Wang D, Huang S, Yuan X, Liang J, Xu R, Yao G, et al. The regulation of the Treg/Th17 balance by mesenchymal stem cells in human systemic lupus erythematosus. Cell Mol Immunol 2017;14:423–31.10.1038/cmi.2015.89Search in Google Scholar PubMed PubMed Central
11 Greish S, Abogresha N, Abdel-Hady Z, Zakaria E, Ghaly M, Hefny M. Human umbilical cord mesenchymal stem cells as treatment of adjuvant rheumatoid arthritis in a rat model. World J Stem Cells 2012;4:101–9.10.4252/wjsc.v4.i10.101Search in Google Scholar PubMed PubMed Central
12 Ahmed EA, Ahmed OM, Fahim HI, Ali TM, Elesawy BH, Ashour MB. Potency of Bone Marrow-Derived Mesenchymal Stem Cells and Indomethacin in Complete Freund’s Adjuvant-Induced Arthritic Rats: Roles of TNF-α, IL-10, iNOS, MMP-9, and TGF-β1. Stem Cells Int 2021;2021:6665601.10.1155/2021/6665601Search in Google Scholar PubMed PubMed Central
13 Haikal SM, Abdeltawab NF, Rashed LA, Abd El-Galil TI, Elmalt HA, Amin MA. Combination Therapy of Mesenchymal Stromal Cells and Interleukin-4 Attenuates Rheumatoid Arthritis in a Collagen-Induced Murine Model. Cells 2019;8:823.10.3390/cells8080823Search in Google Scholar PubMed PubMed Central
14 Liu LN, Wang G, Hendricks K, Lee K, Bohnlein E, Junker U, et al. Comparison of drug and cell-based delivery: engineered adult mesenchymal stem cells expressing soluble tumor necrosis factor receptor II prevent arthritis in mouse and rat animal models. Stem Cells Transl Med 2013;2:362–75.10.5966/sctm.2012-0135Search in Google Scholar PubMed PubMed Central
15 Chen M, Su W, Lin X, Guo Z, Wang J, Zhang Q. Adoptive transfer of human gingiva-derived mesenchymal stem cells ameliorates collagen-induced arthritis via suppression of Th1 and Th17 cells and enhancement of regulatory T cell differentiation. Arthritis Rheum 2013;65:1181–93.10.1002/art.37894Search in Google Scholar PubMed PubMed Central
16 Chen J, Wang Q, Feng X, Zhang Z, Geng L, Xu T, et al. Umbilical Cord-Derived Mesenchymal Stem Cells Suppress Autophagy of T Cells in Patients with Systemic Lupus Erythematosus via Transfer of Mitochondria. Stem Cells Int 2016;2016:4062789.10.1155/2016/4062789Search in Google Scholar PubMed PubMed Central
17 Djouad F, Fritz V, Apparailly F, Louis-Plence P, Bony C, Sany J, et al. Reversal of the immunosuppressive properties of mesenchymal stem cells by tumor necrosis factor alpha in collagen-induced arthritis. Arthritis Rheum 2005;52:1595–603.10.1002/art.21012Search in Google Scholar PubMed
18 Barhanpurkar-Naik A, Mhaske ST, Pote ST, Singh K, Wani MR. Interleukin-3 enhances the migration of human mesenchymal stem cells by regulating expression of CXCR4. Stem Cell Res Ther 2017;8:168.10.1186/s13287-017-0618-ySearch in Google Scholar PubMed PubMed Central
19 Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25 high FOXP3+ regulatory T cells. Stem Cells 2008;26:212–22.10.1634/stemcells.2007-0554Search in Google Scholar PubMed
20 Zheng B, Zhang P, Yuan L, Chhetri RK, Guo Y, Deng D. Effects of human umbilical cord mesenchymal stem cells on inflammatory factors and miR-181a in T lymphocytes from patients with systemic lupus erythematosus. Lupus 2020;29:126–35.10.1177/0961203319896417Search in Google Scholar PubMed
21 Gonzalo-Gil E, Pérez-Lorenzo MJ, Galindo M, Díaz de la Guardia R, López-Millán B, Bueno C, et al. Human embryonic stem cell-derived mesenchymal stromal cells ameliorate collagen-induced arthritis by inducing host-derived indoleamine 2,3 dioxygenase. Arthritis Res Ther 2016;18:77.10.1186/s13075-016-0979-0Search in Google Scholar PubMed PubMed Central
22 Lopez-Santalla M, Mancheño-Corvo P, Menta R, Lopez-Belmonte J, DelaRosa O, Bueren JA, et al. Human Adipose-Derived Mesenchymal Stem Cells Modulate Experimental Autoimmune Arthritis by Modifying Early Adaptive T Cell Responses. Stem Cells 2015;33:3493–503.10.1002/stem.2113Search in Google Scholar PubMed
23 González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum 2009;60:1006–19.10.1002/art.24405Search in Google Scholar PubMed
24 Kim KW, Kim HJ, Kim BM, Kwon YR, Kim HR, Kim YJ. Epigenetic modification of mesenchymal stromal cells enhances their suppressive effects on the Th17 responses of cells from rheumatoid arthritis patients. Stem Cell Res Ther 2018;9:20810.1186/s13287-018-0948-4Search in Google Scholar PubMed PubMed Central
25 Darlan DM, Munir D, Putra A, Jusuf NK. MSCs-released TGFβ1 generate CD4+CD25+Foxp3+ in T-reg cells of human SLE PBMC. J Formos Med Assoc 2021;120:602–8.10.1016/j.jfma.2020.06.028Search in Google Scholar PubMed
26 Geng L, Tang X, Zhou K, Wang D, Wang S, Yao G, et al. MicroRNA-663 induces immune dysregulation by inhibiting TGF-β1 production in bone marrow-derived mesenchymal stem cells in patients with systemic lupus erythematosus. Cell Mol Immunol 2019;16:260–74.10.1038/cmi.2018.1Search in Google Scholar PubMed PubMed Central
27 Li X, Lu C, Fan D, Lu X, Xia Y, Zhao H, et al. Human Umbilical Mesenchymal Stem Cells Display Therapeutic Potential in Rheumatoid Arthritis by Regulating Interactions Between Immunity and Gut Microbiota via the Aryl Hydrocarbon Receptor. Front Cell Dev Biol 2020;8:131.10.3389/fcell.2020.00131Search in Google Scholar PubMed PubMed Central
28 Alunno A, Bistoni O, Montanucci P, Basta G, Calafiore R, Gerli R, et al. Umbilical cord mesenchymal stem cells for the treatment of autoimmune diseases: beware of cell-to-cell contact. Ann Rheum Dis 2018;77:e14.10.1136/annrheumdis-2017-211790Search in Google Scholar PubMed
29 Cosenza S, Toupet K, Maumus M, Luz-Crawford P, Blanc-Brude O, Jorgensen C, et al. Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics 2018;8:1399–410.10.7150/thno.21072Search in Google Scholar PubMed PubMed Central
30 Piper C, Pesenacker AM, Bending D, Thirugnanabalan B, Varsani H, Wedderburn LR, et al. T cell expression of granulocyte-macrophage colony-stimulating factor in juvenile arthritis is contingent upon Th17 plasticity. Arthritis Rheumatol 2014;66:1955–60.10.1002/art.38647Search in Google Scholar PubMed PubMed Central
31 Che N, Li X, Zhang L, Liu R, Chen H, Gao X, et al. Impaired B cell inhibition by lupus bone marrow mesenchymal stem cells is caused by reduced CCL2 expression. J Immunol 2014;193:5306–14.10.4049/jimmunol.1400036Search in Google Scholar PubMed
32 Schena F, Gambini C, Gregorio A, Mosconi M, Reverberi D, Gattorno M, et al. Interferon-γ-dependent inhibition of B cell activation by bone marrow-derived mesenchymal stem cells in a murine model of systemic lupus erythematosus. Arthritis Rheum 2010;62:2776–86.10.1002/art.27560Search in Google Scholar PubMed
33 Traggiai E, Volpi S, Schena F, Gattorno M, Ferlito F, Moretta L, et al. Bone marrow-derived mesenchymal stem cells induce both polyclonal expansion and differentiation of B cells isolated from healthy donors and systemic lupus erythematosus patients. Stem Cells 2008;26:562–9.10.1634/stemcells.2007-0528Search in Google Scholar PubMed
34 Alunno A, Montanucci P, Bistoni O, Basta G, Caterbi S, Pescara T, et al. In vitro immunomodulatory effects of microencapsulated umbilical cord Wharton jelly-derived mesenchymal stem cells in primary Sjögren’s syndrome. Rheumatology (Oxford) 2015;54:163–8.10.1093/rheumatology/keu292Search in Google Scholar PubMed
35 Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 2009;15:42–9.10.1038/nm.1905Search in Google Scholar PubMed PubMed Central
36 Vasandan AB, Jahnavi S, Shashank C, Prasad P, Kumar A, Prasanna SJ. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE(2)-dependent mechanism. Sci Rep 2016;6:38308.10.1038/srep38308Search in Google Scholar PubMed PubMed Central
37 Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 2005;105:4120–6.10.1182/blood-2004-02-0586Search in Google Scholar PubMed
38 Yuan X, Qin X, Wang D, Zhang Z, Tang X, Gao X, et al. Mesenchymal stem cell therapy induces FLT3L and CD1c+ dendritic cells in systemic lupus erythematosus patients. Nat Commun 2019;10:2498.10.1038/s41467-019-10491-8Search in Google Scholar PubMed PubMed Central
39 Zhang B, Liu R, Shi D, Liu X, Chen Y, Dou X, et al. Mesenchymal stem cells induce mature dendritic cells into a novel Jagged-2-dependent regulatory dendritic cell population. Blood 2009;113:46–57.10.1182/blood-2008-04-154138Search in Google Scholar PubMed
40 Gao L, Bird AK, Meednu N, Dauenhauer K, Liesveld J, Anolik J, et al. Bone Marrow-Derived Mesenchymal Stem Cells From Patients With Systemic Lupus Erythematosus Have a Senescence-Associated Secretory Phenotype Mediated by a Mitochondrial Antiviral Signaling Protein-Interferon-β Feedback Loop. Arthritis Rheumatol 2017;69:1623–35.10.1002/art.40142Search in Google Scholar PubMed PubMed Central
41 Wang D, Feng X, Lu L, Konkel JE, Zhang H, Chen Z, et al. A CD8 T cell/indoleamine 2,3-dioxygenase axis is required for mesenchymal stem cell suppression of human systemic lupus erythematosus. Arthritis Rheumatol 2014;66:2234–45.10.1002/art.38674Search in Google Scholar PubMed PubMed Central
42 Tan W, Gu Z, Shen B, Jiang J, Meng Y, Da Z, et al. PTEN/Akt-p27(kip1) Signaling Promote the BM-MSCs Senescence and Apoptosis in SLE Patients. J Cell Biochem 2015;116:1583–94.10.1002/jcb.25112Search in Google Scholar PubMed
43 Guo G, Meng Y, Tan W, Xia Y, Cheng C, Chen X, et al. Induction of Apoptosis Coupled to Endoplasmic Reticulum Stress through Regulation of CHOP and JNK in Bone Marrow Mesenchymal Stem Cells from Patients with Systemic Lupus Erythematosus. J Immunol Res 2015;2015:183738.10.1155/2015/183738Search in Google Scholar PubMed PubMed Central
44 Zhou T, Liao C, Li HY, Lin W, Lin S, Zhong H. Efficacy of mesenchymal stem cells in animal models of lupus nephritis: a meta-analysis. Stem Cell Res Ther 2020;11:48.10.1186/s13287-019-1538-9Search in Google Scholar PubMed PubMed Central
45 Chang JW, Hung SP, Wu HH, Wu WM, Yang AH, Tsai HL, et al. Therapeutic effects of umbilical cord blood-derived mesenchymal stem cell transplantation in experimental lupus nephritis. Cell Transplant 2011;20:245–57.10.3727/096368910X520056Search in Google Scholar PubMed
46 Makino Y, Yamaza H, Akiyama K, Ma L, Hoshino Y, Nonaka K, et al. Immune therapeutic potential of stem cells from human supernumerary teeth. J Dent Res 2013;92:609–15.10.1177/0022034513490732Search in Google Scholar PubMed PubMed Central
47 Zhang Z, Niu L, Tang X, Feng R, Yao G, Chen W, et al. Mesenchymal stem cells prevent podocyte injury in lupus-prone B6.MRL-Faslpr mice via polarizing macrophage into an anti-inflammatory phenotype. Nephrol Dial Transplant 2019;34:597–605.10.1093/ndt/gfy195Search in Google Scholar PubMed
48 M Ma L, Aijima R, Hoshino Y, Yamaza H, Tomoda E, Tanaka Y, et al. Transplantation of mesenchymal stem cells ameliorates secondary osteoporosis through interleukin-17-impaired functions of recipient bone marrow mesenchymal stem cells in MRL/lpr mice. Stem Cell Res Ther 2015;6:104.10.1186/s13287-015-0091-4Search in Google Scholar PubMed PubMed Central
49 Bárcia RN, Santos JM, Filipe M, Teixeira M, Martins JP, Almeida J, et al. What Makes Umbilical Cord Tissue-Derived Mesenchymal Stromal Cells Superior Immunomodulators When Compared to Bone Marrow Derived Mesenchymal Stromal Cells? Stem Cells Int 2015;2015:583984.10.1155/2015/583984Search in Google Scholar PubMed PubMed Central
50 Collins E, Gu F, Qi M, Molano I, Ruiz P, Sun L, et al. Differential efficacy of human mesenchymal stem cells based on source of origin. J Immunol 2014;193:4381–90.10.4049/jimmunol.1401636Search in Google Scholar PubMed PubMed Central
51 Gu Z, Tan W, Ji J, Feng G, Meng Y, Da Z, et al. Rapamycin reverses the senescent phenotype and improves immunoregulation of mesenchymal stem cells from MRL/lpr mice and systemic lupus erythematosus patients through inhibition of the mTOR signaling pathway. Aging (Albany NY) 2016;8:1102–14.10.18632/aging.100925Search in Google Scholar PubMed PubMed Central
52 Jang SG, Lee J, Hong SM, Kwok SK, Cho ML, Park SH. Metformin enhances the immunomodulatory potential of adipose-derived mesenchymal stem cells through STAT1 in an animal model of lupus. Rheumatology (Oxford) 2020;59:1426–38.10.1093/rheumatology/kez631Search in Google Scholar PubMed
53 Xu J, Chen J, Li W, Lian W, Huang J, Lai B, et al. Additive Therapeutic Effects of Mesenchymal Stem Cells and IL-37 for Systemic Lupus Erythematosus. J Am Soc Nephrol 2020;31:54–65.10.1681/ASN.2019050545Search in Google Scholar PubMed PubMed Central
54 Ma X, Che N, Gu Z, Huang J, Wang D, Liang J, et al. Allogenic mesenchymal stem cell transplantation ameliorates nephritis in lupus mice via inhibition of B-cell activation. Cell Transplant 2013;22:2279–90.10.3727/096368912X658692Search in Google Scholar PubMed
55 Lee HK, Kim EY, Kim HS, Park EJ, Lee HJ, Lee TY, et al. Effect of Human Mesenchymal Stem Cells on Xenogeneic T and B Cells Isolated from Lupus-Prone MRL.Fas (lpr) Mice. Stem Cells Int 2020;2020:5617192.10.1155/2020/5617192Search in Google Scholar PubMed PubMed Central
56 Liu S, Liu D, Chen C, Hamamura K, Moshaverinia A, Yang R, et al. MSC Transplantation Improves Osteopenia via Epigenetic Regulation of Notch Signaling in Lupus. Cell Metab 2015;22:606–18.10.1016/j.cmet.2015.08.018Search in Google Scholar PubMed PubMed Central
57 Chen R, Lee WY, Zhang XH, Zhang JT, Lin S, Xu LL, et al. Epigenetic Modification of the CCL5/CCR1/ERK Axis Enhances Glioma Targeting in Dedifferentiation-Reprogrammed BMSCs. Stem Cell Reports 2017;8:743–57.10.1016/j.stemcr.2017.01.016Search in Google Scholar PubMed PubMed Central
58 Lin S, Lee WYW, Xu L, Wang Y, Chen Y, Ho KKW, et al. Stepwise preconditioning enhances mesenchymal stem cell-based cartilage regeneration through epigenetic modification. Osteoarthritis Cartilage 2017;25:1541–50.10.1016/j.joca.2017.05.008Search in Google Scholar PubMed
59 Sun Y, Deng W, Yao G, Chen W, Tang X, Feng X, et al. Citrullinated fibrinogen impairs immunomodulatory function of bone marrow mesenchymal stem cells by triggering toll-like receptor. Clin Immunol 2018;193:38–45.10.1016/j.clim.2018.01.008Search in Google Scholar PubMed
60 Feng Z, Zhai Y, Zheng Z, Yang L, Luo X, Dong X, et al. Loss of A20 in BM-MSCs regulates the Th17/Treg balance in Rheumatoid Arthritis. Sci Rep 2018;8:427.10.1038/s41598-017-18693-0Search in Google Scholar PubMed PubMed Central
61 Liu L, Wong CW, Han M, Farhoodi HP, Liu G, Liu Y, et al. Meta-analysis of preclinical studies of mesenchymal stromal cells to treat rheumatoid arthritis. EBioMedicine 2019;47:563–77.10.1016/j.ebiom.2019.08.073Search in Google Scholar PubMed PubMed Central
62 Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum 2007;56:1175–86.10.1002/art.22511Search in Google Scholar PubMed
63 Schurgers E, Kelchtermans H, Mitera T, Geboes L, Matthys P. Discrepancy between the in vitro and in vivo effects of murine mesenchymal stem cells on T-cell proliferation and collagen-induced arthritis. Arthritis Res Ther 2010;12:R31.10.1186/ar2939Search in Google Scholar PubMed PubMed Central
64 Sullivan C, Murphy JM, Griffin MD, Porter RM, Evans CH, O’Flatharta C, et al. Genetic mismatch affects the immunosuppressive properties of mesenchymal stem cells in vitro and their ability to influence the course of collagen-induced arthritis. Arthritis Res Ther 2012;14:R167.10.1186/ar3916Search in Google Scholar PubMed PubMed Central
65 Liu Y, Mu R, Wang S, Long L, Liu X, Li R, et al. Therapeutic potential of human umbilical cord mesenchymal stem cells in the treatment of rheumatoid arthritis. Arthritis Res Ther 2010;12:R210.10.1186/ar3187Search in Google Scholar PubMed PubMed Central
66 Sullivan C, Murphy JM, Griffin MD, Porter RM, Evans CH, O’Flatharta C, et al. The Secretome Derived From 3D-Cultured Umbilical Cord Tissue MSCs Counteracts Manifestations Typifying Rheumatoid Arthritis. Front Immunol 2019;10:18.10.3389/fimmu.2019.00018Search in Google Scholar PubMed PubMed Central
67 Okamura A, Matsushita T, Komuro A, Kobayashi T, Maeda S, Hamaguchi Y, et al. Adipose-derived stromal/stem cells successfully attenuate the fibrosis of scleroderma mouse models. Int J Rheum Dis 2020;23:216–25.10.1111/1756-185X.13764Search in Google Scholar PubMed
68 Jin J, Ou Q, Wang Z, Tian H, Xu JY, Gao F, et al. BMSC-derived extracellular vesicles intervened the pathogenic changes of scleroderma in mice through miRNAs. Stem Cell Res Ther 2021;12:327.10.1186/s13287-021-02400-ySearch in Google Scholar PubMed PubMed Central
69 Maria ATJ, Toupet K, Maumus M, Rozier P, Vozenin MC, Le Quellec A, et al. Fibrosis Development in HOCl-Induced Systemic Sclerosis: A Multistage Process Hampered by Mesenchymal Stem Cells. Front Immunol 2018;9:2571.10.3389/fimmu.2018.02571Search in Google Scholar PubMed PubMed Central
70 Aluri HS, Samizadeh M, Edman MC, Hawley DR, Armaos HL, Janga SR, et al. Delivery of Bone Marrow-Derived Mesenchymal Stem Cells Improves Tear Production in a Mouse Model of Sjögren’s Syndrome. Stem Cells Int 2017;2017:3134543.10.1155/2017/3134543Search in Google Scholar PubMed PubMed Central
71 Tian J, Hong Y, Zhu Q, Zhou H, Zhang Y, Shen Z, et al. Mesenchymal Stem Cell Enhances the Function of MDSCs in Experimental Sjögren Syndrome. Front Immunol 2020;11:604607.10.3389/fimmu.2020.604607Search in Google Scholar PubMed PubMed Central
72 Akiyama K, You YO, Yamaza T, Chen C, Tang L, Jin Y, et al. Characterization of bone marrow derived mesenchymal stem cells in suspension. Stem Cell Res Ther 2012;3:40.10.1186/scrt131Search in Google Scholar PubMed PubMed Central
73 Cheng T, Ding S, Liu S, Li Y, Sun L. Human umbilical cord-derived mesenchymal stem cell therapy ameliorates lupus through increasing CD4+ T cell senescence via MiR-199a-5p/Sirt1/p53 axis. Theranostics 2021;11:893–905.10.7150/thno.48080Search in Google Scholar PubMed PubMed Central
74 Choi EW, Lee M, Song JW, Shin IS, Kim SJ. Mesenchymal stem cell transplantation can restore lupus disease-associated miRNA expression and Th1/Th2 ratios in a murine model of SLE. Sci Rep 2016;6:38237.10.1038/srep38237Search in Google Scholar PubMed PubMed Central
75 Choi EW, Shin IS, Park SY, Park JH, Kim JS, Yoon EJ, et al. Reversal of serologic, immunologic, and histologic dysfunction in mice with systemic lupus erythematosus by long-term serial adipose tissue-derived mesenchymal stem cell transplantation. Arthritis Rheum 2012;64:243–53.10.1002/art.33313Search in Google Scholar PubMed
76 Dang J, Xu Z, Xu A, Liu Y, Fu Q, Wang J , et al. Human gingiva-derived mesenchymal stem cells are therapeutic in lupus nephritis through targeting of CD39-CD73 signaling pathway. J Autoimmun 2020;113:102491.10.1016/j.jaut.2020.102491Search in Google Scholar PubMed
77 Papadopoulou A, Yiangou M, Athanasiou E, Zogas N, Kaloyannidis P, Batsis I, et al. Mesenchymal stem cells are conditionally therapeutic in preclinical models of rheumatoid arthritis. Ann Rheum Dis 2012;71:1733–40.10.1136/annrheumdis-2011-200985Search in Google Scholar PubMed
78 Park MJ, Park HS, Cho ML, Oh HJ, Cho YG, Min SY, et al. Transforming growth factor β-transduced mesenchymal stem cells ameliorate experimental autoimmune arthritis through reciprocal regulation of Treg/Th17 cells and osteoclastogenesis. Arthritis Rheum 2011;63:1668–80.10.1002/art.30326Search in Google Scholar PubMed
79 Rui K, Zhang Z, Tian J, Lin X, Wang X, Ma J, et al. Olfactory ecto-mesenchymal stem cells possess immunoregulatory function and suppress autoimmune arthritis. Cell Mol Immunol 2016;13:401–8.10.1038/cmi.2015.82Search in Google Scholar PubMed PubMed Central
80 Shin TH, Kim HS, Kang TW, Lee BC, Lee HY, Kim YJ , et al. Human umbilical cord blood-stem cells direct macrophage polarization and block inflammasome activation to alleviate rheumatoid arthritis. Cell Death Dis 2016;7:e2524.10.1038/cddis.2016.442Search in Google Scholar PubMed PubMed Central
81 Wei ST, Huang YC, Chiang JY, Lin CC, Lin YJ, Shyu WC, et al. Gain of CXCR7 function with mesenchymal stem cell therapy ameliorates experimental arthritis via enhancing tissue regeneration and immunomodulation. Stem Cell Res Ther 2021;12:314.10.1186/s13287-021-02402-wSearch in Google Scholar PubMed PubMed Central
82 Wu W, Xiao ZX, Zeng D, Huang F, Wang J, Liu Y, et al. B7-H1 Promotes the Functional Effect of Human Gingiva-Derived Mesenchymal Stem Cells on Collagen-Induced Arthritis Murine Model. Mol Ther 2020;28:2417–29.10.1016/j.ymthe.2020.07.002Search in Google Scholar PubMed PubMed Central
83 Yu Y, Yoon KA, Kang TW, Jeon HJ, Sim YB, Choe SH, et al. Therapeutic effect of long-interval repeated intravenous administration of human umbilical cord blood-derived mesenchymal stem cells in DBA/1 mice with collagen-induced arthritis. J Tissue Eng Regen Med 2019;13:1134–42.10.1002/term.2861Search in Google Scholar PubMed
84 Jiang M, Yu Y, Luo J, Gao Q, Zhang L, Wang Q, et al. Bone Marrow-Derived Mesenchymal Stem Cells Expressing Thioredoxin 1 Attenuate Bleomycin-Induced Skin Fibrosis and Oxidative Stress in Scleroderma. J Invest Dermatol 2017;137:1223–33.10.1016/j.jid.2017.01.011Search in Google Scholar PubMed
85 Maria AT, Toupet K, Maumus M, Fonteneau G, Le Quellec A, Jorgensen C, et al. Human adipose mesenchymal stem cells as potent anti-fibrosis therapy for systemic sclerosis. J Autoimmun 2016;70:31–9.10.1016/j.jaut.2016.03.013Search in Google Scholar PubMed
86 Yang Y, Zhu S, Li Y, Lu Q, Zhang Q, Su L, et al. Human umbilical cord mesenchymal stem cells ameliorate skin fibrosis development in a mouse model of bleomycin-induced systemic sclerosis. Exp Ther Med 2020;20:257.10.3892/etm.2020.9387Search in Google Scholar PubMed PubMed Central
87 Xu J, Wang D, Liu D, Fan Z, Zhang H, Liu O, et al. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjögren syndrome. Blood 2012;120:3142–51.10.1182/blood-2011-11-391144Search in Google Scholar PubMed PubMed Central
88 Sun L, Akiyama K, Zhang H, Yamaza T, Hou Y, Zhao S, et al. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells 2009;27:1421–32.10.1002/stem.68Search in Google Scholar PubMed PubMed Central
89 Wang D, Li J, Zhang Y, Zhang M, Chen J, Li X, et al. Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: a multicenter clinical study. Arthritis Res Ther 2014;16:R79.10.1186/ar4520Search in Google Scholar PubMed PubMed Central
90 Wang D, Zhang H, Liang J, Wang H, Hua B, Feng X, et al. A Long-Term Follow-Up Study of Allogeneic Mesenchymal Stem/Stromal Cell Transplantation in Patients with Drug-Resistant Systemic Lupus Erythematosus. Stem Cell Reports 2018;10:933–41.10.1016/j.stemcr.2018.01.029Search in Google Scholar PubMed PubMed Central
91 Wang D, Zhang H, Liang J, Wang H, Hua B, Feng X, et al. Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell Transplant 2013;22:2267–77.10.3727/096368911X582769cSearch in Google Scholar PubMed
92 Wang D, Niu L, Feng X, Yuan X, Zhao S, Zhang H, et al. Long-term safety of umbilical cord mesenchymal stem cells transplantation for systemic lupus erythematosus: a 6-year follow-up study. Clin Exp Med 2017;17:333–40.10.1007/s10238-016-0427-0Search in Google Scholar PubMed
93 Deng D, Zhang P, Guo Y, Lim TO. A randomised double-blind, placebo-controlled trial of allogeneic umbilical cord-derived mesenchymal stem cell for lupus nephritis. Ann Rheum Dis 2017;76:14369.10.1136/annrheumdis-2017-211073Search in Google Scholar PubMed
94 Ma H, Liu C, Shi B, Zhang Z, Feng R, Guo M, et al. Mesenchymal Stem Cells Control Complement C5 Activation by Factor H in Lupus Nephritis. EBioMedicine 2018;32:21–30.10.1016/j.ebiom.2018.05.034Search in Google Scholar PubMed PubMed Central
95 Zhang Z, Huang S, Wu S, Qi J, Li W, Liu S, et al. Clearance of apoptotic cells by mesenchymal stem cells contributes to immunosuppression via PGE2. EBioMedicine 2019;45:341–50.10.1016/j.ebiom.2019.06.016Search in Google Scholar PubMed PubMed Central
96 Wang D, Wang S, Huang S, Zhang Z, Yuan X, Feng X, et al. Serum IFN-γ Predicts the Therapeutic Effect of Mesenchymal Stem Cells Transplantation in Systemic Lupus Erythematosus Patients. Stem Cells Transl Med 2017;6:1777–85.10.1002/sctm.17-0002Search in Google Scholar PubMed PubMed Central
97 Lv X, Wang L, Zou X, Huang S. Umbilical Cord Mesenchymal Stem Cell Therapy for Regenerative Treatment of Rheumatoid Arthritis: Opportunities and Challenges. Drug Des Devel Ther 2021;15:3927–36.10.2147/DDDT.S323107Search in Google Scholar PubMed PubMed Central
98 Ra JC, Kang SK, Shin IS, Park HG, Joo SA, Kim JG, et al. Stem cell treatment for patients with autoimmune disease by systemic infusion of culture-expanded autologous adipose tissue derived mesenchymal stem cells. J Transl Med 2011;9:181.10.1186/1479-5876-9-181Search in Google Scholar PubMed PubMed Central
99 Wang L, Huang S, Li S, Li M, Shi J, Bai W, et al. Efficacy and Safety of Umbilical Cord Mesenchymal Stem Cell Therapy for Rheumatoid Arthritis Patients: A Prospective Phase I/II Study. Drug Des Devel Ther 2019;13:4331–40.10.2147/DDDT.S225613Search in Google Scholar PubMed PubMed Central
100 Wang L, Wang L, Cong X, Liu G, Zhou J, Bai B, et al. Human umbilical cord mesenchymal stem cell therapy for patients with active rheumatoid arthritis: safety and efficacy. Stem Cells Dev 2013;22:3192–202.10.1089/scd.2013.0023Search in Google Scholar PubMed
101 Álvaro-Gracia JM, Jover JA, García-Vicuña R, Carreño L, Alonso A, Marsal S, et al. Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): results of a multicentre, dose escalation, randomised, single-blind, placebo-controlled phase Ib/IIa clinical trial. Ann Rheum Dis 2017;76:196–202.10.1136/annrheumdis-2015-208918Search in Google Scholar PubMed
102 Shadmanfar S, Labibzadeh N, Emadedin M, Jaroughi N, Azimian V, Mardpour S, et al. Intra-articular knee implantation of autologous bone marrow-derived mesenchymal stromal cells in rheumatoid arthritis patients with knee involvement: Results of a randomized, triple-blind, placebo-controlled phase 1/2 clinical trial. Cytotherapy 2018;20:499–506.10.1016/j.jcyt.2017.12.009Search in Google Scholar PubMed
103 Ghoryani M, Shariati-Sarabi Z, Tavakkol-Afshari J, Mohammadi M. The Sufficient Immunoregulatory Effect of Autologous Bone Marrow-Derived Mesenchymal Stem Cell Transplantation on Regulatory T Cells in Patients with Refractory Rheumatoid Arthritis. J Immunol Res 2020;2020:3562753.10.1155/2020/3562753Search in Google Scholar PubMed PubMed Central
104 Ghoryani M, Shariati-Sarabi Z, Tavakkol-Afshari J, Ghasemi A, Poursamimi J, Mohammadi M. Amelioration of clinical symptoms of patients with refractory rheumatoid arthritis following treatment with autologous bone marrow-derived mesenchymal stem cells: A successful clinical trial in Iran. Biomed Pharmacother 2019;109:1834–40.10.1016/j.biopha.2018.11.056Search in Google Scholar PubMed
105 Park EH, Lim HS, Lee S, Roh K, Seo KW, Kang KS, et al. Intravenous Infusion of Umbilical Cord Blood-Derived Mesenchymal Stem Cells in Rheumatoid Arthritis: A Phase Ia Clinical Trial. Stem Cells Transl Med 2018;7:636–42.10.1002/sctm.18-0031Search in Google Scholar PubMed PubMed Central
106 Ross CL, Ang DC and Almeida-Porada G. Targeting Mesenchymal Stromal Cells/Pericytes (MSCs) With Pulsed Electromagnetic Field (PEMF) Has the Potential to Treat Rheumatoid Arthritis. Front Immunol 2019;10:266.10.3389/fimmu.2019.00266Search in Google Scholar PubMed PubMed Central
107 He X, Yang Y, Yao M, Yang L, Ao L, Hu X, et al. Combination of human umbilical cord mesenchymal stem (stromal) cell transplantation with IFN-γ treatment synergistically improves the clinical outcomes of patients with rheumatoid arthritis. Ann Rheum Dis 2020;79:1298–304.10.1136/annrheumdis-2020-217798Search in Google Scholar PubMed
108 Keyszer G, Christopeit M, Fick S, Schendel M, Taute BM, Behre G, et al. Treatment of severe progressive systemic sclerosis with transplantation of mesenchymal stromal cells from allogeneic related donors: report of five cases. Arthritis Rheum 2011;63:2540–2.10.1002/art.30431Search in Google Scholar PubMed
109 Park Y, Lee YJ, Koh JH, Lee J, Min HK, Kim MY, et al. Clinical Efficacy and Safety of Injection of Stromal Vascular Fraction Derived from Autologous Adipose Tissues in Systemic Sclerosis Patients with Hand Disability: A Proof-Of-Concept Trial. J Clin Med 2020;9:3023.10.3390/jcm9093023Search in Google Scholar PubMed PubMed Central
110 Zhang H, Liang J, Tang X, Wang D, Feng X, Wang F, et al. Sustained benefit from combined plasmapheresis and allogeneic mesenchymal stem cells transplantation therapy in systemic sclerosis. Arthritis Res Ther 2017;19:165.10.1186/s13075-017-1373-2Search in Google Scholar PubMed PubMed Central
111 Wang D, Zhang H, Cao M, Tang Y, Liang J, Feng X, Wang H, et al. Efficacy of allogeneic mesenchymal stem cell transplantation in patients with drug-resistant polymyositis and dermatomyositis. Ann Rheum Dis 2011;70:1285–8.10.1136/ard.2010.141804Search in Google Scholar PubMed
112 Barbado J, Tabera S, Sánchez A, García-Sancho J. Therapeutic potential of allogeneic mesenchymal stromal cells transplantation for lupus nephritis. Lupus 2018;27:2161–5.10.1177/0961203318804922Search in Google Scholar PubMed
113 Wen L, Labopin M, Badoglio M, Wang D, Sun L, Farge-Bancel D. Prognostic Factors for Clinical Response in Systemic Lupus Erythematosus Patients Treated by Allogeneic Mesenchymal Stem Cells. Stem Cells Int 2019;2019:7061408.10.1155/2019/7061408Search in Google Scholar PubMed PubMed Central
114 Sun L, Wang D, Liang J, Zhang H, Feng X, Wang H, et al. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum 2010;62:2467–75.10.1002/art.27548Search in Google Scholar PubMed
115 Liang J, Zhang H, Kong W, Deng W, Wang D, Feng X, et al. Safety analysis in patients with autoimmune disease receiving allogeneic mesenchymal stem cells infusion: a long-term retrospective study. Stem Cell Res Ther 2018;9:312.10.1186/s13287-018-1053-4Search in Google Scholar PubMed PubMed Central
116 Liang J, Zhang H, Hua B, Wang H, Lu L, Shi S, et al. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Ann Rheum Dis 2010;69:1423–9.10.1136/ard.2009.123463Search in Google Scholar PubMed
117 Li X, Wang D, Liang J, Zhang H, Sun L. Mesenchymal SCT ameliorates refractory cytopenia in patients with systemic lupus erythematosus. Bone Marrow Transplant 2013;48:544–50.10.1038/bmt.2012.184Search in Google Scholar PubMed
118 Yang Y, He X, Zhao R, Guo W, Zhu M, Xing W, et al. Serum IFN-γ levels predict the therapeutic effect of mesenchymal stem cell transplantation in active rheumatoid arthritis. J Transl Med 2018;16:165.10.1186/s12967-018-1541-4Search in Google Scholar PubMed PubMed Central
119 Del Papa N, Di Luca G, Sambataro D, Zaccara E, Maglione W, Gabrielli A, et al. Regional implantation of autologous adipose tissue-derived cells induces a prompt healing of long-lasting indolent digital ulcers in patients with systemic sclerosis. Cell Transplant 2015;24:2297–305.10.3727/096368914X685636Search in Google Scholar PubMed
120 Takagi G, Miyamoto M, Tara S, Kirinoki-Ichikawa S, Kubota Y, Hada T, et al. Therapeutic vascular angiogenesis for intractable macroangiopathy-related digital ulcer in patients with systemic sclerosis: a pilot study. Rheumatology (Oxford) 2014;53:854–9.10.1093/rheumatology/ket432Search in Google Scholar PubMed
121 Kamata Y, Takahashi Y, Iwamoto M, Matsui K, Murakami Y, Muroi K, et al. Local implantation of autologous mononuclear cells from bone marrow and peripheral blood for treatment of ischaemic digits in patients with connective tissue diseases. Rheumatology (Oxford) 2007;46:882–4.10.1093/rheumatology/kel436Search in Google Scholar PubMed
© 2023 Yue Shi, Nan Jiang, Mengtao Li, Xiaofeng Zeng, Xinping Tian, published by Sciendo
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Perspective
- Revising the hemodynamic criteria for pulmonary hypertension: A perspective from China
- Animal models: An essential tool to dissect the heterogeneity of chronic obstructive pulmonary disease
- Effective albumin – A novel paradigm in the management of decompensated liver cirrhosis
- Monkeypox: A real new warning or just a sign of times?
- Severe acute hepatitis of unknown origin in children: Clinical issues of concern
- Commentary
- Manipulating cell motility by Legionella: Speeding up or slowing down?
- Standardized inhalation capability assessment: A key to optimal inhaler selection for inhalation therapy
- Review Article
- Mesenchymal stem cells and connective tissue diseases: From bench to bedside
- Predictors of progression in idiopathic inflammatory myopathies with interstitial lung disease
- Original Article
- Moderate-intensity continuous training has time-specific effects on the lipid metabolism of adolescents
- Point-of-care ultrasound-guided submucosal paclitaxel injection in tracheal stenosis model
- Gas chromatography-mass spectrometry pilot study to identify volatile organic compound biomarkers of childhood obesity with dyslipidemia in exhaled breath
- Letter to Editor
- Efficacy and safety of avatrombopag in aplastic anemia patients with liver disease
- Retraction Note
- Retraction note: Hydrogel: A promising new technique for treating Alzheimer’s disease (in Volume 10 Issue 3)
Articles in the same Issue
- Perspective
- Revising the hemodynamic criteria for pulmonary hypertension: A perspective from China
- Animal models: An essential tool to dissect the heterogeneity of chronic obstructive pulmonary disease
- Effective albumin – A novel paradigm in the management of decompensated liver cirrhosis
- Monkeypox: A real new warning or just a sign of times?
- Severe acute hepatitis of unknown origin in children: Clinical issues of concern
- Commentary
- Manipulating cell motility by Legionella: Speeding up or slowing down?
- Standardized inhalation capability assessment: A key to optimal inhaler selection for inhalation therapy
- Review Article
- Mesenchymal stem cells and connective tissue diseases: From bench to bedside
- Predictors of progression in idiopathic inflammatory myopathies with interstitial lung disease
- Original Article
- Moderate-intensity continuous training has time-specific effects on the lipid metabolism of adolescents
- Point-of-care ultrasound-guided submucosal paclitaxel injection in tracheal stenosis model
- Gas chromatography-mass spectrometry pilot study to identify volatile organic compound biomarkers of childhood obesity with dyslipidemia in exhaled breath
- Letter to Editor
- Efficacy and safety of avatrombopag in aplastic anemia patients with liver disease
- Retraction Note
- Retraction note: Hydrogel: A promising new technique for treating Alzheimer’s disease (in Volume 10 Issue 3)

