Legionella pneumophila is a Gram-negative intracellular parasite whose host in nature is some aquatic unicellular protists. When humans come into contact with infected water, opportunistic infections can occur, resulting in a severe type of pneumonia called Legionella pneumonia.[1,2] After entering the host cells, L. pneumophila transports about 330 effector proteins through the type IV secretion system (T4SS), targeting important intracellular life processes to evade the host immune response, which is essential for bacterial survival and proliferation in cells.[3,4] However, the functions of most of the effectors are unknown. It is expected to provide new therapeutic targets for the treatment of infectious diseases if the biological activities of these effectors are discovered.
As an important cell component, the cytoskeleton plays a key role in L. pneumophila infection and replication.[5] A previous study reported that the Legionella effector LegG1 promotes microtubule polymerization by acting as a guanine nucleotide exchange factor (GEF) for the Ran GTPase, thereby enhancing the migratory capacity of host cells.[6,7] It seems that L. pneumophila may speed up the host cell to facilitate its intracellular replication. Paradoxically, L. pneumophila inhibits the motility of infected cells dependent on the Dot/Icm system, suggesting the existence of other effector proteins that can regulate cell motility.[7] However, the mechanism remains unclear. Recently, Song et al.[8] reported that the Legionella effector protein Lem8 (Lpg1290) is a cysteine protease containing a Cys-His-Asp domain. Further research found that Lem8 showed protease activity only when it is bound to the host protein 14-3-3ζ, and self-cleaved at the carbon end to form a smaller molecular weight cleavage body. Similar to the full-length protein, the cleavage product activates its protease activity after binding to 14-3-3ζ, cleaves and degrades the host cell protein Phldb2 involved in cell motility, thereby inhibiting the migration of the host cell (Figure 1). They also revealed that Lem8 binds to 14-3-3ζ in a non-phosphorylated form through its own coiled coil domain. Migration to sites of disease (e.g., infection and inflammation) is an important mechanism by which immune cells perform functions, and their results suggest that L. pneumophila may slow down the ability of macrophages in clearing pathogens or other damaged cells.
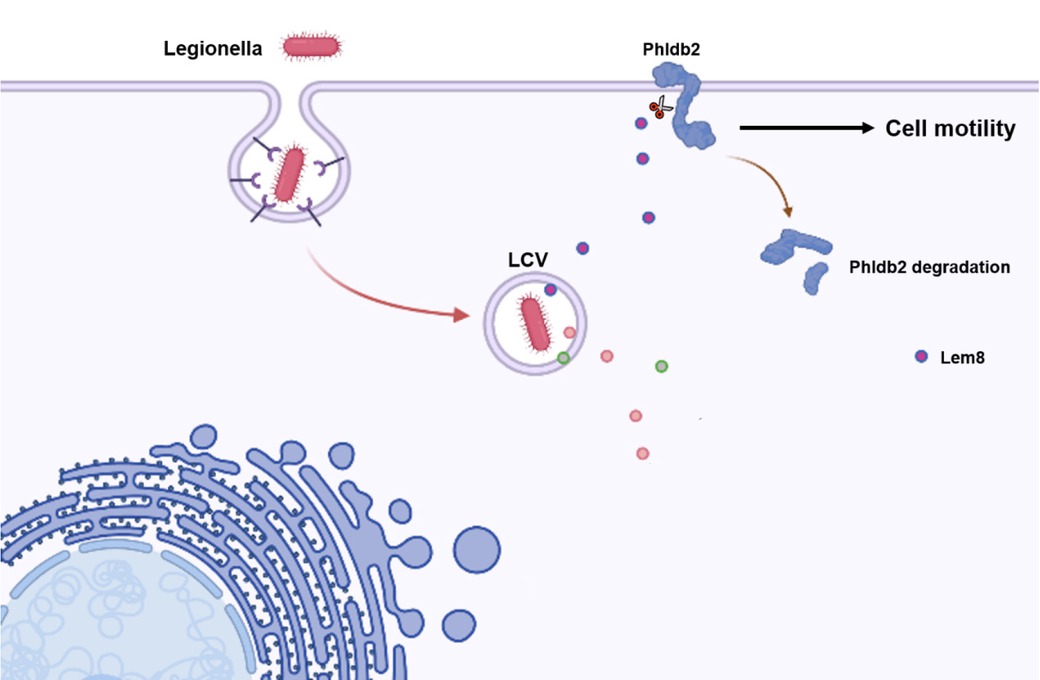
Mechanism underlying the inhibition of host cell motility by Legionella. LCV: legionella containing vacuole.
The results of this study revealed the interaction and functional correlation between effector proteins and host chaperone proteins (Lem8 and 14-3-3ζ), suggesting that bacteria use this mechanism to construct fine-tuned regulatory network to facilitate its successful infection. In addition, the researchers also reported a novel 14-3-3ζ-binding non-phosphorylated protein (Lem8), and revealed for the first time the mechanism by which the non-phosphorylated protein binds to the 14-3-3ζ protein family. In summary, this work has important implications for the study of bacterial gene evolution and co-evolution with host genes.
-
Conflict of Interest
The authors declare no conflict of interest.
References
1 Cunha BA, Burillo A, Bouza E. Legionnaires’ disease. Lancet 2016;387:376–85.10.1016/S0140-6736(15)60078-2Search in Google Scholar PubMed
2 Fu J, Zhou M, Gritsenko MA, Nakayasu ES, Song L, Luo ZQ. Legionella pneumophila modulates host energy metabolism by ADP-ribosylation of ADP/ATP translocases. Elife 2022;11:e73611.Search in Google Scholar
3 Fu J, Zhou M, Gritsenko MA, Nakayasu ES, Song L, Luo ZQ. Legionella pneumophila modulates host energy metabolism by ADP-ribosylation of ADP/ATP translocases. Elife 2022;11:e73611.10.7554/eLife.73611Search in Google Scholar PubMed PubMed Central
4 Song L, Xie Y, Li C, Wang L, He C, Zhang Y, et al. The Legionella Effector SdjA Is a Bifunctional Enzyme That Distinctly Regulates Phosphoribosyl Ubiquitination. mBio 2021;12:e0231621.10.1128/mBio.02316-21Search in Google Scholar PubMed PubMed Central
5 Charpentier X, Gabay JE, Reyes M, Zhu JW, Weiss A, Shuman HA. Chemical genetics reveals bacterial and host cell functions critical for type IV effector translocation by Legionella pneumophila. PLoS Pathog 2009;5:e1000501.10.1371/journal.ppat.1000501Search in Google Scholar PubMed PubMed Central
6 Rothmeier E, Pfaffinger G, Hoffmann C, Harrison CF, Grabmayr H, Rep-nik U, et al. Activation of Ran GTPase by a Legionella effector promotes microtubule polymerization, pathogen vacuole motility and infection. PLoS Pathog 2013;9:e1003598.10.1371/journal.ppat.1003598Search in Google Scholar PubMed PubMed Central
7 Simon S, Wagner MA, Rothmeier E, Muller-Taubenberger A, Hilbi H. Icm/Dot-dependent inhibition of phagocyte migration by Legionella is antagonized by a translocated Ran GTPase activator. Cell Microbiol 2014;16:977–92.10.1111/cmi.12258Search in Google Scholar PubMed
8 Song L, Luo J, Wang H, Huang D, Tan Y, Liu Y, et al. Legionella pneumophila regulates host cell motility by targeting Phldb2 with a 14-3-3zeta-dependent protease effector. Elife 2022;11: e73220.10.7554/eLife.73220Search in Google Scholar PubMed PubMed Central
© 2023 You Xu, Tong Jin, published by Sciendo
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Perspective
- Revising the hemodynamic criteria for pulmonary hypertension: A perspective from China
- Animal models: An essential tool to dissect the heterogeneity of chronic obstructive pulmonary disease
- Effective albumin – A novel paradigm in the management of decompensated liver cirrhosis
- Monkeypox: A real new warning or just a sign of times?
- Severe acute hepatitis of unknown origin in children: Clinical issues of concern
- Commentary
- Manipulating cell motility by Legionella: Speeding up or slowing down?
- Standardized inhalation capability assessment: A key to optimal inhaler selection for inhalation therapy
- Review Article
- Mesenchymal stem cells and connective tissue diseases: From bench to bedside
- Predictors of progression in idiopathic inflammatory myopathies with interstitial lung disease
- Original Article
- Moderate-intensity continuous training has time-specific effects on the lipid metabolism of adolescents
- Point-of-care ultrasound-guided submucosal paclitaxel injection in tracheal stenosis model
- Gas chromatography-mass spectrometry pilot study to identify volatile organic compound biomarkers of childhood obesity with dyslipidemia in exhaled breath
- Letter to Editor
- Efficacy and safety of avatrombopag in aplastic anemia patients with liver disease
- Retraction Note
- Retraction note: Hydrogel: A promising new technique for treating Alzheimer’s disease (in Volume 10 Issue 3)
Articles in the same Issue
- Perspective
- Revising the hemodynamic criteria for pulmonary hypertension: A perspective from China
- Animal models: An essential tool to dissect the heterogeneity of chronic obstructive pulmonary disease
- Effective albumin – A novel paradigm in the management of decompensated liver cirrhosis
- Monkeypox: A real new warning or just a sign of times?
- Severe acute hepatitis of unknown origin in children: Clinical issues of concern
- Commentary
- Manipulating cell motility by Legionella: Speeding up or slowing down?
- Standardized inhalation capability assessment: A key to optimal inhaler selection for inhalation therapy
- Review Article
- Mesenchymal stem cells and connective tissue diseases: From bench to bedside
- Predictors of progression in idiopathic inflammatory myopathies with interstitial lung disease
- Original Article
- Moderate-intensity continuous training has time-specific effects on the lipid metabolism of adolescents
- Point-of-care ultrasound-guided submucosal paclitaxel injection in tracheal stenosis model
- Gas chromatography-mass spectrometry pilot study to identify volatile organic compound biomarkers of childhood obesity with dyslipidemia in exhaled breath
- Letter to Editor
- Efficacy and safety of avatrombopag in aplastic anemia patients with liver disease
- Retraction Note
- Retraction note: Hydrogel: A promising new technique for treating Alzheimer’s disease (in Volume 10 Issue 3)

