To the editor
Immune checkpoint inhibitors (ICIs) are widely used forms of immunotherapy that target different immune check points in the body such as programmed cell death protein 1 (PD-1), programmed death-ligand 1 (PDL-1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). Despite treatment application in numerous malignancies, these drugs can cause various side effects affecting different organ systems, due to their broad spectrum of action. In this article, we review a case of ICI-mediated hypophysitis, which is one of the rarer forms of ICI-mediated toxicities. This case is specifically unique due to the vague and nonspecific presentation that led to variable differentials before reaching the correct diagnosis.
A 66-year-old woman with metastatic melanoma of the right chest presented with a month history of intermittent abdominal pain, diarrhea, nausea, and fatigue, which worsened in the last week. Patient did not endorse any fever, vomiting, cough, or dysuria. Her last immunotherapy regimen with ipilimumab and nivolumab started two months ago. On presentation, she was hypotensive with a blood pressure 85/53 mmHg and heart rate 56/min. She was afebrile and was maintaining normal oxygen saturation in room air. During hospitalization, a new-onset headache in the forehead with blurry vision had developed in the patient. Laboratory investigations demonstrated a serum sodium 124 mmol/L (low), potassium 4 mmol/L, and a negative Clostridium difficile stool assay. Further tests revealed a serum osmolality of 259 mOsm/ kg (low), urine osmolality of 195 mOsm/ kg, urine sodium of 59 mmol/L (high), and a thyroid-stimulating hormone (TSH) level of 0.404 μIU/mL (low). Final investigations included a morning serum cortisol of 0.1 μg/dL (low), adrenocorticotropic hormone (ACTH) level of 3.4 pg/mL (low), T3 of 2.01 pg/mL (low), free T4 of 0.50 ng/ dL (low), luteinizing hormone (LH) of 1.1 mIU/mL (low), follicular stimulating hormone (FSH) of 6.8 mIU/mL (low), and a prolactin level of 1.3 ng/mL (low). A magnetic resonance imaging (MRI) of the brain demonstrated an enlarged pituitary gland suggesting hypophysitis (Figure 1). The patient was immediately treated with methylprednisolone at 2 mg/ kg/day, and endocrinology was consulted. Hyponatremia and neurologic symptoms improved drastically with treatment. Methylprednisolone was transitioned to hydrocortisone, and levothyroxine was added to the regimen. Upon discharge, the patient was given emergency hydrocortisone pens and follow-up appointment with the endocrinologist.
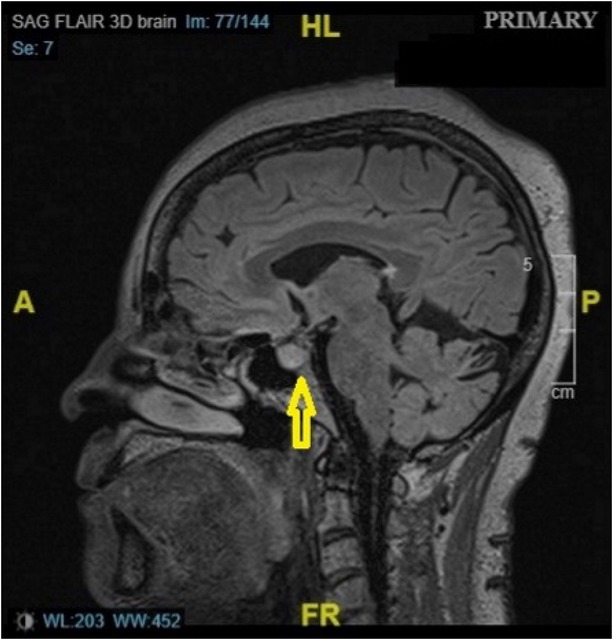
MRI scan of the patient showing pituitary enhancement (yellow arrow). MRI: magnetic resonance imaging. A: anterior; P: posterior; HL: head length; FR: frequency.
Hypophysitis is a relatively rare adverse effect of immunotherapy. Combination therapy (6.4%) with ipilimumab (3.2%) and nivolumab (0.4%) has higher incidence of hypophysitis compared to a single agent. The onset of symptoms is generally seen 6–12 weeks after treatment initiation. These were well demonstrated in our patient.[1] ICI-mediated hypophysitis usually presents as fatigue, headache, nausea, and visual disturbances. These symptoms are due to pituitary enlargement and optic chiasma compression. Our patient, however, presented with vague complaints initially, which could lead to various differential diagnosis and even misdiagnosis. Her nausea and fatigue could be easily mistaken as a consequence of her metastatic cancer. The abdominal pain and diarrhea could be due to the other side effects of immunotherapy such as ICI-mediated colitis or infections such as Clostridium difficile, which is very common in such patients. However, she did not meet the diagnostic criteria for ICI colitis, and Clostridium difficile was negative on stool analysis. Later in her hospital stay, she complained of headache and blurry vision which pointed toward the diagnosis of hypophysitis. Other symptoms due to hypophysitis may include those of hypogonadism (loss of libido, menstrual irregularities, and sexual dysfunction), central diabetes insipidus (polydipsia and polyuria), hypothyroidism (myxedema, cold intolerance, and constipation), and hyperprolactinemia (galactorrhea and menstrual irregularities), which were not demonstrated by our patient.[2]
The patient was consistently hypotensive and bradycardic despite adequate fluid resuscitation. Hyponatremia was key evidence that led to the diagnosis of secondary adrenal insufficiency, after ruling out other causes. Further investigations in that direction revealed decreased levels of cortisol, ACTH, FSH, LH, TSH, and prolactin, confirming the diagnosis of hypophysitis. In acute stage of hypophysitis, pituitary enhancement and swelling can be observed on MRI, and the same was demonstrated in our patient.[3]
After diagnosis of ICI-mediated hypophysitis, it is essential to get an endocrine consultation and discontinue the immunotherapy. Patients should be hospitalized and advised to use a medic alert band. Management of hypophysitis depends on the clinical grading as per the National Comprehensive Cancer Network (NCCN) guidelines, which includes corticosteroids and appropriate hormone replacement.[4, 5, 6]
Patients should be educated to tackle emergency situations such as sudden adrenal insufficiency needing instant hydrocortisone injections.[7] In most cases, treatment with ICIs is resumed once the acute phase is under control and corticosteroid therapy is tapered to low dose with resolution of symptoms. Studies have shown that normalization of pituitary–adrenal axis is quite rare. Therefore, most patients may require a life-long hormone replacement therapy.[8,9]
ICI-mediated toxicities can manifest in various forms and affect any organ system of the body leading to increased difficulty in diagnosis. Because of the generalized and nonspecific symptoms, as found in our patient, it is likely to miss the diagnosis of ICI-mediated hypophysitis, due to a lack of vigilance. Hence, it is essential to be aware of the immunotherapy toxicities and to timely recognize these adverse effects. Clinically, ICI-mediated hypophysitis can be considered as a diagnosis of exclusion, until specific lab work is obtained in that direction. A coordinated multidisciplinary approach with collaboration between primary physicians, oncologists, and endocrinologists is fundamental for management of ICI-mediated hypophysitis. Prompt diagnosis and following of appropriate treatment guidelines can result in a good initial response and a better long-term prognosis.
-
Conflict of Interest
None declared.
-
Consent to Participate
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
References
1 Postow M. Toxicities associated with checkpoint inhibitor immunotherapy. Available at: https://www.uptodate.com/contents/toxicities-associated-with-checkpoint-inhibitor-immunotherapy.Accessed on May 1, 2021.Search in Google Scholar
2 Albarel F, Castinetti F, Brue T. Immune check point inhibitors-induced hypophysitis. European Journal of Endocrinology 2019;181:R107–18.10.1530/EJE-19-0169Search in Google Scholar PubMed
3 Castinetti F, Albarel F, Archambeaud F, Bertherat J, Bouillet B, Buffier P, et al. French Endocrine Society Guidance on endocrine side effects of immunotherapy. Endocrine-Related Cancer 2019;26:G1–18.10.1530/ERC-18-0320Search in Google Scholar PubMed PubMed Central
4 NCCN guidelines for patients. Side effects: Immune checkpoint inhibitors. Available at: https://www.nccn.org/patients/guidelines/content/PDF/immunotherapy-se-ici-patient.pdf Accessed on January 22, 2022.Search in Google Scholar
5 Castillero F, Castillo-Fernández O, Jiménez-Jiménez G, Fallas-Ramírez J, Peralta-Álvarez MP, Arrieta O. Cancer immunotherapy-associated hypophysitis. Future Oncol 2019;15:3159–69.10.2217/fon-2019-0101Search in Google Scholar PubMed
6 Brahmer JR, Lacchetti C, Thompson JA. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline Summary. J Oncol Pract 2018;14:247–9.10.1200/JOP.18.00005Search in Google Scholar PubMed
7 Cooksley T, Knight T, Gupta A, Higham C, Lorigan P, Adam S. Emergency ambulatory outpatient management of immune-mediated hypophysitis. Support Care Cancer 2020;28:3995–9.10.1007/s00520-020-05581-zSearch in Google Scholar PubMed
8 Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 2017;5:95.10.1186/s40425-017-0300-zSearch in Google Scholar PubMed PubMed Central
9 Farkas. J. Immune-related adverse events from checkpoint inhibitors. The Internet Book of Critical Care. Available at: https://emcrit.org/ibcc/checkpoint/ Accessed on January 22, 2022.Search in Google Scholar
© 2022 Akankcha Alok, Kieun Seok, Jacqueline Wesolow, published by Sciendo
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Articles in the same Issue
- Editorial
- Research progress on the role of probiotics in acute liver failure
- Commentary
- A bifunctional enzyme of Legionella that distinctly regulates phosphoribosyl ubiquitination of the SidE family effectors
- The potential role of the brain–gut axis in the development and progression of Alzheimer's disease
- Review Article
- Novel insights into alcoholic liver disease: Iron overload, iron sensing and hemolysis
- Right ventricle remodeling in chronic thromboembolic pulmonary hypertension
- Original Article
- Prevalence and risk factors of hyperuricemia and gout: a cross-sectional survey from 31 provinces in mainland China
- Binding domain characterization of growth hormone secretagogue receptor
- Pan-cancer landscape of the RUNX protein family reveals their potential as carcinogenic biomarkers and the mechanisms underlying their action
- Letter to Editor
- Extracorporeal membrane oxygenation using a modified cardiopulmonary bypass system
- A Case of abdominal pain and diarrhea post immunotherapy: Hypophysitis associated with immune checkpoint inhibitors
Articles in the same Issue
- Editorial
- Research progress on the role of probiotics in acute liver failure
- Commentary
- A bifunctional enzyme of Legionella that distinctly regulates phosphoribosyl ubiquitination of the SidE family effectors
- The potential role of the brain–gut axis in the development and progression of Alzheimer's disease
- Review Article
- Novel insights into alcoholic liver disease: Iron overload, iron sensing and hemolysis
- Right ventricle remodeling in chronic thromboembolic pulmonary hypertension
- Original Article
- Prevalence and risk factors of hyperuricemia and gout: a cross-sectional survey from 31 provinces in mainland China
- Binding domain characterization of growth hormone secretagogue receptor
- Pan-cancer landscape of the RUNX protein family reveals their potential as carcinogenic biomarkers and the mechanisms underlying their action
- Letter to Editor
- Extracorporeal membrane oxygenation using a modified cardiopulmonary bypass system
- A Case of abdominal pain and diarrhea post immunotherapy: Hypophysitis associated with immune checkpoint inhibitors

