Abstract
Nanostructures have distinctive chemical and physical features owing to their surface area and nanoscale size. In this study, silver nanoparticles were synthesized using curcumin, a medicinally valuable natural product. The structure of curcumin-mediated silver nanoparticles (c-AgNPs) was identified by extensive spectroscopic techniques. The maximum absorption was observed at 430 nm in UV–Vis spectrum. The crystal structure of c-AgNPs was identified by XRD. The morphology of the structure was determined by SEM image. The particle size was found as 51.13 nm. The functional groups of curcumin and c-AgNPs were established by FTIR spectroscopy. Cytotoxic activity of c-AgNPs was carried out using A549, DLD-1, and L929 with MTT assay. c-AgNPs revealed excellent activity on DLD-1 cell lines and A549 cell lines at 1.0 mg/mL concentration with the lethal effect of 80%. However, nanoparticles did not show the considerable effect on L929. Moreover, they induced apoptosis. Consequently, c-AgNPs are a promising material for anticancer drugs candidate.
1 Introduction
Nanotechnology is a rapidly growing new field for producing valuable materials at the nanoscale with its applications in science and technology [1]. Due to their mechanical, optical, chemical, and pharmaceutical properties, nanoparticles are gaining considerable interest. Among the various noble metals, silver metal has received special attention due to its distinctive properties such as good electrical conductivity, chemical stability, catalytic and biological activity [2]. There are some synthetic methods including physicochemical, electrochemical, photochemical, and radiation to produce silver nanoparticles [3]. These methods are not convenient due to their environmental contamination, harsh reaction condition, and toxic residues. Therefore, natural products mediated nanoparticles synthesis has attracted great attention recently due to its eco-friendly, cheap, easy production approach [4]. Natural products such as extracts, microorganisms, oilcake, vegetable waste, seaweed, enzymes, arthropods, algae, panchakavya have been widely used for the synthesis of silver nanoparticles. Plant-based materials have been suggested to be promising substrates for AgNPs synthesis, as the process is generally easy to apply [5].
Natural products have gained considerable interest for drug development due to their bioactive compound contents [6], [7], [8]. After the breakthrough of spectroscopy in the 19th century, secondary metabolites have been begun to be isolated and they have revealed outstanding biological activities [9], [10], [11]. Synthetic chemists were inspired by these active compounds and began to work intensively to synthesize them [12].
Curcumin (1,7-bis(4-hydroxy-3-metholxyphenyl)-1,6-heptadiene-3,5-dione) is a natural polyphenol, isolated from Curcuma longa L. belongs to the Zingiberaceae family. This yellow spice has been used in traditional medicines for years. C. longa rhizome has been powdered and employed in medicine, cosmetics, cookery, and textile coloring for a long time [13]. Curcumin was reported to reveal significant biological effects such as antioxidant, antitumor, anti-HIV, antibacterial effects [14]. Most of the effects of curcumin are related to its ability to suppress acute and chronic inflammation [15]. Curcumin has chemopreventive, chemotherapeutic, and chemosensitizing effects. It inhibits cancer development and progression, aiming the several steps in the pathway to the malignant tumor. Curcumin has activity as blocking and suppressing way, in the former, it prevents the carcinogen activation, in the latter, it inhibits the cancerous cell proliferation through the progress of cancer formation [16]. Various animal surveys have displayed that curcumin has a chemo-preventive effect on gastric, colon, stomach, and oral carcinogenesis [17]. The main challenge of curcumin is water insolubility, degradation in alkaline, and photodegradation. Synthetic analogy, chemical modification, and a combination of other herbs have been used to overcome this challenge [18]. The nanoparticles which enable to drug delivery system could be a solution to this problem [19]. Dendritic nanoparticles of curcumin enhanced the solubility invitro. Moreover, in an in vivo study in rats with asthma, the curcumin lipid nanocarriers increase the concentration of curcumin in plasma and tissue [20]. Moreover, the addition of piperine to the curcumin increased the serum concentration of curcumin in rats and humans as well as improved bioavailability [21].
Cancer is a deadly disease in which cells in the body grow or divide in an abnormal and uncontrolled way, killing normal cells and often causing death [22]. Cancer is the second terrible and very serious disease in the world. Cancer is a major public health burden, causing almost 7 million deaths worldwide each year in developed countries. Advances in cancer treatment in the last 30 years have led to the survival of cancer patients and a rise in the quality of life [23]. Natural products play a very important role in the drug discovery and development process. 60% of approved cancer drugs for the treatment of human diseases are of natural origin [12].
Herein, silver nanoparticles were synthesized from curcumin, a valuable natural product, and cytotoxic, apoptotic, and necrotic effects were investigated using A549, DLD-1, and L929 cell lines. Although different synthetic methods such as aqueous curcumin:hydroxypropyl-β-cyclodextrin complex has been used for the production of silver nanoparticles [24], no studies have been reported for the synthesis of silver nanoparticles to which this method has been applied. Moreover, the corresponding effects of curcumin mediated silver nanoparticles were first investigated.
2 Materials and methods
2.1 Chemicals and cell lines
Silver nitrate, curcumin, solvents, FBS, L-glutamine, and trypsin-EDTA were bought from Merck (Darmstadt, Germany). L929 fibroblast cells, A-549 lung cancer cells, DLD-2 colorectal cancer cells were supplied from Kirikkale University, Turkey.
2.2 Synthesis of nanoparticles
Curcumin (3.0 g) was dissolved in acetone (80.0 mL). The silver nitrate was dissolved in water (0.39 M, 40 mL) and this solution was added to the curcumin solution slowly. The reaction mixture was kept at 50 °C for 2.5 h. Later, the nanoparticles were subjected to centrifugation at 4000 rpm for 20 min, washed with deionized water, and dried by lyophilization [25].
2.3 Characterisation of silver nanoparticles
The characterization of synthesized c-AgNPs was executed by spectroscopic techniques. The structure of c-AgNPs was presented by XRD pattern (Empyrean, Malvern Panalytical). SEM analysis displayed the morphology of c-AgNPs (Quanta Feg450). The functional groups of curcumin which were responsible for reducing and stabilization were indicated by FTIR (4700) spectrometer. Ultraviolet-visible (UV-2600) spectrophotometer was employed to ascertain of maximum absorption of nanoparticles.
2.4 Cell culture
The frozen cells were dissolved at 37 °C and then transferred to the falcon tube (15 mL) in a sterile Laminar flow cabinet. The cells in the falcon were centrifuged for 3 min. After the addition of 3 mL DMEM (L-glutamine, 10% FBS, 1% antibiotic), the cells were placed to the flasks which were kept in incubation for 48 h, at 37 °C, and 5% CO2 atmosphere. Subsequently, the media were discharged, and then the cells were treated with trypsin-EDTA. They were poured to the Eppendorf (15 mL) and centrifugated at 5000 rpm for 5 min to obtain cells to be used in the study [26].
2.5 MTT assay for cytotoxicity
The 96-well plate was used for the cytotoxic effect of curcumin and c-AgNPs. 100 μL of cells (10 × 103 per well) were placed in media that incubated for 24 h. The media of the well were removed. Afterward, curcumin and c-AgNPs (1.0, 0.5, 0.25, 0.125 and 0.0625 mg/mL) were added to the well and incubated for 24 h. Media and latex rubber were used for negative and positive control respectively. The media were substituted with MTT solution (50 μL, 1.0 mg/mL) and were incubated for 2.5 h, at 37 °C. Later, MTT solution was removed and a new MTT solution (100 μL, in isopropanol) was added. The viability of cells was determined by ELISA at 570 nm. The control cell viability was assumed as 100% and the cell viability of each group was calculated as given Eq. (1)
A1 is the sample of optical density. A 2 is the control [11].
2.6 Apoptotic and necrotic analysis (double staining assay)
Double staining Hoechst dye and propidium iodide (PI) were employed to quantify the apoptotic and necrotic cells. The A549 cells, DLD-1 cells and L929 cells (15 × 103 per well) were cultivated in DMEM with L-glutamine supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin for 24 h, at 37 °C, and 5% CO2 atmosphere in a 48-well plate. After treatment of different concentrations of c-AgNPs (50–500 µg/mL) for 24 h, the cells which were attached and detached were collected, then washed with phosphate-buffered saline (PBS) and stained with Hoechst dye (33,342), PI and DNAse free-RNAse for 20 min at ambient temperature. The control group included only A549, DLD-1, and L929 cells treated with the cell medium. The cell suspension (20 µL) was placed on the glass for the image of the fluorescence microscope. The normal cell nuclei were stained with blue fluorescence using Hoechst dye; however, apoptotic cells were stained with a stronger blue fluorescence. Apoptotic cells were characterized by morphological variation in the nucleus such as nuclear fragmentation and chromatin condensation. PI dye could penetrate to the cell membrane of necrotic cells lacking plasma membrane integrity. Hence, necrotic cell nuclei were stained to red by PI which could not penetrate to the non-necrotic cell membrane. Apoptotic and necrotic cells were determined by selecting microscopic fields. Apoptotic and necrotic cells were assigned by a Fluorescence Inverted Microscopy (Leica DMI 6000 B, Germany) with DAPI and FITCH filters, respectively [27].
2.7 Statistical analysis
GraphPad Prism (8.0.1), one-way ANOVA with Tukey’s multiple comparisons test were used for statistical analysis. The results were expressed as mean values ± SDs of three independent assays (p < 0.05).
3 Results
3.1 Synthesis and UV–Vis spectral analysis of nanoparticles
The signal of the UV–Vis spectrum in the visible region at 350–550 nm approved the formation of the silver nanoparticles. In this study, the maximum absorption peak at 430 nm proved the nanoparticles. Ag+1 ions were reduced while the oxidation of hydroxyl groups of curcumin (Figure 1) and color change also revealed the nanostructures.
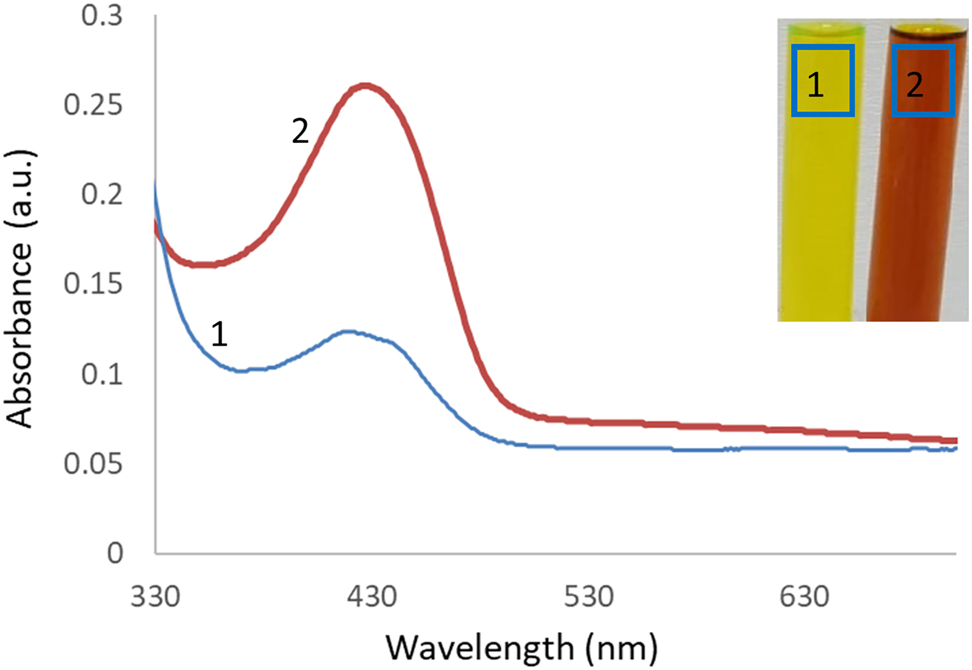
UV–Vis spectrum of curcumin (1) and c-AgNPs (2). Aqueous solution of curcumin (1) and c-AgNPs (2). Maximum absorption was observed at 417 nm.
3.2 Fourier-transform infrared spectroscopy
The reduction of silver ions and stabilization of c-AgNPs synthesized from curcumin was presented by FTIR spectroscopy (Figure 2). The difference between extract and c-AgNPs proved the nanoparticle formation. The signal at 3387 cm−1 represented the OH stretching and the signals at 2923 cm−1 and 2851 cm−1 could be due to the CH stretching. The signal observed at 1703 cm−1 represented the C=O stretching. The peaks at 1625 cm−1, 1585 cm−1 1510 cm−1 corresponded the C=C stretching of alkene. The absorption signal at 1450 cm−1 and 1429 cm−1 could be attributed to the C–C stretching of the aromatic ring. The strong peak at 1372 cm−1 is due to the C–H bending of alkane. The absorption peaks at 1280 cm−1, 1208 cm−1, 1165 cm−1, 1137 cm−1, and 1030 cm−1 corresponded to the C–O stretching of the aromatic ring.
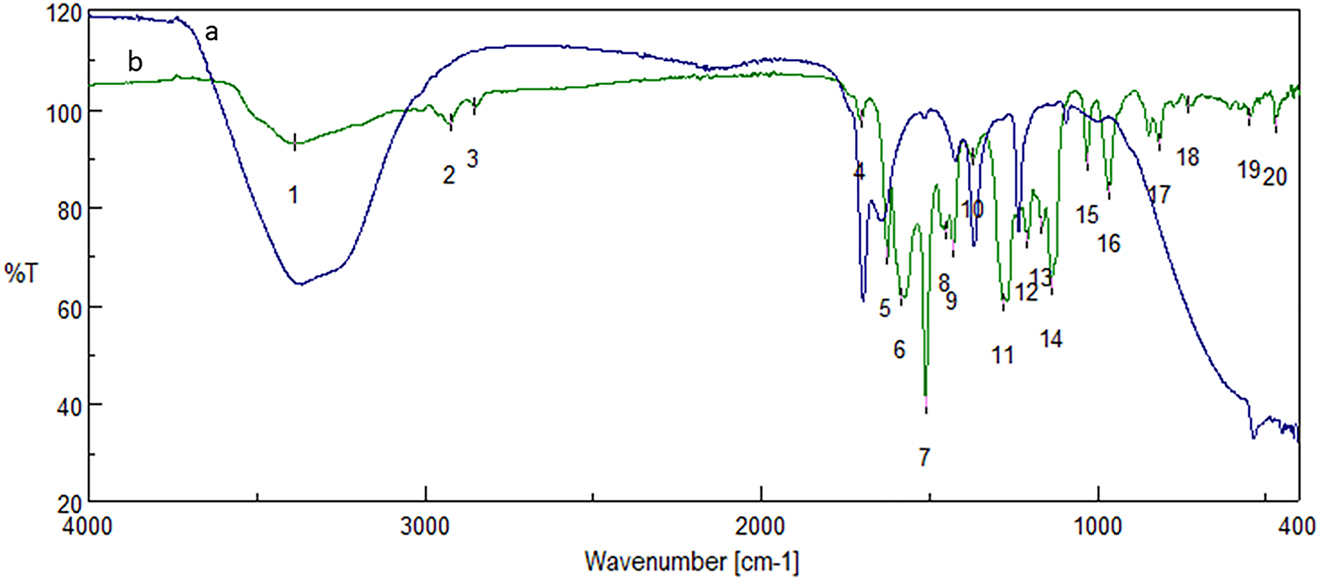
FTIR spectrum of c-AgNPs (a) and curcumin (b). Spectral values of curcumin, 1: 3387, 2: 2923, 3: 2851, 4: 1703, 5: 1625, 6: 1584, 7: 1510, 8: 1450, 9: 1429, 10: 1372, 11: 1280, 12: 1208, 13: 1165, 14: 1137, 15: 1030, 16: 966, 17: 816, 18: 730, 19: 549, 20: 470.
3.3 X-ray diffraction
XRD pattern described the crystalline structure of nanoparticles (Figure 3). The diffraction peaks (2θ) at angles of 38.14°, 44.28°, 64.48°, and 77.38° can be attributed to crystalline lattices (111), (200), (220), and (311), respectively of the face-centered cubic crystalline structure that accorded with the standard silver card values (JCPDS No. 87-0720) (Figure 3). The average crystalline size was calculated by Debye-Scherrer Eq. (2)
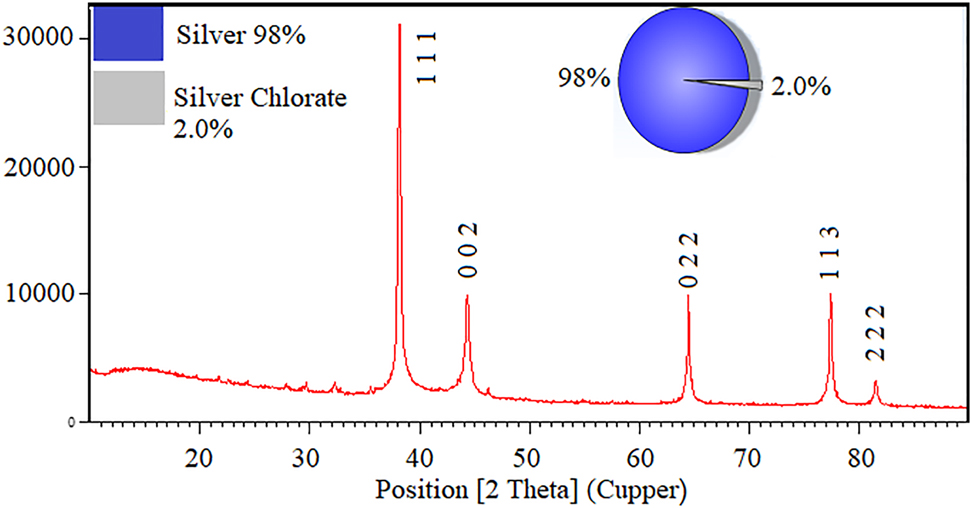
XRD pattern of c-AgNPs.
D is the average crystalline size (°A), λ, X-ray wavelength (nm), β, angular line with at half maximum intensity (radians) and θ, angle (degree).
3.4 Scanning electron microscope
The morphology of synthesized c-AgNPs was described by SEM spectral analysis (Figure 4). The average particle size was calculated as 51.13 nm. Moreover, the results indicated that the nanoparticles had spherical shapes with some agglomeration. SEM image further revealed that the synthesized c-AgNPs were uniformly distributed.
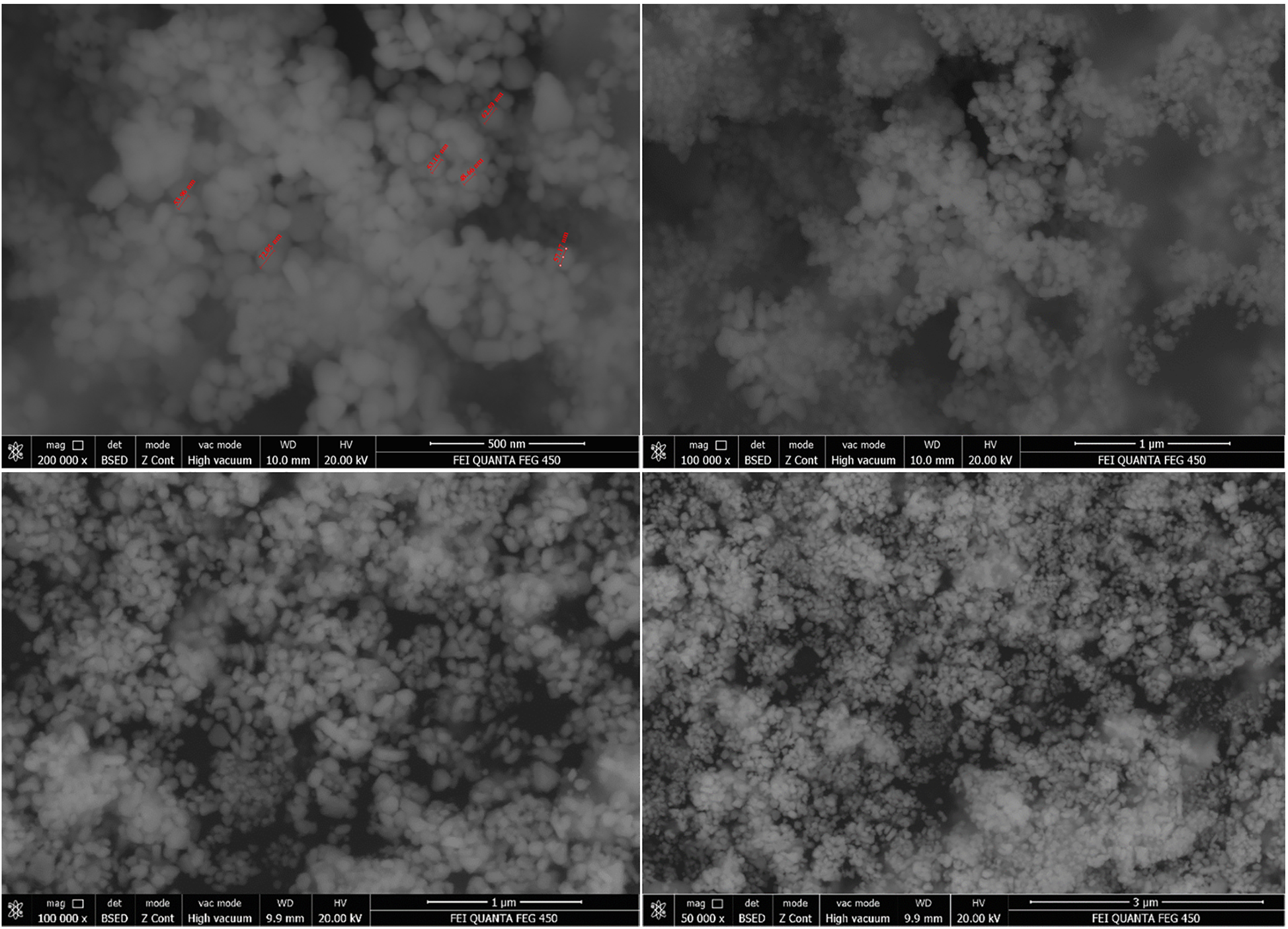
SEM image of c-AgNPs.
3.5 Cytotoxic effect
The most impact of nanostructures was observed at 1.0 mg/mL. Moreover, significant differences were not observed at 0.5 mg/mL concentration on A549 cell lines. In DLD-1 cell lines, the concentration-dependent effect was indicated in these cell lines as well. In that, the viability was 21.9% at 1.0 mg/mL. The viabilities were defined as 35.1, 36.5, 58.4%, and 82.5 at the concentrations of 0.5, 0.25, 0.125, and 0.0625 mg/mL, respectively. In concern to L929 cell lines which is the reference healthy cell lines, the viability was observed 71.2% at 1.0 mg/mL, which means, the toxicity of c-AgNPs was 29.8%. In addition, the toxicity of c-AgNPs was determined as 3.6% at 0.25 mg/mL concentration and nontoxic effect was assigned at 0.125 and 0.0625 mg/mL (Figure 5).
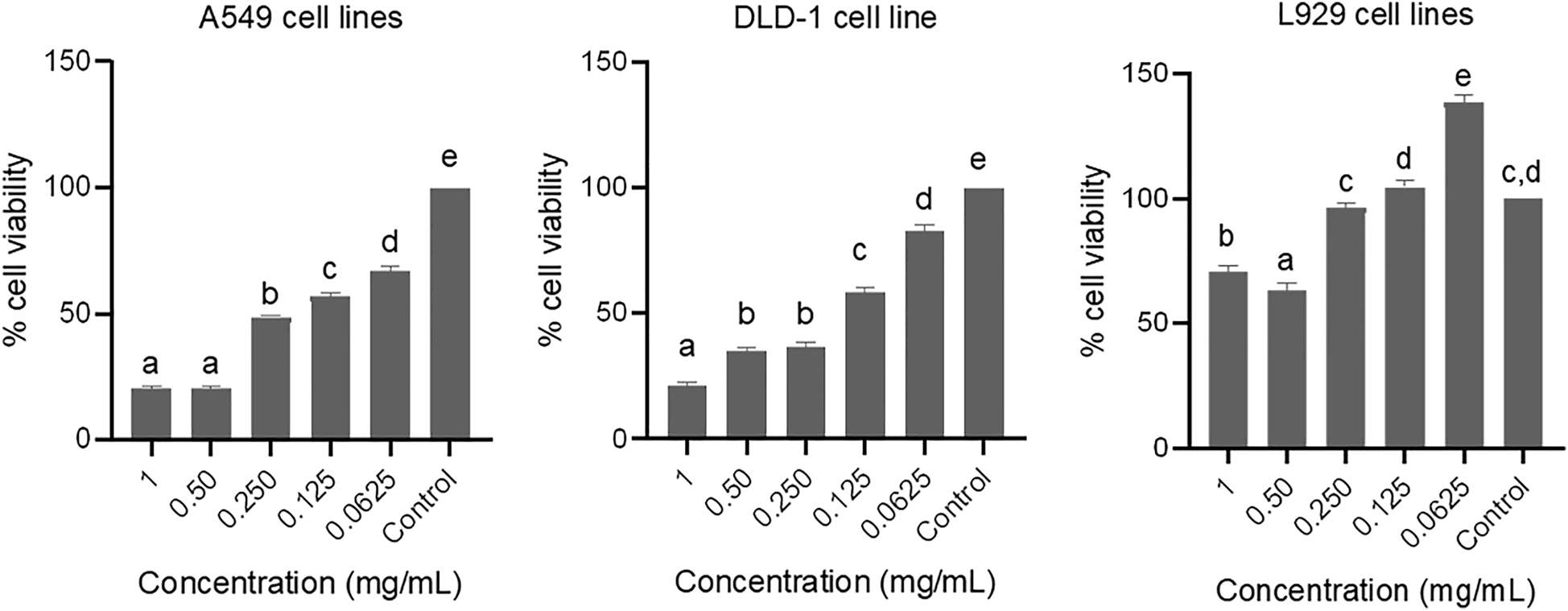
Cytotoxic effect of curcumin mediated silver nanoparticles on A549, DLD-1 and L929 cell lines. Data are shown as % viable cells, different letters indicate the significantly difference (p < 0.05).
3.6 Apoptotic and necrotic cells analysis
Apoptotic and necrotic index of curcumin mediated-silver nanoparticles on A549, DLD-1 and L929 cell lines were given in Table 1. The apoptotic index of c-AgNPs varied depending on the concentration. In A549 cell lines, apoptotic index was found as 37 ± 1.6 at 1.0 mg/mL. However, necrotic index was 12 ± 0.60 at the same concentration. Likewise, apoptotic index was calculated as 35 ± 1.2 (1.0 mg/mL) for DLD-1 cell lines and necrotic index was 18.5 ± 1.5 for this cell line. At the least concentration same trends were observed. The apoptotic and necrotic index of A549 cell lines were found as 3.2 ± 0.20 and 0.6 ± 0.10 at 0.0625 mg/mL, respectively. In DLD-1 cell lines, the apoptotic and necrotic values were 11 ± 1.7 and 4.6 ± 0.8 at the same concentration, respectively. In concerning L929 cell lines, apoptotic and necrotic values were calculated as 2.04 ± 0.2 and 0.5 ± 0.10, respectively. Hence, the results showed that the c-AgNPs have a promising effect on the corresponding cancerous cell lines.
Apoptotic index and necrotic index of c-AgNPs on A549, DLD-1 and L929 cell lines at different concentration (mg/mL).
| Concentration | A549 | DLD-1 | L929 | |||
|---|---|---|---|---|---|---|
| A | N | A | N | A | N | |
| 1.0 | 37 ± 1.6d | 12 ± 0.60de | 35 ± 1.2d | 18.5 ± 1.5c | 9.5 ± 0.5c | 0.72 ± 0.20b |
| 0.5 | 36.6 ± 1.6d | 11.3 ± 2.6cd | 36 ± 1.4d | 17.5 ± 2.3c | 16.03 ± 1.4d | 7.24 ± 1.61c |
| 0.25 | 25 ± 1.20c | 10.42 ± 1.5c | 28 ± 2.4c | 15.3 ± 2.5b | 3.07 ± 0.2ab | 0.75 ± 0.20b |
| 0.125 | 10.04 ± 2.5b | 6.2 ± 1.20b | 24 ± 2.3b | 14.3 ± 3.1b | 4.02 ± 0.4b | 0.82 ± 0.20b |
| 0.0625 | 3.2 ± 0.20a | 0.6 ± 0.10a | 11 ± 1.7a | 4.6 ± 0.8a | 2.04 ± 0.2a | 0.5 ± 0.10a |
-
A, apoptotic index (%); N, necrotic index (%). Statistical analysis was carried out for each column as multiple comparison test. Different letters in each column indicated the statistically different (p < 0.05).
4 Discussion
Curcumin’s wide biological activity, its importance in medicine, the coating and stabilization of silver nanoparticles by curcumin will increase the current importance of curcumin and the nanoparticles will have the potential to be used as a drug development process. The color change from yellow to dark brown indicates the formation of silver nanoparticles. The intense signal of the UV–Vis spectrum in the visible region ranging from 350 to 550 nm confirmed the formation of the silver nanoparticles [28]. XRD analysis proved that the formation of the nanoparticles took place in a high yield (98%). In addition, the structures were determined as the face centered cubic unit cell.
The double staining method was used to determine the pathway of cell death. When the apoptotic and necrotic indexes were examined, it was found out that the apoptotic index was higher than the necrotic index in both cancer cell lines (A549, DLD-1) that presented the cells death took place by apoptosis (Table 1). The Hoechst (33342) fluorescent dye in the double staining solution binded to DNA causing the blue color of the cell nuclei. Apoptotic cell nuclei were deformed, brightened, and distorted border, so it could be distinguished from the other nuclei which was the blue one. The necrotic cell nuclei were dyed by propodium iodide that appeared red under the fluorescent light (Figure 6). In the control groups, morphological change was not detected in the cell nuclei. However, the nuclei of the apoptotic cell were stained by a strong blue fluorescence in comparison with the nonapoptotic cells. The apoptotic index revealed that the c-AgNPs had the apoptotic effect in a concentration-dependent manner. The necrotic effect was induced by c-AgNPs on corresponding cell lines. The necrotic effect was concentration-dependent as well.
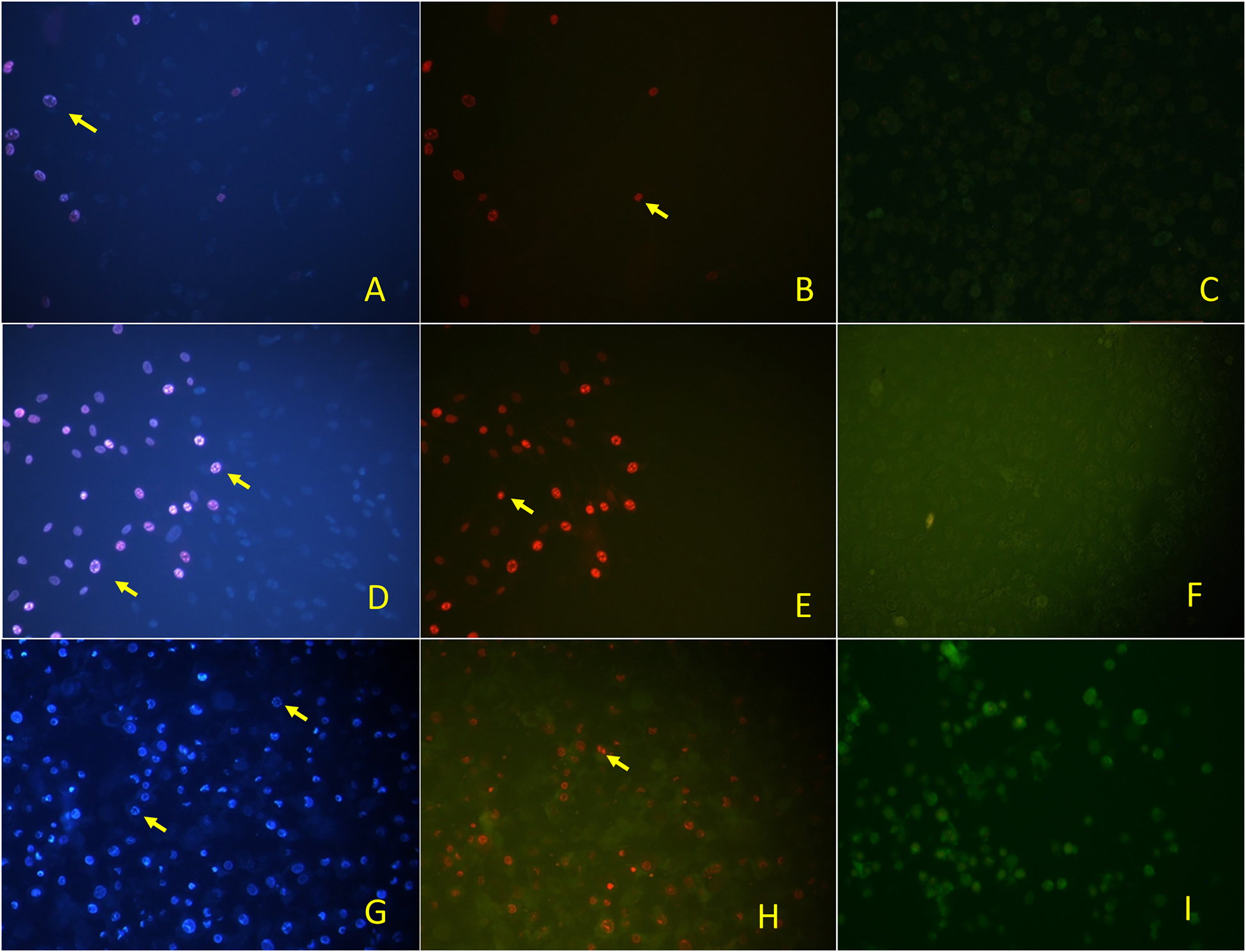
Fluorescence inverted microscopy image of c-AgNPs on L929 cells, DAPI filter (A), FITC filter (B), and control (C), on A549 cells, DAPI (D), FITC (E), and control (F) on DLD-1 cells, DAPI (G), FITC (H), and control (I). DAPI images indicated the apoptotic cells and FITC images indicated the necrotic cells.
Different methods were used for the fabrication of curcumin nanostructures that revealed good biological activities. It has been reported that curcumin inhibits the cell cycle in some cancer types by affecting various processes in the cell cycle and has chemotherapeutic activity by inducing apoptosis [29]. Another research indicated that synthesized nano-curcumin had a therapeutic effect on human pancreatic cancer cells and induced apoptosis [30]. Curcumin nanoparticles were prepared by the wet-milling method and they increased the aqueous phase solubility. Moreover, the anticancer activity of curcumin nanodispersion was executed on lung (A549), liver (HepG2), and skin (A431) cancer cell lines, the results indicated that curcumin nanoparticles revealed a similar or stronger antiproliferative effect on the cancer cells compared to normal curcumin [31]. It was reported that curcumin encapsulated in PLGA enhanced the lysosome activity, apoptosis, androgen receptor inhibition, and nuclear beta-catenin activity resulting from growth obstruction in prostate cancer cells [32]. Silver nanoparticles synthesized from curcumin were reported to reveal considerable inhibition effect against respiratory syncytial virus infection [33]. Gelatin-loaded curcumin/silver composite could be used as a therapeutic agent for the treatment of infected wounds due to its antibacterial and antioxidant effects and low toxicity [34]. Synthesis of PVP-modified silver nanoparticles was achieved by ultrasonic process and they revealed excellent antibacterial activity [35]. Silver nanoparticles synthesized from curcumin with PVP and PVA polymers displayed good antioxidant and antimicrobial activity [36]. Silver nanoparticles were synthesized using oxidized amylose, and curcumin was then added to this mixture. The prepared hybrid antibacterial agent revealed high solubility in an aqueous solution and enhanced antibacterial activity [37].
In the present study, silver nanoparticles synthesized from curcumin could be an effective agent for lung carcinoma and colon adenocarcinoma.
5 Conclusions
Silver nanoparticles were synthesized from curcumin which is a pharmaceutically and medicinally valuable compound. Curcumin acted both reducing and capping agent. c-AgNPs revealed the excellent antiproliferative effect on human lung carcinoma (A549), and colon adenocarcinoma (DLD-1). Moreover, c-AgNPs induced the cells to apoptosis. Cytotoxic effects of curcumin-mediated silver nanoparticles were performed. Viability (%) was calculated by comparison of absorbance values of control cells. The effective products should be nontoxic to the L929 cells, and they should be toxic against the cancer cells. Moreover, the pathway of death should be apoptotic and should not be necrotic. Hence, c-AgNPs satisfied the corresponding conditions. The concentration of AgNPs significantly affected the cell viability in a concentration dependant manner. The silver nanoparticles synthesized from curcumin could be promising for drug development process especially, the drugs for lung cancer and colon cancer. An in vivo and clinical study should be performed to reveal the drug potential of c-AgNPs.
Abbreviations
- A549
-
Human lung carcinoma
- DLD-1
-
Colon adenocarcinoma cell lines
- DMEM
-
Dulbecco’s Modified Eagle’s Medium
- EDTA
-
Ethylenediaminetetraacetic acid tetrasodium salt dihydrate
- FBS
-
Fetal bovine serum
- FTIR
-
Fourier transform infrared
- L929
-
Mouse fibroblasts
- MTT
-
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- SEM
-
Scanning electron microscope
- UV–Vis
-
Ultraviolet–Visible
- XRD
-
X-ray diffraction
-
Author contributions: All authors are responsible for the entire content and approved submission.
-
Research funding: None declared.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Ahmed, RH, Mustafa, DE. Green synthesis of silver nanoparticles mediated by traditionally used medicinal plants in Sudan. Int Nano Lett 2020;10:1. https://doi.org/10.1007/s40089-019-00291-9.Search in Google Scholar
2. Akintelu, SA, Bo, Y, Folorunso, AS. A review on synthesis, optimization, mechanism, characterization, and antibacterial application of silver nanoparticles synthesized from plants. J Chem 2020;2020:3189043. https://doi.org/10.1155/2020/3189043.Search in Google Scholar
3. Callegari, A, Tonti, D, Chergui, M. Photochemically grown silver nanoparticles with wavelength-controlled size and shape. Nano Lett 2003;3:1565. https://doi.org/10.1021/nl034757a.Search in Google Scholar
4. Ameen, F, Srinivasan, P, Selvankumar, T, Kamala-Kannan, S, Al Nadhari, S, Almansob, A, et al.. Phytosynthesis of silver nanoparticles using Mangifera indica flower extract as bioreductant and their broad-spectrum antibacterial activity. Bioorg Chem 2019;88:102970. https://doi.org/10.1016/j.bioorg.2019.102970.Search in Google Scholar
5. Govarthanan, M, Selvankumar, T, Manoharan, K, Rathika, R, Shanthi, K, Lee, K-J, et al.. Biosynthesis and characterization of silver nanoparticles using panchakavya, an Indian traditional farming formulating agent. Int J Nanomed 2014;9:1593. https://doi.org/10.2147/ijn.s58932.Search in Google Scholar
6. Topçu, G, Erenler, R, Çakmak, O, Johansson, CB, Çelik, C, Chai, H-B, et al.. Diterpenes from the berries of Juniperus excelsa. Phytochemistry 1999;50:1195.10.1016/S0031-9422(98)00675-XSearch in Google Scholar
7. Aksit, H, Çelik, SM, Sen, Ö, Erenler, R, Demirtas, I, Telci, I, et al.. Complete isolation and characterization of polar portion of Mentha dumetorum Water Extract. Record Nat Prod 2014;8:277.Search in Google Scholar
8. Erenler, R, Yilmaz, S, Aksit, H, Sen, O, Genc, N, Elmastas, M, et al.. Antioxidant activities of chemical constituents isolated from Echinops orientalis Trauv. Record Nat Prod 2014;8:32.Search in Google Scholar
9. Elmastaş, M, Telci, İ, Akşit, H, Erenler, R. Comparison of total phenolic contents and antioxidant capacities in mint genotypes used as spices. Turk J Biochem 2015;40:456.10.1515/tjb-2015-0034Search in Google Scholar
10. Erenler, R, Telci, I, Ulutas, M, Demirtas, I, Gul, F, Elmastas, M, et al.. Chemical constituents, quantitative analysis and antioxidant activities of E. chinacea purpurea (L.) Moench and E. chinacea pallida (Nutt.) Nutt. J Food Biochem 2015;39:622. https://doi.org/10.1111/jfbc.12168.Search in Google Scholar
11. Aydin, A, Erenler, R, Yılmaz, B, Tekin, Ş. Antiproliferative effect of Cherry laurel. J Turk Chem Soc, Sec A: Inside Chem 2016;3:217. https://doi.org/10.18596/jotcsa.21204.Search in Google Scholar
12. Newman, DJ, Cragg, GM, Snader, KM. Natural products as sources of new drugs over the period 1981−2002. J Nat Prod 2003;66:1022. https://doi.org/10.1021/np030096l.Search in Google Scholar PubMed
13. Hatcher, H, Planalp, R, Cho, J, Torti, F, Torti, S. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci 2008;65:1631. https://doi.org/10.1007/s00018-008-7452-4.Search in Google Scholar PubMed PubMed Central
14. Pourbagher-Shahri, AM, Farkhondeh, T, Ashrafizadeh, M, Talebi, M, Samargahndian, S. Curcumin and cardiovascular diseases: Focus on cellular targets and cascades. Biomed Pharmacother 2021;136:111214. https://doi.org/10.1016/j.biopha.2020.111214.Search in Google Scholar PubMed
15. Shishodia, S, Sethi, G, Aggarwal, BB. Curcumin: getting back to the roots. Ann N Y Acad Sci 2005;1056:206. https://doi.org/10.1196/annals.1352.010.Search in Google Scholar PubMed
16. Duvoix, A, Blasius, R, Delhalle, S, Schnekenburger, M, Morceau, F, Henry, E, et al.. Chemopreventive and therapeutic effects of curcumin. Cancer Lett 2005;223:181. https://doi.org/10.1016/j.canlet.2004.09.041.Search in Google Scholar PubMed
17. Maheshwari, RK, Singh, AK, Gaddipati, J, Srimal, RC. Multiple biological activities of curcumin: a short review. Life Sci 2006;78:2081. https://doi.org/10.1016/j.lfs.2005.12.007.Search in Google Scholar PubMed
18. Tang, H, Murphy, CJ, Zhang, B, Shen, Y, Van Kirk, EA, Murdoch, WJ, et al.. Curcumin polymers as anticancer conjugates. Biomaterials 2010;31:7139. https://doi.org/10.1016/j.biomaterials.2010.06.007.Search in Google Scholar PubMed
19. Wang, S, Tan, M, Zhong, Z, Chen, M, Wang, Y. Nanotechnologies for curcumin: an ancient puzzler meets modern solutions. J Nanomater 2011;2011:723178. https://doi.org/10.1155/2011/723178.Search in Google Scholar
20. Wang, W, Zhu, R, Xie, Q, Li, A, Xiao, Y, Li, K, et al.. Enhanced bioavailability and efficiency of curcumin for the treatment of asthma by its formulation in solid lipid nanoparticles. Int J Nanomed 2012;7:3667. https://doi.org/10.2147/ijn.s30428.Search in Google Scholar
21. Shoba, G, Joy, D, Joseph, T, Majeed, M, Rajendran, R, Srinivas, P. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med 1998;64:353. https://doi.org/10.1055/s-2006-957450.Search in Google Scholar PubMed
22. Jhanwar, YS, Divgi, C. Current status of therapy of solid tumors. J Nucl Med 2005;46:141S.Search in Google Scholar
23. Patel, D, Shukla, S, Gupta, S. Apigenin and cancer chemoprevention: progress, potential and promise (review). Int J Oncol 2007;30:233. https://doi.org/10.3892/ijo.30.1.233.Search in Google Scholar
24. Gupta, A, Briffa, SM, Swingler, S, Gibson, H, Kannappan, V, Adamus, G, et al.. Synthesis of silver nanoparticles using curcumin-cyclodextrins loaded into bacterial cellulose-based hydrogels for wound dressing applications. Biomacromolecules 2020;21:1802. https://doi.org/10.1021/acs.biomac.9b01724.Search in Google Scholar PubMed PubMed Central
25. Genc, N, Yildiz, I, Chaoui, R, Erenler, R, Temiz, C, Elmastas, M. Biosynthesis, characterization and antioxidant activity of oleuropein-mediated silver nanoparticles. Inorg Nano-Met Chem 2021;51:411. https://doi.org/10.1080/24701556.2020.1792495.Search in Google Scholar
26. Karan, T, Erenler, R. Fatty acid constituents and anticancer activity of Cladophora fracta (OF Müller ex Vahl) Kützing. Trop J Pharm Res 2018;1977:17.10.4314/tjpr.v17i10.12Search in Google Scholar
27. Ulu, M, Ouztuuml, S, Menemen, Y. Apoptotic and necrotic effects of carboxylated quercetin/polyethylenimine complex on HeLa cells. Afr J Pharm Pharmacol 2011;5:894. https://doi.org/10.5897/AJPP11.285.Search in Google Scholar
28. Zuas, O, Hamim, N, Sampora, Y. Bio-synthesis of silver nanoparticles using water extract of Myrmecodia pendan (Sarang Semut plant). Mater Lett 2014;123:156. https://doi.org/10.1016/j.matlet.2014.03.026.Search in Google Scholar
29. Sa, G, Das, T. Anti cancer effects of curcumin: cycle of life and death. Cell Div 2008;3:1. https://doi.org/10.1186/1747-1028-3-14.Search in Google Scholar PubMed PubMed Central
30. Bisht, S, Feldmann, G, Soni, S, Ravi, R, Karikar, C, Maitra, A, et al.. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. J. Nanobiotechnol 2007;5:1. https://doi.org/10.1186/1477-3155-5-3.Search in Google Scholar PubMed PubMed Central
31. Basniwal, RK, Khosla, R, Jain, N. Improving the anticancer activity of curcumin using nanocurcumin dispersion in water. Nutr Cancer 2014;66:1015. https://doi.org/10.1080/01635581.2014.936948.Search in Google Scholar PubMed
32. Yallapu, MM, Nagesh, PKB, Jaggi, M, Chauhan, SC. Therapeutic applications of curcumin nanoformulations. AAPS J 2015;17:1341. https://doi.org/10.1208/s12248-015-9811-z.Search in Google Scholar PubMed PubMed Central
33. Yang, XX, Li, CM, Huang, CZ. Curcumin modified silver nanoparticles for highly efficient inhibition of respiratory syncytial virus infection. Nanoscale 2016;8:3040. https://doi.org/10.1039/c5nr07918g.Search in Google Scholar PubMed
34. Khanh, LL, Truc, NT, Dat, NT, Nghi, NTP, Van Toi, V, Hoai, NTT, et al.. Gelatin-stabilized composites of silver nanoparticles and curcumin: characterization, antibacterial and antioxidant study. Sci Technol Adv Mater 2019;20:276. https://doi.org/10.1080/14686996.2019.1585131.Search in Google Scholar PubMed PubMed Central
35. Song, Z, Wu, Y, Wang, H, Han, H. Synergistic antibacterial effects of curcumin modified silver nanoparticles through ROS-mediated pathways. Mater Sci Eng C 2019;99:255. https://doi.org/10.1016/j.msec.2018.12.053.Search in Google Scholar PubMed
36. Alves, TF, Chaud, MV, Grotto, D, Jozala, AF, Pandit, R, Rai, M, et al.. Association of silver nanoparticles and curcumin solid dispersion: antimicrobial and antioxidant properties. AAPS PharmSciTech 2018;19:225. https://doi.org/10.1208/s12249-017-0832-z.Search in Google Scholar PubMed
37. Lyu, Y, Yu, M, Liu, Q, Zhang, Q, Liu, Z, Tian, Y, et al.. Synthesis of silver nanoparticles using oxidized amylose and combination with curcumin for enhanced antibacterial activity. Carbohydr Polym 2020;230:115573. https://doi.org/10.1016/j.carbpol.2019.115573.Search in Google Scholar PubMed
© 2022 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Research Articles
- In vitro anticancer and antibacterial potentials of selected medicinal plants and isolation and characterization of a natural compound from Withania coagulans
- New compounds from Sarcophyton glaucom-derived Penicillium sp.
- Novel benzofurane-pyrazole derivatives with anti-inflammatory, cyclooxygenase inhibitory and cytotoxicity evaluation
- Evaluation and enzyme-aided enhancement of anti-photoaging properties of Camellia japonica in UVA-irradiated keratinocytes
- Electrochemical quantification of biomarker myeloperoxidase
- Scopoletin: a review of its source, biosynthesis, methods of extraction, and pharmacological activities
- Occurrence of Z-2-oxo-4-methyl-3-pentene-1,5-dioic acid and its regioisomer 4-methylene-2-oxo-glutaric acid in tulip tissues
- Phytochemical analysis, antioxidant, cytotoxic, and antimicrobial activities of golden chamomile (Matricaria aurea (Loefl.) Schultz Bip)
- Synthesis and characterization of silver nanoparticles using curcumin: cytotoxic, apoptotic, and necrotic effects on various cell lines
- Review Article
- A comparative analysis on the safety and efficacy of Covaxin versus other vaccines against COVID-19: a review
- Corrigendum
- Corrigendum to: an update on the progress of microbial biotransformation of commercial monoterpenes
Articles in the same Issue
- Frontmatter
- Research Articles
- In vitro anticancer and antibacterial potentials of selected medicinal plants and isolation and characterization of a natural compound from Withania coagulans
- New compounds from Sarcophyton glaucom-derived Penicillium sp.
- Novel benzofurane-pyrazole derivatives with anti-inflammatory, cyclooxygenase inhibitory and cytotoxicity evaluation
- Evaluation and enzyme-aided enhancement of anti-photoaging properties of Camellia japonica in UVA-irradiated keratinocytes
- Electrochemical quantification of biomarker myeloperoxidase
- Scopoletin: a review of its source, biosynthesis, methods of extraction, and pharmacological activities
- Occurrence of Z-2-oxo-4-methyl-3-pentene-1,5-dioic acid and its regioisomer 4-methylene-2-oxo-glutaric acid in tulip tissues
- Phytochemical analysis, antioxidant, cytotoxic, and antimicrobial activities of golden chamomile (Matricaria aurea (Loefl.) Schultz Bip)
- Synthesis and characterization of silver nanoparticles using curcumin: cytotoxic, apoptotic, and necrotic effects on various cell lines
- Review Article
- A comparative analysis on the safety and efficacy of Covaxin versus other vaccines against COVID-19: a review
- Corrigendum
- Corrigendum to: an update on the progress of microbial biotransformation of commercial monoterpenes

