Abstract
The naphthalene glycosidecurviflorside [1,5-dihydroxy-8-methoxynaphthalene-2-O-β-D-xylopyranoside] (3) and the flavanol curviflorin [(+)-catechin-7-O-3″,4″-dihydroxybenzoate] (4), along with two known flavonoids: (+)-catechin (1) and quercetin (2) were isolated from the shoots of Plicosepalu scurviflorus Benth. (Loranthaceae) growing in Saudi Arabia and the chemical structures were elucidated by 2D-NMR spectroscopy.
1 Introduction
Naphthalenes are reported from fungi, plants, insects, and liverworts. Naphthalenes possess a wide range of bioactivities: antimicrobial, antioxidant, cytotoxic, anti-inflammatory, anti-platelet aggregation, and antiprotozoal [1]. Several naphthalene-containing drugs are available, such as nafacillin, naftifine, tolnaftate, and terbinafine, which play a vital role in controlling microbial infection [2]. Flavan-3-ols are the most common group of flavonoids in dietare 3-ring phenolic compounds having multiple hydroxyl groups on the A, B, and C rings. They are considered functional ingredients of fruits, beverages, vegetables, food grains, dietary supplements, herbal remedies, and dairy products [3]. Many studies indicated that they exhibited several health beneficial effects by acting as antioxidant, anti-diabetic, anti-proliferative, neuro- and cardio-protective, antimicrobial, and antiviral agents [3], [4], [5]. Family Loranthaceae plays an important and complex role in the biological system in which species of this family live by interacting with insects, birds, and mammals [6]. This family comprises four genera: Phragmanthera, Oncocalyx, Tapinanthus, and Plicosepalus, which grow naturally in Saudi Arabia. These genera include six species, Plicosepalus acacia, Plicosepalus curviflorus, Phragmanthera austroarabica, Oncocalyx schimperi, Oncocalyx glabratus, and Tapinanthus globiferus spread in the north, west, and south of the Saudi Arabia [7]. Earlier investigations on genus Plicosepalus reported various biological activities such as antioxidant [8], [9], [10], antihepatotoxic [11], anti-diabetic [12], [13], [14], antiviral [10], [15], antimicrobial [8], [10], [16], [17], and cytotoxic [10], [18], [19] activities. A literature survey revealed that very little phytochemical work has been carried out on the genus Plicosepalus. Flavonoids [8], [14], [19], phenolic acids [5], triterpenes, sterols [14], and sesquiterpene lactones [20] have been isolated from different Plicosepalus species. Flavane gallates, triterpenes, and sterols have been isolated from P. curviflorus Benth. [8], [15]. P. curviflorus is a parasitic plant which is generally known as “Enam ElTalh” (in Arabic). It is found in north East Africa, East Africa, Yemen, and Saudi Arabia [21]. The stems of P. curviflorus are used for the treatment of cancer in Yemen [10] and traditionally used in Saudi Arabia for increasing lactation in cattle [21]. Moreover, P. curviflorus is used for the treatment of diabetes in Saudi Arabia folk medicine [22], [23]. The present work reports the isolation and structural elucidation of two new compounds (3 and 4), along with two known ones (Figure 1) from the shoots of P. curviflorus. Their structures were assigned by extensive spectroscopic methods as well as comparison with the literature.
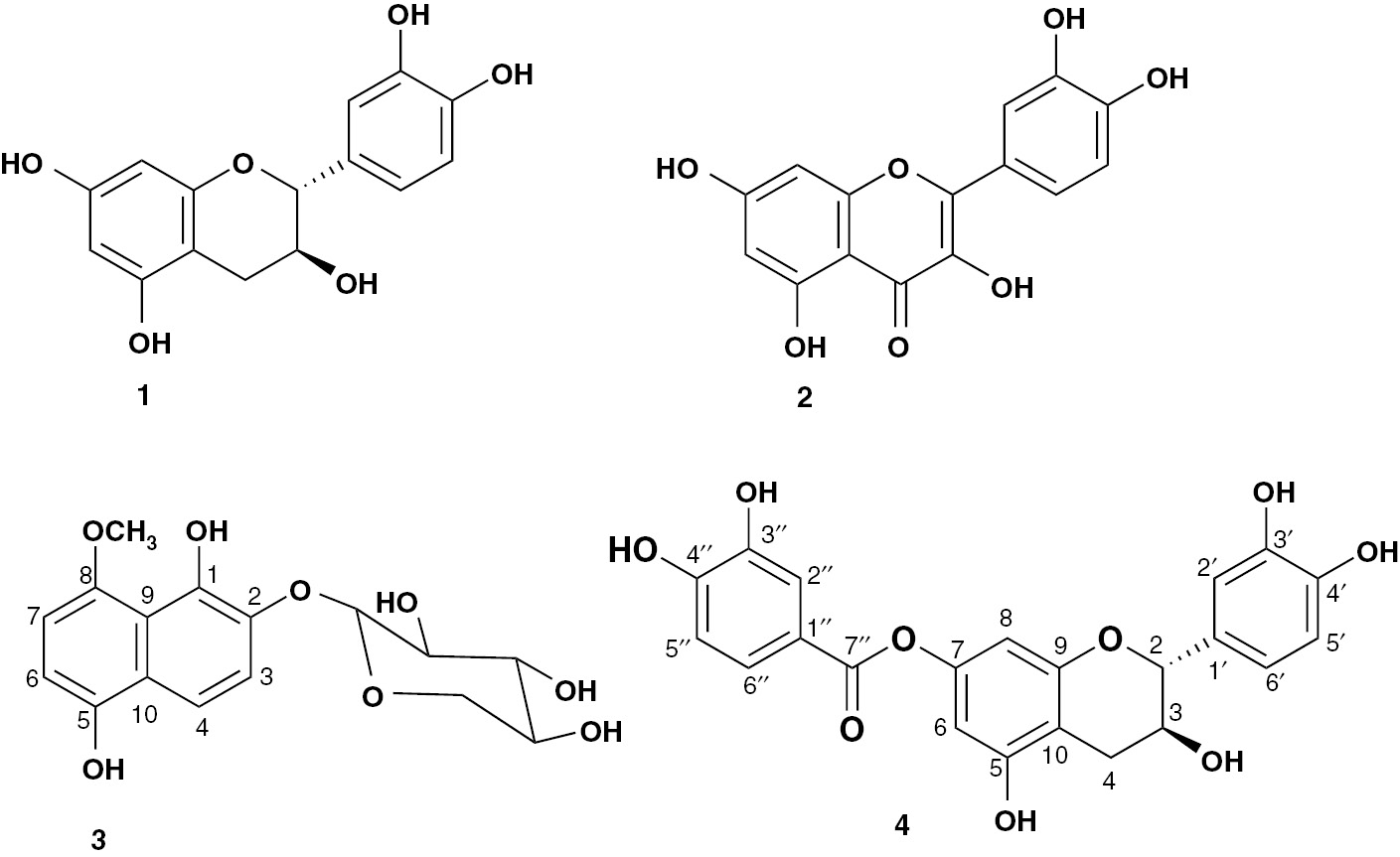
Structure of isolated compounds 1–4.
2 Materials and methods
2.1 General
Optical rotations were measured on a Perkin-Elmer Model 341 LC polarimeter (Perkin-Elmer, Waltham, MA, USA). Infrared (IR) spectra were measured with a Shimadzu Infrared-400 spectrophotometer (Shimadzu, Kyoto, Japan). The UV spectra were carried out in MeOH using a Perkin-Elmer Lambda 25 UV/VIS spectrophotometer (Perkin-Elmer, Waltham, MA, USA). Electrospray ionization mass spectrometry (ESIMS) spectra were recorded on a Finnigan MAT TSQ-7000 triple stage quadrupole mass spectrometer (Thermo Finnigan, Bremen, Germany). High-resolution electrospray ionization mass spectrometry (HRESIMS) spectra were obtained using an LTQ Orbitrap mass spectrometer (Thermo Fisher, Waltham, MA, USA). 1D and 2D nuclear magnetic resonance (NMR) spectra were measured on Bruker DRX 700 spectrometers (Bruker, Rheinstetten, Germany). Vacuum liquid chromatography (VLC) was performed using silica gel 60 (0.04–0.063 mm, Merck, Darmstadt, Germany). Column chromatographic separations were performed on silica gel 60 (0.04–0.063 mm, Merck, Darmstadt, Germany). Thin-layer chromatography (TLC) analyses were conducted on pre-coated silica gel F254 aluminum sheets (Merck, Darmstadt, Germany). Compounds were detected by spraying the sheets with p-anisaldehyde/H2SO4 reagent (Sigma-Aldrich Chemical Co., Taufkirchen, Germany) followed by heating at 110°C for 1–2 min.
2.2 Plant material
Shoots of P. curviflorus were collected in March 2013 from Abha, Saudi Arabia. The plant was identified by Dr. Mohamed Yousef, Prof. of Pharmacognosy, College of Pharmacy, King Saud University, Saudi Arabia. A voucher specimen (PC-3-2010) was deposited at the Department’s herbarium.
2.3 Extraction and isolation
The air-dried powdered shoots (500 g) were extracted with MeOH (2×3 L, each) using soxhlet apparatus for 8 h at room temperature. The combined extracts were concentrated under reduced pressure to afford a dark green residue (14.0 g). The latter was suspended in distilled water (150 mL) and partitioned between n-hexane (3×500 mL), EtOAc (3×500 mL), and n-BuOH (3×500 mL) successively. Each fraction was concentrated to give n-hexane (4.1 g), EtOAc (2.9 g), n-BuOH (1.8 g), and aqueous (4.6 g) fractions. The EtOAc fraction (2.9 g) was subjected to VLC using CHCl3:MeOH gradient to afford five subfractions: PC-1 to PC-5. Subfraction PC-2 (232 mg) was chromatographed over silica gel column (50 g×50×2 cm) using n-hexane:EtOAc gradients to get 1 (11.6 mg, yellow amorphous powder). Subfraction PC-2 (345 mg) was similarly treated as subfraction PC-1 to give 2 (34.7 mg, yellow amorphous powder). Subfraction PC-3 (541 mg) was chromatographed over sephadex LH-20 column (100 g×50×3 cm) using MeOH as an eluent to give two major subfractions: PC-3A (261 mg) and PC-3B (207 mg). Subfraction PC-3A was subjected to RP18 column (50 g×50×2 cm) using MeOH:H2O gradient to afford 3 (4.6 mg, white amorphous powder). Subfraction PC-3B (207 mg) was similarly treated as subfraction PC-3A to give 4 (6.2 mg, yellow amorphous powder).
Curviflorside (3): White amorphous powder (4.6 mg). [α]D: 52.3 (c 0.06, MeOH). UV (MeOH) λmax (log ε): 237 (5.36), 258 (4.04), 297 (3.78) nm. IR (KBr) νmax: 3425, 2934, 1625, 1518, 1070 cm−1. NMR data (DMSO-d6, 700 and 175 MHz), see Table 1. HRESIMS m/z 339.1075 (calcd for C16H19O8, [M+H]+, 339.1080).
NMR spectral data of compound 3 (DMSO-d6, 700 and 175 MHz).
| Position | δH [multiplicity, J (Hz)] | δC (multiplicity) | HMBC |
|---|---|---|---|
| 1 | – | 152.6 C | – |
| 2 | – | 140.1 C | – |
| 3 | 7.55 d (8.2) | 112.0 CH | 1, 10 |
| 4 | 7.70 d (8.2) | 112.8 CH | 1, 2, 3, 5 |
| 5 | – | 149.0 C | – |
| 6 | 7.47 d (8.5) | 110.7 CH | 8, 10 |
| 7 | 7.47 d (8.5) | 109.1 CH | 5, 9 |
| 8 | – | 148.9 C | – |
| 9 | – | 108.1 C | – |
| 10 | – | 123.5 C | – |
| 1′ | 5.00 d (7.5) | 103.3 CH | 2 |
| 2′ | 3.41 m | 73.5 CH | |
| 3′ | 3.25 m | 76.0 CH | |
| 4′ | 3.43 m | 69.8 CH | |
| 5′ | 3.86 m 3.38 m | 66.1 CH2 | |
| 8-OCH3 | 4.05 s | 61.4 CH3 | 8 |
Curviflorin (4): Yellow amorphous powder (6.2 mg). [α]D: + 15.3 (c 0.5, MeOH). UV (MeOH) λmax (log ε): 218 (4.86), 277 (2.96) nm. IR (KBr) νmax: 3356, 1732, 1614, 1519, 1463 cm−1. NMR data (DMSO-d6, 700 and 175 MHz), see Table 2. HRESIMS m/z 427.1033 (calcd for C22H19O9, [M+H]+, 427.1029).
NMR spectral data of compound 4 (DMSO-d6, 700 and 175 MHz).
| Position | δH [multiplicity, J (Hz)] | δC (multiplicity) | HMBC |
|---|---|---|---|
| 2 | 4.64 d (7.2) | 81.6 CH | 3, 4, 9, 1′, 2′, 6′ |
| 3 | 3.94 m | 66.4 CH | 2, 5, 10, 1′ |
| 4 | 2.67 dd (16.1, 5.2) 2.45 dd (16.1, 8.2) | 28.1 CH2 | 2, 3, 5, 10 |
| 5 | – | 155.6 C | – |
| 6 | 6.13 d (2.0) | 100.6 CH | 5, 7, 8, 10, 7″ |
| 7 | – | 150.7 C | – |
| 8 | 6.21 d (2.0) | 101.2 CH | 6, 7, 9, 10, 7″ |
| 9 | – | 156.6 C | – |
| 10 | – | 106.2 C | – |
| 1′ | – | 130.7 C | – |
| 2′ | 6.75 brs | 114.9 CH | 2, 4′, 6′ |
| 3′ | – | 145.4 C | – |
| 4′ | – | 146.1 C | – |
| 5′ | 6.68 d (7.8) | 115.6 CH | 1′, 3′, 4′ |
| 6′ | 6.58 brd (7.8) | 118.9 CH | 2, 1′, 2′, 4′ |
| 1″ | – | 139.7 C | – |
| 2″ | 7.09 d (2.2) | 109.5 CH | 1″, 4″, 6″, 7″ |
| 3″ | – | 145.9 C | – |
| 4″ | – | 146.1 C | – |
| 5″ | 6.95 d (8.2) | 108.0 CH | 1″, 3″, 6″ |
| 6″ | 7.06 dd (8.2, 2.2) | 109.5 CH | 1″, 2″, 4″, 7″ |
| 7″ | – | 164.9 C | – |
| 3-OH | 5.75 brs | – | – |
| 5-OH | 9.75 s | – | – |
| 3″-OH | 8.87 s | – | – |
| 4″-OH | 9.38 s | – | – |
3 Results and discussion
Compound 3 was obtained as white amorphous powder, and its molecular formula was defined as C16H18O8 by HRESIMS pseudo-molecular ion peak at m/z 339.1075 [M+H]+ (calcd for C16H19O8, 339.1080), requiring eight degrees of unsaturation (see Supplementary Material, Figure S17). Seven of degrees of unsaturation were attributed to the naphthalene moiety and one for sugar moiety. The ESIMS revealed a fragment ion peak at m/z 190 [(M+H)-149]+, indicating the loss of a pentose moiety. Its IR spectrum exhibited absorption bands for hydroxyl (3425 cm−1) and aromatic (1625 and 1518 cm−1) functionalities. The 13C NMR and heteronuclear single quantum coherence (HSQC) data showed resonances for 16 carbon signals: 10 carbon signals in the range δC 108.1–152.6 for a naphthalene ring, five oxygen-bonded aliphatic carbons in the range δC 66.1–103.3 for pentose moiety, and methoxy group (δC 61.4) (see Supplementary Material, Figures S12 and S15). The 1H NMR spectrum of 3 exhibited two pairs of ortho-coupled aromatic protons at δH 7.55 (d, J=8.2 Hz, H-3), 7.70 (d, J=8.2 Hz, H-4), and 7.47 (2H, d, J=8.5 Hz, H-6, 7), correlating to the carbons at δC 112.0, 112.8, 110.7, and 109.1, respectively, in the HSQC spectrum characteristic for a tetra-substituted naphthalene moiety (Table 1). The heteronuclear multiple bond correlations (HMBC) of H-3 to C-1 and C-10; H-4 to C-1, C-2, C-3, and C-5; H-6 to C-8 and C-10; and H-7 to C-5 and C-9 confirmed the presence of such moiety (Figures 2 and S16). Moreover, the 1H NMR spectrum showed characteristic signals of a sugar moiety between δH 3.25 and 5.00, including an anomeric proton signal at δH 5.00 (d, J=7.5 Hz, H-1′). The sugar was assigned as xylopyranose according to 13C NMR data (Table 1), where signals belonging to the sugar moiety were three oxymethines at δC 69.8 (C-4′), 73.5 (C-2′), and 76.0 (C-3′), one oxymethylene at δC 66.1 (C-5′), and one anomeric oxymethine at δC 103.3 (C-1′) [1], [24]. The configuration at the anomeric center of the xylopyranosyl moiety (C-1′) was determined to be β based on the coupling constant value (J1′,2′=7.5 Hz). The sugar moiety was placed at C-2 on the basis of the 3J HMBC correlation of the anomeric proton to C-2 (δC 140.1). Moreover, signals for methoxy group at δH 4.05/δC 61.4 were observed. Its attachment at C-8 was established by the HMBC cross peak of the methoxy group to C-8 (δC 148.9). On the basis of these data, compound 3 was identified as 1,5-dihydroxy-8-methoxynaphthalene-2-O-β-D-xylopyranoside. The trivial name curviflorside was given to it.
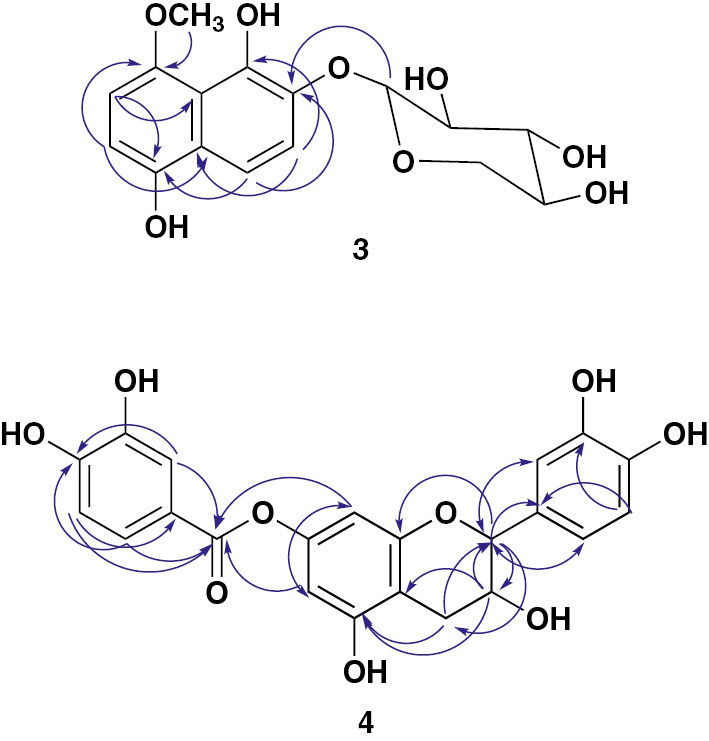
Some key HMBC correlations of 3 and 4.
Compound 4 was obtained as yellow amorphous powder and gave positive tests for flavonoids [25], [26]. The HRESIMS spectrum of 4 gave a pseudo-molecular ion peak at m/z 427.1033 [M+H]+, corresponding to the molecular formula C22H18O9. The ESIMS spectrum showed a significant fragment ion peak at m/z 290 [M + H-(3,4-dihydroxybenzoyl)]+. Its UV spectrum displayed absorption bands at 218 and 277 nm, which are characteristic for the presence of a flavan-3-ol moiety in 4 [27]. Its IR spectrum exhibited absorption bands for hydroxyl (3356 cm−1), ester carbonyl (1732 cm−1), and phenyl (1614, 1519, and 1463 cm−1) moieties. The 13C, distortionless enhancement by polarization transfer, and HSQC spectra of 4 showed the presence of 22 carbons, consisting of methylene, 8 aromatic methines, 2 oxymethines at δC 81.6 (C-2) and 66.4 (C-3), and 11 quaternary carbons, including one carbonyl at δC 164.9 (C-7″) (see Supplementary Material, Figures S19 and S20). The 1H NMR spectrum showed resonances for two meta-coupled protons at δH 6.13 (d, J=2.0 Hz, H-6) and 6.21 (d, J=2.0 Hz, H-8) (Table 2). These signals showed HSQC cross peaks to the carbons, resonating at δC 100.6 and 101.2, respectively, consistent with a 5,7-dioxygenated A ring of flavane [28]. The 1H NMR and 1H-1H correlation spectroscopy (COSY) also displayed an ABX system for 1,3,4-tri-substituted ring B at δH 6.75 (brs, H-2′), 6.68 (d, J=7.8 Hz, H-5′), and 6.58 (brd, J=7.8 Hz, H-6′) [29]. They showed HSQC cross peaks to carbon signals at δC 114.9, 115.6, and 118.9, respectively (Table 2). Moreover, two oxymethine protons at δH 4.64 (d, J=7.2 Hz, H-2) and 3.94 (m, H-3) and methylene group at δH2.67 (dd, J=16.1, 5.2 Hz, H-4A) and 2.45 (dd, J=16.1, 8.2 Hz, H-4B) were observed, suggesting that 4 contained a catechin moiety. This moiety was confirmed by the observed 1H-1H COSY cross peaks and HMBC correlations of H-2 to C-4, C-9, C-1′, C-2′, and C-6′; H-3 to C-5, C-10, and C-1′; H-4 to C-2, C-3, C-5, and C-10; H-6 and H-8 to C-7 and C-10; H-2′ and H-6′ to C-2 and C-4′; and H-5′ to C-1′ and C-3′ (Figure 2). This was further secured by the ESIMS fragment ion peak at m/z 290 [M + H-(3,4-dihydroxybenzoyl)]+. The 1HNMR spectrum of 4 also displayed three coupled proton signals for a tri-substituted phenyl moiety at δH 7.09 (d, J=2.2 Hz, H-2″), 6.95 (d, J=8.2 Hz, H-5″), and 7.06 (dd, J=8.2, 2.2 Hz, H-6″), indicating the presence of a 3,4-dihydroxybenzoyl moiety [30]. This was confirmed by 13C NMR signals at δC 139.7 (C-1″), 109.5 (C-2″), 145.9 (C-3″), 146.1 (C-4″), 108.0 (C-5″), 109.5 (C-6″), and 164.9 (C-7″) and further secured by the observed HMBC correlations (Figure 2). Moreover, the four singlet signals at δH 5.75, 9.75, 8.87, and 9.38 were assigned to 3, 5, 3′, and 4′-OH groups, respectively. The connectivity of 3,4-dihydroxybenzoyl moiety at C-7 was established by the HMBC correlations of H-6 and H-8 to C-7″ at δC 164.9. Upon the hydrolysis of 4, catechin and 3,4-dihydroxybenzoic acid were identified by co-TLC alongside authentic samples. On the basis of the above evidences, the structure of 4 was assigned as (+)-catechin-7-O-3″,4″-dihydroxybenzoate and named curviflorin.
The known compounds were identified as (+)-catechin (1) [31] and quercetin (2) [32], [33] by comparing their NMR spectral and physical data with the literature.
4 Conclusions
Four compounds (1–4) were isolated and characterized from the shoots of P. curviflorus; two of them are new natural products (3 and 4). Their structures were determined on the basis of extensive spectroscopic data analysis.
Acknowledgments
This research was supported by the Research Center of the Female Scientific and Medical Colleges, Deanship of Scientific Research, King Saud University.
References
1. Ibrahim SR, Mohamed GA. Naturally occurring naphthalenes: chemistry, biosynthesis, structural elucidation, and biological activities. Phytochem Rev 2016;15:279–95.10.1007/s11101-015-9413-5Suche in Google Scholar
2. Wilson CO, Gisvolds O. Textbook of organic medicinal and pharmaceutical chemistry. Lippincott, Williams and Wilkins, Philadelphia 2004:255.Suche in Google Scholar
3. Aron PM, Kennedy JA. Flavan-3-ols: nature, occurrence and biological activity. Mol Nutr Food Res 2008;52:79–104.10.1002/mnfr.200700137Suche in Google Scholar
4. Braicu C, Pilecki V, Balacescu O, Irimie A, Neagoe IB. The relationships between biological activities and structure of flavan-3-ols. Int J Mol Sci 2011;12:9342–53.10.3390/ijms12129342Suche in Google Scholar
5. Abdallah HM, El-Bassossy HM, Mohamed GA, El-halawany AM, Alshali KZ, Banjar ZM. Phenolics from Garcinia mangostana alleviate exaggerated vasoconstriction in metabolic syndrome through direct vasodilatation and nitric oxide generation. BMC Complement Altern Med 2016;16:359.10.1186/s12906-016-1340-5Suche in Google Scholar
6. Watson DM. Mistletoe – a keystone resource in forests and woodlands worldwide. Annu Rev Ecol Syst 2001;32:219–49.10.1146/annurev.ecolsys.32.081501.114024Suche in Google Scholar
7. Waly NM. Anatomical and statistical analysis of six parasitic Loranthaceae species. Am J Res Commun 2013;1:317–32.Suche in Google Scholar
8. Bar JM, Ibrahim SR, Abou-Hussein DR. Plicosepalin A, a novel catechin-gallic acid derivative of inositolfrom Plicosepalus curviflorus. Z Naturforsch 2016;71:375–80.10.1515/znc-2015-0231Suche in Google Scholar
9. Bamane FH, Badr JM, Amin OA. Antioxidant activities and flavonoid contents of selected plants belonging to family loranthaceae. Afr J Biotechnol 2012;11:14380–5.10.5897/AJB12.2093Suche in Google Scholar
10. Al-Fatimi M, Wurster M, Schroder G, Lindequist U. Antioxidant, antimicrobial and cytotoxic activities of selected medicinal plants from Yemen. J Ethnopharmacol 2007;111:657–6.10.1016/j.jep.2007.01.018Suche in Google Scholar
11. Yang LL, Yen KY, Kiso Y, Hikino H. Antihepatotoxic actions of Formosan plant drugs. J Ethnopharmacol 1987;19:103–10.10.1016/0378-8741(87)90142-5Suche in Google Scholar
12. Osadebe PO, Okide GB, Akabogu IC. Study on anti-diabetic activities of crude methanolic extracts of Loranthus micranthus sourced from five different host trees. J Ethnopharmacol 2004;95:133–8.10.1016/j.jep.2004.06.029Suche in Google Scholar PubMed
13. Aldawsari HM, Hanafy A, Labib GS, Badr JM. Antihyperglycemic activities of extracts of the Mistletoes Plicosepalusacacia and P. curviflorus in comparison to their solid lipid nanoparticle suspension formulations. Z Naturforsch C. 2015;69:391–8.10.5560/znc.2014-0047Suche in Google Scholar
14. Al-Taweel AM, Perveen S, Fawzy GA, Alqasoumi SI, El Tahir KE. New flavane gallates isolated from the leaves of Plicosepalus curviflorus and their hypoglycemic activity. Fitoterapia 2012;83:1610–5.10.1016/j.fitote.2012.09.010Suche in Google Scholar
15. Lohezic-Le FD, Bakhtiar A, Bezivin C, Amoros M, Boustie J. Antiviral and cytotoxic activities of some Indonesian plants. Fitoterapia 2002;73:400–5.10.1016/S0367-326X(02)00125-9Suche in Google Scholar
16. Daud A, Gallo A, Sánchez AR. Antimicrobial properties of Phrygilanthus acutifolius. J Ethnopharmacol 2005;99:193–7.10.1016/j.jep.2005.01.043Suche in Google Scholar
17. Elegami AA, Elnima EI, Muddathir AK, Omer ME. Antimicrobial activity of Plicosepalus acacia. Fitoterapia 2001;72:431–4.10.1016/S0367-326X(01)00268-4Suche in Google Scholar
18. Fawzy GA, Al-Taweel AM, Perveen S. Anticancer activity of flavane gallates isolated from Plicosepalus curviflorus. Pharmacogn Mag 2014;10:S519–23.10.4103/0973-1296.139787Suche in Google Scholar PubMed PubMed Central
19. Kim YK, Kim YS, Choi SU, Ryu SY. Isolation of flavonol rhamnosides from Loranthus tanakae and cytotoxic effect of them on human tumor cell lines. Arch Pharm Res 2004;27:44–7.10.1007/BF02980044Suche in Google Scholar PubMed
20. Okuda T, Yoshida T, Chen XM, Xie JX, Fukushima M. Corianin from Coriaria japonica A. Gray, and sesquiterpene lactones from Loranthus parasiticus Merr. used for treatment of schizophrenia. Chem Pharm Bull 1987;35:182–7.10.1248/cpb.35.182Suche in Google Scholar PubMed
21. Sher H, Alyemeni MN. Pharmaceutically important plants used in traditional system of Arab medicine for the treatment of livestock ailments in the Kingdom of Saudi Arabia. Afr J Biotechnol 2011;10:9153–9.10.5897/AJB10.1570Suche in Google Scholar
22. Elshanawani MA. Plants Used in Saudi Folk-Medicine, King Abdulaziz City for Science and Technology (KACST) publishing, Kingdom of Saudi Arabia 1996:236.Suche in Google Scholar
23. Mossa JS. A study on the crude antidiabetic drugs used in Arabian folk medicine. Int J Crude Drug Res 1985;23:137–45.10.3109/13880208509069018Suche in Google Scholar
24. Yang Q, Yao C, Fang W. A new triglucosylated naphthalene glycoside from Aloe vera L. Fitoterapia 2010;81:59–62.10.1016/j.fitote.2009.07.006Suche in Google Scholar PubMed
25. Mohamed GA, Ibrahim SR, Ross SA. New ceramides and isoflavone from the Egyptian Iris germanica L. rhizomes. Phytochem Lett 2013;6:340–4.10.1016/j.phytol.2013.04.009Suche in Google Scholar
26. Markham K. Techniques of flavonoid identification. New York: Academic Press, 1982.Suche in Google Scholar
27. Mabry TJ, Markham KR, Thomas MB. The Systematic identification of flavonoids. New York, NY: Springer, 1970.10.1007/978-3-642-88458-0Suche in Google Scholar
28. Harborne JB. The flavonoids: advances in research since 1986. London: Chapman and Hall, 1994.10.1007/978-1-4899-2911-2Suche in Google Scholar
29. Mohamed GA, Ibrahim SR, Al-Musayeib NM, Ross SA. New anti-inflammatory flavonoids from Cadaba glandulosa Forssk. Arch Pharm Res 2014;37:459–66.10.1007/s12272-013-0305-1Suche in Google Scholar PubMed
30. El-Shanawany MA, Sayed HM, Ibrahim SR, Fayed MA, Radwan MM, Ross SA. A new isoflavone from Blepharis ciliaris of an Egyptian origin. Med Chem Res 2012:19:2346–50.10.1007/s00044-012-0228-2Suche in Google Scholar
31. Sang S, Lapsley K, Rosen RT, Ho C. New prenylated benzoic acid and other constituents from almond hulls (Prunus amygdalus Batsch). J Agric Food Chem 2002;50:607–9.10.1021/jf0110194Suche in Google Scholar PubMed
32. Al-Musayeib NM, Mohamed GA, Ibrahim SR, Ross SA. Lupeol-3-O-decanoate, a new triterpene ester from Cadaba farinosa Forsk. growing in Saudi Arabia. Med Chem Res 2013;22:5297–302.10.1007/s00044-013-0536-1Suche in Google Scholar
33. Mohamed GA, Ibrahim SR, Elkhayat ES, Ross SA, Sayed HM, El-Moghazy SA, et al. Blepharisides A and B, new flavonol glycosides from Blepharis ciliaris growing in Saudi Arabia. Phytochem Lett 2015;11:177–82.10.1016/j.phytol.2014.12.018Suche in Google Scholar
Supplemental Material:
The online version of this article (DOI: https://doi.org/10.1515/znc-2016-0180) offers supplementary material, available to authorized users.
©2017 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Aspernolides L and M, new butyrolactones from the endophytic fungus Aspergillus versicolor
- Synthesis, in-vitro cytotoxicity of 1H-benzo[f]chromene derivatives and structure–activity relationships of the 1-aryl group and 9-position
- Biodegradation of micropollutant naproxen with a selected fungal strain and identification of metabolites
- A comparative proteomic analysis for adventitious root formation in lotus root (Nelumbo nucifera Gaertn)
- Curviflorside and curviflorin, new naphthalene glycoside and flavanol from Plicosepalus curviflorus
- Termiglaucescin, a new polyhydroxy triterpene glucoside from Terminalia glaucescens with antioxidant and anti-inflammatory potential
- Influence of structure of lactones with the methylcyclohexene and dimethylcyclohexene ring on their biotransformation and antimicrobial activity
- Effects of Hypericum perforatum extract on oxaliplatin-induced neurotoxicity: in vitro evaluations
- Bioenergetic strategy of microalgae for the biodegradation of tyrosol and hydroxytyrosol
Artikel in diesem Heft
- Frontmatter
- Aspernolides L and M, new butyrolactones from the endophytic fungus Aspergillus versicolor
- Synthesis, in-vitro cytotoxicity of 1H-benzo[f]chromene derivatives and structure–activity relationships of the 1-aryl group and 9-position
- Biodegradation of micropollutant naproxen with a selected fungal strain and identification of metabolites
- A comparative proteomic analysis for adventitious root formation in lotus root (Nelumbo nucifera Gaertn)
- Curviflorside and curviflorin, new naphthalene glycoside and flavanol from Plicosepalus curviflorus
- Termiglaucescin, a new polyhydroxy triterpene glucoside from Terminalia glaucescens with antioxidant and anti-inflammatory potential
- Influence of structure of lactones with the methylcyclohexene and dimethylcyclohexene ring on their biotransformation and antimicrobial activity
- Effects of Hypericum perforatum extract on oxaliplatin-induced neurotoxicity: in vitro evaluations
- Bioenergetic strategy of microalgae for the biodegradation of tyrosol and hydroxytyrosol

