Abstract
A new cytochalasin, named cytochalasin P1 (1), together with four known analogs (2–5) was isolated from marine-derived fungus Xylaria sp. SOF11 from the South China Sea. The structure of the new compound was elucidated on the basis of MS and NMR (1H, 13C, HSQC, HMBC, and NOESY) data analyses. Compounds 1–5 were tested for their cytotoxicities against four tumor cell lines (SF-268, MCF-7, NCI-H460, and HepG-2). Compounds 1–5 showed significant cytotoxicity against two tumor cell lines MCF-7 and SF-268, with the IC50 values varying between 0.33 and 4.17 μM.
1 Introduction
Marine-derived fungus has been recognized as a productive and important source of novel natural compounds that could potentially be used or modified as anticancer, antibacterial, and antiviral agents. Up to date, more than 1000 structurally interesting compounds have been isolated and characterized from marine fungi [1]. Among these compounds, a promising example is halimide, which demonstrated potent cytotoxic activities against human cancer cells [2]. A derivative of halimide, plinabulin (NPI-2358), is undergoing clinical trials as potential anticancer drug [3].
We focused our efforts on the investigation of bioactive secondary metabolites from the South China Sea-derived fungi with the intention of developing drug candidates. In the past few years, we have reported new cytochalasins from Xylaria sp. SCSIO 156, cytotoxic cycloheptapeptides from Acremonium persicinum SCSIO 115, immunosuppressive mycophenolic acid derivatives from Penicillium sp. SOF07 and halogenated anthraquinones from Aspergillus sp. SCSIO F063 [4], [5], [6], [7]. Recently, a marine-derived fungus strain Xylaria sp. SOF11 attracted our attention because that the crude extract of Xylaria sp. SOF11 was lethal to brine shrimp (Artemia salina) and displayed cytotoxicity against three human tumor cell lines (A549, HCT15, and HEP3B). Chemical investigation of the fermentation products of Xylaria sp. SOF11 has resulted in the isolation of a new cytochalasin, named cytochalasin P1 (1), as well as four known analogs, cytochalasin P (2) [8], 19, 20-epoxycytochalasin N (3), 19, 20-epoxycytochalasin D (4) [9], and zygosporin G (5) [10] (Figure 1). Herein, we report the isolation, structure elucidation, and cytotoxicity of the new compound.
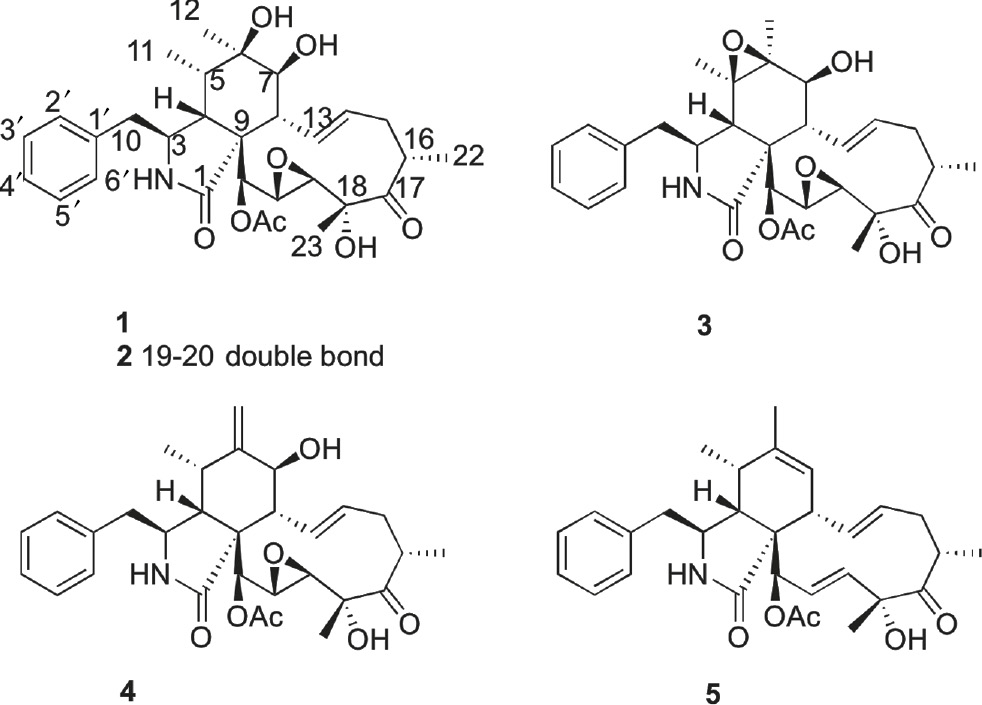
Structures of compounds 1–5.
2 Results and discussion
Compound 1 was obtained as a white powder. Its molecular formula was determined to be C30H39NO8 based on the quasimolecular ion peak at m/z 540.2612 [M–H]− (calculated 540.2603) in HR-ESI-MS spectrum, indicating 12 degrees of unsaturation. The 1H NMR spectrum of 1 displayed five methyl group proton signals at δH 0.91 (d, J=6.5 Hz, H-11), 1.13 (d,J=7.0 Hz, H-22), 1.17 (s, H-12), 1.49 (s, H-23), 2.09 (s, acetyl), 2 olefinic proton signals at δH 5.63 (dd, J=15.8, 10.5 Hz, H-13), 5.60 (m, H-14), 2 pair of ortho-coupled aromatic proton signals at δH 7.13 (2H, d, J=7.0 Hz, H-2′, 6′), 7.29 (2H, d, J=7.0 Hz, H-3′, 5′), and several aliphatic proton signals between δH 1.89–3.55 (Table 1). Analysis of the 13C NMR and HSQC data for 1 revealed 3 carbonyls (C-1, C-17, and acetyl), 4 quaternary carbons, including 2 oxygen-bearing quaternary carbons (C-6 and C-18), 16 methines, including 3 oxymethines (C-7, C-19, and C-20) and 7 aromatic methines (C-2′, C-3′, C-4′, C-5′, C-6′, C-13, and C-14), 2 methylenes (C-10 and C-15) and five methyls (C-11, C-12, C-22, C-23, and acetyl).
1H and 13C NMR spectroscopic data for 1 in CDCl3 at 500/125 MHz.
| Position | δC, mult. | δH (J in Hz) | Position | δC, mult. | δH (J in Hz) |
|---|---|---|---|---|---|
| 1 | 173.5 s | 16 | 42.0 d | 3.15 (m) | |
| 3 | 54.2 d | 3.55 (m) | 17 | 215.5 s | |
| 4 | 50.3 d | 2.17 t (10.0) | 18 | 76.4 s | |
| 5 | 38.5 d | 1.89 t (6.5) | 19 | 54.3 d | 3.48 (br s) |
| 6 | 71.9 s | 20 | 59.1 d | 3.09 d (1.5) | |
| 7 | 71.4 d | 3.02 d (12.0) | 21 | 74.5 d | 5.26 (br s) |
| 8 | 42.3 d | 2.70 t (10.0) | 22 | 18.9 q | 1.13 d (7.0) |
| 9 | 54.4 s | 23 | 21.6 q | 1.49 (s) | |
| 10 | 45.6 t | 2.88 dd (13.5, 3.5) | 1′ | 136.6 s | |
| 2.55 (m) | 2′, 6′ | 128.9 d | 7.13 d (7.0) | ||
| 11 | 13.3 q | 0.91 d (6.5) | 3′, 5′ | 129.3 d | 7.29 t (7.0) |
| 12 | 24.8 q | 1.17 (s) | 4′ | 127.1 d | 7.23 t (7.0) |
| 13 | 129.9 d | 5.63 dd (15.8, 10.5) | CH3CO | 20.5 q | 2.09 (s) |
| 14 | 133.7 d | 5.60 (m) | CH3CO | 170.0 s | |
| 15 | 37.6 t | 2.52 (m) | |||
| 2.08 (m) |
Chemical shifts (δ) are given in ppm and J in Hz.
The 1H and 13C NMR data of 1 were similar to those of the known cytochalasin P (2), except that the signals of the double bond at δH 5.10 (1H, dd, J=16.2, 2.0 Hz, H-19), 6.08 (1H, dd, J=15.5, 3.0 Hz, H-20) and at δC 128.8 (C-19), 135.0 (C-20) in 2 were absent in 1. Instead, two oxymethine signals were observed at δH 3.48 (1H, br s, H-19) and δC 54.3 (C-19), 3.09 (1H, d, J=1.5 Hz, H-20), and δC 59.1 (C-20), suggesting the double bond at C-19 and C-20 in 2 was replaced by an epoxide bond in 1. In the HMBC spectrum (Figure 2), the correlations of H-21/C-19, C-20, and H-23/C-19 substantiated the above conclusion.
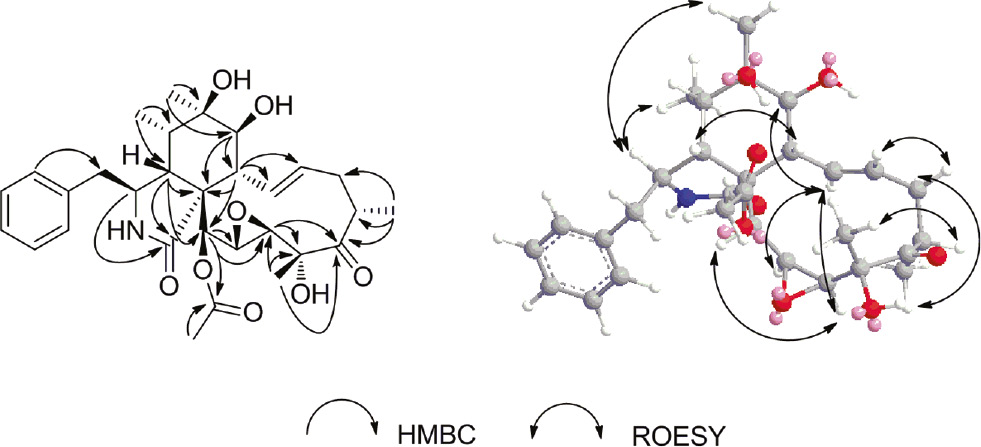
Key HMBC and NOESY correlations for compound 1.
The relative configuration of 1 was established by a combination of coupling constant and NOESY experiment. In the 1H NMR spectrum, the large coupling constant (J=12.0 Hz) for H-7 and H-8 suggested the trans orientation of the proton pair. The E-geometry of the Δ13-double bond was deduced from the large coupling constant observed for H-13 and H-14 (J=15.8 Hz). In the NOESY spectrum (Figure 2), the NOE correlation of H-3/H3-11 and H3-12, H3-12/H-7, H-4/ H-5 and H-8 indicated that H-3, H3-11, H-7, and H3-12 were in the same orientation (α); while H-4, H-5 and H-8 were in the opposite direction (β). The NOE correlation of H-7/H-13, H-14/H-8 and H-15b, H-15a/H-22, H-16/H-23 indicated that H-13 and H-22 were α-oriented; H-16 and H-23 were β-oriented. The NOE correlation of H-19/H-13 and H-21, H-13/H-20 indicated that H-19, H-20, and H-21 were α-oriented. Thus, compound 1 was established to be a new cytochalasin, and named cytochalasin P1.
Compounds 1–5 were biologically evaluated for in vitro cytotoxicity against four human tumor cell lines (SF-268, MCF-7, NCI-H460, and HepG-2) by using the SRB method with cisplatin as the positive control. Compounds 1–5 showed cytotoxic activities against SF-268 and MCF-7 cell lines, with the IC50 values varying between 0.33 and 4.17 μM, as listed in Table 2. However, Compounds 1–5 exhibited no cytotoxic activities against NCI-H460 and HepG-2 cell lines, those tentative results revealed that the tested compounds had excellent selective cytotoxic activities.
Cytotoxicities of compounds 1–5 against four tumor cell lines (IC50, μM).
| Compounds | SF-268 | MCF-7 | NCI-H460 | HepG-2 |
|---|---|---|---|---|
| 1 | 1.37 ±0.06 | 0.71±0.05 | >100 | >100 |
| 2 | 4.17±0.08 | 3.28±0.25 | >100 | >100 |
| 3 | 1.57±0.10 | 2.67±0.13 | >100 | >100 |
| 4 | 0.33±0.04 | 0.53±0.07 | 49.38±2.47 | >100 |
| 5 | 1.38±0.30 | 4.11±0.29 | >100 | >100 |
| Cisplatina | 1.93±0.16 | 5.87±0.45 | 1.32±0.15 | 1.75±0.17 |
aPositive control.
3 Experimental
3.1 General experimental procedures
Optical rotations were recorded on an Anton-Paar-MCP-300 polarimeter. 1H, 13C, and 2D NMR spectra were measured on a Bruker Avance-500 spectrometer; δ in ppm relative to Me4Si as internal standard, J in Hz. ESI-MS was recorded on a Bruker Esquire 3000plus spectrometer. HR-ESI-MS was recorded on a Waters-Q-TOF-micro-mass spectrometer. Column chromatography (CC) was carried out using silica gel (SiO2, 100–200 mesh; Qingdao Haiyang Chemical Co., China), ODS (40–63 μm; YMC, Japan), and Sephadex LH-20 (GE Healthcare, Sweden). Thin layer chromatography was conducted using precoated SiO2 GF254 plates (10–40 μm; Qingdao Haiyang Chemical Co., China). Semipreparative HPLC was operated on a Varian ProStar 210 solvent delivery system equipped with a 335-PDA detector, using a YMC-Pack ODS-A column (250×10 mm, 5 μm), flow rate at 2.5 mL/min.
3.2 Fungal material
The strain of Xylaria sp. SOF11 was isolated from a marine sediment collected in the South China Sea (E114°14.709′, N20°44.239′). The fungus was identified by observing the morphological characteristics and analysis of the internal transcribed spacer (ITS) regions (GenBank accession number JF703668). A voucher strain of this fungus has been preserved at the RNAM Center for Marine Microbiology, South China Sea Institute of Oceanology, Chinese Academy of Sciences. The fungal strain was cultured on slants of potato dextrose agar at 25 °C for 10 days. Agar plugs were inoculated in 250-mL Erlenmeyer flask containing 50 mL of potato dextrose broth supplemented with 3% sea salt. Flask cultures were incubated at 28 °C on a rotary shaker at 200 rpm for two days as seed culture. Then 10 mL seed culture was inoculated to a 500-mL Erlenmeyer flask containing 100 g of rice, 100 mL of H2O, and 3 g of sea salt. Totally, twenty 500 mL Erlenmeyer flasks were used. The Statistical incubation was carried out at 25 °C for 20 days.
3.3 Extraction and isolation
The fermented rice substrate was extracted repeatedly with ethanol (3×2 L), and the ethanol solvent was evaporated to dryness under vacuum to afford the crude extract. The crude extract was then suspended in water (1 L) and extracted repeatedly with butanone (3×1 L). The butanone-soluble fraction (24.0 g) was separated into six fractions (Fr.1~Fr.6) on a silica gel CC using gradient elution of CH2Cl2–MeOH (from 100:0 to 0:100, V/V). Fr.2, eluted with CH2Cl2–MeOH (95:5), was separated into six subfractions (Fr.2A~Fr.2F) on a silica gel CC using gradient elution of petroleum ether – EtOAc (from 70:30 to 0:100, V/V). Frs. 2F was further separated into seven subfractions (Fr.2F1~Fr.2F7) by silica gel CC eluting with CHCl3–MeOH (100:0 to 95:5, V/V). Fr.2F5 was recrystallized with MeOH to yield compound 4 (63.0 mg). Fr. 2F6 was further purified by semipreparative HPLC to yield compounds 1 (5.6 mg), 2 (7.1 mg), 3 (8.3 mg), and 5 (7.5 mg).
Cytochalasin P1 (1): a white powder.
4 Cytotoxicity assay
The cell growth inhibitory activities of compounds 1–5 against the human tumor cell lines (SF-268, MCF-7, NCI-H460, and HepG-2) were determined using the SRB method [11]. Briefly, cells (180 μL) with a density of 3×104 cells/mL of media were seeded onto 96-well plates and incubated for 24 h at 37 °C, 5% CO2. Various concentrations of compounds (20 μL) were added to the plate wells, and plates were further incubated for 72 h. After incubation, cell monolayers were fixed with 50% (w/v) trichloroacetic acid (50 μL) and stained for 30 min with 0.4% (w/v) SRB dissolved in 1% acetic acid. Unbound dye was removed by washing repeatedly with 1% acetic acid. The protein-bound dye was dissolved in 10 mM Tris base solution (200 μL) for OD determination at 570 nm using a microplate reader. Cisplatin was used as a positive control. All data were obtained in triplicate and are presented as means±S.D. IC50 values were calculated with the Sigma Plot 10.0 software using a nonlinear curve-fitting method.
Acknowledgments
This study was supported by the Research Foundation for Advanced Talents, Lingnan Normal University (ZL1403), Natural Science Foundation of Guangdong Province (2016A030307021 and 2015A030310406), and the China Spark Program (2015GA780036).
References
1. Rateb ME, Ebel R. Secondary metabolites of fungi from marine habitats. Nat Prod Rep 2011;28:290–344.10.1039/c0np00061bSuche in Google Scholar
2. Fenical W, Jensen PR, Cheng XC. Halimide, a Cytotoxic Marine Natural Product, and Derivatives Thereof. US Pat 2000;6069146.Suche in Google Scholar
3. Kingston DG. Tubulin-interactive natural products as anticancer agents. J Nat Prod 2009;72:507–15.10.1021/np800568jSuche in Google Scholar
4. Chen ZM, Huang HB, Chen YC, Wang ZW, Ma JY, Wang B, et al. New Cytochalasins from the Marine-Derived Fungus Xylaria sp. SCSIO 156. Helv Chim Acta 2011;94:1671–6.10.1002/hlca.201100051Suche in Google Scholar
5. Chen ZM, Song YX, Chen YC, Huang HB, Zhang WM, Ju JH. Cyclic heptapeptides, cordyheptapeptides C-E, from the marine-derived fungus Acremonium persicinum SCSIO 115 and their cytotoxic activities. J Nat Prod 2012;75:1215–9.10.1021/np300152dSuche in Google Scholar
6. Chen ZM, Zheng ZH, Huang HB, Song YX, Zhang XL, Ma J, et al. Penicacids A-C, three new mycophenolic acid derivatives and immunosuppressive activities from the marine-derived fungus Penicillium sp. SOF07. Bioorg Med Chem Lett 2012;22:3332–5.10.1016/j.bmcl.2012.02.106Suche in Google Scholar
7. Huang HB, Wang FZ, Luo MH, Chen YC, Song YX, Zhang W, et al. Halogenated anthraquinones from the marine-derived fungus Aspergillus sp. SCSIO F063. J Nat Prod 2012;75:1346–52.10.1021/np3002699Suche in Google Scholar
8. Edwards RL, Maitland DJ. Metabolites of the higher fungi. Part 24. Cytochalasin N, O, P, Q, and R. New cytochalasins from the fungus Hypoxylon terricola Mill. J Chem Soc Perkin 1 1989;1: 57–65.10.1039/p19890000057Suche in Google Scholar
9. Espada A, Rivera-Sagredo S, De La Fuente JM, Hueso-Rodriguez JA, Elson SW. New cytochalasins from the fungus Xylaria hypoxylon. Tetrahedron 1997;53:6485–92.10.1016/S0040-4020(97)00305-0Suche in Google Scholar
10. Minato H, Katayama T. Studies on the metabolites of Zygosporium masonii. Part II. Structures of zygosporins D, E, F, and G. J Chem Soc Perkin 1 1970;1:45–7.10.1039/j39700000045Suche in Google Scholar PubMed
11. Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 1990;82:1107–12.10.1093/jnci/82.13.1107Suche in Google Scholar PubMed
©2017 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Research Article
- Environmental alterations in biofuel generating molecules in Zilla spinosa
- Short Communication
- Antinociceptive effect of Aristolochia trilobata stem essential oil and 6-methyl-5-hepten-2yl acetate, its main compound, in rodents
- Research Articles
- Bioenergetics of lactate vs. acetate outside TCA enhanced the hydrogen evolution levels in two newly isolated strains of the photosynthetic bacterium Rhodopseudomonas
- Leaves of Cordia boissieri A. DC. as a potential source of bioactive secondary metabolites for protection against metabolic syndrome-induced in rats
- Rapid Communication
- The evaluation of the synergistic effect of 3-(2,4-dihydroxyphenyl)propionic acid and l-ascorbic acid on tyrosinase inhibition
- Research Articles
- Virus inactivation under the photodynamic effect of phthalocyanine zinc(II) complexes
- Cytochalasin P1, a new cytochalasin from the marine-derived fungus Xylaria sp. SOF11
- Synthesis and biological evaluation of newer 1,3,4-oxadiazoles incorporated with benzothiazepine and benzodiazepine moieties
- Caspase-1 from the silkworm, Bombyx mori, is involved in Bombyx mori nucleopolyhedrovirus infection
Artikel in diesem Heft
- Frontmatter
- Research Article
- Environmental alterations in biofuel generating molecules in Zilla spinosa
- Short Communication
- Antinociceptive effect of Aristolochia trilobata stem essential oil and 6-methyl-5-hepten-2yl acetate, its main compound, in rodents
- Research Articles
- Bioenergetics of lactate vs. acetate outside TCA enhanced the hydrogen evolution levels in two newly isolated strains of the photosynthetic bacterium Rhodopseudomonas
- Leaves of Cordia boissieri A. DC. as a potential source of bioactive secondary metabolites for protection against metabolic syndrome-induced in rats
- Rapid Communication
- The evaluation of the synergistic effect of 3-(2,4-dihydroxyphenyl)propionic acid and l-ascorbic acid on tyrosinase inhibition
- Research Articles
- Virus inactivation under the photodynamic effect of phthalocyanine zinc(II) complexes
- Cytochalasin P1, a new cytochalasin from the marine-derived fungus Xylaria sp. SOF11
- Synthesis and biological evaluation of newer 1,3,4-oxadiazoles incorporated with benzothiazepine and benzodiazepine moieties
- Caspase-1 from the silkworm, Bombyx mori, is involved in Bombyx mori nucleopolyhedrovirus infection


