Abstract
Caspase-1 is one of the effector caspases in mammals that plays a central role in apoptosis. However, the lepidopteran caspase-1, especially the Bombyx mori caspase-1 (Bm-caspase-1), has not been investigated in detail. In this study, Bm-caspase-1 was identified from an expressed sequence tag database in B. mori by BLAST search. The open reading frame of Bm-caspase-1 contained 879 nucleotides and encoded 293 amino acids with a predicted molecular mass of 33 kDa. Bm-caspase-1 contained two consensus amino acid motifs of caspase cleavage sites, DEGDA and TETDG. Caspase activity assays revealed significant proteolytic activity of the Ac-DEVD-pNA substrate. Bm-caspase-1 can be detected in all tissues and developmental stages by a semi quantitative polymerase chain reaction assay. More importantly, the expression level of Bm-caspase-1 is increased upon baculovirus infection and up-regulated in BmNPV-resistant silkworms. Taken together, these results indicate that Bm-caspase-1 plays an important role during baculovirus infection.
1 Introduction
Apoptosis is a tightly regulated programmed cell death process leading to the elimination of unwanted cells. It plays a key role in embryonic development and tissue homeostasis [1]. Apoptosis is also a highly evolutionarily conserved process in different species. Diverse apoptotic stimuli, including viral infections, trigger apoptosis through several apoptotic pathways that converge on the activation of the caspase cascade. Caspases are a family of cysteine proteases that have long been considered to play important roles in programmed cell death [2]. They can be further classified into initiator and effector caspases based on their structure and order in the apoptosis pathways [3].
Since the identification of caspase-1 in 1992, several classes of initiator and effector caspases have been identified in different species ranging from vertebrate to invertebrate [2]. Three initiators and four effectors have been characterized in Drosophila melanogaster [4], [5]. Since the sequencing for the genome of the silkworm, Bombyx mori in 2004, it has become a key model organism for lepidopteran gene function and genetics studies [6]. Recently, five caspase family homologues have been identified in the B. mori genome, including two initiators (BmDredd and BmDronc) and three effectors (Bm-caspase-1, Bm-ICE, and Bm-caspase-N) [7]. However, the functions of B. mori’s caspases have not yet been clearly defined. BmDronc has been shown to be an initiator caspase responsible for the induction of caspase-dependent apoptosis [8]. BmICE-2 was identified as a novel pro-apoptotic gene with caspase-9 activity [9]. Caspase-1 is one of principal effector caspases and has been identified in multicellular organisms. It was recently reported that Bm-caspase-1 likely played a role in B. mori nucleopolyhedrovirus (BmNPV) infection in a study using comparative proteomic analysis [10]. However, the function of Bm-caspase-1 was not investigated in detail, especially in the context of baculovirus infection.
In this study, we cloned and characterized Bm-caspase-1 from the silkworm and found high similarity to the previously reported lepidopteran caspase-1. To examine its caspase activity, the full-length Bm-caspase-1 gene was amplified and expressed in Escherichia coli. Cell lysates from E. coli expressing Bm-caspase-1 were examined using different synthetic substrates. The results showed that Bm-caspase-1 had significant proteolytic activity for the caspase-3 Ac-DEVD substrate. Furthermore, BmNPV infection increased Bm-caspase-1 expression levels and activated caspase-1-like protease activity. Most importantly, real-time polymerase chain reaction (PCR) analysis indicated that Bm-caspase-1 expression levels were much higher in BmNPV-resistant silkworms than in BmNPV-susceptible silkworms.
2 Materials and methods
2.1 Animals and real-time PCR analysis
Silkworms (NB, BC8, and 306 strains) were reared on fresh mulberry leaves at 25°C under 12:12-h light/dark cycles. Total RNA from B. mori larval tissues was extracted using the RNeasy® Mini Kit (Qiagen, Valencia, CA, USA). The extracted RNA was used to synthesize cDNA using the PrimeScript™ RT reagent Kit (Takara, Kyoto, Japan) following the manufacturer’s instructions. Semiquantitative PCR was performed according to the standard protocol using gene-specific primes and internal control Bm-rp49 primers. Gene expression was evaluated by real-time PCR as previously described [11]. Bm-rp49 primers were used as internal control.
2.2 Cloning of Bm-caspase-1 and sequence analysis
Tn-caspase-1 sequence was used as target sequence to identify the Bm-caspase-1 from B. mori expressed sequence tags (EST) database. Multiple sequence alignment was performed with BioEdit software (http://www.ebi.ac.uk/clustaw/). The phylogenetic tree was constructed in MEGA 5 software using neighbor-joining (NJ) method.
2.3 Bm-caspase-1 expression in E. coli
Gene-specific primers for Bm-caspase-1 (forward: 5′-CGCGGATCCATGGCTGATGAAGAAAAGAAAACC-3′; reverse: 5′-AGTAGCGGCCGCCAAACAAGAGAAGGCGTGTCAG-3′) containing BamHI and XhoI restriction sites were synthesized (Shanghai Shenggong, Shanghai, China). The full length of Bm-caspase-1 was amplified by PCR using pfu polymerase (Takara) and cloned into the pET-30a expression vector. After ligation, the recombinant vector was transformed into E. coli BL21. The expression plasmid was induced with 0.4 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) at 28°C for 4 h.
Subsequently, the bacterial cells were centrifuged and resuspended in 50 mM phosphate buffer (pH 8.0). The bacterial cells were harvested by sonication and centrifugation and then they were boiled for 10 min. Protein samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then stained with Coomassie brilliant blue.
2.4 Caspase activity assay
Caspase activity was measured using a caspase activity kit according to the manufacturer’s protocol (Beyotime, Haimen, China). After IPTG induction, cell lysates were prepared and the supernatants were incubated in a 96-well plate with Ac-DEVD-pNA, Ac-LEHD-pNA, or Ac-LETD-pNA at 37°C overnight. The absorbance values of pNA were measured at 405 nm using plate reader (BioTek, Santa Barbara, CA, USA).
3 Results
3.1 Isolation and characterization of Bm-caspase-1
The cDNA sequence showed high similarity to lepidopteran caspase-1 that was identified from the B. mori EST database using Trichoplusia ni caspase-1 sequence as a template. The open reading frame of this cDNA is 879 bp and encodes 293 amino acids with a putative molecular mass of 33 kDa (Figure 1). The Bm-caspase-1 protein sequence contained the highly conserved amino acid motif QACQG surrounding the catalytic site. It also contained two consensus motifs of caspase cleavage, DEGDA and TETDG (Figure 1). Protein sequence alignment results revealed that Bm-caspase-1 shared 83% identity with Manduca sexta, 81% identity with T. ni, 80% identity with Chilo suppressalis, and 79% identity with Helicoverpa armigera orthologues (Figure 2). As shown in Figure 2, the Bm-caspase-1 amino acid sequence shared a high level of identity among reported lepidopteran effector caspase-1 genes by multiple sequence alignment (Figure 2). All of these caspase-1 proteins displayed the highly conserved amino acid QACQG and two consensus caspase-cleavage motifs DEGDA and TETDG (Figure 2). Next, CLUSTALX and MEGA 5.0 phylogenetic analyses were performed to compare the Bm-caspase-1 with other species by the NJ method. As shown in Figure 3, Bm-caspase-1 was most closely related to Hm-caspase-1. The phylogenetic relationship analysis revealed that Bm-caspase-1 had closer orthologous relationships with other lepidopteran caspase-1 genes (Figure 3).

Sequence analysis of Bm-caspase-1: open reading frame sequence and amino acid sequences of Bm-caspase-1. Filled boxes, the two cleavage sites, DEGDA and TETDG, used to generate the large and small subunits; black lines, pentapeptide QACQG active site.
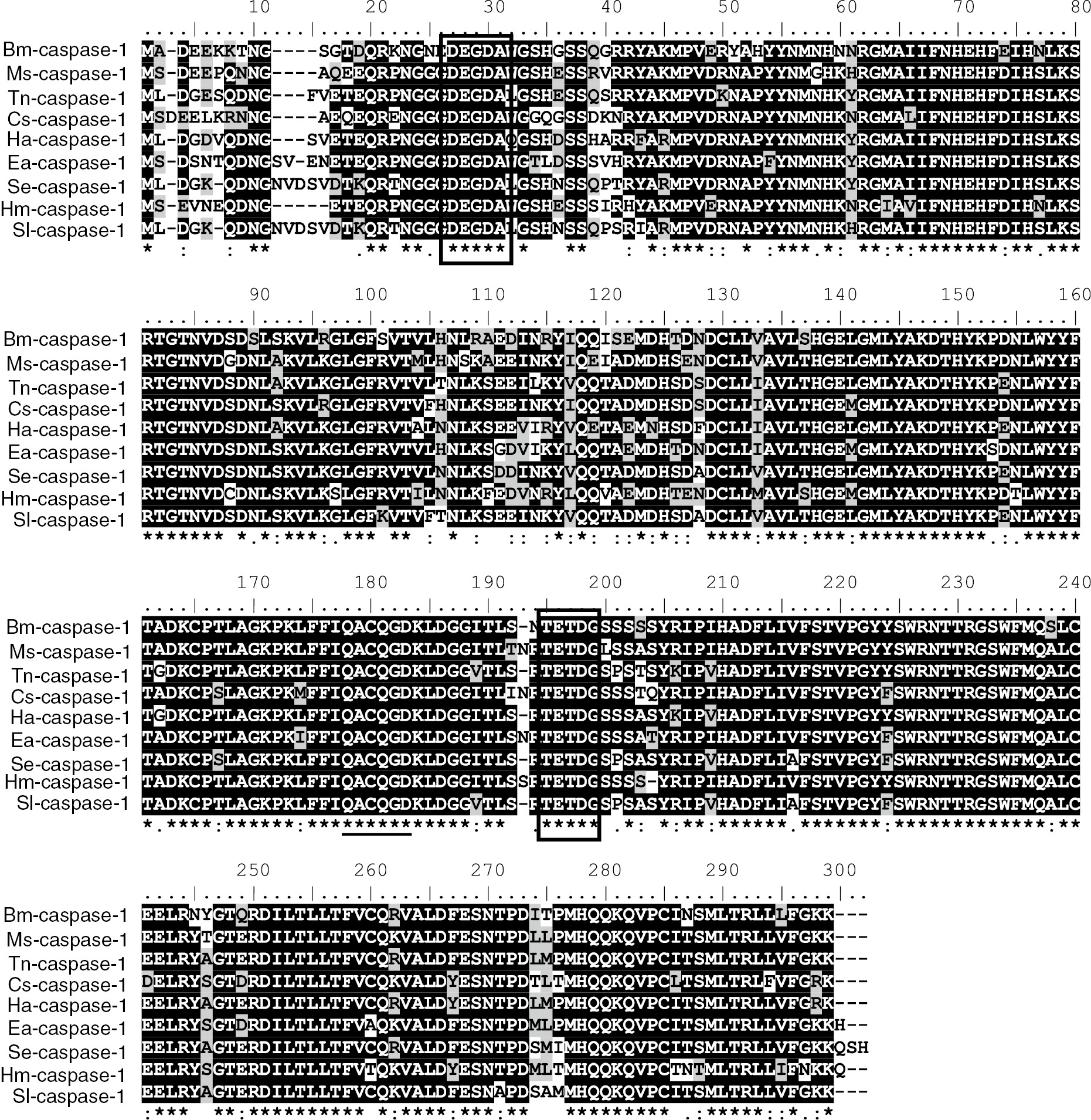
Amino sequence alignment of the Bm-caspase-1 with other lepidopteran caspase-1: multiple sequence alignment of caspase-1 from Manduca sexta (AEF30493), Trichoplusia ni (AAO17788), Chilo suppressalis (AFJ97219), Helicoverpa armigera (ABS18284), Euphydryas aurinia (AEF30498), Spodoptera exigua (AEK12771), Heliconius melpomene (ACU11588) and Spodoptera litura (BAM62940). Filled boxes, the two cleavage sites DEGDA and TETDG, used to generate the large and small subunits; black lines, pentapeptide QACQG active site.
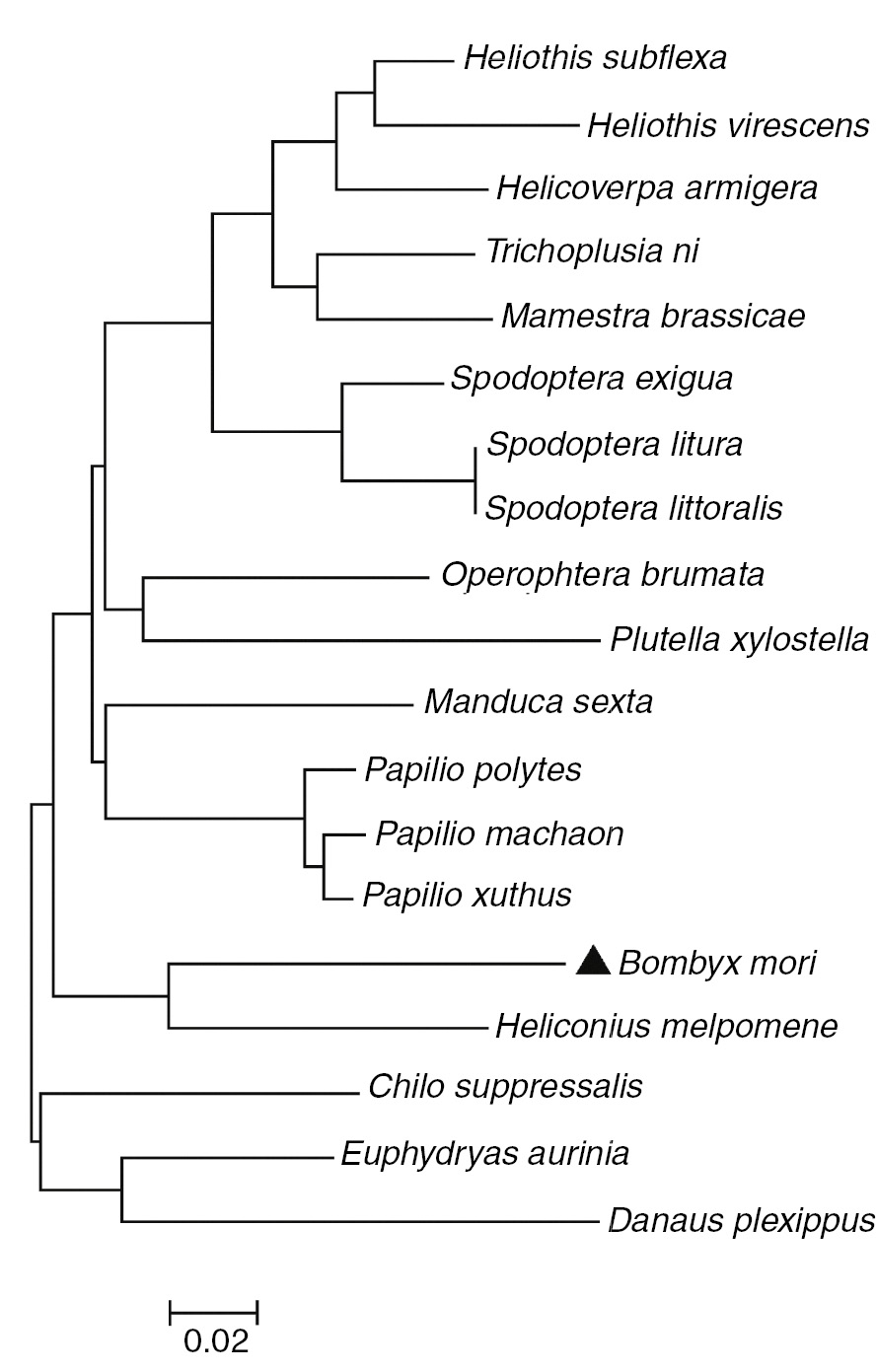
NJ phylogram showing the relationships between Bm-caspase-1 with respect to the other caspase-1. The phylogenetic tree was constructed in MEGA 5 software using the NJ method. ▲, Bm-caspase-1.
3.2 Expression and caspase activity analysis of Bm-caspase-1
We then expressed the complete ORF of Bm-caspase-1 in E. coli BL21 using the vector pET-30a. After induction with 0.5 mM IPTG for 3 h, the recombinant protein was detected using SDS-PAGE analysis (Figure 4A). Cell lysates were prepared from E. coli BL21 and centrifuged at 16,200 g for 10 min at 4°C. The resultant supernatants were examined using different synthetic caspase substrates. The results showed significant proteolytic activity for the caspase-3 Ac-DEVD-pNA substrate. However, only negligible proteolytic activity was observed for the caspase-8 Ac-LETD-pNA and caspase-9 Ac-LEHD-pNA substrates (Figure 4B). Similar results were also observed using purified proteins as shown in Figure 4C. The results demonstrated that Bm-caspase-1 protein expressed in E. coli BL21 functions as an effector caspase.
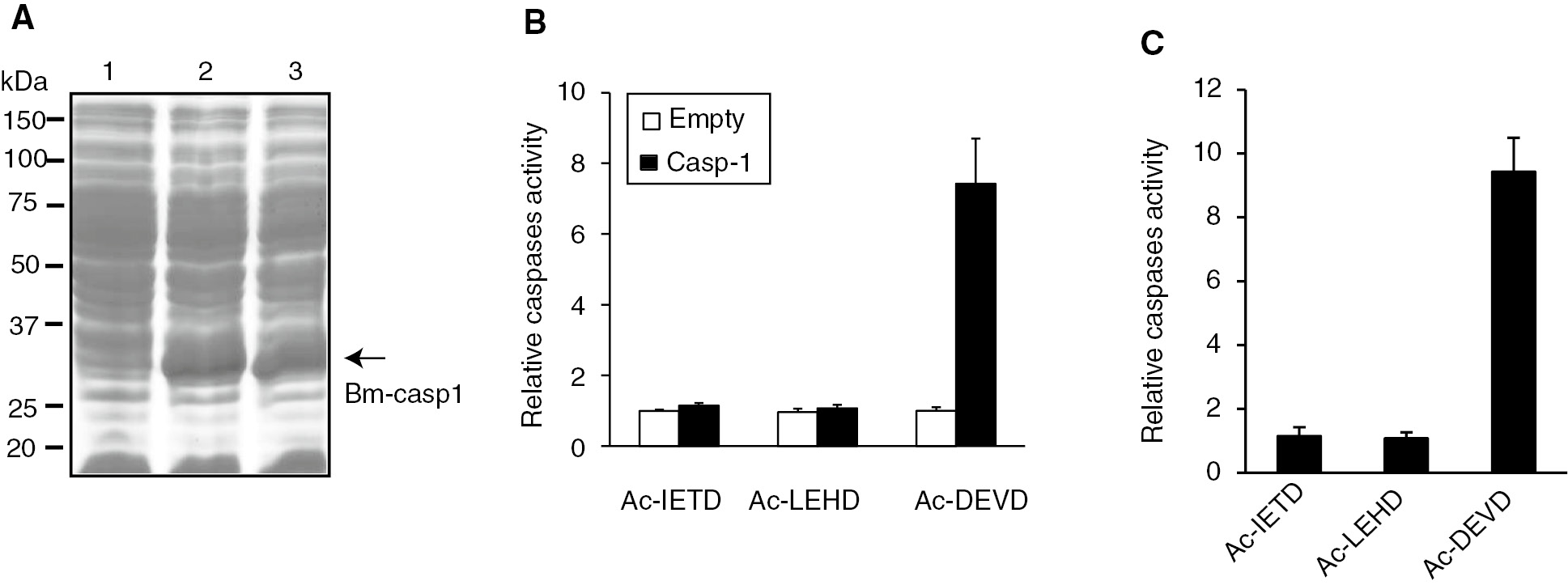
Caspase activity assay of Bm-caspase-1 in Escherichia coli BL21 cells. (A) Protein samples were separated by SDS-PAGE and stained with Coomassie brilliant blue. Lane 1, proteins from BL21 cells transformed with pET30a plasmid. Lanes 2 and 3, proteins from BL21 cells transformed with pET-30a-Bm-caspase-1. (B, C) Supernatants from BL21 cells or purified proteins were incubated with Ac-DEVD-pNA, Ac-IEHD-pNA, or Ac-LEHD-pNA at 37°C overnight. The absorbance values of pNA were measured at 405 nm. Relative caspase activity was calculated from the pNA standard curve.
3.3 Expression profiles of Bm-caspase-1 in different tissues and developmental stages
Reverse transcription-PCR (RT-PCR) was used to determine the Bm-caspase-1 mRNA expression levels in different tissues and developmental stages. Bm-caspase-1 could be detected in all of the examined tissues including fat body, integument, head, midgut, silk gland, trachea, and hemocytes (Figure 5A). Next, the mRNA from day 1 of egg, pupae, silkworm moth, and first to fifth instars was isolated, and the Bm-caspase-1 mRNA expression levels were examined. RNA from RT-PCR revealed that Bm-caspase-1 was expressed in egg, pupae, silkworm moth, and first to fifth instars (Figure 5B). These results indicate that Bm-caspase-1 is universally expressed in different tissues and at different developmental stages.
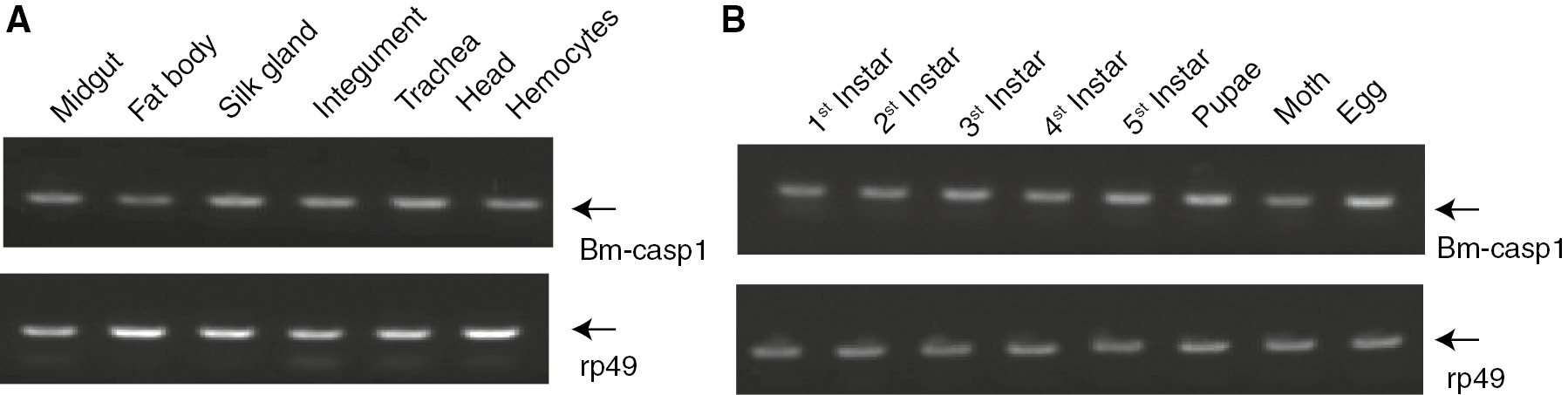
Transcriptional levels of Bm-caspase-1 in different tissues and developmental stages. (A) Bm-caspase-1 transcription levels in fat body, skin, head, midgut, silk gland, trachea, and hemocytes were measured by RT-PCR analysis. Rp49 was used as an internal control. (B) mRNA expression levels of Bm-caspase-1 in the egg, pupae, silkworm moth, and first to fifth instars were measured by RT-PCR.
3.4 Bm-caspase-1 mRNA expression levels increased during baculovirus infection
Previous studies have demonstrated that AcMNPV infection induced the expression and maturation of SI-caspase-1 in SL2 cells [12]. Silkworm larvae infected with BmNPV died with in 110 h post-infection [13]. However, no study has examined the caspase activity after infection by BmNPV in vivo. When fifth-instar B. mori larvae were infected with BmNPV, the mRNA expression levels of Bm-caspase-1 in the midgut from different strains were significantly increased from 24 to 72 h compared with the control (Figure 6A). Next, the caspase activity was analyzed during the same 24 to 72 h post-infection. As shown in Figure 6B, the caspase activity of infected silkworms increased greatly after 72 h post-infection, but was not heightened at the time of infection. Taken together, these results demonstrated that Bm-caspase-1 was likely involved in BmNPV infection of the silkworm.
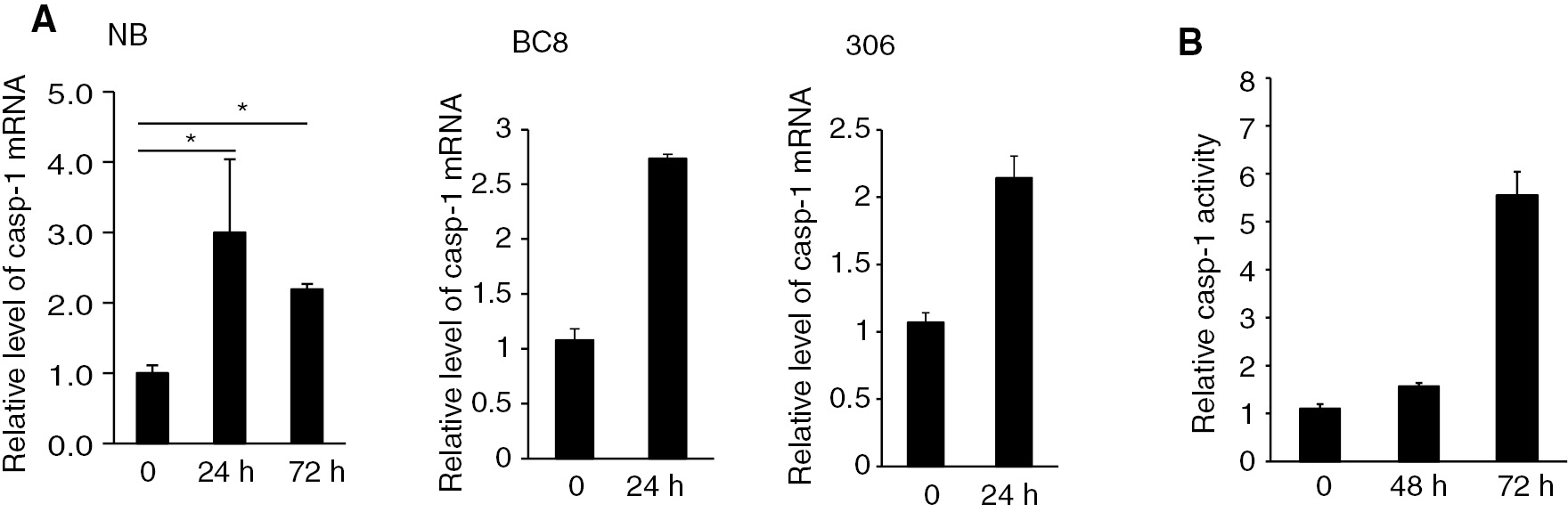
Bm-caspase-1 transcription levels and caspase-1 activity in the midgut of silkworms after BmNPV infection. (A) BmNPV-resistant strain NB, BC8, and susceptible strain 306 were infected with BmNPV for 24 h or 72 h. Then, the midguts were isolated and mRNA was extracted for RT-PCR analysis. Rp49 was used as an internal control. (B). Protein samples from the midgut in the BmNPV-resistant strain NB were isolated and supernatants were incubated with Ac-DEVD-pNA at 37°C overnight. The absorbance values of pNA were measured at 405 nm. Error bars and asterisks indicate standard deviation and statistical significance, respectively. *p<0.05.
3.5 Bm-caspase-1 transcriptional level up-regulated in BmNPV-resistant silkworms
The differences in the 50% lethal dosage (LD50) values among silkworm populations were defined to be the level of resistance to BmNPV infection. A silkworm strain named NB, with high resistance to BmNPV infection, was previously established. There was an approximately 1000-fold LD50 difference between NB and susceptible strain 306 after infection. A near-isogenic line named BC8 with resistance to BmNPV was also established [10], [14]. We then compared the mRNA expression levels of Bm-caspase-1 between the two strains. As shown in Figure 7A, the Bm-caspase-1 transcriptional level in NB was much higher than in the susceptible strain 306, even at 0 h infection. At 48 and 72 h post-infection, Bm-caspase-1 transcriptional levels were much higher in NB compared with the susceptible strain 306. Similar results were also observed in another resistant strain BC8 (Figure 7B). These results suggested that Bm-caspase-1 probably played a critical role in silkworm resistance against BmNPV infection.
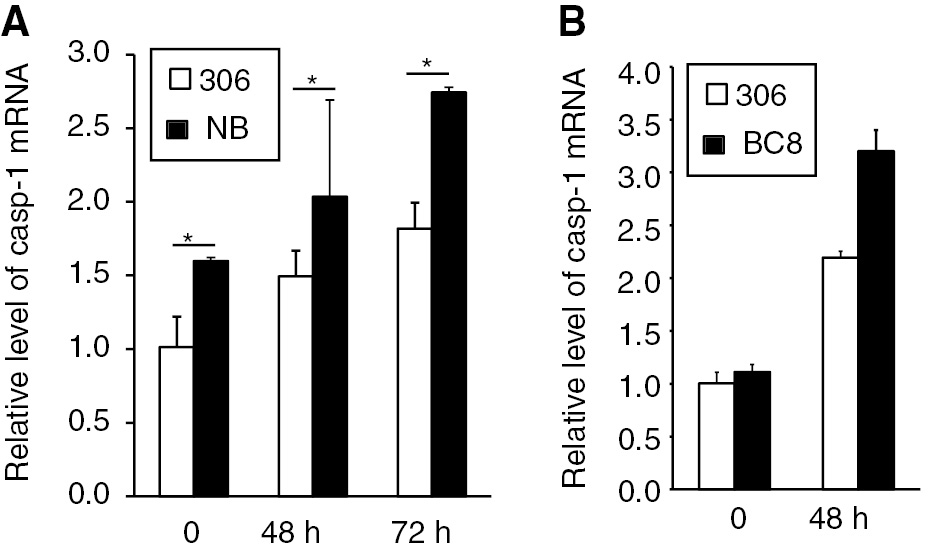
Relative expression levels of Bm-caspase-1 in the BmNPV-resistant strain NB, BC8, and susceptible strain 306. (A, B) The BmNPV-resistant strain NB, BC8, and susceptible strain 306 were infected with BmNPV for 48 or 72 h. Fat bodies in the silkworm were isolated, and mRNA was extracted for RT-PCR analysis. Rp49 was used as an internal control. Error bars and asterisks indicate standard deviation and statistical significance, respectively. *p<0.05.
4 Discussion
In this study, after searching the silkworm genome database, we cloned and expressed Bm-caspase-1 into E. coli as a recombinant protein. The caspase enzyme activity assay revealed significant proteolytic activity for the caspase-3 Ac-DEVD-pNA substrate, but not the caspase-8 Ac-LETD-pNA or caspase-9 Ac-LEHD-pNA substrates.
We further found that the Bm-caspase-1 expression level in the midgut was up-regulated during BmNPV infection of the silkworm. Furthermore, Bm-caspase-1 transcriptional levels in resistant strain NB were much higher than in the susceptible strain 306. Taken together, the data indicate that Bm-caspase-1 plays an important role during baculovirus infection.
Apoptosis of host cells is known to play a critical role during baculovirus infection. At the early stage of infection, apoptosis serves as an antiviral defensive strategy to inhibit baculovirus replication and dissemination within the host. However, at the later stages of infection, apoptosis serves as part of the virus’ strategy to self-propagate and promotes dissemination within the host [15]. A wild range of baculovirus infections induce apoptosis in different types of host cells, including B. mori BmN cells [16]. The apoptosis pathways in mammals have been extensively investigated; however, very little is known about apoptotic pathways in lepidopterans, especially during baculovirus infection. Inhibition of Sf-caspase-1 or Tn-caspase-1 expression significantly increased the production of recombinant proteins in the baculovirus expression vector system, suggesting that the apoptotic pathway mediated by caspase-1 plays critical role during baculovirus infection. Through an extensive survey of lepidopteran-derived EST datasets, Bm-caspase-1 was identified as an effector caspase; however, to date, the true function of Bm-caspase-1 remains obscure [7]. We found that Bm-caspase-1 contained two consensus motifs for caspase cleavage sites, DEGDA and TETDG. These two motifs were completely conserved among the lepidopteran caspase-1 proteins. Phylogenetic relationship analysis revealed that Bm-caspase-1 had closer orthologous relationships with lepidopteran caspase-1, suggesting that these caspase-1 proteins have similar functions. Both Ld-caspase-1 and Sf-caspase-1 were reported as effector caspases during baculovirus infection. Our results also confirmed that Bm-caspase-1 was involved in BmNPV infection.
Many studies have investigated the genes involved in resistance against baculovirus infection. Reports show that the silkworm genes for serine proteases, BmNOX, and red fluorescent proteins had certain anti-BmNPV capabilities [17], [18], [19]. Although several genes, such as gloverin 3 and 4, arylphorin, and cathepsin B, were significantly up-regulated in the resistant silkworm strain after NPV infection, whether these genes have anti-BmNPV ability remains unknown. In this study, we also found that the caspase-1 transcriptional level in the resistant strain NB was much higher than in the susceptible strain 306. However, the full details of the molecular mechanisms involved in silkworm resistance against BmNPV infection need to be further investigated.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 31572467
Award Identifier / Grant number: 81502621
Award Identifier / Grant number: 81502088
Funding source: Natural Science Foundation of Jiangsu Province
Award Identifier / Grant number: BK20140539
Funding statement: This work was supported by the National Natural Science Foundation of China (31572467, 81502621, and 81502088), the Natural Science Foundation of Jiangsu Province (BK20140539), the Postdoctoral Science Foundation of China (2015M571677), the Natural Science Fund for Colleges and Universities in Jiangsu Province of China (14KJB180004), and the Start-Up Research Funding of Jiangsu University for Distinguished Scholars (14JDG065 and 15JDG021).
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31572467, 81502621, and 81502088), the Natural Science Foundation of Jiangsu Province (BK20140539), the Postdoctoral Science Foundation of China (2015M571677), the Natural Science Fund for Colleges and Universities in Jiangsu Province of China (14KJB180004), and the Start-Up Research Funding of Jiangsu University for Distinguished Scholars (14JDG065 and 15JDG021).
References
1. Duprez L, Wirawan E, Vanden Berghe T, Vandenabeele P. Major cell death pathways at a glance. Microbes Infect 2009;11:1050–62.10.1016/j.micinf.2009.08.013Search in Google Scholar PubMed
2. Degterev A, Boyce M, Yuan J. A decade of caspases. Oncogene 2003;22:8543–67.10.1038/sj.onc.1207107Search in Google Scholar PubMed
3. Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem 1999;68:383–424.10.1146/annurev.biochem.68.1.383Search in Google Scholar PubMed
4. Accorsi A, Zibaee A, Malagoli D. The multifaceted activity of insect caspases. J Insect Physiol 2015;76:17–23.10.1016/j.jinsphys.2015.03.007Search in Google Scholar PubMed
5. Cooper DM, Granville DJ, Lowenberger C. The insect caspases. Apoptosis 2009;14:247–56.10.1007/s10495-009-0322-1Search in Google Scholar PubMed
6. Xia Q, Li S, Feng Q. Advances in silkworm studies accelerated by the genome sequencing of Bombyx mori. Annu Rev Entomol 2014;59:513–36.10.1146/annurev-ento-011613-161940Search in Google Scholar PubMed
7. Courtiade J, Pauchet Y, Vogel H, Heckel DG. A comprehensive characterization of the caspase gene family in insects from the order Lepidoptera. BMC Genomics 2011;12:357.10.1186/1471-2164-12-357Search in Google Scholar PubMed PubMed Central
8. Suganuma I, Ushiyama T, Yamada H, Iwamoto A, Kobayashi M, Ikeda M. Cloning and characterization of a dronc homologue in the silkworm, Bombyx mori. Insect Biochem Mol Biol 2011;41:909–21.10.1016/j.ibmb.2011.08.005Search in Google Scholar PubMed
9. Yi HS, Pan CX, Pan C, Song J, Hu YF, Wang L, et al. BmICE-2 is a novel pro-apoptotic caspase involved in apoptosis in the silkworm, Bombyx mori. Biochem Biophys Res Commun 2014;445:100–6.10.1016/j.bbrc.2014.01.139Search in Google Scholar PubMed
10. Qin L, Xia H, Shi H, Zhou Y, Chen L, Yao Q, et al. Comparative proteomic analysis reveals that caspase-1 and serine protease may be involved in silkworm resistance to Bombyx mori nuclear polyhedrosis virus. J Proteomics 2012;75:3630–8.10.1016/j.jprot.2012.04.015Search in Google Scholar PubMed
11. Wang Q, Zhou Y, Chen K, Ju X. Suppression of Bm-caspase-1 expression in BmN cells enhances recombinant protein production in a baculovirus expression vector system. Mol Biotechnol 2016;58:319–27.10.1007/s12033-016-9931-4Search in Google Scholar PubMed
12. Liu Q, Qi Y, Chejanovsky N. Spodoptera littoralis caspase-1, a lepidopteran effector caspase inducible by apoptotic signaling. Apoptosis 2005;10:787–95.10.1007/s10495-005-0365-xSearch in Google Scholar PubMed
13. Suzuki T, Kanaya T, Okazaki H, Ogawa K, Usami A, Watanabe H, et al. Efficient protein production using a Bombyx mori nuclear polyhedrosis virus lacking the cysteine proteinase gene. J Gen Virol 1997;78:3073–80.10.1099/0022-1317-78-12-3073Search in Google Scholar PubMed
14. Liu X, Yao Q, Wang Y, Chen K. Proteomic analysis of nucleopolyhedrovirus infection resistance in the silkworm, Bombyx mori (Lepidoptera: Bombycidae). J Invertebr Pathol 2010;105:84–90.10.1016/j.jip.2010.05.007Search in Google Scholar PubMed
15. Ikeda M, Yamada H, Hamajima R, Kobayashi M. Baculovirus genes modulating intracellular innate antiviral immunity of lepidopteran insect cells. Virology 2013;435:1–13.10.1016/j.virol.2012.10.016Search in Google Scholar PubMed
16. Wu Y, Wu Y, Hui T, Wu H, Wu Y, Wang W. Reaper homologue IBM1 in silkworm Bombyx mori induces apoptosis upon baculovirus infection. FEBS Lett 2013;587:600–6.10.1016/j.febslet.2013.01.072Search in Google Scholar PubMed
17. Nakazawa H, Tsuneishi E, Ponnuvel KM, Furukawa S, Asaoka A, Tanaka H, et al. Antiviral activity of a serine protease from the digestive juice of Bombyx mori larvae against nucleopolyhedrovirus. Virology 2004;321:154–62.10.1016/j.virol.2003.12.011Search in Google Scholar PubMed
18. Selot R, Kumar V, Sekhar SC, Kumar PG. Molecular characterization and expression analysis of BmNOX in two strains of Bombyx mori with contrasting viral resistance phenotype. Arch Insect Biochem Physiol 2010;73:163–75.10.1002/arch.20348Search in Google Scholar
19. Sunagar SG, Savanurmath CJ, Hinchigeri SB. The profiles of red fluorescent proteins with antinucleopolyhedrovirus activity in races of the silkworm Bombyx mori. J Insect Physiol 2011;57:1707–14.10.1016/j.jinsphys.2011.09.009Search in Google Scholar PubMed
©2017 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Research Article
- Environmental alterations in biofuel generating molecules in Zilla spinosa
- Short Communication
- Antinociceptive effect of Aristolochia trilobata stem essential oil and 6-methyl-5-hepten-2yl acetate, its main compound, in rodents
- Research Articles
- Bioenergetics of lactate vs. acetate outside TCA enhanced the hydrogen evolution levels in two newly isolated strains of the photosynthetic bacterium Rhodopseudomonas
- Leaves of Cordia boissieri A. DC. as a potential source of bioactive secondary metabolites for protection against metabolic syndrome-induced in rats
- Rapid Communication
- The evaluation of the synergistic effect of 3-(2,4-dihydroxyphenyl)propionic acid and l-ascorbic acid on tyrosinase inhibition
- Research Articles
- Virus inactivation under the photodynamic effect of phthalocyanine zinc(II) complexes
- Cytochalasin P1, a new cytochalasin from the marine-derived fungus Xylaria sp. SOF11
- Synthesis and biological evaluation of newer 1,3,4-oxadiazoles incorporated with benzothiazepine and benzodiazepine moieties
- Caspase-1 from the silkworm, Bombyx mori, is involved in Bombyx mori nucleopolyhedrovirus infection
Articles in the same Issue
- Frontmatter
- Research Article
- Environmental alterations in biofuel generating molecules in Zilla spinosa
- Short Communication
- Antinociceptive effect of Aristolochia trilobata stem essential oil and 6-methyl-5-hepten-2yl acetate, its main compound, in rodents
- Research Articles
- Bioenergetics of lactate vs. acetate outside TCA enhanced the hydrogen evolution levels in two newly isolated strains of the photosynthetic bacterium Rhodopseudomonas
- Leaves of Cordia boissieri A. DC. as a potential source of bioactive secondary metabolites for protection against metabolic syndrome-induced in rats
- Rapid Communication
- The evaluation of the synergistic effect of 3-(2,4-dihydroxyphenyl)propionic acid and l-ascorbic acid on tyrosinase inhibition
- Research Articles
- Virus inactivation under the photodynamic effect of phthalocyanine zinc(II) complexes
- Cytochalasin P1, a new cytochalasin from the marine-derived fungus Xylaria sp. SOF11
- Synthesis and biological evaluation of newer 1,3,4-oxadiazoles incorporated with benzothiazepine and benzodiazepine moieties
- Caspase-1 from the silkworm, Bombyx mori, is involved in Bombyx mori nucleopolyhedrovirus infection


