Abstract
Now days, production of fuels and petrochemicals from renewable lignocellulosic biomass is an indispensable issue to meet the growing energy demand. Meanwhile, the changes in the climate and soil topography influence the growth and development as well as canopy level of the lignocellulosic biomass. In this study, Zilla spinosa Turr (Zilla) plants with similar age and size were collected from three main sectors (upstream, midstream, and downstream) of Wadi Hagul during spring (April) and summer (July) seasons. Environmental stresses evoked reduction in the energy trapping pigments concomitant with increments in chlorophyll fluorescence in summer harvested plants particularly at downstream. Furthermore, the biofuels generating compounds including carbohydrate, lignin, and lipid making the plant biomasses are greatly affected by environmental conditions. Greater amount of lignin was estimated in summer harvested Z. spinosa shoots particularly at downstream. Moreover, the total oil content which is a promising source of biodiesel was considerably decreased during summer season particularly at downstream. The physical properties of the lipids major constituent fatty acid methyl esters determine the biofuel properties and contribute in the adaptation of plants against environmental stresses. Hence, the analysis of fatty acid profile showed significant modifications under combined drought and heat stress displayed in the summer season. The maximum increase in saturated fatty acid levels including tridecanoic acid (C13:0), pentadeanoic acid (C15:0), palmitic acid (C16:0), and stearic acid (C18:0) were estimated in spring harvested Z. spinosa aerial portions particularly at midstream. In spite of the reduction in the total oil content, a marked increase in the value of unsaturated to saturated fatty acids ratio and thereby the unsaturation index were achieved during the dry summer period. Henceforth, these seasonal and spatial variations in fatty acids profiles may contribute in the acclimatization of Z. spinosa plants to soil water scarcity associated with heat stress experienced during summer. In addition, the alterations in the fatty acid profiles may match biofuel requirements. In conclusion, the most adequate growing season (spring) will be decisive for achieving high lipid productivity associated with improved biofuel quality in terms of high saturated fatty acids percentage that improves its cetane number. However, the dry summer season enhanced the accumulation of greater amount of lignin that may enhance the biodiesel quantity.
1 Introduction
The Bio-energy from natural photosynthetic biomass can substitute the fossil fuels in providing clean and reliable renewable energy resources [1]. Meanwhile, the natural green biomass particularly in the desert is regularly face adverse growth conditions, such as drought, salinity, and high temperatures [2]. These stresses can reduce growth and development, productivity, and cause plant death under severe abiotic stress condition [3]. Similarly, environmental stresses can interrupt plant cellular structures and impair the physiological functions [4]. The relative concentrations of energy trapping pigments are known to be altered under abiotic stresses, and therefore, they can be used as indicator for interaction between plants and their environments [5]. Severe drought stress causes changes in chlorophyll content, affecting chlorophyll components, damaging the photosynthetic apparatus, and inhibits the photosynthesis [6]. In addition, the pigment ratio of chlorophylls (a+b)/carotenoids can be used as an indicator for stress. Thus, greater values of chlorophylls (a+b)/carotenoids ratio up to 5–6 indicated intact photosynthetic apparatus. It was deduced that chlorophyll is broken down faster than carotenoids under stress, and the pigment ratio declined to be about 2–3 [7].
Indeed, photosynthesis is one of the most sensitive physiological processes in stressed plants [8], which thereby directly contribute in biomass production. Boughalleb and Hajlaoui [9] postulated that the photosynthetic pigments, net photosynthetic rate, stomatal conductance, transpiration rate, the maximal photochemical efficiency of PSII, and the intrinsic efficiency of PSII reaction centers decreased in Olea europaea plants exposed to water scarcity. The sensitivity of photosynthesis to heat and drought is related to the damage of photosystem (PS) II components located in the thylakoid membranes [10], [11]. Consequently, the reduction in photosynthesis will reduce the production of metabolites and hence the plant biomass. Indeed, the greatest component of the plant biomass is the cell walls, which comprise lignin and polysaccharides as cellulose and hemicellulose. The utilization of the lignocellulosic plant materials could be considered as a source for the intermediate chemicals and the second generation biofuels [12]. It was recorded that the utilization of lignocellulosic plant residues could provide about 10–20% of the current world energy demand [13]. However, the reduction in lignin is sometimes accompanied by increased cellulose and hemicellulose deposition. Thus, the reduction in lignin biosynthesis was associated with cellulose accumulation and growth in some transgenic trees [14]. The variation in lignin content and components could be used to improve the digestibility of biomass [15]. Lignin provides mechanical support to the xylem cells, plays an important role in plant defense against various biotic and abiotic stresses [16], [17], in seed dispersal and in the formation of an apoplastic diffusion barrier in the roots [18].
Meanwhile, environmental stresses can induce oxidative stress in the plant cell due to overproduction of reactive oxygen species (ROS, [19], [20]). The ROS can directly damage the cellular macromolecules including lipids, metabolic enzymes, and the nucleic acids leading to cell death [21]. Henceforth, the decomposition product of polyunsaturated fatty acids hydroperoxides such as malondialdehyde (MDA) can be used as an evidence for lipid peroxidation extent [22]. Consequently, the change in membranes will usually be reflected by corresponding alterations in plant total lipids content which represents about 80% of the total lipid of leaf tissue [23].
Moreover, the vegetable oil can be used as a renewable biological sources for biodiesel which substitute diesel fuel [24]. Recently, the vegetable oil was transformed into green diesel or renewable biofuel by transesterification [24]. Several researchers reported that vegetable oils are a promising fuel that can substitute petroleum fuels [25]. The vegetable oil composition of arid inhabiting plants is considerably affected by the changes in environmental conditions beside their genetic factors [26]. The nature and the structure of the fatty acid methyl ester determine the biofuel properties. The distribution of fatty acids in the vegetable oil or fat determines the cetane number of the produced biodiesel. In general, the exposure of various crop species to long periods of water deficits lead to reductions in the levels of phospholipid, glycolipid and linoleic acid contents and increased the triacylglycerol [27], [28]. The relative amounts of the different fatty acid radicals determine the properties of fats.
Saturated fatty acids including C14:0, myristic acid; C16:0, palmitic acid; and C18:0, stearic acid have higher cetane numbers and are less susceptible to oxidation than unsaturated ones but they tend to crystallize at very high temperatures [29]. A variety of ester-based fatty esters can be used as biodiesel (or biofuel) beside its roles in the adaptation of plants against environmental stresses. Moreover, the adaptive role of lipid modifications evoked by environmental stresses is frequently depend on physical properties of the lipids involved in membrane structure and affected the permeability of biomembranes [23]. So, the knowledge of the lipid composition in plant cells is important issue. The fatty acid composition of all acyl lipids changed during stress in the direction of increased saturation of the fatty acids [30]. It was reported that short chain fatty acids particularly C16 and C18 are nonspecific and exist in the plant cell membranes and cuticle or wax [31]. Pham Thi et al. [27] pointed out that water deficits inhibit fatty acid desaturation, resulting in a sharp decrease in linoleic and linolenic acid biosynthesis. However, water stress induces an increase in fatty acid chain length in Arabidopsis thaliana, maintains the saturation level of fatty acids through a reduction in 7,10,13-hexadecatrienoic acid, and induces an increase in the proportion of linolenic acid which may help in drought-stress tolerance [32]. It was reported that the unsaturation level of polar lipids decreased in drought-sensitive plants, whereas it persisted unchanged or even increased in drought-resistant plants [33], [34]. The capacity of a plant to maintain (or increase) its polyunsaturated fatty acid contents was related to its resistance to drought stress [32], [34]. In most plants, the five major fatty acids including palmitic acid (C16:0), stearic acid (C18:0), oleic acid (C18:1), linoleic acid (C18:2), and linolenic acid (C18: 3), forming about 95–98% of the total fatty acids [35]. The proportions of these fatty acids are strongly influenced by high temperatures [36] and drought [37], [38].
Accordingly, wild plants such as Zilla spinosa (Brassicaceae or Cruciferae) one of the most common lignocellulosic wild plant species inhabiting deserts has considerable economic importance to local people. Plants acclimate the dry environments by modifying their phenology, morphology, physiology, and metabolism [6], [39]. Hence, studying the physiological and biochemical changes including bioenergetics molecules as carbohydrates, cellulose, lignin, and lipids may help in better understanding the tolerance strategies against the harsh environmental conditions and well explore the benefits of using Zilla plants for producing renewable energy that may be utilized as a second generation biofuel material.
2 Materials and methods
Wadi Hagul situated in the northern portion of the Eastern Desert of Egypt within Cairo-Suez district and is restricted by latitudes 29°48′28″–29°57′43″N and longitudes 32°09′32″–32°17′27″E. Wadi Hagul occupied the valley depression between Gebel Ataqa to the north and Gebel Kahaliya to the south. Its main channel extends for about 35 km and collects drainage water on both sides. With reference to the vegetation and geological features of Wadi Hagul, three main sectors may be distinguished, upstream, middle, and downstream (Figure 1). Moreover, the climate of the Wadi area has been described as arid to extremely arid [40].
Map of Cairo-Suez road showing the locations of the studied area of Wadi Hagul.
2.1 Soil sampling
Soil (sand) samples from up-, mid-, and downstream of Wadi Hagul were collected from the surface layer (0–20 cm) and subsurface layer (20–40 cm) during the flowering (May, spring) and fruiting (July, summer) seasons, air dried, and then large gravels and plant fragments were excluded and made ready for the mechanical and chemical analysis.
Soil–water extract (1:1) was prepared, shaking well for 2 h and leaving overnight. The filtrate was used for measuring the chemical properties of the soil.
2.2 Soil reaction (pH)
Soil reaction (pH) was measured by using a portable pH-meter (Model, ion lab pH level 1) [41].
2.3 Electrical conductivity (E.C.)
The electrical conductivity (E.C.) was measured by using a portable conductivity meter (YSI Model, 35, Yellow Springs instrument, Co. Inc., USA). The results were expressed as dS/m (dS/m=mmhos/cm) [41], [42].
2.4 Plant material
Zilla spinosa plants with similar age and size were collected from the three main sectors upstream, midstream, and downstream of Wadi Hagul during spring (April) and summer (July) seasons. The aerial parts of Zilla spinosa were dried under shade for 15 days, then ground to a powder and stored in dark bottles until used in the extraction and estimation of total soluble carbohydrates, lignocellulosic substances, and total lipids. In addition, the fresh aerial parts were used in the determination of photosynthetic pigments and MDA contents.
2.5 Extraction and estimation of photosynthetic pigments
The photosynthetic pigments (chlorophyll a, chlorophyll b, and carotenoids) were extracted from freshly harvested aerial parts of Z. spinosa in 80% acetone and determined spectrophotometrically by the method described by Metzner et al. [43].
2.6 Fluorescence spectroscopy
The fluorescence emission spectra analyses were performed using total pigments extracted from one g plant tissue. Fluorescence spectroscopy of acetone extracted pigments was performed in a Perkin Elmer LS 50B spectroflourometer using the indicated excitation and emission wavelengths [44]. Red fluorescence of chlorophyll a was recorded between 650 and 800 nm. The extracts were excited at 435 nm. Blue-green fluorescence emission spectra were recorded between 380 and 600 nm. Moreover, the extracts were excited at 337 nm. The spectral slit widths were set at 3 and 1.5 nm (excitation and emission, respectively).
2.7 Determination of total soluble carbohydrates
Total soluble carbohydrates were extracted following the method of Homme et al. [45] and determined using anthrone reagent according to the method described by Fairbairn [46].
2.8 Determination of cellulose and lignin contents
The cellulose percentage was determined according to the method of Jenkin [47], and the lignin content was determined by the method described by Rittler et al. [48].
2.9 Lipid peroxidation
The lipid peroxidation product MDA was assayed by using thiobarbituric acid (TBA) protocol described by Cakmak and Horst [49]. The amount of MDA was calculated from the absorbance at 532 nm after subtracting the nonspecific absorption at 600 nm. The extinction coefficient 155 mmol/L−1 cm−1 for MDA was used.
2.10 Extraction and determination of lipids and fatty acid profiles
The dried powder (10 g) was extracted with ethyl acetate for 18 h by using Soxhlet apparatus [50]. The extract was then quantitatively transferred to a weighed flask and the solvent was evaporated using an electric fan. The flask was then reweighed and the increase in weight was equivalent to the weight of total lipids.
Methylation was done according to the method described by Metcalfe et al. [51] using boron trifluoride (BF3)-methanol. Then, the fatty acid esters were extracted from the BF3-methanol solution by using hexane. The concentrated extract of fatty acid esters was used for gas liquid chromatography (GLC). The GLC analysis was carried out on HP-5 system equipped with a DB-5 fused silica column (30 m×0.35 mm×0.88 μm films); oven temperature was 40–240 °C at a rate of 4 °C/min, injector temperature 260 °C, detector temperature 280 °C, carrier gas helium with a linear velocity of 31.5 cm/s, split ratio 1/60, flow rate 1.1 mL/min, rate 4 °C/min, final temperature 260 °C, final time 8 min, run time 30 min, and injected amount 1 μL.
Peaks identification and quantification was carried out by using UP 4810 computing integrator (Perkins Elmer XL, USA). The percentage of each fatty acid was calculated by the following equation:
The unsaturation level of all fatty acids was estimated according to Pham Thi et al. [27], the unsaturated index can be calculated from the following equation:
where 18:1, 18:2, and 18:3, represent the oleic, linoleic, and linolenic acids, respectively.
2.11 Statistical analysis
Statistical analysis was performed by two-way analysis of variance (ANOVA) using SAS software (SAS Institute, Cary, N.C.) at a significance level of 5%. Duncan’s multiple range test was applied to assess the differences between the three sites during the two investigated seasons.
3 Results
3.1 Characteristics of Wadi Hagul habitats
Soil analysis showed a significant decrease in Wadi Hagul soil moisture particularly at the third location (downstream) during the dry season (July), whereas the EC rises compared with the Spring season. However, the pH of the soil remained more or less alkaline throughout the study period and did not seasonally fluctuate (Tables 1 and 2). The high percentage of soil moisture content was more pronounced during the wet period of April which was related to the fall of rain; however, it significantly reduced in the dry season (July). Soil moisture scarcity in Wadi Hagul was intensified during summer particularly at the edges due to low precipitation concomitant with high temperature (Table 3).
Soil reaction, electrical conductivity (EC), and chemical analysis of soil pastes of Wadi Hagul habitats during summer (Sum) and spring (Spr).
| Location | Depth | pH | EC | Soluble anions meq/L (milliequivalents/liter) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SO4−2 | CI- | HCO3− | |||||||||
| Spr | Sum | Spr | Sum | Spr | Sum | Spr | Sum | Spr | Sum | ||
| Upstream | 0–20 | 8.5 | 7.76 | 1.053 | 1.379 | 4.97 | 3.813 | 4.1 | 8.33 | 0.8 | 1.6 |
| 20–40 | 8.5 | 7.75 | 0.87 | 1.118 | 1.4 | 3.238 | 3.3 | 6.24 | 0.9 | 1.66 | |
| Midstream | 0–20 | 8.4 | 7.52 | 1.80 | 2.36 | 9.1 | 5.588 | 19.5 | 14.31 | 2.1 | 3.744 |
| 20–40 | 8.4 | 7.98 | 0.198 | 0.245 | 2.92 | 0.728 | 1.4 | 0.944 | 0.5 | 0.777 | |
| Downstream | 0–20 | 8.3 | 7.96 | 0.123 | 0.252 | 8.2 | 0.885 | 0.42 | 0.95 | 0.62 | 0.78 |
| 20–40 | 8.3 | 8.09 | 0.88 | 0.139 | 2.65 | 0.34 | 0.22 | 0.65 | 0.36 | 0.4 | |
The percentage of soil water content and the chemical analysis of Wadi Hagul soil pastes during spring (Spr) and summer (Sum).
| Location | Soil water content (%) | Soluble cations (meq/L milliequivalents/liter) | CaCO3 (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spr | Sum | Mg2+ | Na+ | Ca2+ | K+ | |||||||
| Spr | Sum | Spr | Sum | Spr | Sum | Spr | Sum | Spr | Sum | |||
| Upstream | 1.45 | 0.75 | 0.86 | 2.45 | 2.80 | 8.10 | 2.06 | 2.94 | 0.214 | 0.226 | 7.9 | 15.5 |
| 0.53 | 1.56 | 1.10 | 6.33 | 2.42 | 3.12 | 0.100 | 0.133 | 5.7 | 8.10 | |||
| Midstream | 2.90 | 2.75 | 1.98 | 4.16 | 5.43 | 12.86 | 5.40 | 6.24 | 0.214 | 0.346 | 17.3 | 19.3 |
| 0.12 | 0.56 | 0.37 | 0.95 | 0.296 | 0.777 | 0.100 | 0.171 | 13.1 | 14.9 | |||
| Downstream | 0.90 | 0.36 | 0.26 | 0.60 | 0.37 | 0.96 | 0.41 | 0.81 | 0.143 | 0.154 | 40.4 | 41.7 |
| 0.73 | 0.21 | 0.10 | 0.68 | 0.15 | 0295 | 0.100 | 0.105 | 48.7 | 51.2 | |||
The meteorological data (climatic condition) of Wadi Hagul at the 4 years 2010–2014.
| Months | Wind velocity (km/h) | Relative humidity (%) | Rain fall (mm/month) | Mean minimum temperature (°C) | Mean maximum temperature (°C) |
|---|---|---|---|---|---|
| January | 7.02 | 59 | 3.4 | 10 | 20 |
| February | 7.65 | 58 | 3.7 | 11 | 21 |
| March | 7.46 | 54 | 3.2 | 13 | 25 |
| April | 8.36 | 47 | 17.7 | 16 | 28 |
| May | 7.46 | 45 | 0 | 19 | 32 |
| June | 8.81 | 48 | 0 | 22 | 36 |
| July | 7.89 | 52 | 0.5 | 24 | 38 |
| August | 7.78 | 54 | 0 | 24 | 38 |
| September | 8.3 | 56 | 0 | 22 | 34 |
| October | 8.56 | 58 | 0.2 | 19 | 30 |
| November | 6.8 | 60 | 1.7 | 15 | 26 |
| December | 6.21 | 62 | 3.9 | 11 | 22 |
3.2 The alteration in photosynthetic pigments
The values of chlorophylls a, b and carotenoids are greater in the wet season collected Zilla spinosa shoots compared to those of dry season (Table 4). The greatest levels of chlorophylls a and b, a/b, a/b, and total carotenoids as well as carotenoids was measured in Zilla spinosa shoot tops inhabiting the midstream of Wadi Hagul bed during the wet season followed by that collected during the dry season. Moreover, the minimum values of chlorophylls a and b, a/b ratio, and carotenoids were recorded in plants inhabiting downstream the bed particularly during the dry season.
Seasonal changes in pigment levels in Zilla spinosa aerial portions collected from different habitats of Wadi Hagul.
| Season | Pigment content Location | Chl a | Chl b | Chl a+b | Chl a/b | Total carotenoids | Chl (a+b)/carotenoids |
|---|---|---|---|---|---|---|---|
| Spring | Upstream | 2.23±0.013b | 0.60±0.020b | 2.83 | 3.72 | 1.20±0.013b | 2.36 |
| Midstream | 3.00±0.010a | 0.8±0.017a | 3.80 | 3.8 | 1.38±0.010a | 2.75 | |
| Downstream | 0.92±0.011e | 0.42±0.023c | 1.33 | 2.19 | 0.50±0.0008e | 2.66 | |
| Summer | Upstream | 1.10±0.011d | 0.421±0.016c | 1.52 | 2.62 | 0.57±0.0002d | 2.71 |
| Midstream | 1.70±0.009c | 0.61±0.010b | 2.31 | 2.79 | 0.84±0.0004c | 2.75 | |
| Downstream | 0.81±0.007f | 0.41±0.011c | 1.22 | 1.98 | 0.47±0.0001e | 2.60 |
Each value is a mean of three replicates±SD. a,b,c,d,eChanges indicated by similar letters are not significantly different. fChange is significantly different.
Measurements of fluorescence emission spectra of chlorophyll a have been an early indicator of stress conditions in plants. Hot dry summer conditions provoke an increase in F675 which is more distinct in downstream inhabiting Zilla shoots harvested in dry summer season (Figure 2 A–D). Furthermore, a slight increase in chlorophyll a fluorescence emission of upstream and downstream located plants was observed with a red shift at 676 of about 1 nm during Spring (Table 5). Moreover, the maximum chlorophyll a fluorescence emission around 675 nm of Summer plants was greater compared to Spring ones at the three studied locations.
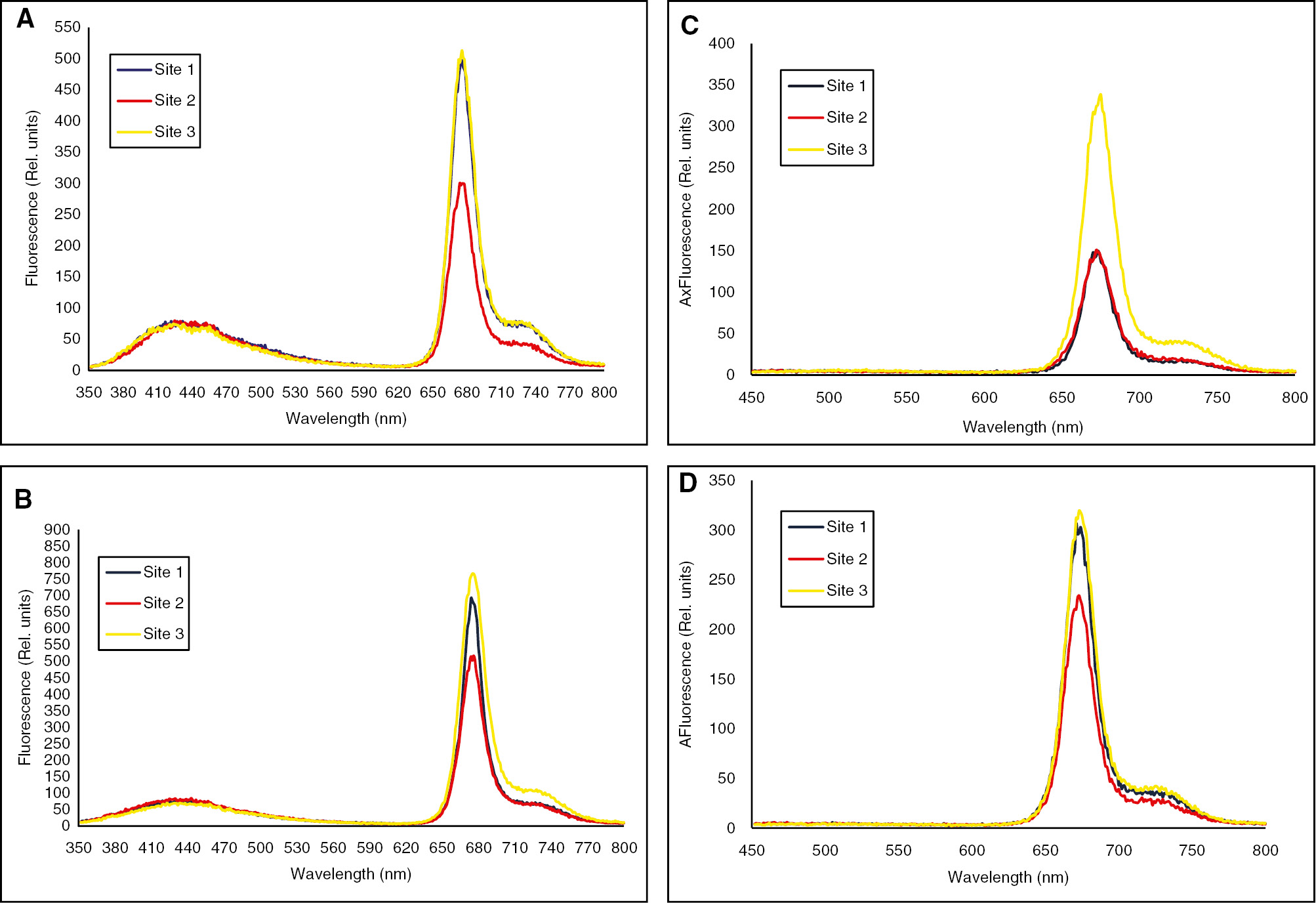
(A and B) Seasonal changes in blue-green fluorescence emission spectra of Zilla spinosa green tops inhabiting Wadi Hagul during the spring (A) and summer (B) seasons. Exλ=337 nm (Slit width for both excitation and emission was 3 and 1.5 nm, respectively). Each value is a mean of three replicates. (C and D) Seasonal changes in red fluorescence emission spectra of Zilla spinosa green tops inhabiting Wadi Hagul during the spring (C) and summer (D) seasons. Exλ=435 nm (Slit width for both excitation and emission was 3 and 1.5 nm, respectively). Each value is a mean of three replicates.
Seasonal changes in the peak position of fluorescence spectra around 450 and 680 nm in Zilla spinosa shoot extract grown in different habitats of Wadi Hagul.
| Season | Location | Peak position around 675 nm | Peak position around 730 nm | F 450/F 675 | F 675/F 730 |
|---|---|---|---|---|---|
| Spring | Upstream | 676 | 727 | 0.11 | 7.3 |
| Midstream | 674 | 725 | 0.16 | 6.9 | |
| Downstream | 676 | 728 | 0.08 | 9.7 | |
| Summer | Upstream | 677 | 730 | 0.15 | 6.6 |
| Midstream | 676 | 728 | 0.25 | 6.5 | |
| Downstream | 677 | 732 | 0.14 | 9.9 |
Values are means of three replicates.
It is of interesting to note here that a longer shift at F730 with lengths of 2–4 nm was displayed in summer harvested shoots as compared with those of spring. The increase in the fluorescence emission and the red shift are parallel to the reduction in chlorophyll content. Furthermore, the ratio of F450/F675 was markedly reduced in Zilla shoots inhabiting the edges of Wadi Hagul particularly, downstream. In contrary, the ratio of F675/F730 was markedly increased in Zilla inhabiting Wadi’s edges particularly, in downstream inhabiting shoot tops (Table 5).
3.3 The alteration in total soluble carbohydrates
The greatest level of total soluble carbohydrates accumulation attained in edges of Wadi Hagul inhabiting plants during the wet spring season (Table 6). However, during the dry summer season, the total soluble carbohydrates reached the highest level in Zilla inhabiting downstream Wadi Hagul compared to the other locations. The minimum values of total soluble carbohydrates accumulation were estimated in midstream grown plants during the wet and dry seasons as compared with those of the other Wadi’s beds.
Seasonal changes in lipid peroxidation product (malondialdehyde, MDA), total soluble sugars, % of cellulose, % of lignin, and total oil content of Zilla spinosa shoots grown in different habitats of Wadi Hagul.
| Season | Parameter Location | MAD (mmol) | Total soluble sugars (mg/gfw) | Cellulose (%) | Lignin (%) | Total lipids (%) |
|---|---|---|---|---|---|---|
| Spring | Upstream | 3.46±0.13b | 14.0±0.20b | 52.0±0.020b | 43.1±0.020b | 3.90±0.013b |
| Midstream | 1.90±0.10a | 6.76±0.17a | 50.4±0.017a | 41.7±0.017a | 7.24±0.010a | |
| Downstream | 5.79±0.11e | 10.31±0.23c | 56.3±0.023c | 42.3±0.023c | 3.60±0.0008e | |
| Summer | Upstream | 6.820±0.11d | 4.19±0.16c | 62.1±0.016c | 62.1±0.016c | 3.20±0.0002d |
| Midstream | 5.98±0.9c | 2.64±0.10b | 60.6±0.010b | 60.6±0.010b | 3.60±0.0004c | |
| Downstream | 7.17±0.7f | 6.42± 0.11c | 66.0±0.011c | 66.0±0.011c | 2.90±0.0001e |
Each value is a mean of three replicates±SD. a,b,c,d,eChanges indicated by similar letters are not significantly different. fChange is significantly different.
3.4 The alteration in cellulose and lignin
The amount of both cellulose and lignin was significantly increased in Zilla shoots inhabiting Wadi Hagul edges (Table 6). Such effect was more pronounced in summer harvested Zilla shoots. The maximum increases in cellulose (61.6 g/100 g dw) and lignin (66 g/100 g dw) was recorded at the third location during summer season. The lowest amount of either cellulose or lignin was measured in spring harvested Zilla inhabits the second location.
3.5 Lipd peroxidation product molondialdehyde (MDA)
The intensification of lipid peroxidation is one of the main reactions for lipid damage by ROS. When the unsaturated fatty acids are peroxidized, molondialdehyde (MDA) is produced. The increments in MDA levels in Zilla tops inhabiting the edges of Wadi Hagul during spring and summer was positively related to the intensity of the soil water scarcity (Table 6).
3.6 The alteration in total lipids contents
The percentage of total lipid content was positively related to soil moisture content along Wadi Hagul (Table 6). The greatest lipid percentage displayed in the areal parts of Zilla inhabiting the second location during spring (7.24%) followed by summer (3.6%) season (Table 6). The lowest lipid content (2.9%) displayed by summer harvested Zilla inhabiting the third location (downstream).
3.7 The alteration in fatty acid composition in Zilla shoots
The GLC analysis of the fatty acid methyl esters resulted in the identification of 12 fatty acids in which palmitic (C16:0) and stearic acids (C18:0) are the main saturated acids and linoleic acid (C18:2) is the main unsaturated acid in Zilla spinosa aerial parts inhabiting Wadi Hagul (Table 7). The proportion of tridecanoic acid (C13:0), pentadecanoic (C15:0), palmitic acid (C16:0), and stearic acid (C18:0) were markedly increased in spring harvested Zilla aerial parts particularly those inhabiting midstream of Wadi Hagul. The fatty acid profile of Zilla characterized also, by a greater amount of unsaturated fatty acids which was detected in summer harvested plants particularly in plants inhabiting downstream Wadi Hagul. The increments in percentage of unsaturated fatty acids such as linoleic acid (C18:2) and linolenic (C18:3) were about 37.33% and 11.1%, respectively, in harvested Zilla aerial parts inhibiting downstream Wadi Hagul.
Seasonal changes in composition of lipid content, percentage of saturated and unsaturated fatty acids, unsaturation index, carbon preference index (CPI), and carboxylic acid ratio (CAR) of Zilla spinosa shoots grown in different habitats of Wadi Hagul.
| Season | Location | C10:0 | C11:0 | C13:0 | C14:0 | C15:0 | C16:0 | C16:1 | C17:0 | C18:0 | C18:1 | C18:2 | C18:3 | Unsaturated fatty acids (%) | Saturated fatty acids (%) | Ratio of saturated/unsaturated | Unsaturation index |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spring | Upstream | – | 9.55 | 10.34 | 1.13 | 7.02 | 21.98 | 1.72 | 1.26 | 19.13 | 7.20 | 10.96 | 9.71 | 29.59 | 70.41 | 2.38 | 0.583 |
| Midstream | 0.25 | 9.34 | 13.78 | – | 10.39 | 28.46 | 0.18 | 6.28 | 23.16 | – | 7.98 | – | 08.16 | 91.66 | 11.23 | 0.161 | |
| Downstream | – | 2.44 | 6.47 | – | 8.14 | 23.25 | – | 3.88 | 16.29 | 13.26 | 16.04 | 10.10 | 39.53 | 60.47 | 1.53 | 0.655 | |
| Summer | Upstream | – | 8.04 | 6.38 | 1.08 | 3.25 | 21.88 | 2.31 | 1.91 | 14.48 | 5.33 | 25.09 | 10.25 | 42.98 | 57.02 | 1.33 | 0.853 |
| Midstream | – | 9.24 | 6.28 | 1.17 | 8.23 | 24.25 | 1.06 | 1.78 | 23.76 | – | 24.23 | – | 25. 29 | 74.71 | 2.95 | 0.578 | |
| Downstream | – | 5.79 | 4.79 | 1.11 | 2.77 | 17.71 | 1.41 | 2.01 | 9.25 | 7.00 | 37.33 | 11.10 | 56.57 | 43.43 | 0.768 | 1.150 |
C10:0, Capric acid; C11:0, Undecylic acid; C13:0, Tridecylic acid; C14:0, Myristic acid; C15:0, Pentadecanoic acid; C16:0, Palmitic acid; C16:1, Palmitoleic acid; C17:0, Margaric acid; C18:0, Stearic acid; C18:1, Oleic acid; C18:2, Linoleic acid; and C18:3, Linolenic acid.
4 Discussion
The geologic alterations impact soil type and hence, the habitat as well as weather condition including fluctuations in seasonal temperature and precipitation crossways the landscape [52]. Moreover, the spatial pattern plays a central role in plant community dynamics, such as succession, adaptation, maintenance of species density, and competition [53].
Indeed, soil moisture scarcity in arid environment affects plant community’s occurrence due to low-precipitation-induced drought stress [4]. Soil analysis showed a significant decrease in Wadi Hagul soil moisture content particularly at the third location (downstream) during the dry season (July), whereas the EC rises compared with the spring season. However, the pH of the soil remained more or less alkaline throughout the study period and did not cause significant seasonal alterations. The high percentage of soil moisture content was attained during the wet period of April which was related to the fall of rain. The distinct depletion in soil moisture content at the edges of Wadi Hagul, particularly during summer was attributed to the inclination associated with effects of high temperature combined with low precipitation.
Henceforth, only the arid plant communities such Zilla spinosa are able to survive and can either avoid or tolerate drought periods [54]. Meanwhile, Zilla inhabiting midstream can survive and tolerate the hot summer under the availability of water (Table 1). Consequently, soil moisture level seems to be the limiting factor for the permanent and continuous growth of Zilla populations in Wadi Hagul habitats. Moreover, Zilla biomass seems to be more sensitive to water scarcity than heat stress.
Similarly, the environmental stresses alter the metabolic processes in stressed plants particularly, the synthesis of chlorophyll (energy trapping pigment) which was positively related to the availability of moisture in the soil. The values of chlorophylls a, b, and a/b ratios as well as carotenoids in Z. spinosa shoots were markedly increased during the wet season particularly in plants inhabiting midstream. In addition, the higher levels of chlorophylls in Zilla shoots at the second location reflected also that the plants did not have much problem to survive in the moist arid habitats that help it to withstand heat stress. However, the reduction in chlorophylls in plants inhabiting the Wadi’s edges particularly during the dry summer season perhaps related to retardation in pigments production and/or increase in their degradation which may be due to the reduction in soil moisture content that comes from the variations in topographic factor and changes in climatic factors, the edaphic factors and organic substances [55], [56], which causes reductions in water use efficiency and plant water potential [57]. However, recently, it was deduced that the reduction in chlorophyll was attributed to the acceleration of chlorophyll breakdown rather than its slow synthesis [58]. Similar seasonal trends were reported for desert shrubs by Aziz [59]. Similarly, it was reported that the altitudinal variation induced changes in pigment content in Arnica montana and Porphyra yezoensis [60], [61].
Similarly, hot dry summer induced reduction in chlorophyll a/b ratio particularly at the edges of the Wadi. It was postulated that chlorophyll a/b ratio slightly increased in drought tolerant wheat cultivars and significantly decreased in the susceptible ones under water deficit conditions [62]. Such differences could be due to a shift in an occurrence of photosynthetic systems toward a lower ratio of photosystem (PS) II to PSI [63] or/and reduction in Chl biosynthesis as reported for several plant species [64], [65], [66].
Similarly, the ratio of chlorophylls (a+b) to carotenoids decreased in Zilla inhabiting the edges particularly during the dry season. Such effect was negatively related to soil moisture and stress intensity and could be used as a stress indicator [7]. The substantial reduction in chlorophyll content assayed in Zilla inhabiting the edges particularly during the dry summer season suggested a possible influence of drought stress on the reduction of stomatal conductance and photosynthetic rates during the dry periods [57], [67] and thereby the biomass.
Furthermore, the spectroscopic methods have been used to characterize the physiological state of plant as an early stress indicator. The relationships between the intensity of fluorescence emission bands or band ratios to plant health and stress condition were investigated by Buschmann [68] and Lichtenthaler et al. [69]. The intensity and the form of the fluorescence emission spectra are influenced by environmental conditions [70]. The chlorophyll fluorescence emission of far red values and ratio of F680 to F730 and blue to red (F450/F680) are very suitable to describe the seasonal and spatial variation in photosynthetic activity in Zilla shoot tops. The fluorescence emission reflects the intactness of the internal photosynthetic activity [71]. The greatest increase in the chlorophyll a fluorescence emission and the ratio of F675/F730 associated with the decline in F450/F675 of upstream and downstream collected Zilla during dry seasons was positively related to the reduction in the level of chlorophylls which displayed by the variation in the intensity of soil water deficit and the EC of the soil. Moreover, the observed increase in red fluorescence emission particularly in downstream inhabiting plants during summer and spring occurs at the expense of the photosynthetic conversion of the absorbed light and is related to damage of PSII and light harvesting system complex (LHC) [71]. Furthermore, excitation at 337 nm shows the presence of blue fluorescence with an emission near 425 nm, smaller shoulder around 520 nm region (green fluorescence), and the red chlorophyll fluorescence with emission near 675 nm. The increase in the intensity of red fluorescence emission near the 680 nm region was attributed to the reduction in chlorophyll content, which might be results from the injuries of antenna and reaction centres of chlorophyll a in PSII [72]. The increase in the fluorescence emission and the red shift due to varying chlorophyll content was reported also by Krause and Weis [73] and Kancheva et al. [74].
The plants response to water stress depends mainly on the severity and duration of the stress and growth stage of the plant [75]. Thus, different physiological and biochemical processes are altered by stress such as water relation [76], gas exchange, photosynthesis [77], and carbohydrates, protein, amino acids, and other organic compounds metabolism [78], which may contribute in stress tolerance and thereby biomass production. The total soluble carbohydrates were quite different among Zilla shoots grown in different habitats during the wet and dry seasons. Higher values of soluble carbohydrates were measured in Zilla shoots during the wet season particularly in upstream inhabiting plants. The greater accumulation of soluble sugars attained in spring collected Zilla shoots was positively related to chlorophyll content and chlorophyll molecules efficiency [79]. The accumulation of carbohydrates depends on the plant species, soil topography, and moisture content. Some plants as palms and some leguminous species accumulated high carbohydrate content during the wet season as to reinitiate the growth of new tissues [80], [81] and survive the dry season characterized by low soil moisture content [82]. Subsequently, the accumulated carbohydrates involved in the adaptation of arid deciduous species, for maintenance of metabolism under long drought period [83]. Soluble sugars can serve as osmoprotectant and a carbon source for biomass production [84].
On the other hand, the decline in soluble carbohydrates in summer collected Zilla shoots might be due the decrease in chlorophyll content which may result in lower rate of photosynthesis and minimal metabolic activity under extreme conditions [85], [86] or allocation to the underground roots [87] to promote root growth to search of water [88].
Furthermore, under the harsh environmental stressful conditions, the woody plants tend to spend large amounts of carbon in the production of lignified support tissues [89]. The greatest amounts of lignin displayed in Zilla shoots inhabiting downstream the Wadi particularly during summer was concomitant with the increases in total phenols particularly P-coumaric and caffeic acids and the activities of PAL and peroxidases which involved in lignin biosynthesis parallel with reduction in feruelic acid (Khattab et al., Unpublished). Such biochemical alterations induced lignin deposition and cell wall stiffness as well as reduced cell wall extensibility and consequently enhanced plant stress tolerance [90]. The accumulation of lignin in response to biotic and abiotic stresses is an important defence mechanism in plants [91] to cope with the severe stresses. It confers stability to xylem vessels required for efficient water transport [92]. In addition, the carbohydrate polymers including cellulose, hemicellulose, and lignin forms the largest portion of “lignocellulosic” plant materials which have recently been utilized as a source of feedstock for bioenergy production [93], [94]. Similarly, the accumulation of lignin in response to biotic and abiotic stress was recorded in many plants [91], [95].
In addition, plant nutrient deficiency induced by different stressors during summer may result in a considerable reduction in total lipid content. The total lipids content of Zilla spinosa inhabiting different habitats of Wadi Hagul was positively related to the soil moisture level. On the other hand, the reduction in the total lipids content in Zilla inhabiting the edges particularly during summer might be attributed to the inhibition of lipid biosynthesis [96] and/or stimulation of lipolytic and peroxidative activities [97], [98], concomitant with decline in membrane lipid content [99]. Similarly, the total lipid content generally exhibits a decline in response to either drought or temperature stress in various plant species [32], [100], [101], [102], [103].
Indeed, the fatty acid pattern which composes plants lipids depends mainly on the temperature and water availability [104], [105]. Meanwhile, the alteration of fatty acid composition particularly in membrane lipids is critical for plant adaptation against drought stress [100], [106]. The fatty acid profile analysis showed the occurrence of the saturated fatty acids including undecylic acid (C11:0), trideclic acid (C13:0), pentadecanoic acid (C15:0), palmitic acid (C16:0), margaric acid (C17:0), stearic acid (C18:0), and unsaturated fatty acids as linoleic acid (C18:2) in Zilla aerial portions inhabiting Wadi Hagul. However, oleic acid (C18:1) and linolenic acid (C18:3) were absent in midstream inhabiting plants during the two investigated seasons. Hence, the greatest levels of total saturated fatty acids have been exhibited in Zilla aerial portions during the spring growing season; however, the total amount of unsaturated fatty acids was attained during the dry summer period concomitant with increments in the proportions of oleic acid (C18:1), linoleic acid (C18:2), and linolenic acid (C18:3) and decline in saturated fatty acids palmitic acid (C16:0) and stearic acid (C18:0). The downstream inhabiting plants exhibited the greatest level of unsaturation index (DBI) during the dry summer period (Table 7). The stress-induced changes in unsaturated fatty acids may play a role in the defense mechanism and it reflects the deleterious effects in Zilla plants. Such increments in the unsaturated fatty acids and the double bond index (DBI) might be due to the effect of heat and drought stresses which may speed up the kinetic energy and molecules movement across membranes, thus cause loosening of chemical bonds inside molecules of biological membranes. Such effect increases the membrane fluidity by either denaturation of proteins or an increase in unsaturated fatty acids [107]. Meanwhile, the extent of fatty acids unsaturation varies by the plant species and the drought intensity [108]. It was reported that the unsaturation level of lipids decreased in sensitive plants, whereas it stayed unchanged or even increased in resistant cultivars under drought stress conditions [33], [34]. Therefore, the specific adjustments in the fatty acid composition and unsaturated lipid level under drought stress could help plant maintain membrane integrity [108], [109] and plant dehydration tolerance [110], [111]. Henceforth, the greatest increase in the unsaturated level and DBI in Zilla inhabiting downstream seems to be concomitant with the superior extent of stresses which stimulate the activities of desaturases enzymes and thus the production of unsaturated fatty acids. The increases in desaturases activities improved drought and salt stress tolerance in transgenic tobacco and mutants Synechocystis [112], [113], which suggest that drought and salt tolerance of plants depends on the levels of unsaturated fatty acids [113], [114]. Similarly, Sui et al [115] and Sui and Han [116] showed that the increments in unsaturated fatty acids in halophytes were concomitant with the increased tolerance of the photosystem to salt stress.
In addition, many reports pointed out that environmental stresses such as heat, drought, and salt induce changes in FA composition, mainly in the content of linolenic acid (18:3) [34], [117]. In the present investigation, the observed predominant accumulation of free linolenic acid (C18:3) in summer growing Zilla inhabiting Wadi Hagul edges particularly at the third location may serve as a stress signal and precursor for phyto-oxylipin biosynthesis [118] and involved in formation of cellular membranes, suberin and cutin waxes which act as protectors against stressful environmental conditions [119]. Furthermore, such fatty acids could reduce the structural and functional damages of cellular membranes induced by stresses [99], [120]. It was reported that the increase in C18:3 fatty acids was associated with enhanced plants tolerance against abiotic stresses which dependent on the inherent level of fatty acid unsaturation and/or the ability to maintain or adjust fatty acid unsaturation [114], [115]. Similarly, the greater unsaturation level induced by drought stress was reported in Arabidopsis thaliana [32] and kentucky bluegrass [121].
On the other hand, both salt and drought stress were found to reduce the amount of 18:3, in rape leaves, cruciferous herbs (Crambe sp.), pea (Pisum sativum), the legume Pachyrhizus ahipa, and in salt-tolerant but not salt-sensitive citrus cells [122], [123], [124].
Similarly, the integrity and functions of cell membranes are sensitive to stresses such as drought and heat. Membranes are the main targets of degradative processes induced by drought and it has been shown that water scarcity decreases membrane lipid content [27], [33] concomitant with inhibition of lipid biosynthesis [98] and stimulation of lipolytic and peroxidative activities [97], [125]. Hence for, the reduction in the total lipid content is concomitant with increments in MDA (a lipid peroxidation product) levels in Zilla tops inhabiting the edges of Wadi Hagul particularly during hot–dry summer season. Moreover, the substantial greatest increase in the MDA suggests more ROS in summer collected Zilla shoots, reflected the greater membranes damages and decreased cell membrane stability which serves as an indirect measure of stress tolerance in diverse plant species [126], [127], [128], [129].
5 Conclusion
The hot dry summer associated with water scarcity in the arid habitats of Wadi Hagul markedly modified the quantity and composition of infochemicals which in turn influence the occurrence and density of Zilla population and thereby biomass production, meanwhile contribute in plant tolerance. Thus, deficiency in soil water content and plant nutrient induced by different stressors during dry hot summer season evoked a considerable increase in the lignin content concomitant with reduction in total oil level in Zilla aerial portions inhabiting different habitats of Wadi Hagul. The environmental stressors not only limited resources of vegetable oil but also modulate the oil comprises fatty acids in favor of increasing the percentage of unsaturated fatty acids particularly in downstream inhabiting plants which is undesirable for biodiesel generation (greater cetane number). Zilla spinosa is a lignocellulosic woody shrub with promising biofuel sources including lignocellulosic compounds beside the vegetable oil content. Finally, the biomass and chemical composition of Zilla spinosa are greatly affected by water scarcity compared to the upraised temperatures displayed in summer season.
References
1. Dash M, Dasu VV, Mohanty K. Physico-chemical characterization of miscanthus, castor, and jatropha towards biofuel production, J Renew Sust Energy 2015;7:043124.10.1063/1.4926577Search in Google Scholar
2. Pereira JS, Chaves MM. Plant responses to drought under climate change in mediterranean-type ecosystems. In global change and mediterranean-type ecosystems 1995. New York: Springer, 1995:140–160.10.1007/978-1-4612-4186-7_7Search in Google Scholar
3. Krasensky J, Jonak C. Drough, salt, and temperature stress induced metabolic rearrangements and regulatory networks, J Exp Bot 2012;63:1593–608.10.1093/jxb/err460Search in Google Scholar PubMed PubMed Central
4. Larcher W. Physiological plant ecology. (plant growth regulation, The Netherlands) the 4th ed. Berlin Heidelberg; Springer-Verlag, 2003:513. Available at: http://refhub.elsevier.com/S1364-0321(14)00067-7/sbref38.Search in Google Scholar
5. Richardson AD, Duigan SP, Berlyn GP. An evaluation of non-invasive methods to estimate foliar chlorophyll content. New Phytol 2002;153:185–94.10.1046/j.0028-646X.2001.00289.xSearch in Google Scholar
6. Nikolaeva MK, Maevskaya SN, Shugaev AG, Bukhov NG. Effect of drought on chlorophyll content and antioxidant enzyme activities in leaves of three wheat cultivars varying in productivity. Russian J Plant Physiol 2010;57:87–95.10.1134/S1021443710010127Search in Google Scholar
7. Lichtenthaler HK, Rinderle U. The role of chlorophyll fluorescence in the detection of stress conditions in plants. CRC Crit Rev Anal Chem 1988;19(suppl 1):529–85.10.1080/15476510.1988.10401466Search in Google Scholar
8. Crafts-Brandner SJ, Salvucci ME. Sensitivity of photosynthesis in a C4 plant maize to heat stress. Plant Physiol 2002;129:1773–80.10.1104/pp.002170Search in Google Scholar PubMed PubMed Central
9. Boughalleb F, Hajlaoui H. Physiological and anatomical changes induced by drought in two olive cultivars (cv Zalmati and Chemlali). Acta Physiol Plant 2011;650:53–65.10.1007/s11738-010-0516-8Search in Google Scholar
10. Al-Khatib K, Paulsen GM. High-temperature effects on photosynthetic processes in temperate and tropical cereals. Crop Sci 1999;39:119–25.10.2135/cropsci1999.0011183X003900010019xSearch in Google Scholar
11. Thebud R, Santarius KA. Effects of high-temperature stress on various biomembranes of leaf cells in situ and in vitro. Plant Physiol 1982;70:200–205.10.1104/pp.70.1.200Search in Google Scholar PubMed PubMed Central
12. Carroll A, Somerville C. Cellulosic biofuels. Annu Rev Plant Biol 2008;60:165–182.10.1146/annurev.arplant.043008.092125Search in Google Scholar PubMed
13. Lange JP. Lignocellulose conversion: an introduction to chemistry, process and economics. In: Centi G, van Santen R, editors. Catalysis for renewables. Weinheim: Wiley-VCH, 2007.10.1002/9783527621118.ch2Search in Google Scholar
14. Hu WJ, Harding SA, Lung J, Popko J, Ralph J, Stokke DD, et al. Repression of lignin biosynthesis promotes cellulose accumulation and growth in transgenic trees. Nat Biotechnol 1999;17:808–12.10.1038/11758Search in Google Scholar PubMed
15. Fu C, Mielenz JR, Xiao X,Ge Y, Hamilton CY, Rodriguez M Jr, et al. Genetic Manipulation of Lignin Reduces Recalcitrance and Improves Ethanol Production from Switchgrass. Proc Natl Acad Sci USA 2011;108:3803–8.10.1073/pnas.1100310108Search in Google Scholar PubMed PubMed Central
16. Weng JK, Chapple C. The origin and evolution of lignin biosynthesis. New Phytol 2010;187:273–85.10.1111/j.1469-8137.2010.03327.xSearch in Google Scholar PubMed
17. Uzal EN, Gomez Ros LV, Pomar F, Bernal MA, Paradela A, Albar JP, et al. The presence of sinapyl lignin in ginkgo biloba cell cultures changes our views of the evolution of lignin biosynthesis. Physiol Plant 2009;135:196–213.10.1111/j.1399-3054.2008.01185.xSearch in Google Scholar PubMed
18. Nawrath C, Schreiber L, Franke RB, Geldner N, Reina-Pinto JJ, Kunst L. Apoplastic diffusion barriers in arabidopsis. Arab Book 2013;11: e0167.10.1199/tab.0167Search in Google Scholar PubMed PubMed Central
19. Asada K. The water-water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annu Rev Plant Biol 1999;50:601–39.10.1146/annurev.arplant.50.1.601Search in Google Scholar PubMed
20. Stepien P, Klobus G. Antioxidant defense in the leaves of C3 and C4 plants under salinity stress. Physiol Plant 2005;125:31–40.10.1111/j.1399-3054.2005.00534.xSearch in Google Scholar
21. Sharma P, Jha BA, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012;2012. Article ID 217037, 26 pp. doi:10.1155/2012/217037.Search in Google Scholar
22. Bailly C, Benamar A, Corbineau F, Dome D. Changes in malondialdehyde content and in superoxide dismutase, catalase and glutathione reductase activities in sunflower seed as related to deterioration during accelerated aging. Physiol Plant 1996;97:104–10.10.1111/j.1399-3054.1996.tb00485.xSearch in Google Scholar
23. Harwood JL, Russell NJ. Lipids in plants and microbes. London: George Allen & Unwin, 1984, pp. 162.10.1007/978-94-011-5989-0Search in Google Scholar
24. Tiwari AK, Kumar A, Raheman H. Biodiesel production from jatropha oil (Jatropha curcas L.) with high free fatty acid: an optimized process. Biomass Bioenergy 2007;31:569–75.10.1016/j.biombioe.2007.03.003Search in Google Scholar
25. Schwab AW, Bagby MO, Freedman B. Preparation and properties of diesel fuels from vegetable oils. Fuel 1987;66:1372–8.10.1016/0016-2361(87)90184-0Search in Google Scholar
26. Bamgboye AI, Hansen AC. Prediction of cetane number of biodiesel fuel from the fatty acid methyl ester (FAME) composition. Int Agrophys 2008;22:21–9.Search in Google Scholar
27. Pham-Thi AT, Borrel-Flood C, Vieira da Silva J, Justin AM, Mazliak P. Effects of water stress on lipid metabolism in cotton leaves. Phytochemistry 1985;24:23–7.10.1016/S0031-9422(00)84884-0Search in Google Scholar
28. Navari-Izzo F, Quartacci MF, Izzo, R. Lipid changes in maize seedlings in response to field water deficits. J Exp Bot 1989;40:675–80.10.1093/jxb/40.6.675Search in Google Scholar
29. Canakci M, Van Gerpen J. Biodiesel production from oils and fats with high free fatty acids. Trans Am Soc Agric Eng (ASAE) 2001;44:1429–36.10.13031/2013.7010Search in Google Scholar
30. Wilson C, Gilmore R, Morrison T. Translation and membrane insertion of the hemagglutinin-neuraminidase glycoprotein of newcastle disease virus. Mol Cell Biol 1987;7:1386–92.10.1128/MCB.7.4.1386Search in Google Scholar
31. Wiesenberg GL, Schneckenberger K, Schwark L, Kuzyakov Y. Use of molecular ratios to identify changes in fatty acid composition of Miscanthus giganteus (Greef et Deu.) plant tissue, rhizosphere and root-free soil during a laboratory experiment. Org Geochem 2012;46:1–11.10.1016/j.orggeochem.2012.01.010Search in Google Scholar
32. Gigon A, Matos AR, Laffray D, Zuily-Fodil Y, Pham-Thi AT. Effect of drought stress on lipid metabolism in the leaves of Arabidopsis thaliana (Ecotype Columbia). Ann Bot 2004;94:345–51.10.1093/aob/mch150Search in Google Scholar
33. Monteiro de Paula F, Pham Thi AT, Vieira da Silva J, Justin AM, Demandre C, Mazliak P. Effects of water stress on the molecular species composition of polar lipids from Vigna unguiculata leaves. Plant Sci 1990;66:185–93.10.1016/0168-9452(90)90203-ZSearch in Google Scholar
34. Repellin A, Pham-Thi AT, Tashakorie A, Sahsah Y, Daniel C, Zuily-Fodil Y. Leaf membrane lipids and drought tolerance in young coconut palms (Cocos nucifera L.). Eur J Agron 1997;6: 25–33.10.1016/S1161-0301(96)02034-5Search in Google Scholar
35. Quinn PJ. Regulation of membrane fluidity in plants. Advances in membrane fluidity, physiological regulation of membrane fluidity. New York: Alan R. Liss, Inc., 1988:293–321.Search in Google Scholar
36. Matsuzaki F, Matsumoto S, Yahara I. Truncation of the carboxy-terminal domain of yeast beta-tubulin causes temperature-sensitive growth and hypersensitivity to antimitotic drugs. J Cell Biol 1988;107:1427–35.10.1083/jcb.107.4.1427Search in Google Scholar PubMed PubMed Central
37. Hamrouni I, Salah HB, Marzouk B. Effects of water-deficit on lipids of safflower aerial parts. Phytochemistry 2001;58:277–80.10.1016/S0031-9422(01)00210-2Search in Google Scholar
38. Bettaieb I, Zakhama N, Wannes WA, Kchouk ME, Marzouk B. Water deficit effects on salvia officinalis fatty acids and essential oils composition. Sci Hortic 2009;120:271–5.10.1016/j.scienta.2008.10.016Search in Google Scholar
39. Hanson AD, Hitz WD. Metabolic responses of mesophytes to plant water deficits. Annu Rev Plant Physiol 1982;33:163–203.10.1146/annurev.pp.33.060182.001115Search in Google Scholar
40. Zaki VA. Ecophsiological studies on plant-soil relationships in an african arid environment under some stress conditions, ph.D. thesis, Natural. Resources Department Institute of African Research and studies; Cairo, Egypt, 1995.Search in Google Scholar
41. Richards LA. Diagnosis and improvement of saline and alkali soils. USDA Handbook 1954; No. 60.10.1097/00010694-195408000-00012Search in Google Scholar
42. Ryan J, Garabet S, Rashid A, El-Gharous M. Assessment of soil and plant analysis laboratories in the West Asia – North Africa region. Commun Soil Sci Plant Anal 1999;30:885–94.10.1080/00103629909370253Search in Google Scholar
43. Metzener H, Rau H, Senger H. Untersuchungen Zur Synchronisierbarteit Einzelnerpigment-Mangel-Mutantebvon Chlorella. Planta (Berl.) 1965;65:186–94.10.1007/BF00384998Search in Google Scholar
44. Reinbothe C, Lebedev N, Reinbothe S. A protochlorophyllide light-harvesting complex involved in de-etiolation of higher plants. Nature 1999;397:80–4.10.1038/16283Search in Google Scholar
45. Homme PM, Gonalez B, Billard J. Carbohydrate content, fructane and sucrose enzyme activities in roots, stubble and leaves of rye grass (Lolium perenne L.) as affected by source/sink modification after cutting. J Plant Physiol 1992;140:282–29.10.1016/S0176-1617(11)81080-1Search in Google Scholar
46. Fairbairn NJ. A modified anthrone reagent. Chem Ind 1953; 31:86.Search in Google Scholar
47. Jenkins SH. The determination of cellulose in straws. Biochem J 1930;24:1428–32.10.1042/bj0241428Search in Google Scholar PubMed PubMed Central
48. Ritter GJ, Seborg RM, Mitchell RL. Factors affecting quantitative determination of lignin by 72% sulfuric acid method. Ind Eng Chem Anal Ed 1932;4:202–4.10.1021/ac50078a017Search in Google Scholar
49. Cakmak I, Horst JH. Effects of aluminum on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant 1991;83:463–8.10.1111/j.1399-3054.1991.tb00121.xSearch in Google Scholar
50. AOAC, Official methods of analysis. Association Of Official Analytical Chemist International, (ed) 17th. Gaithersburg MD, USA. Rosenthal A, Pyle 2002.Search in Google Scholar
51. Metcalfe LD, Schemitz AA, Pelka JR. Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal Chem 1966;38:514–5.10.1021/ac60235a044Search in Google Scholar
52. Ruggiero LF, Hayward GD, Squires JR. Viability analysis in biological evaluations: concepts of population viability analysis, biological population, and ecological scale. Conserv Biol 1994;8:364–72.10.1046/j.1523-1739.1994.08020364.xSearch in Google Scholar
53. Legendre P, Legendre LF. Numerical ecology, 3rd English edition. In: Developments in environmental modelling, Vol. 24. Amsterdam: Elsevier Science BV, 2012:990.Search in Google Scholar
54. Ehleringer JR, Cooper TA. On the role of orientation in reducing photoinhibitory damage in photosynthetic-twig desert shrubs. Plant Cell Environ 1992;15:301–6.10.1111/j.1365-3040.1992.tb00977.xSearch in Google Scholar
55. Jin VL, Haney RL, Fay PA, Polley HW. Soil type and moisture regime control microbial C and N mineralization in grassland soils more than atmospheric CO2-induced changes in litter quality virginia L. Soil Biol Biochem 2013;58:172–80.10.1016/j.soilbio.2012.11.024Search in Google Scholar
56. Rhizopoulou S, Meletiou-Christou MS, Diamantoglou S. Water relations for sun and shade leaves of four mediterranean evergreen sclerophylls. J Exp Bot 1991;42:627–35.10.1093/jxb/42.5.627Search in Google Scholar
57. Aziz I, Ayoob M, Jite PK. Response of Solanum melongena l. to inoculation with arbuscular mycorrhizal fungi under low and high phosphate condition. Not Sci Biol 2011;3:70–4.10.15835/nsb336106Search in Google Scholar
58. Kaewsuksaeng S. Chlorophyll degradation in horticultural crops. Walailak J Sci Technol 2011;8:9–19.Search in Google Scholar
59. Aziz I. Seasonal flux in water potential, proline and chlorophyll content in desert shrubs at Ziarat valley, Balochistan, Pakistan. Pak J Bot 2007;39:1995–2002.Search in Google Scholar
60. Spitaler R, Schlorhaufer PD, Ellmerer EP, Merfort I, Bortenschlager S, Stuppner H, et al. Altitudinal variation of secondary metabolite profiles in flowering heads of Arnica montanacv. ARBO. Phytochemistry 2006;67:409–17.10.1016/j.phytochem.2005.11.018Search in Google Scholar PubMed
61. Zhang T, Shen Z, Xu P, Zhu J, Lu Q, Shen Y, et al. Analysis of photosynthetic pigments and chlorophyll fluorescence characteristics of different strains of Porphyra yezoensis. J Appl Phycol 2012;24:881–6.10.1007/s10811-011-9708-xSearch in Google Scholar
62. Ashraf MY, Azmi AR, Khan AH, Ala SA. Effect of water stress on total phenol, peroxidase activity and chlorophyll contents in wheat (Triticum aestivum L.). Acta Physiol Plant 1994;16:185–91.Search in Google Scholar
63. Estill K, Delaney RH, Smith WK, Ditterline RL. Water relations and productivity of alfalfa leaf chlorophyll variants. Crop Sci 1991;31:1229–33.10.2135/cropsci1991.0011183X003100050030xSearch in Google Scholar
64. Efeoglu B, Terzioglu S. Photosynthetic responses of two wheat varieties to high temperature. EurAsia J BioSci 2009;3:97–106.10.5053/ejobios.2009.3.0.13Search in Google Scholar
65. Balouchi HR. Screening wheat parents of mapping population for heat and drought tolerance, detection of wheat genetic variation. Int J Biol Life Sci 2010;6:56–66.Search in Google Scholar
66. Reda F, Mandoura HM. Response of enzymes activities, photosynthetic pigments, proline to low or high temperature stressed wheat plant (Triticum aestivum L.) in the presence or absence of exogenous proline or cysteine. Int J Acad Res 2011;3:108–15.Search in Google Scholar
67. Koyro HW. Effect of salinity on growth, photosynthesis, water relations and solute composition of the potential cash crop halophyte Plantago coronopusL. Environ Exper Bot 2006;56:136–46.10.1016/j.envexpbot.2005.02.001Search in Google Scholar
68. Buschmann C. Plant responses to environmental stresses. J Plant Physiol 2000;157:243.10.1016/S0176-1617(00)80207-2Search in Google Scholar
69. Lichtenthaler HK, Babani F, Langsdorf G, Buschmann C. Measurement of differences in red chlorophyll fluorescence and photosynthetic activity between sun and shade leaves by fluorescence imaging. Photosynthetica 2000;38:523–31.10.1023/A:1012453205740Search in Google Scholar
70. Theisen AF. Fluorescence changes of a drying marple leaf observed in the visible and nearinfrared. In: Lichtenthaler HK, editor. Applications of chlorophyll fluorescence in photosynthesis research, stress physiology, hydrobiology and remote sensing. Dordrecht: Kluwer Academic Publishers, 1988:197–201.10.1007/978-94-009-2823-7_24Search in Google Scholar
71. Lichtenthaler HK. The stress concept in plants: an introduction. In: “Stress of Life: from Molecules to Man. (P. Csermely Ed.)”, Ann. N. Y. Acad. Sci. 1998;851:187–98.10.1111/j.1749-6632.1998.tb08993.xSearch in Google Scholar PubMed
72. Hura T, Grzesiak S, Hura K, Grzesiak M, Rzepka A. Differences in the physiological state between triticale and maize plants during drought stress and followed rehydration expressed by the leaf gas exchange and spectrofluorimetric methods. Acta Physiol Plant 2006;28:433–43.10.1007/BF02706626Search in Google Scholar
73. Krause GH, Weis E. Chlorophyll Fluorescence and Photosynthesis: the Basics. Annu. Rev. Plant Biol 1991;42:313–49.10.1146/annurev.pp.42.060191.001525Search in Google Scholar
74. Kancheva R, Iliev I, Borisova D, Chankova S, et al. Detection of plant physiological stress using spectral data. Ecol Eng Environ Protect 2005;1:4–9.Search in Google Scholar
75. Levitt J. Responses of plants to environmental stresses. Volume II. Water, radiation, salt, and other stresses (No. ed. 2). New York: Academic Press, 1980.Search in Google Scholar
76. Silva EC, Nogueira RJ, Vale FH, Araújo FP, Pimenta MA. Stomatal changes induced by intermittent drought in four umbu tree genotypes. Braz J Plant Physiol 2009;21:33–42.10.1590/S1677-04202009000100005Search in Google Scholar
77. Pagter M, Bragato C, Brix H. Tolerance and physiological responses of Phragmites australis to water deficit. Aquat Bot 2005;81:285–99.10.1016/j.aquabot.2005.01.002Search in Google Scholar
78. Sircelj H, Tausz M, Grill D, Batic F. Biochemical responses in leaves of two apple tree cultivars subjected to progressing drought. J Plant Physiol 2005;162:1308–18.10.1016/j.jplph.2005.01.018Search in Google Scholar PubMed
79. Rayan A. Seasonal variation in chlorophyll and sugar contents in some desert species. Bulletin of the Faculty of Science, Assiut University; 2004;33:61–70.Search in Google Scholar
80. Mattson NS, Lieth JH, Kim WS. Temporal dynamics of nutrient and carbohydrate distribution during crop cycles of rosa spp. ‘Kardinal’ in response to light availability. Sci Hortic 2008;118:246–54.10.1016/j.scienta.2008.06.009Search in Google Scholar
81. Vilela AE, Agüero PR, Ravetta D, González-Paleo R. Long-term effect of carbohydrate reserves on growth and reproduction of Prosopis denudans (Fabaceae): implications for conservation of woody perennials. Conserv Physiol 2016;4:cov068.10.1093/conphys/cov068Search in Google Scholar PubMed PubMed Central
82. Scarano FR, Cattânio JH, Crawford RMM, root carbohydrate storage in young saplings of an Amazonian Tidal VáRzea forest before the onset of the wet season. Acta Bot Bras 1994;8: 129–39.10.1590/S0102-33061994000200002Search in Google Scholar
83. McDowell N, Pockman WT, Allen CD, Breshears DD, Coob N, Kolb T, et al. mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 2008;178:719–39.10.1111/j.1469-8137.2008.02436.xSearch in Google Scholar PubMed
84. David D, Sundarababu S, Gerst JE. Involvement of long chain fatty acid elongation in the trafficking of secretory vesicles in yeast. J Cell Biol 1998;143:1167–82.10.1083/jcb.143.5.1167Search in Google Scholar PubMed PubMed Central
85. Li T, Zhang Y, Liu H, et al. Stable expression of Arabidopsis vacuolar Na+/H+ antiporter gene AtNHX1 and salt tolerance in transgenic soybean for over six generations. Chinese Sci Bull 2010;55:1127–34.10.1007/s11434-010-0092-8Search in Google Scholar
86. Netting AG. PH, abscisic acid and the integration of metabolism in plants under stressed and non-stressed conditions: cellular responses to stress and their implication for plant water relations. J Exp Bot 2000;51:147–158.10.1093/jexbot/51.343.147Search in Google Scholar PubMed
87. Lambers H, Atkin OK, Millenaar FF. Respiratory patterns in roots in relation to their functioning. In: Waisel Y, Eshel A, Kafkafi U, editors. Plant roots, the hidden half, 3rd ed. New York, NY, USA: Marcel Dekker, 2002:521–52.10.1201/9780203909423.pt6Search in Google Scholar
88. Kafkafi U. Root growth under stress: salinity. In: Waisel Y, Eshel A, Kafkafi U, editors. Plant roots. The hidden half. New York: Marcel Dekker, Inc., 1991:375–91.Search in Google Scholar
89. Dietze MC, Sala A, Carbone MS, Czimczik CI, Mantooth JA, Richardson AD, et al. Nonstructural carbon in woody plants. Annu Rev Plant Biol 2014;65:667–87.10.1146/annurev-arplant-050213-040054Search in Google Scholar PubMed
90. Ride JP. Cell wall and other structural barriers. In: Callow JA, editor. Biochemical plant pathology. New York: Jhon Wiley & Sons, Ltd., 1983:215–35.Search in Google Scholar
91. Baxter HL, Stewart Jr CN. Effects of altered lignin biosynthesis on phenylpropanoid metabolism and plant stress. Biofuels 2013;4:635–50.10.4155/bfs.13.56Search in Google Scholar
92. Voelker L, Lachenbruch B, Meinzer FC, Kitin P, Strauss SH, et al. Transgenic Poplars with Reduced Lignin Show Impaired Xylem Conductivity, Growth Efficiency and Survival. Plant Cell Environ 2011;34:655–68.10.1111/j.1365-3040.2010.02270.xSearch in Google Scholar
93. Somerville C, Youngs H, Taylor C, Davis SC, Long SP. Feedstocks for lignocellulosic biofuels. Science 2010;329:790–92.10.1126/science.1189268Search in Google Scholar
94. Frei M. Lignin: characterization of a multifaceted crop component. Sci World J 2013;2013:25.10.1155/2013/436517Search in Google Scholar
95. Vance CP, Kirk TK, Sherwood RT. Lignification as a mechanism of disease resistance. Annu Rev Phytopath 1980;18:259–88.10.1146/annurev.py.18.090180.001355Search in Google Scholar
96. Monteiro de Paula F, Thi ATP, Zuily-Fodil Y, Ferrari-Iliou R, et al. Effects of Water stress on the biosynthesis and degradation of polyunsaturated lipid molecular species in leaves of Vigna unguiculata. Plant Physiol Biochem 1993;31:707–15.Search in Google Scholar
97. Matos AR, d’Arcy-Lameta A, França M, Pêtres S, Edelman L, Kader J, et al. A Novel Patatin-like Gene Stimulated by Drought Stress Encodes a Galactolipid Acyl Hydrolase. Febs Lett. 2001;491:188–92.10.1016/S0014-5793(01)02194-9Search in Google Scholar
98. Liu X, Huang B. Heat stress injury in relation to membrane lipid peroxidation in creeping bentgrass. Crop Sci 2000;40: 503–10.10.2135/cropsci2000.402503xSearch in Google Scholar
99. Upchurch RG. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol Lett 2008;30:967–77.10.1007/s10529-008-9639-zSearch in Google Scholar PubMed
100. Martins-Júnior RR, Oliveira MS, Baccache MA, de Paula FM. Effects of water deficit and rehydration on the polar lipid and membranes resistance leaves of Phaseolus vulgaris L. cv. Pérola. Braz Arch Biol Technol 2008;51:361–7.10.1590/S1516-89132008000200016Search in Google Scholar
101. Silva R. Effect of planting date and planning distance on growth of flaxseed. Agron J 2005;136:113–8.Search in Google Scholar
102. Werteker M, Lorenz A, Johannes H, Berghofer E, Findlay CS. Environmental and varietal influences on the fatty acid composition of rapeseed, soybeans and sunflowers. J Agron Crop Sci 2010;196:20–7.10.1111/j.1439-037X.2009.00393.xSearch in Google Scholar
103. Mirshekari M, Amiri R, Iran Nezhad H, Sadat Noori SA, Zandvakili OR. Effects of planting date and water deficit on quantitative and qualitative traits of flax Seed. Am Eur J Agric Environ Sci 2012;12:901–13.Search in Google Scholar
104. Ahmed FE, Hall A E, Madore MA. Interactive effects of high temperature and elevated carbon dioxide concentration on cowpea Vigna unguiculata (L.)Walp. Plant Cell Environ 1993;16:835–42.10.1111/j.1365-3040.1993.tb00505.xSearch in Google Scholar
105. Kizis D, Lumbreras V, Pagès M. Role of AP2/EREBP transcription factors in gene regulation during abiotic stress. FEBS Lett 2001;498:187–9.10.1016/S0014-5793(01)02460-7Search in Google Scholar
106. Yordanov V, Velikova I, Tsonev T. Plant responses to drought, acclimation and stress tolerance. Photosynthesis 2000;38:171–86.10.1023/A:1007201411474Search in Google Scholar
107. Savchenko GE, Klyuchareva EA, Abramchik LM, Serdyuchenko EV. Effect of periodic heat shock on the inner membrane system of etioplasts. Russ J Plant Physiol 2002;49:349–59.10.1023/A:1015592902659Search in Google Scholar
108. Zhong DH, Du HM, Wang ZL, Huang BR. Genotypic variation in fatty acid composition and unsaturation levels in bermudagrass associated with leaf dehydration tolerance. J Am Soc Hortic Sci 2011;136:35–40.10.21273/JASHS.136.1.35Search in Google Scholar
109. Toumi I, Gargouri M, Nouairi I, Moschou PN, Ben Salem-Fnayou A, Mliki A, et al. Water stress induced changes in the leaf lipid composition of four grapevine genotypes with different drought tolerance. Biol Plant 2008;52:161–4.10.1007/s10535-008-0035-2Search in Google Scholar
110. Rachmilevitch S, Da Costa M, Huang B. Physiological and biochemical indicators for stress tolerance. Plant–environment interactions, 3rd ed. Boca Raton, FL: CRC Press, 2006: 321–356.10.1201/9781420019346.ch11Search in Google Scholar
111. Zhang M, Barg R, Yin M, Gueta-Dahan Y, Leikin-Frenkel A, Salts Y, et al. Modulated fatty acid desaturation via overexpression of two distinct omega-3 desaturases differentially alters tolerance to various abiotic stresses in transgenic tobacco cells and plants. Plant J 2005;44:361–71.10.1111/j.1365-313X.2005.02536.xSearch in Google Scholar
112. Allakhverdiev SI, Kinoshita M, Inaba M, Suzuki I, Murata N. Unsaturated fatty acids in membrane lipids protect the photosynthetic machinery against salt-induced damage in Synechococcus. Plant Physiol 2001;125:1842–53.10.1104/pp.125.4.1842Search in Google Scholar
113. Berberich T, Harada M, Sugawara K, Kodama H, Iba K, Kusano T. Two maize genes encoding omega-3 fatty acid desaturase and their differential expression to temperature. Plant Mol Boil 1998;36:297–306.10.1023/A:1005993408270Search in Google Scholar
114. Mikami K, Murata N. Membrane fluidity and the perception of environmental signals in cyanobacteria and plants. Prog Lipid Res 2003;42:527–43.10.1016/S0163-7827(03)00036-5Search in Google Scholar
115. Sui N, Li M, Li K, Song J, Wang BS. Increase in unsaturated fatty acids in membrane lipids of Suaeda salsa L. enhances protection of photosystem II under high salinity. Photosynthetica, 2010;48:623–9.10.1007/s11099-010-0080-xSearch in Google Scholar
116. Sui N, Han G. Salt-induced photoinhibition of PSII is alleviated in halophyte Thellungiellahalophila by increases of unsaturated fatty acids in membrane lipids. Acta Physiol Plant 2014;36:983–92.10.1007/s11738-013-1477-5Search in Google Scholar
117. Anai T, Koga M, Tanaka H, Kinoshita T, Rahman SM, Takagi Y, et al. Improvement of rice (Oryza sativa L.) seed oil quality through introduction of a soybean microsomal omega-3 fatty acid desaturase gene. Plant Cell Rep 2003;21:988–92.10.1007/s00299-003-0609-6Search in Google Scholar
118. Blee E. Impact of phyto-oxylipins in plant defense. Trends Plant Sci 2002;7:315–22.10.1016/S1360-1385(02)02290-2Search in Google Scholar
119. Beisson F, Li Y, Bonaventure G, Pollard M, Ohlrogge JB. The acyltransferase GPAT5 is required for the synthesis of suberin in seed coat and root of arabidopsis. Plant Cell Online 2007;19:351–68.10.1105/tpc.106.048033Search in Google Scholar
120. Yuan X, Li Y, Liu S, Xia F, Li X, Qi B. Accumulation of eicosapolyenoic acids enhances sensitivity to abscisic acid and mitigates the effects of drought in transgenic Aarabidopsis thaliana. J Exper Bot 2014;65:1637–49.10.1093/jxb/eru031Search in Google Scholar
121. Xu L, Han L, Huang B. Membrane fatty acid composition and saturation levels associated with leaf dehydration tolerance and post-drought rehydration in kentucky bluegrass. Crop Sci 2011;51:273–81.10.2135/cropsci2010.06.0368Search in Google Scholar
122. Dakhma WS, Zarrouk M, Cherif, A. Effects of drought stress on lipids in rape leaves. Phytochemistry 1995;40:1383–6.10.1016/0031-9422(95)00459-KSearch in Google Scholar
123. Francois LE, Kleiman R. Salinity effects on vegetative growth, seed yield, and fatty acid composition of crambe. Agron J 1990;82:1110–4.10.2134/agronj1990.00021962008200060017xSearch in Google Scholar
124. Gueta-Dahan Y, Yaniv Z, Zilinskas BA, Ben-Hayyim G. Salt and oxidative stress: similar and specific responses and their relation to salt tolerance in citrus. Planta 1997;203:460–9.10.1007/s004250050215Search in Google Scholar PubMed
125. El-Maarouf H, Zuily-Fodil Y, Gareil M, Arcy-Lameta A, Pham-Thi AT. Enzymatic activity and gene expression under water stress of phospholipase D in two cultivars of Vigna unguiculata L. walp. differing in drought tolerance. Plant Mol Biol 1999;39:1257–65.10.1023/A:1006165919928Search in Google Scholar
126. Xu ZZ, Zhou GS. Combined effects of water stress and high temperature on photosynthesis, nitrogen metabolism and lipid peroxidation of a perennial grass Leymus chinensis. Planta 2006;224:1080–90.10.1007/s00425-006-0281-5Search in Google Scholar PubMed
127. Gülen H, Çetinkaya C, Kadıoğlu M, Kesici M, Cansev A, Eri A. Peroxidase activity and lipid peroxidation in strawberry (Fragaria×ananassa) plants under low temperature. J Biol Environ Sci 2008;2:95–100.Search in Google Scholar
128. Amini H, Arzani A, Karami M. Effect of water deficiency on seed quality and physiological traits of different safflower genotypes. Turk J Biol 2014;38:271–82.10.3906/biy-1308-22Search in Google Scholar
129. Aldesuquy H, Ghanem H. Exogenous salicylic acid and trehalose ameliorate short term drought stress in wheat cultivars by up-regulating membrane characteristics and antioxidant defense system. J Horticult 2015;2:1–10.10.4172/2376-0354.1000139Search in Google Scholar
©2017 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Research Article
- Environmental alterations in biofuel generating molecules in Zilla spinosa
- Short Communication
- Antinociceptive effect of Aristolochia trilobata stem essential oil and 6-methyl-5-hepten-2yl acetate, its main compound, in rodents
- Research Articles
- Bioenergetics of lactate vs. acetate outside TCA enhanced the hydrogen evolution levels in two newly isolated strains of the photosynthetic bacterium Rhodopseudomonas
- Leaves of Cordia boissieri A. DC. as a potential source of bioactive secondary metabolites for protection against metabolic syndrome-induced in rats
- Rapid Communication
- The evaluation of the synergistic effect of 3-(2,4-dihydroxyphenyl)propionic acid and l-ascorbic acid on tyrosinase inhibition
- Research Articles
- Virus inactivation under the photodynamic effect of phthalocyanine zinc(II) complexes
- Cytochalasin P1, a new cytochalasin from the marine-derived fungus Xylaria sp. SOF11
- Synthesis and biological evaluation of newer 1,3,4-oxadiazoles incorporated with benzothiazepine and benzodiazepine moieties
- Caspase-1 from the silkworm, Bombyx mori, is involved in Bombyx mori nucleopolyhedrovirus infection
Articles in the same Issue
- Frontmatter
- Research Article
- Environmental alterations in biofuel generating molecules in Zilla spinosa
- Short Communication
- Antinociceptive effect of Aristolochia trilobata stem essential oil and 6-methyl-5-hepten-2yl acetate, its main compound, in rodents
- Research Articles
- Bioenergetics of lactate vs. acetate outside TCA enhanced the hydrogen evolution levels in two newly isolated strains of the photosynthetic bacterium Rhodopseudomonas
- Leaves of Cordia boissieri A. DC. as a potential source of bioactive secondary metabolites for protection against metabolic syndrome-induced in rats
- Rapid Communication
- The evaluation of the synergistic effect of 3-(2,4-dihydroxyphenyl)propionic acid and l-ascorbic acid on tyrosinase inhibition
- Research Articles
- Virus inactivation under the photodynamic effect of phthalocyanine zinc(II) complexes
- Cytochalasin P1, a new cytochalasin from the marine-derived fungus Xylaria sp. SOF11
- Synthesis and biological evaluation of newer 1,3,4-oxadiazoles incorporated with benzothiazepine and benzodiazepine moieties
- Caspase-1 from the silkworm, Bombyx mori, is involved in Bombyx mori nucleopolyhedrovirus infection


