Abstract
In work of the mid-19th century, it has been shown that the physical properties of cellulose and related materials are changed drastically upon their immersion in “aqueous zinc chloride solutions”, followed by washing to remove the salt and drying the resulting fabric, whose chemical composition did not change, but had a different structure. It was soon recognized that the process worked only at very high concentrations of zinc chloride, but it was only in the last decade that the “solutions” were finally identified to be in fact ionic liquids with an optimum performance for the trihydrate ZnCl2(H2O)3 which is built up of [Zn(H2O)6]2+ cations and [ZnCl4]2− anions, both in the solid state and in the melt, and which has a melting point of 6.5 °C. The production of Vulcanised Fibre has long been an important industrial process based on the treatment of cellulose with zinc chloride following established protocols. In the present paper the consequences of the ionic liquid concept for the underlying processes are discussed on the molecular level, proposing a sequence of reversible reactions, which allow the reorganization of the cellulose structure under surprisingly mild conditions. Due to its advantageous properties as a biomaterial, the all-cellulose, self-reinforced composite is currently being rediscovered for newly considered applications within several industries such as the construction industry as one example. Its strength, lightweight and plasticity present unique opportunities for architectural as well as structural applications.
1 Introduction
New work of the last two decades has shown, that the many processes for the conversion of polysaccharides into modified products with properties that serve special applications are based on some unique reactions which must be conducted under very special conditions. The products include materials which are obtained by partial or complete dissolution and reprecipitation of cellulose in/from various reactive and non-reactive solvents.
In the former, the cellulose is converted into derivatives different in composition from the native material (cellulose dissolution by chemical modification). Derivatizations like nitration (to “nitrocellulose”) or xanthation (to “viscose”) are among the classical forms in the cellulose industry.
In the latter, cellulose is dissolved without chemical modification using a broad range of solvents, which induce an easily reversible solvation, such that the composition of the recovered cellulose - but not its structure - are largely unchanged. Solvents for this dissolution are aqueous alkali hydroxides or ammonia, mixtures of organic solvents, or aqueous solutions of inorganic salts (mainly of copper, lithium and zinc). However, at high concentrations the “solutions” of these salts are actually devoid of any solvent water molecules, because all water molecules are part of the cations (below). The crystal structures of these salts show only very weak interionic forces and thus have low lattice energies, which give rise to very low melting points. The melts show the characteristics of “ionic liquids”.
In the cellulose fiber industry, the inorganic salts in these “solutions” have some important advantages owing to e. g. the low cost of the salts, their low vapor pressure and their easy recovery (recycling) associated with a tolerable energy economy. Dissolution and reprecipitation of cellulose in/from ionic liquids therefore has become a process of choice in the cellulose fiber industry. Currently, zinc chloride hydrates are often the preferred medium.
However, the authors of a review dedicated to the dissolution of cellulose in general and in ionic liquids in particular published in 2012, 1 as those of previous articles, 2 , 3 , 4 , 5 , 6 , 7 have not yet considered zinc chloride hydrates as ionic liquids, because the true nature of this medium was recognized only shortly thereafter. 8 , 9 , 10
It is therefore surprising, that other industries, where the cellulose is not dissolved and not chemically modified, but only surface-treated with salt hydrates (below) have not truly considered the role of ionic liquids. This is particularly true for the industry of Vulcanised Fibre materials, where zinc chloride hydrates are used as the “parchmentation media” (below). The present review is dedicated to the chemistry of the processes that afford Vulcanised Fibre.
The currently re-emerging interest in the material by a wide range of industries makes a thorough understanding of the chemical processes involved in the production of Vulcanized Fibre all the more relevant. Here, life cycle, reusability or biodegradability of reusable or waste/stock materials are the most important new aspects in the present context.
2 A brief history of developments in the paper industry in the 19th and 20th century which led the way to Vulcanised Fibre with its unique properties
Following his attempts to prepare papers with properties resembling those of classical “parchment” by treatment of cotton-based papers with various chemicals, the British chemist Thomas Taylor in 1856 filed a patent with the title “Improved Means of Giving Increased Strength to Paper”, 11 followed by a US Patent in 1871 for “Improvement in the treatment of paper and paper-pulp”. 12 The papers prepared according to his patent were initially taken as “modified papers with properties close to those of parchment”, and the process he introduced was called “parchmentation”. 13 , 14
In order to gain from the enormous economic success and publicity of the process discovered in 1839 by the US chemist James Goodyear, who heated rubber obtained from the sap of the rubber tree with elemental sulfur and called the process “vulcanization”, the “parchmented paper” was later addressed as “Vulcanised Fibre”, even though the materials and processes used for their preparation have nothing in common.
In the ancient world, the “parchment” used in the form of sheets or rolls (“scrolls”) to write or draw on was obtained from animal skins, and these scripts were preserved in libraries as in the City of Pergamon, which is the origin of their name. These parchments replaced the earlier scripts which were written on “papyros”, prepared from the marrow of the papyros leaves, of which the name “paper“ is derived. When in later centuries new qualities of papers based on cellulose materials were introduced, these were initially also addressed as “parchments”.
In his early experiments, Taylor used cellulose materials based on cotton wool, but later other natural products like wood, straw, linen, hemp etc. were also employed. For many “Vulcanised Fibre” products, cotton wool in its various forms (fibers, linters and rags) is still the material of choice. The length and the order, or the random distribution of the cotton fibers determine the properties of the materials.
Cotton wool, as all other cellulose materials, consists of polysaccharide macromolecules composed of chains of β(1 → 4) linked cyclic d-glucose molecules which exhibit three hydroxy functions (–OH) at each of the rings (Figure 1).

The molecular structure of a short segment of a polysaccharide chain in a cellulose fiber (https://commons.wikimedia.org/wiki/File:Cellulose_Sessel.svg, public domain image).
These –OH functions are engaged in hydrogen bonding –O–H⋯O both within and between the polysaccharide chains (Figure 2). The hydrogen bonding linkages between the polysaccharide molecules lead to their parallel aggregation into bundles and fibers. Depending on the stereochemistry of the association, cellulose exists in various polymorphs, but this aspect is not considered here. In an enthalpy-entropy consideration, the organization of cellulose fibers is also partly assigned to the amphiphilic nature of the polysaccharide chains, i. e. hydrophilic and hydrophobic properties contribute to the stabilization. 15
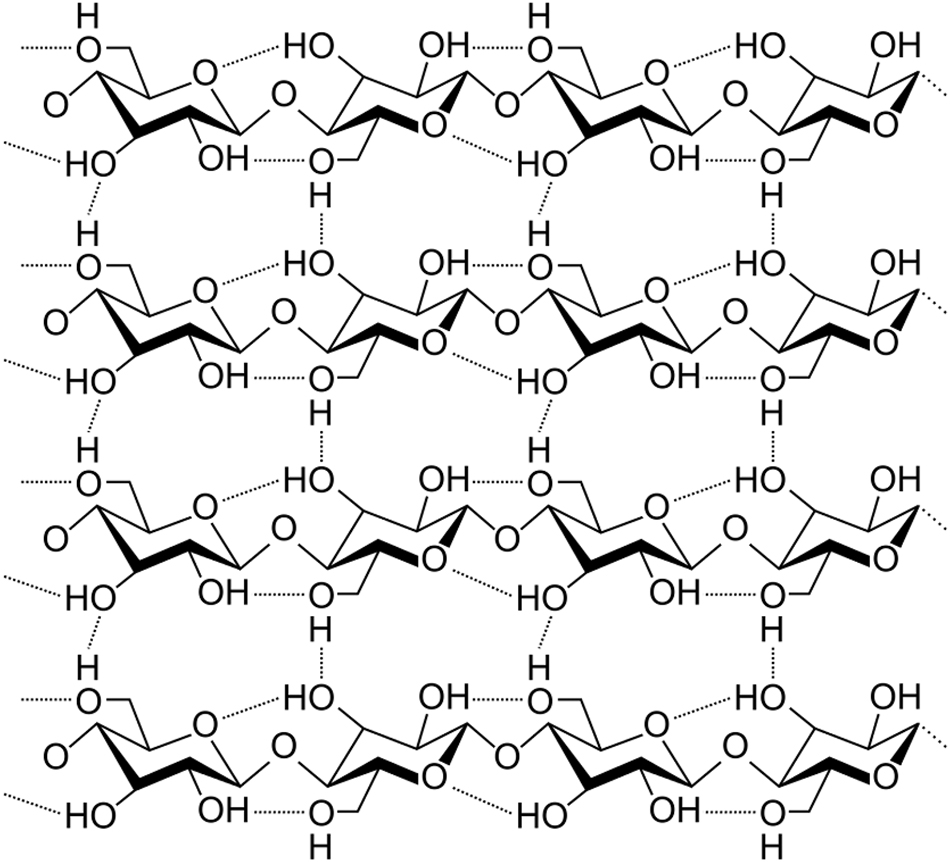
Parallel aggregation of polysaccharide macromolecules through hydrogen bonding (https://commons.wikimedia.org/wiki/File:Cellulose_strand.svg, file licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license, free to copy, distribute and transmit. No changes were made. Author: Luca Laghi).
The polysaccharide molecules are highly ordered within the multichain aggregates as required by the spatial orientation of the –OH functions. Their hydrogen bonding optimizes the overall bonding energy.
In the various forms of cellulose, amorphous or ordered (crystalline) domains are observed, which allow a more detailed characterization of the materials. In the amorphous domains, the chains and fibers are strongly disordered and tangled, while the crystalline domains have at least a short-range order. 16
Owing to their peripheral –OH functions, cellulose materials adsorb and absorb water molecules which become attached to the surface of the fibers and between its chain molecules, respectively. Previously compressed material swells up and increases in size and weight when immersed into water or exposed to humid air, and loses its mechanical strength. These sorption properties were the basis for the production of blotting paper, which has been in use for centuries on the writing desks where the letters were written with ink and pen.
In the 19th century, it was tried to reduce these sorption properties of paper by various post-treatments aiming at products which do not undergo the quick disintegration and defibration upon contact with water, or do not show the penetration by oily and fatty materials. In both French and British laboratories, it was found that a short treatment of cellulose paper with sulfuric acid, followed by extraction of the acid by washing with water and finally a careful drying, led to “parchment papers” (“Pergamentpapiere” in German) with properties familiar to everybody from the widely used food packaging papers in the first half of the 20th century.
In this process, which is now called “parchmentation” (“Pergamentierung”), the catalytic action of the acid causes the breaking of parts of the peripheral and internal hydrogen bonds of the fibers (Figure 2) depending on acid concentration, temperature and reaction time. Upon removal of the inter-chain water molecules by drying, the chains are reconnected and reorganized in tighter forms of connectivity. The resulting reduced pore sizes and the poorer wettability of the fiber material prevent the access of water molecules, but also of oil and fat. Parchmented paper is more transparent owing to reduced light scattering of the tight fiber material.
For a variety of applications, later variants of parchment paper have been developed using special additives. In contrast to pure cellulose, these products and also the single-sided coated materials and their waste are no longer compostable and require separate disposal.
In the second half of the 20th century, parchment paper for some time has largely disappeared from the packaging markets and has been replaced by PE and PP films and bags, but this development is reversed now as environmentally conscious customers again prefer the standard “brown paper bags” made of native or recycled cellulose, or yesterday’s newspapers.
3 The use of zinc chloride hydrates for the parchmentation of cellulose materials to produce Vulcanised Fibre
The inventor of the process leading to Vulcanised Fibre (above) disclosed in his patents that treatment of cotton and other cellulose papers with “aqueous solutions of zinc chloride” also leads to products which show not only improved tear resistance and structural stability, but also reduced sorption properties for water and oily liquids. These properties become most obvious for compressed multilayer materials which are produced in two-dimensional forms and in various thicknesses. Since its discovery, this process has continuously been modified into variants which serve certain applications. The principle of the currently practiced process is briefly summarized here, preceded by a simplified flow-scheme in Figure 3. 17
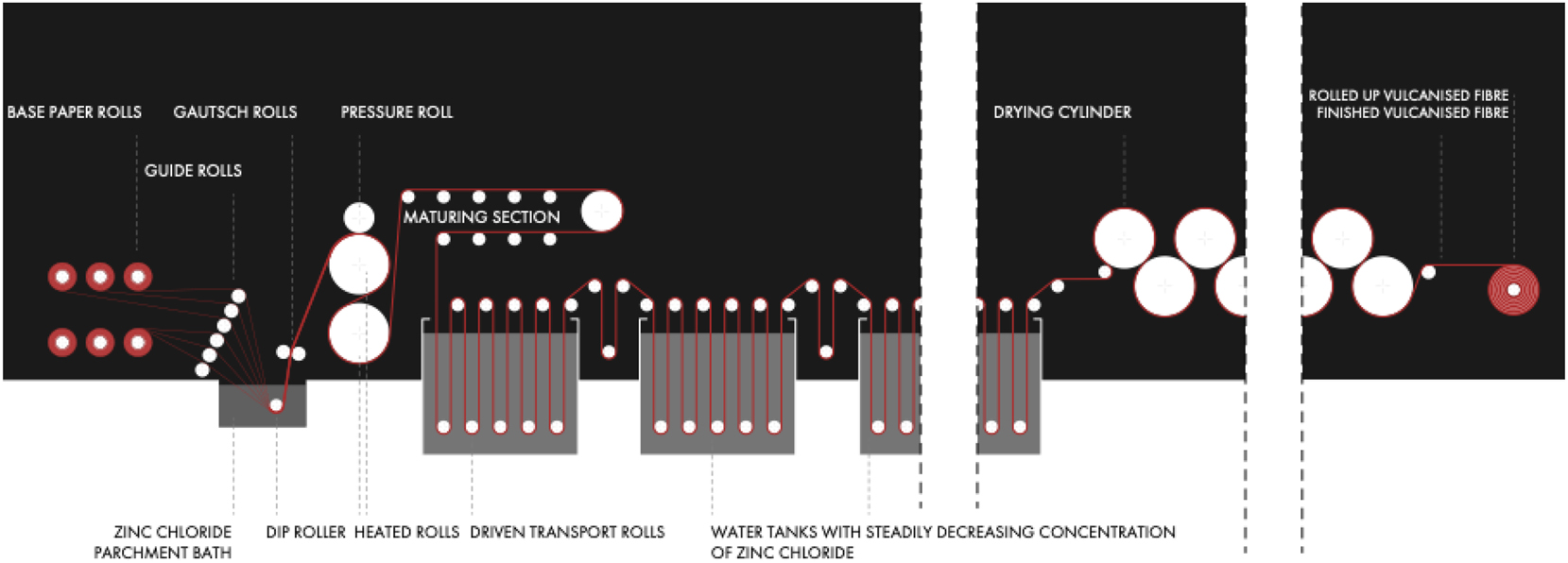
Simplified flow-scheme of the continuous Vulcanised Fibre process.
Thin sheets of Vulcanised Fibre with a thickness of 6–3 mm (wet or dry) or even less, are produced in a continuous process. In this variant, belt-like fabric panels made of cellulose (“Vulcanised Fibre base papers”, prepared e. g. from cotton pulp) are assembled and compressed via calender rolls to multilayer belts which are carried into a parchmentation bath containing “a concentrated solution of zinc chloride” (approx. 70 w%) which is strongly acidic and kept at a temperature of up to 40°. The dwelling time of the multilayer belts – generally less than a minute - in the bath is controlled by the running speed of the transport rolls (Figures 4 and 5).

Insertion of layers of base material into the parchmentation bath. Photo ©Hornex, Ernst Krüger GmbH & Co. KG. (Reproduced with written permission from M. Joseph).

Transport of the parchmented multilayer material belts into the washing baths. Photo ©Hornex, Ernst Krüger GmbH & Co. KG. (Reproduced with written permission from M. Joseph).
The multilayer belts which are still loaded with the zinc chloride parchmentation liquid are allowed to undergo a ripening for a time span which is adapted to the expected thickness and quality of the product. The remaining liquid is then stripped and pressed off, and the material is washed with water to remove remaining zinc chloride and to reach neutrality. Residual water is pressed out of the formerly multilayer belt, which at this stage has reached a compact structure through newly established interlayer connectivity (Figure 6). The intergrowth of new bonding between original base papers has led to a virtually compact structure. In cross-sections, the boundaries between the original base papers are no longer visible. The moist belt is still flexible and kept under its own vapor pressure for further processing.
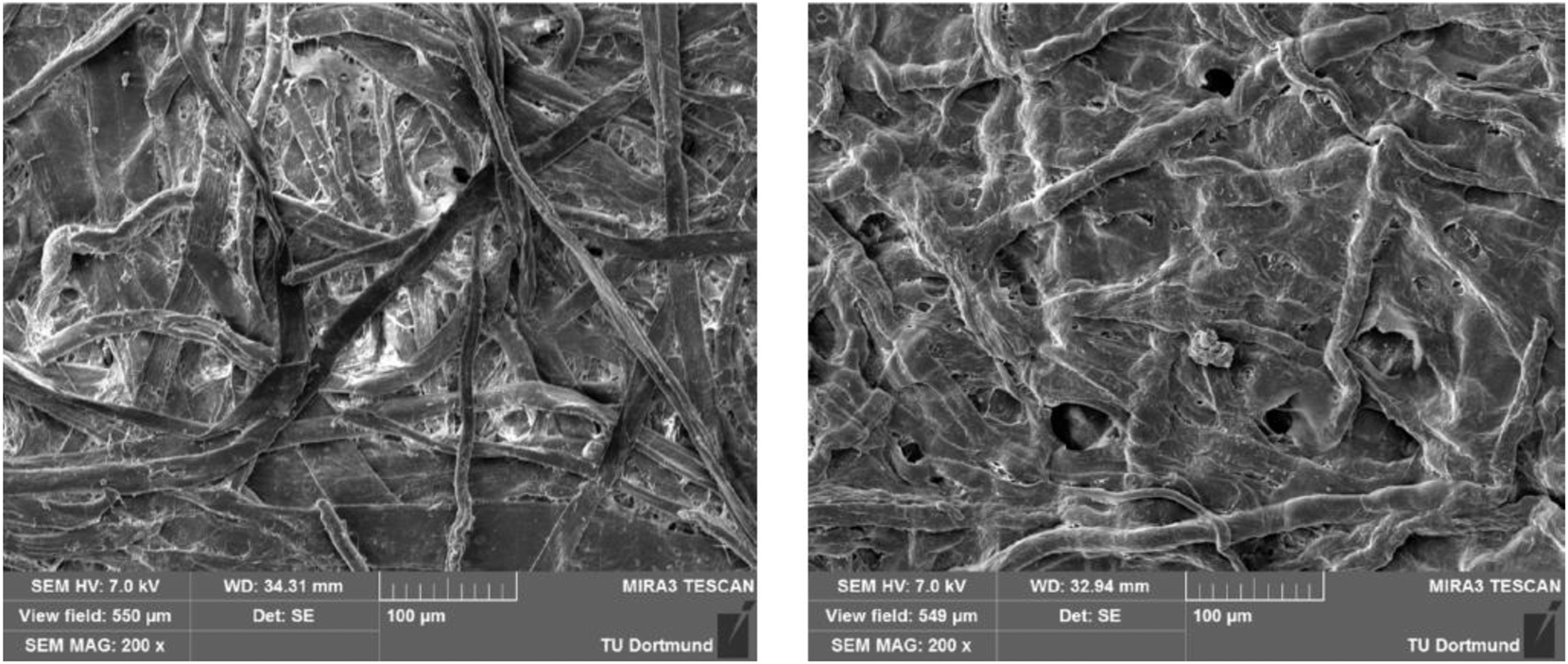
Electron microscopic images of raw paper (left) in comparison to Vulcanised Fibre (right). 35
Upon further drying, the layers shrink and harden to leave the multilayer material in a strongly modified morphology as compared to the original base material. Without any external pressure, which could e. g. preserve the planarity of the sheets, a multidimensional deformation takes place because after the parchmentation the fibers of the material have modified preferences for their organization. A new linkage pattern is required for an intermolecular organization which has become the energy minimum of the Vulcanised Fibre material. The deformation of the panels also proceeds differently if in the drying process the access of air is free from all directions or is limited by the support or other barriers. Finally, the temperature and the humidity of the drying air also have an influence.
Thicker sheets of Vulcanized Fibre are produced in a discontinuous process which yields multilayer blankets of various sizes and with thicknesses in the cm range. The parchmentation, ripening, washing and drying follow the same protocol as described for the continuous process.
In this context it needs to be mentioned that the structure of cotton cellulose is also modified upon treatment with aqueous alkaline solutions. Long-fiber cotton drawn through aqueous sodium hydroxide solutions and washed and neutralized has a new appearance in that it has an “added shine” and is “grip-friendly” while still being tear and wear resistant. This method of improving the quality of cotton was discovered by the British chemist John Mercer in 1844, and the material has been termed “Mercerized Cotton”.
An excellent review published in 2022 includes also both the history and the technology of Vulcanised Fibre but has no focus on the chemical aspects. 17 The authors point out that in the second half of the 20th century the Vulcanised Fibre industry had lost much of its market owing to the competition with the plastics industry. With growing concern about the environmental impact of plastics, there may be a renaissance of the Vulcanised Fibre industry because its products consist solely of biodegradable and sustainable biomaterials associated with several other advantages (below). Similar conclusions were drawn in other recent reviews which have a very broad scope. 18 , 19
4 The chemistry of the parchmentation of cellulose to produce Vulcanised Fibre using zinc chloride hydrates
On the molecular level, the specific action of zinc chloride hydrates on cellulose material for a long time has not been fully disclosed. However, there are several analogous processes where other metal salts are used for the reorganization or even full degradation of native polysaccharides and for which mechanistic and structural studies are more advanced. This is true in particular for the treatment of cellulose with copper salts, which in its most common form leads to “copper silk” (“Kupferseide”, Bemberg silk, named after the German chemist J. P. Bemberg). 20 The most common variant is the treatment with copper(II) salts in aqueous ammonia giving “cuprammonium rayon”. In this process the cellulose is completely dissolved in the strongly basic aqueous solution of the copper salt, from which a polysaccharide is subsequently deposited by the addition of acid. Under these reaction conditions, the polysaccharide molecules are not only disintegrated, but also partially degradated to shorter chains, which then – upon acidification - undergo self-assembly to new and modified macromolecules. These can be spun into fibers with the shining appearance of silk. While “true” silk is a polypeptide (a protein) obtained from cocoons of insect larvae, rayon is a polysaccharide, and regarding their composition, both have nothing in common.
As is shown in the models of Figures 7 and 8 , the hydroxyl groups (HO–C) become either coordinated to a metal cation at their oxygen atoms, or their hydrogen atoms are substituted by the metal cation. This deprotonation is supported by the alkalinity of the aqueous ammonia and does not apply to acidic media (below). The process is reversed by acidification which disconnects the metal cations from the polysaccharide chains.
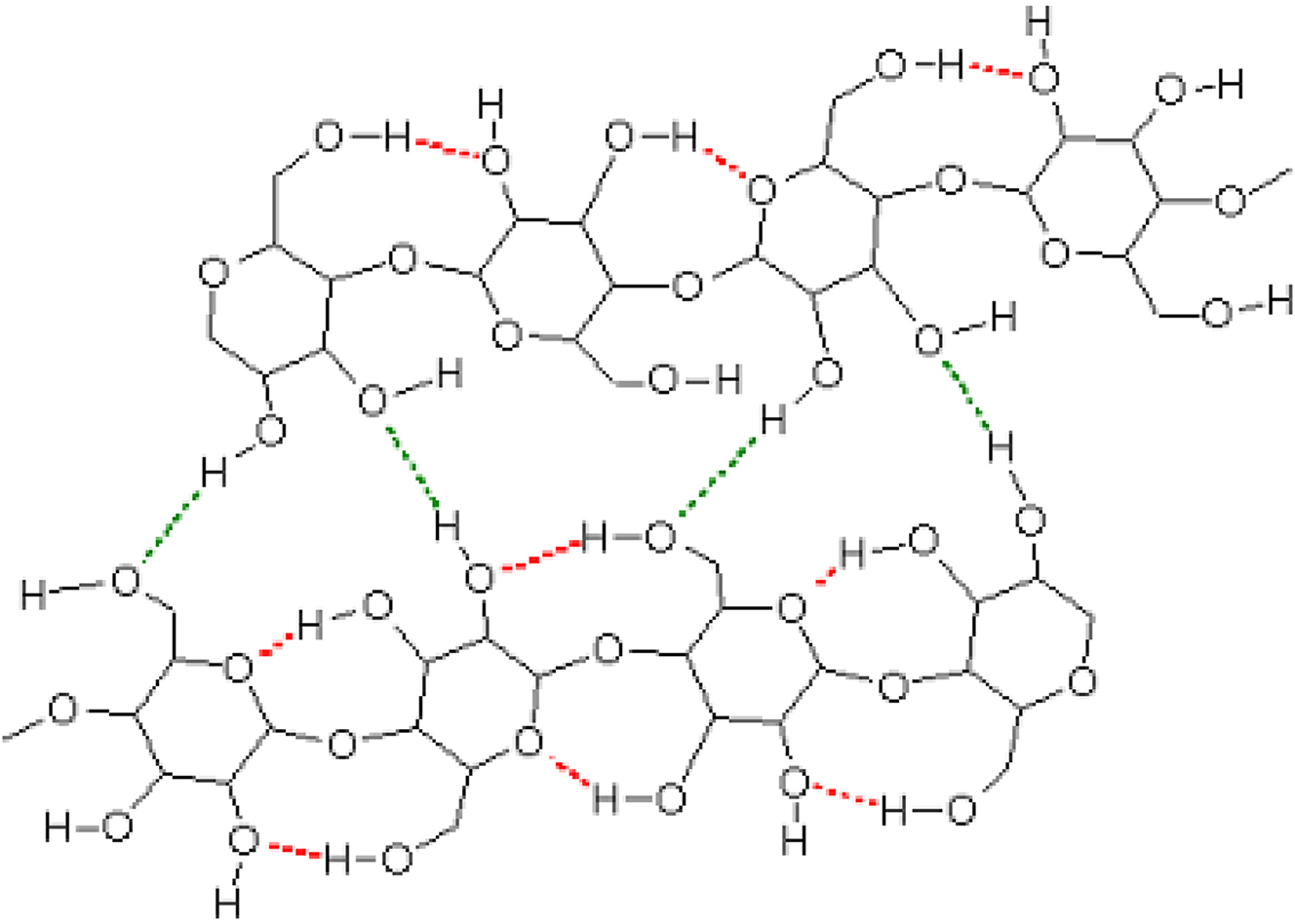
Hydrogen bonding pattern in and between two polysaccharide chains (projected onto a plane) prior to the treatment with an aqueous metal salt solution. 21 Intramolecular hydrogen bonds are shown in red, intermolecular hydrogen bonds are shown in green. (Reproduced with written permission from ACS Publications).
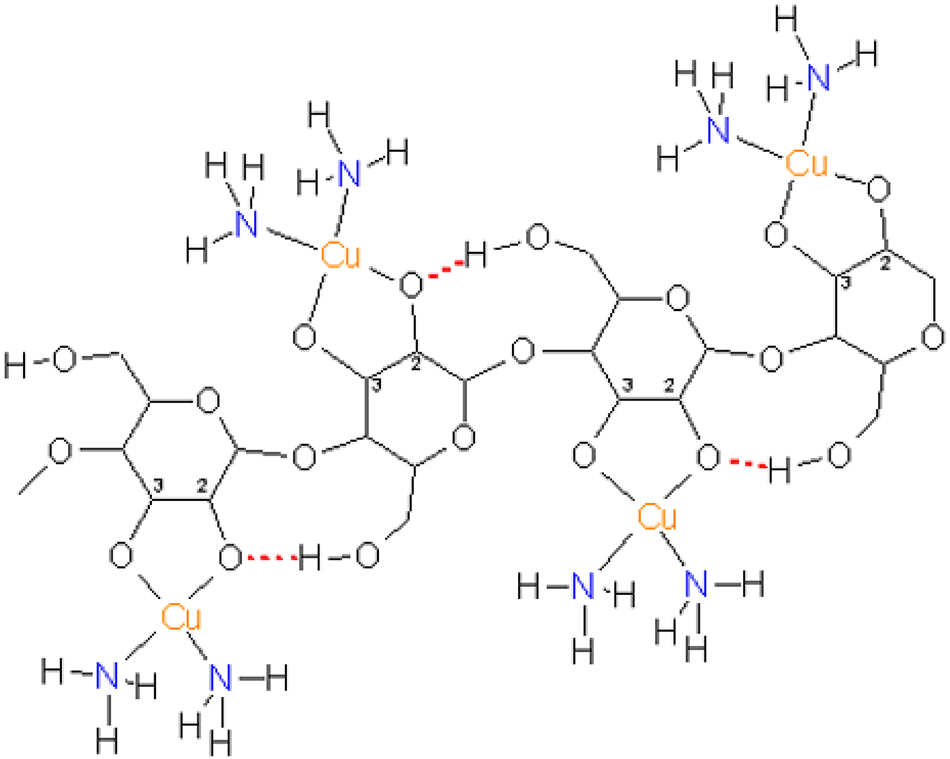
A polysaccharide chain after treatment with a copper(II) salt in aqueous ammonia. The treatment with zinc salts was long believed to lead to an analogous binding pattern (Zn for Cu, OH2 for NH3, but with different coordination numbers and motifs (below)). 21 (Reproduced with written permission from ACS Publications).
As mentioned above, under forcing conditions (higher temperature, longer dwelling times, higher acidity or alkalinity), the treatment of the cellulose leads to a partial degradation of the polysaccharide molecules and finally a complete dissolution of the cellulose in the liquid phase.
In the standard process for the production of Vulcanised Fibre using acidic zinc chloride hydrates described above, the cellulose material is not dissolved with degradation of the polysaccharide chains. Instead, only the inter-chain hydrogen bonds are cleaved and replaced in part by -O-Zn coordinative bonds and by a new pattern of hydrogen bonds. Upon removal of the zinc chloride in the washing process, a new set of inter-chain hydrogen bonds is generated, which determines the modified physical properties of the product.
The zinc chloride concentration of the parchmentation bath is generally in the range between 65 and 80 w%, centering around 72 w%, i. e. near the composition of the trihydrate ZnCl2·3(H2O) with 71.5 w% (75 mol%) ZnCl2.
Following early studies giving only preliminary results, 22 , 23 , 24 phase-analytical studies and a crystal structure determination 8 have shown in 2015 that the trihydrate is a liquid at room temperature, and the compositions in the near neighboring ranges are also liquids. The relevant part of the phase diagram ZnCl2/H2O presented in Figure 9 reveals that the trihydrate has a melting point of 6.5 °C, and even lower melting points are observed for composition ZnCl2(H2O)n with n in the range 2–4. 8
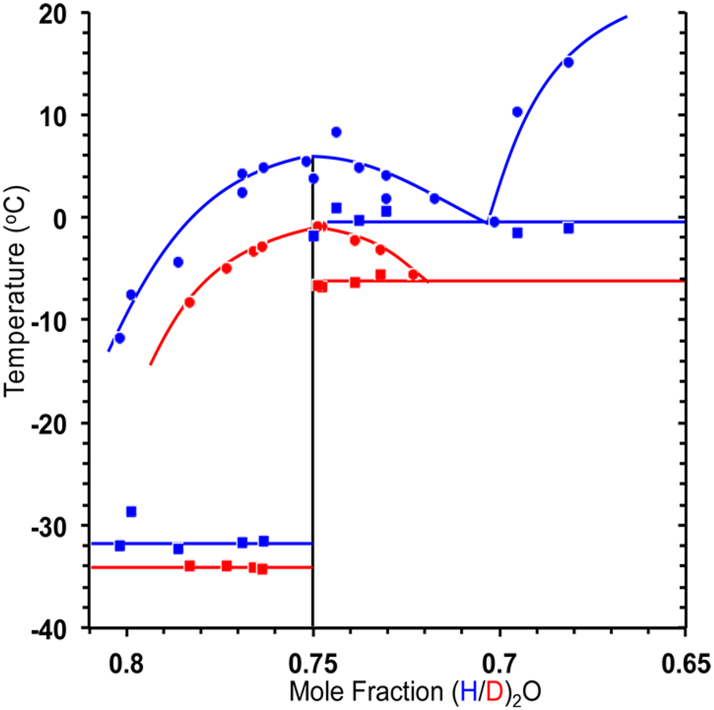
Section of the phase diagram ZnCl2/H2O. The maximum in the melting points is reached at 6.5 °C for the trihydrate ZnCl2·3(H2O) (blue for H2O, red for experiments with D2O). 8 (Reproduced with written permission from ACS Publications).
In order to clarify the assembly of the components (Zn2+, Cl−, H2O), the crystal structure of the trihydrate was examined by single-crystal X-ray diffraction. 8 It has been found that the structure is composed solely of the homoleptic ions [Zn(H2O)6]2+ und [ZnCl4]2− (Figure 10). The compound thus has the zinc cations in both an octahedrally hexa- and a tetrahedrally tetra-coordinated state. No polymorphs were detected for the trihydrate ZnCl2·3(H2O) stoichiometry, and - most importantly - there are no non-coordinated water molecules in its structure and in the melt. 8 The contacts between the cations and anions are established solely by weak O–H⋯Cl hydrogen bonds. The structure is thus closely related to that of [Mg(OH2)6][ZnCl4]. 25
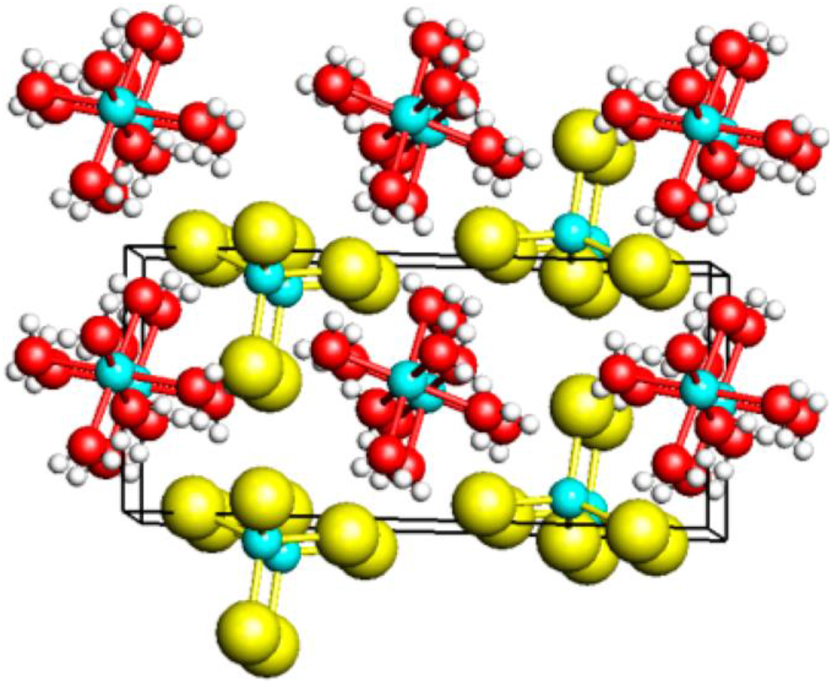
Crystal structure of the zinc chloride trihydrate ZnCl2·3(H2O) (turquoise: Zn; yellow: Cl; red: O). 8 (Reproduced with written permission from ACS Publications).
Extensive vibrational and NMR spectroscopic studies have shown that the liquids that are formed upon melting the solid (i.e. above 6.5 °C for the trihydrate ZnCl2·3(H2O)) have the same set of infrared bands, Raman spectroscopic lines and NMR spectroscopic signals, respectively, which is proof that the homoleptic ions remain unchanged in the room temperature regime. 24 , 26
The zinc chloride parchmentation baths with the trihydrate ZnCl2·3(H2O) stoichiometry thus cannot be simply addressed as aqueous solutions of zinc chloride or zinc chloride hydrates but must instead be classified as ionic liquids .
Similar, but not identical vibrational spectra were observed for systems ZnCl2(H2O) n with n ≤ 3. Differences arise, because a loss of water leads to changes in the ligand distributions between the zinc cations. For the composition ZnCl2(H2O)1.33 the crystal structure has also been determined. 23 These compositions have higher melting points and do not qualify as ionic liquids. For systems ZnCl2(H2O) n with n ≥ 3 the ligand distribution is less clear, since there are no X-ray diffraction data and the vibrational characteristics are more complex. For n = 4, the performance in the parchmentation is different, but the action can be adjusted by variations in the reaction temperature.
5 Proposed reaction mechanism
A reaction mechanism of the process that leads to the parchmentation of polysaccharides en route to Vulcanised Fibre materials is therefore proposed for the trihydrate ZnCl2(H2O)3. Of the two ions of the ionic liquid, only the cations can be assumed to be the reactive components. While the tetracoordinated anions [ZnCl4]2− are most stable, the hexacoordinated cations most readily lose one or two of their water ligands (Equation (1)) if these can be accepted by hydrogen bonding donors or acceptors. In the present system they become integrated in new hydrogen bonds to the polysaccharide chains (Equation (2)). It has been found in earlier studies that the water dynamics in a saturated ZnCl2 solution (i. e. in the ionic liquids) is altered considerably compared with that in dilute solutions. 18 , 19
The thus generated free coordination site at the zinc atom becomes occupied by a hydroxyl group of the chains (Equation (3)). Figure 11a shows the modes proposed for the interaction between the reaction components.
![Figure 11a:
Attachment of a [(Zn(H2O)5]2+ cation to a polysaccharide chain with random attachment of the liberated water molecule. (See Equations (1)–(3)).
8
,
24
The charge-balancing [ZnCl4]2− anion is omitted. Note that there are no solvent molecules.](/document/doi/10.1515/znb-2025-0013/asset/graphic/j_znb-2025-0013_fig_011a.jpg)
Attachment of a [(Zn(H2O)5]2+ cation to a polysaccharide chain with random attachment of the liberated water molecule. (See Equations (1)–(3)). 8 , 24 The charge-balancing [ZnCl4]2− anion is omitted. Note that there are no solvent molecules.
The process thus involves the zinc hexaquo complex as a donor of water molecules, which are lured away by the surface of the polysaccharide chains. (Equations (1) and (2)). In a following step (Equation (4)), the coordination vacancy at the zinc atom accepts another R′–OH function, which may even be chelating and thus adding to the stability of the generated intermediate (R′–OH and R″–OH being neighbors at the same polysaccharide chain or functions of two different chains, the latter being stereochemically preferred). (Equations (4) and (5)). This model suggests that hydrated Zn cations are readily inserted between cellulose chain molecules (Figure 11b).
![Figure 11b:
Insertion of a [(Zn(H2O)4]2+ cation between two polysaccharide chains, with random attachment of the two liberated water molecules. (See Equations (4) and (5)). The charge-balancing [ZnCl4]2− anion is omitted. Note that there are no solvent molecules.](/document/doi/10.1515/znb-2025-0013/asset/graphic/j_znb-2025-0013_fig_011b.jpg)
Insertion of a [(Zn(H2O)4]2+ cation between two polysaccharide chains, with random attachment of the two liberated water molecules. (See Equations (4) and (5)). The charge-balancing [ZnCl4]2− anion is omitted. Note that there are no solvent molecules.
In the washing process, the zinc cations are separated from the chains and obtain their original coordination sphere of hydration accepting the incoming water molecules (Equation (6)). New hydrogen bonds are formed within and between the chains leading to a new bonding pattern and new properties.
Introduction of a large amount of water (in the washing process) means that there is a competition with the few coordinated R–OH groups for zinc coordination sites, leading in the end to complete hydration of the metal cations and their removal from or between the chain molecules. By contrast, in the ionic liquid no free water molecules are present as competitors for the HOR functions of the cellulose.
Conversely, this mechanism explains why dilute aqueous solutions of zinc chloride have no effect on cellulose materials, since the excess of water molecules leads to a complete coverage of all –OH functions of the polysaccharide chains and to a complete hydration of the zinc cations and a complete solvation of the [ZnCl4]2− anions.
There appears to be general agreement that the [ZnCl4]2− anions are not directly involved in the attack of the cellulose substrate owing to the high stability of the Zn–Cl bonds and the comparatively weak Cl⋯H–O hydrogen bonds formed upon hydration.
It is important to note that in the production of Vulcanised Fibre using zinc chloride ionic liquids at low temperatures (approx. 40 °C) and short reaction times (several minutes) no cellulose material is lost, and that the chemical composition of the polysaccharide remains unchanged. Under mild conditions, the cellulose polymer molecules are not hydrolyzed to soluble shorter oligomers. There is also no irreversible derivatization.
By contrast, at higher temperatures and long reaction times, or with small variations of the composition of the ionic liquid [ZnCl2(H2O) n (n = 3, 3.5, 4)], cellulose is indeed partly or completely dissolved in zinc chloride ionic liquids (below).
For the different behavior of copper(II) and zinc(II) salts (above) it is relevant that the two cations prefer different coordination polyhedra: With four ligands, Cu(II) appears in the center of a square, while Zn(II) occupies the center of a tetrahedron. This difference gives rise to different modes of aggregation of the chains once they are loaded with metal cations. A model structure as shown in Figure 8 thus needs to be modified when Cu is replaced by Zn and the ligands NH3 by OH2. Generally, zinc cations reach higher coordination numbers than copper cations, as in [Zn(H2O)6]2+. Therefore, a mechanism involving low-coordinate [Zn(H2O)4]2+ or even [Zn(H2O)3]2+ appears to be less applicable. 27 It is an equally unsupported assumption that complete [Zn(H2O)6]2+ cations are inserted unchanged between the cellulose chains. 28
It is also important that in alkaline (ammoniacal) media the Cu–O(H)–C linkages are deprotonated and converted in metal alkoxide Cu–O–C linkages, a step that does not apply for the acidic media. Therefore, in the acidic zinc chloride ionic liquids the coordination of an alcoholic group to the zinc atom (Eq. (3)) is the only way in which the zinc atoms become – weakly and reversibly – fixed to a cellulose chain (Figure 10).
6 Dissolution of cellulose materials in zinc chloride hydrates
Since the 1990s, the fact that cellulose can be completely dissolved in zinc chloride hydrates has attracted growing interest. 5 Depending on the temperature, the reaction time, the composition and the relative volume of the zinc chloride hydrate solvent, the polysaccharide degradation proceeds via disintegration of the bundles, the shortening of the chain molecules and finally the complete hydrolysis to glucose molecules. 18 , 28 , 29 , 30 , 31 , 32 While in some reports the zinc chloride hydrates were still considered as “zinc chloride aqueous solutions”, 27 , 29 most later studies acknowledged the presence of zinc chloride hydrates as “ionic liquids” mainly with the formula ZnCl2(H2O) n with n = 3, 3.5 and 4. 28 , 30
With the tetrahydrate (n = 4) as an example, 5 the temperature has been set to 80 °C for complete dissolution of the cellulose, while for the salt with n = 3.5 the complete dissolution is achieved already at room temperature with longer reaction times. The dissolved material is precipitated by addition of water, separated by centrifugation and washed to remove the zinc salt and to reach neutrality. The cellulose is not fully recovered due to the loss of the amounts of material that are not reprecipitated, but the composition of the precipitate is unchanged (pure polysaccharide cellulose). The zinc chloride is regained by evaporation of the water in a vacuum at 65 °C to restore the hydrate composition required for the process.
7 Advantageous properties of Vulcanised Fibre products obtained from ionic liquids of zinc chloride hydrates
Upon the final drying, Vulcanised Fibre products shrink considerably yielding a material which is extremely robust to any mechanical deformation. 33 , 34 A remodeling is only possible after soaking the material in water, which now is a very slow process owing to the compact and tight new ordering of the fiber structure. The low permeability for gases makes vulcanized fiber products surprisingly flameproof, even though once ignited the material burns with a hot flame.
It is a great advantage of products of Vulcanised Fibre prepared with the zinc chloride parchmentation that zinc salts are colorless and not toxic for humans, and oral or percutaneous uptake is acceptable within the usual limits, and in trace amounts even essential. Zinc chloride is a low-cost medium for the parchmentation and it can be recovered from the stripped ionic liquid and from the washing solutions either by evaporation of the water to reach the desired concentration or by membrane technology using the principle of reverse osmosis. The bio-cellulose material is cheap and sustainable.
The dry Vulcanised Fibre product has a very low density of 1.0–1.5 g cm−3 and ranks among the less dense of the mechanically robust construction materials. 35 It is biodegradable, and no special treatment of the waste is necessary.
Several of the currently practiced processes for the production of Vulcanised Fibre are therefore still based on the treatment of the cellulose material with zinc chloride hydrate ionic liquids. The parchmentation with sulfuric acid is also widely in use but requires a careful control of the temperature and the acidity of the medium. The zinc chloride process is less sensitive.
8 From enforced forming to the spontaneous self-forming of Vulcanised Fibre products
Industrial products currently made of vulcanized fiber, where specific dimensions and forms are required, are produced by applying mechanical pressure on the still wet material in frameworks meeting these requirements, while being dried to induce hardening and form stability. The reorganization of the partially disintegrated polysaccharide chains generated in the parchmentation process (v. s.), which after washing are still loaded with water, approaches completion upon drying, but has to yield to the external pressure. Thus, a wet planar sheet will keep its planarity upon drying under vertical pressure (Figure 12a) or may be molded under additional constraints (Figure 12b).

Example of a dry planar sheet in red or black (0.5 mm thickness).

Example of a welding helmet made of black molded Vulcanized Fibre (produced by Honeywell Safety Products Slovakia s.r.o. from material by Ernst Krüger GmbH & Co. KG.).
However, if this wet planar sheet is allowed to dry without any external influence of pressure or fixation, shrinkage and hardening lead to strong three-dimensional deformations of the flat sheets producing distinct forms (Figure 12c). The mode and degree of this spontaneous deformation are determined by the material’s inherent preferences and are further influenced by several minor details of the exposition of the object to drying.
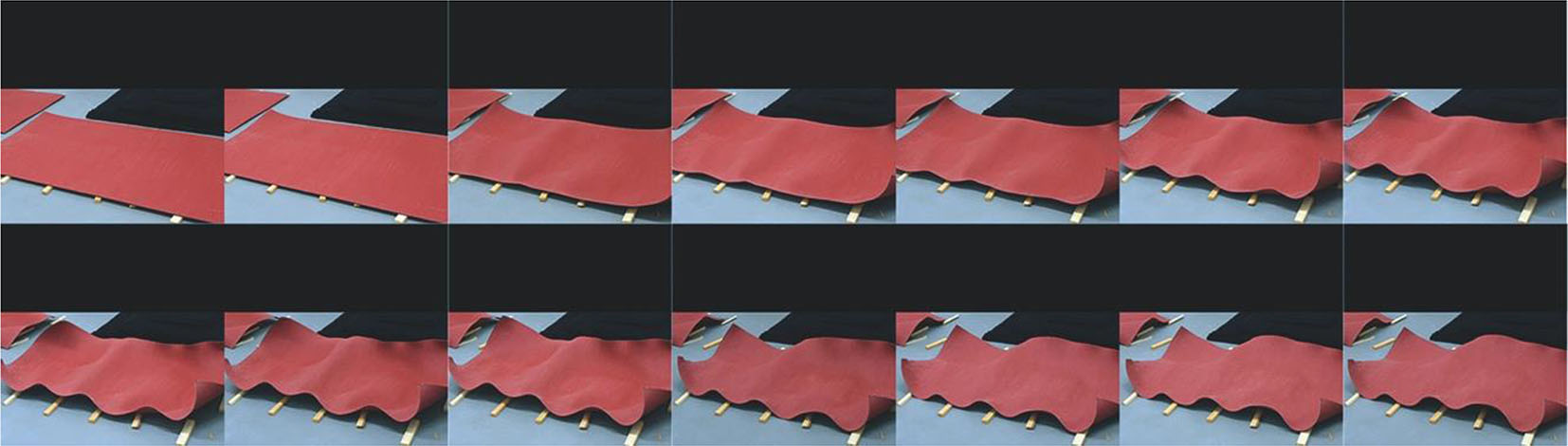
Example of a sheet dried without external constraints.
Some geometric changes can be associated with specific local conditions at the sheet surface such as asymmetric ventilation, or fiber direction in the base material (paper) and the corresponding shrinkage, allowing for a general and vague prediction of a deformation. However, the exact geometry of the forms emerging after drying remains unpredictable and varies across multiple instances of identical specimens (Figure 13).

3D scanned models of nine square shaped sheets (30 × 30 cm in wet state) dried under identical conditions.
Vulcanised Fibre materials can be prepared in various colors, traditionally mainly with traces of metal oxide pigments contained in the bulk of the material, e. g. with iron oxides for tones of red (Figures 4 and 5). Surface coloring can also be applied, and finally, mechanical and chemical treatment of the surface may generate a rough, mat or shiny appearance.
Techniques for surface treatment like polishing, layering, impregnation, and painting of Vulcanised Fibre are well documented in the literature. The same is true for a wealth of data on the mechanical properties of the products. These have been tabulated in several handbooks and resulted in the norms which are to be consulted for final quality assessments which was most important when the Vulcanised Fibre industry was in full swing. 13 , 14 , 15 , 16 , 17 , 18
9 Conclusions
Thomas Taylor’s observations 11 , 12 that zinc chloride hydrates can be used for a structural reorganization of all kinds of cellulose materials have been the start of a development which over more than one and a half centuries has led to a variety of processes which provide modified cellulose materials for many sorts of applications. Most of these processes are - albeit initially not recognized - among the earliest examples of the usage of “ionic liquids”, and this is also true for the production of Vulcanised Fibre.
The Vulcanised Fibre technology is currently undergoing a renaissance because the products are now recognized as being particularly environmentally friendly, being based not only on abundant and sustainable biomaterials, but also biodegradable. Therefore, they are market-competitors of common “plastics” of which there is growing environmental concern.
Vulcanised Fibre is produced by partial disentangling and reorganization of cellulose structures into tighter aggregates, which are lightweight, but of high mechanical strength.
Complete degradation of cellulose using these ionic liquids to produce low molecular weight carbohydrates and finally alcohol is another currently developing area.
In the authors´ ongoing research, Vulcanised Fibre is investigated as a building material with a special interest in its drying behavior and in the resulting forms of the objects. The formal and structural variations that emerge from an interplay of external form-giving and the self-forming and self-reinforcing capacity of the material offer a unique approach to the design of structural and building systems that is specific to the material. The inherent capacity for spontaneous structural rearrangement that results from the parchmentation process of the Vulcanic Fibre materials, and which escapes the designer’s complete determination, suggests the formation of unorthodox structures with unique properties for a wide range of applications. 36 , 37
In this context the inherent reaction mechanisms proposed above are further studied and expanded to identify whether the self-forming behavior leads to an increased stiffness and stability of the material compared to forms produced by applying external pressure.
Funding source: FWF Austrian Science Fund PEEK
Award Identifier / Grant number: AR 808-GBL
Acknowledgement
For process details described in the paper the authors are very grateful to Dr. Manfred Joseph of Hornex, Ernst Krüger GmbH & Co. KG, Geldern, Germany, and to Prof. Dr. Peter Klüfers of Ludwig-Maximilians-Universität München (LMU), Department of Chemistry and Pharmacy, for helpful discussions.
-
Research ethics: Not applicable.
-
Informed consent: All authors understand and consent to their involvement.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: This work is funded by a research grant from the FWF Austrian Science Fund PEEK (Halfforms-Material agency in spatial formations AR 808-GBL).
-
Data availability: Not applicable.
References
1. Wang, H.; Gurau, G.; Rogers, R. D. Ionic Liquid Processing of Cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537; https://doi.org/10.1039/c2cs15311d.Suche in Google Scholar PubMed
2. Swatloski, R. P.; Spear, S. K.; Holbrey, J. D.; Rogers, R. D. Dissolution of Cellulose with Ionic Liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975; https://doi.org/10.1021/ja025790m.Suche in Google Scholar PubMed
3. Pinkert, A.; Marsh, K. N.; Pang, S.; Staiger, M. P. Ionic Liquids and Their Interaction with Cellulose. Chem. Rev. 2009, 109, 6712–6728; https://doi.org/10.1021/cr9001947.Suche in Google Scholar PubMed
4. Fischer, S.; Leipner, H.; Thummler, K.; Brendler, E.; Peters, J. Inorganic Molten Salts as Solvents for Cellulose. Cellulose 2003, 10, 227–236; https://doi.org/10.1023/a:1025128028462.10.1023/A:1025128028462Suche in Google Scholar
5. Leipner, H.; Fischer, S.; Brendler, E.; Voigt, W. Structural Changes of Cellulose Dissolved in Molten Salt Hydrates. Macromol. Chem. Phys. 2000, 201, 2041–2049; https://doi.org/10.1002/1521-3935(20001001)201:15<2041::aid-macp2041>3.3.co;2-5.10.1002/1521-3935(20001001)201:15<2041::AID-MACP2041>3.3.CO;2-5Suche in Google Scholar
6. Sen, S.; Martin, J. D. Argyropoulos, Review of Cellulose Non-Derivatizing Solvent Interactions with Emphasis on Activity in Inorganic Molten Salt Hydrates. ACS Sustainable Chem. Eng. 2013, 1, 858–870; https://doi.org/10.1021/sc400085a.Suche in Google Scholar
7. Braunstein, J. Some Aspects of Solution Chemistry in Liquid Mixtures of Inorganic Salts with Water. Inorg. Chim. Acta, Rev. 1968, 2, 19–30; https://doi.org/10.1016/0073-8085(68)80012-9.Suche in Google Scholar
8. Wilcox, R. J.; Losey, B. P.; J Folmer, W.; Martin, J. D.; Zeller, M.; Sommer, R. Crystalline and Liquid Structure of Zinc Chloride Trihydrate: A Unique Ionic Liquid. Inorg. Chem. 2015, 54, 1109–1119; https://doi.org/10.1021/ic5024532.Suche in Google Scholar
9. Sen, S.; Losey, B. P.; Gordon, E. E.; Elijah, D. S.; Gordon, E.; Argyropoulos, D. S.; Martin, J. D. Ionic Liquid Character of Zinc Chloride Hydrates Define Solvent Characteristics that Afford the Solubility of Cellulose. J. Phys. Chem. 2016, 120, 1134–1141; https://doi.org/10.1021/acs.jpcb.5b11400.Suche in Google Scholar
10. Argyropoulos, D. S.; Martin, J. D.; Gordon, E. E.; Martin, J. D. Ionic Liquid Character of Zinc Chloride Hydrates Define Solvent Characteristics that Afford the Solubility of Cellulose. J. Phys. Chem. B 2016, 120, 1134; https://doi.org/10.1021/acs.jpcb.5b11400.Suche in Google Scholar
11. Taylor, T. Improved Means of Giving Increased Strength to Paper. English Patent, 787, 1859.Suche in Google Scholar
12. Taylor, T. Improvement in the Treatment of Paper and Paper-Pulp. U.S Patent 114880, 1871.Suche in Google Scholar
13. Große-Wilde, S. Werkstoff zwischen den Systemen, eine Stoffgeschichte der Vulkanfiber im 19. und 20. Jahrhundert; Springer: Berlin, 2022.10.1007/978-3-662-65602-0Suche in Google Scholar
14. Kunstoffhandbuch, Band III. Abgewandelte Naturstoffe. Vieweg, R.; Becker, E. Herausg.; Carl Hanser Verlag: München, 1965.Suche in Google Scholar
15. Lindman, B.; Karlstrom, G.; Stigsson, L. On the Mechanism of Dissolution of Cellulose. J. Mol. Liq. 2010, 156, 76–81; https://doi.org/10.1016/j.molliq.2010.04.016.Suche in Google Scholar
16. Klemm, D.; Heublein, B.; Fink, H. P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393; https://doi.org/10.1002/anie.200460587.Suche in Google Scholar PubMed
17. Nemoto, J.; Nakamata, K. All-cellulose Material Prepared Using Aqueous Zinc Chloride Solution. Cellulose 2022, 29, 2795–2803; https://doi.org/10.1007/s10570-021-04344-1.Suche in Google Scholar
18. Qin, Q. S.; Zeng, S.; Duan, G.; Liu, Y.; Han, X.; Yu, R.; Huang, Y.; Zhang, C. H.; Han, J.; Jiang, S. H. “Bottom-up’’ and ‘‘top-Down’’ Strategies toward Strong Cellulose-Based Materials. Chem. Soc. Rev. 2024, 53, 9306–9343; https://doi.org/10.1039/d4cs00387j.Suche in Google Scholar PubMed
19. Burchard, W. Solubility and Solution Structure of Cellulose Derivatives. Cellulose 2003, 10, 213–225; https://doi.org/10.1023/a:1025160620576.10.1023/A:1025160620576Suche in Google Scholar
20. Jentgen, H. Die Entwicklung der Verfahren zur Herstellung von Kupferkunstseide. Chem. Ztg. 1942, 66, 502–503.Suche in Google Scholar
21. Saalwächter, K.; Burchard, W.; Klüfers, P.; Kettenbach, G.; Mayer, P.; Klemm, D.; Fugarmaa, S. Cellulose Solutions in Water Containing Metal Complexes. Macromolecules 2000, 33, 4094–4107; https://doi.org/10.1021/ma991893m.Suche in Google Scholar
22. Mylius, F.; Dietz, R. Über das Chlorzink. Z. Anorg. Chem. 1905, 44, 209–220; https://doi.org/10.1002/zaac.19050440115.Suche in Google Scholar
23. Follner, V. H.; Brehler, B. Die Kristallstruktur des ZnCl2·(H2O)1.33. Acta Crystallogr. 1970, B26, 1679–1682.10.1107/S0567740870004715Suche in Google Scholar
24. Yamaguchi, T.; Hayashi, S.; Ohtaki, S. X-Ray Diffraction and Raman Studies of Zinc(II) Chloride Hydrate Melts, ZnCl2.rH2O (R = 1.8, 2.5, 3.0, 4.0, and 6.2). J. Phys. Chem. 1989, 93, 2620–2625; https://doi.org/10.1021/j100343a074.Suche in Google Scholar
25. Duhlev, R.; Macicek, J. Structure of Magnesium Zinc Tetrachloride Hexahydrate. Acta Crystallogr. 1991, C47, 1573–1575.10.1107/S0108270191001798Suche in Google Scholar
26. Irish, D. E.; McCarroll, B.; Young, T. F. Raman Studies of Zinc Chloride Solutions. J. Chem. Phys. 1963, 39, 3436–3444; https://doi.org/10.1063/1.1734212.Suche in Google Scholar
27. Ma, W.; Li, X.; Zhang, L.; Zheng, Y.; Xi, Y.; Ma, J.; Wang, Zh. Novel Insights on Room Temperature-Induced Cellulose Dissolution Mechanism via ZnCl2 Aqueous Solution: Migration, Penetration, Interaction, and Dispersion. Int. J. Biol. Macromol. 2024, 272, 132912 (13 pages); https://doi.org/10.1016/j.ijbiomac.2024.132912.Suche in Google Scholar PubMed
28. Lara-Serrano, M.; Morales-delaRosa, S.; Campos-Martín, J. M.; Fierro, J. L. G. High Enhancement of the Hydrolysis Rate of Cellulose after Pretreatment with Inorganic Salt Hydrates. Green Chem. 2020, 22, 3860–3866; https://doi.org/10.1039/d0gc01066a.Suche in Google Scholar
29. Lu, X.; Shen, X. Solubility of Bacteria Cellulose in Zinc Chloride Aqueous Solutions. Carbohydr. Polym. 2011, 86, 239–244; https://doi.org/10.1016/j.carbpol.2011.04.042.Suche in Google Scholar
30. Awosusi, A. A.; Ayeni, A.; Adelekec, R.; Daramola, M. O. Effect of Water of Crystallization on the Dissolution Efficiency of Molten Zinc Chloride Hydrate Salts during the Pre-treatment of Corncob Biomass. J. Chem. Technol. Biotechnol. 2017, 92, 2468–2476; https://doi.org/10.1002/jctb.5266.Suche in Google Scholar
31. Cao, N. J.; Xu, Q.; Chen, C. S.; Gong, C. S.; Chen, L. F. Cellulose Hydrolysis Using Zinc-Chloride as a Solvent and Catalyst. Appl. Biochem. Biotechnol. 1994, 45, 521–530; https://doi.org/10.1007/bf02941827.Suche in Google Scholar
32. Cao, N. J.; Xu, Q.; Chen, L. F. Acid-hydrolysis of Cellulose in Zinc-Chloride Solution. Appl. Biochem. Biotechnol. 1995, 51−52, 21–28; https://doi.org/10.1007/bf02933408.Suche in Google Scholar
33. Regel, O. Kunststoffe 1930 (11), 241. 1931 (4) 82, 246 und 273.10.1017/S0003581500039846Suche in Google Scholar
34. Mittendorf, R.-M. Entwicklung einer Richtlinie für die Auslegung von Direktschraubverbindungen in Vulkanfiber. Dissertation; University of Dortmund: Dortmund, 2016.Suche in Google Scholar
35. Scholz, R.; Mittendorf, R.-M.; Engels, J.; Hartmaier, A.; Walther, F. Direction-dependent Mechanical Characterization of Cellulose-Based Composite Vulcanized Fiber. Mater. Test. 2016, 58, 813–817; https://doi.org/10.3139/120.110929.Suche in Google Scholar
36. Allner, L.; Schmidbaur, K.; Vaíllo, G. (Un)fitting Aggregations: Material-dependent Production and Spielraum of Distinct Vulcanized Fiber Pieces. In Proceedings Of the 45th Annual Conference of ACADIA; Banff: Canada, 2025 (in progress).Suche in Google Scholar
37. Penning, B.; Walther, F.; Dumke, D.; Künne, B. Einfluss der Verformungsgeschwindigkeit und des Feuchtegehaltes auf das quasistatische Verformungsverhalten technischer Vulkanfiber. Mater. Test. 2013, 55, 276–284; https://doi.org/10.3139/120.110435.Suche in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- In this issue
- Research Articles
- Chemical aspects of the preparation of Vulcanised Fibre using zinc chloride hydrates: from a brief history to a new consideration of the key reactions in ionic liquids
- On the preparation and spectral properties of some hydroxy-substituted triphenylmethane dyes
- High-pressure synthesis and crystal structure of Ga5Na1–x B12O26–x (OH) x (x = 0.12)
- Intermetallic phases with Ho4Ir13Ge9-type structure
- Interplay between oxidative addition and reductive elimination at a diruthenium complex bearing the bis(diphenylphosphanyl)amine ligand
- Anthracene-d- and l-phenylalanine derivatives: synthesis, dual-state emission, mechanochromic luminescence, chiroptical property and enantioselective recognition of free amino acids
- Synthesis, structures and optical properties of 3,3′-disubstituted biphenyl compounds
Artikel in diesem Heft
- Frontmatter
- In this issue
- Research Articles
- Chemical aspects of the preparation of Vulcanised Fibre using zinc chloride hydrates: from a brief history to a new consideration of the key reactions in ionic liquids
- On the preparation and spectral properties of some hydroxy-substituted triphenylmethane dyes
- High-pressure synthesis and crystal structure of Ga5Na1–x B12O26–x (OH) x (x = 0.12)
- Intermetallic phases with Ho4Ir13Ge9-type structure
- Interplay between oxidative addition and reductive elimination at a diruthenium complex bearing the bis(diphenylphosphanyl)amine ligand
- Anthracene-d- and l-phenylalanine derivatives: synthesis, dual-state emission, mechanochromic luminescence, chiroptical property and enantioselective recognition of free amino acids
- Synthesis, structures and optical properties of 3,3′-disubstituted biphenyl compounds

