Abstract
Tetragonal Ga5Na1–x B12O26–x (OH) x (x = 0.12) was synthesized in a Walker-type multianvil apparatus under high-pressure/high-temperature conditions of 12.4 GPa/1,280 °C. The compound crystallizes in the space group I41/acd (no. 142) with lattice parameters of a = 11.0884(6) and c = 21.730(2) Å. The crystal structure has been determined by single-crystal X-ray diffraction and revealed an occupation with Na+ of about 88 % of the cuboctahedral cavity formed by condensed [BO4] tetrahedra in the related homeotypic structure of Ga5B12O25(OH). The structure refinement is additionally supported by infrared spectroscopy (IR) and energy-dispersive X-ray (EDX) investigations.
1 Introduction
Black Ti5B12O26 1 was discovered by Haberer et al. in 2009 and is the first representative of a structure class characterized by an anionic framework composed of two interpenetrating, three-dimensional networks of corner-sharing [BO4] tetrahedra crystallizing in the space group I41/acd. 1 Subsequently, the mixed valent Ti3+ and Ti4+ cations could be replaced by triel elements with the charge of 3+ (In, Ga), accompanied by the incorporation of hydrogen cations to reach charge neutrality. 2 Surprising photocatalytic properties as well as luminescence of the Eu3+ activated phase In5B12O25(OH), reported by Vitzthum et al., are of particular interest regarding potential future applications. 2 Later on, the great structural diversity of this structure type using Al, Ga, In, V, Cr, Mn, Fe, and Co as metal cations was revealed. 2 , 3 , 4 , 5 Moreover, cubic structures with the same sum formula were also found, which show great similarities with the tetragonal structure type M 5B12O25(OH). 6
The aim of the work described in the present publication was to extend the system M 5B12O25(OH) by structural modifications. First, we started with an optimization of the synthesis conditions and succeeded for Ga5B12O25(OH) 2 with a powder X-ray diffraction pattern without reflections of side-phases, which is representative for the first time in the system M 5B12O25(OH). Subsequently, we sought to modify this phase, Ga5B12O25(OH), 2 with an occupied cuboctahedral site. To avoid excessive distortion of the cuboctahedral site, we considered using an atom with a small ionic radius such as Na+. As in all previously synthesized specimens, we used a multianvil press for high-pressure/high-temperature experiments. We used NaCN as Na+ source, since we assumed from our experience that CN− does not withstand rather extreme synthesis conditions, so it will not be present in the product.
In the following, we discuss the high-pressure/high-temperature synthesis, the crystal structure, as well as the results of infrared (IR) spectroscopy and energy-dispersive X-ray spectroscopy (EDX) of Ga5Na1–x B12O26–x (OH) x (x = 0.12).
2 Experimental section
2.1 Synthesis
The synthesis of the title compound was performed under high-pressure/high-temperature conditions in a multianvil apparatus. A mixture of Ga2O3 (99.99 %, Tradium GmbH, Frankfurt am Main, Germany), H3BO3 (≥99.8 %, Carl Roth GmbH & Co KG, Karlsruhe, Germany), and NaCN (>98 %, Fluka Chemie GmbH, Buchs, Switzerland) in a molar ratio of 5:24:3 (stoichiometric ratio apart from NaCN, which was used abundantly to improve the incorporation of Na+ compared to a previous experiment that was performed with less Na+) was ground in an agate mortar and encapsulated in a platinum foil (0.027 mm, 99.9 %, Chem-PUR, Karlsruhe, Germany). The capsule was placed in a crucible with a lid, both made of α-BN (HeBoSint, P100, Henze Boron Nitride Products AG, Kempten, Germany) and centered in a “14/8” assembly, which was equipped with a graphite furnace (FE 254, Schunk-group GmbH, Vienna, Austria) for resistance heating. The octahedral assembly was arranged in the center of eight beveled tungsten carbide cubes (HA-7% Co, Hawedia, Marklkofen, Germany). The synthesis was performed in a modified Walker-type multianvil device (mavo press LPR 1000-400/50, Max Voggenreiter GmbH, Mainleus, Germany). More detailed information on the experimental setup is available in the literature. 7 , 8 , 9 After compression of the sample to 12.4 GPa, the sample was heated to 1,280 °C within 7 min, kept constant for 5 min, then cooled to 900 °C within 30 min, before the heating was switched off. After decompression, a in most of its parts colorless sample containing crystals of Ga5Na1–x B12O26–x (OH) x with some additional black impurities was isolated.
2.2 X-ray structure determination
The reaction product was analyzed with a STOE Stadi P powder X-ray diffractometer (STOE & Cie GmbH, Darmstadt, Germany) equipped with a Mythen 1 K microstrip detector (Dectris, Baden-Daettwil, Switzerland). The measurement was performed with Ge(111)-monochromatized Mo-Kα 1 radiation (λ = 0.7093 Å) in transmission geometry across a 2θ range of 2.0–60.5°. Figure 1 shows the experimental powder pattern in comparison to a calculated pattern derived from the single-crystal structure data. While the great majority of reflections could be explained by the title compound, a few minor reflections are caused by an unidentified by-product.

Experimental powder pattern (black) of Ga5Na1–x B12O26–x (OH) x compared to a calculated powder pattern based on single-crystal structure data of Ga5Na1–x B12O26–x (OH) x (x = 0.12) (colored blue, inverted). The asterisk-marked reflections refer to an unidentified byproduct.
A colorless single-crystal of Ga5Na1–x B12O26–x (OH) x (x = 0.12) was measured with a Bruker D8 Quest Photon III C14 diffractometer (Bruker Corporation, Billerica, Massachusetts, U.S.). For data collection and processing, the programs Saint (v8.40 B) 10 and Apex4 (v2021.4.0) 11 were used and a multi-scan absorption correction was performed by the program Sadabs (2016/2). 12 For structure solution and parameter refinement, the software tools Shelxt (2018/2) 13 and Shelxl (2018/3) 14 implemented in the program Olex2-1.5 15 were applied. All atoms could be refined anisotropically, except the hydrogen atom, which could not be located.
Details of the data collection are specified in the synoptical Table 1. Tables 2 and 3 show the positional and displacement parameters, the Wyckoff positions, and the site occupancy factors (S.O.F.).
Single-crystal data and structure refinement of Ga5Na1–x B12O26–x (OH) x (x = 0.12). Standard deviations are given in parentheses and refer to the last decimal place.
| Empirical formula | Ga5Na1–x B12O26–x (OH) x (x = 0.12) |
| Molar mass, g mol−1 | 914.59 |
| Crystal system | Tetragonal |
| Space group | I41/acd |
| Single-crystal diffractometer | Bruker D8 Quest |
| Radiation/wavelength λ, Å | Mo-Kα/0.71073 |
| Single-crystal data | |
| a, Å | 11.0884(6) |
| c, Å | 21.730(2) |
| V, Å3 | 2,671.8(4) |
| Formula units per cell Z | 8 |
| Calculated density, g cm−3 | 4.55 |
| Crystal size, mm3 | 0.12 × 0.09 × 0.05 |
| Temperature, K | 183(2) |
| Absorption coefficient, mm−1 | 10.2 |
| F(000), e | 3462 |
| θ range, deg | 3.20–41.14 |
| Range in hkl | ±20; ±20; ±40 |
| Reflections total/independent | 168,453/2,226 |
| R int | 0.0553 |
| Reflections with I ≥ 2 σ(I) | 2,192 |
| R σ | 0.0124 |
| Data/ref. parameters | 2,226/103 |
| Absorption correction | Multi-scan |
| Final R 1/wR 2 [I ≥ 2 σ(I)] | 0.0144/0.0309 |
| Final R 1/wR 2 (all data) | 0.0147/0.0310 |
| Goodness-of-fit on F 2 | 1.229 |
| Largest diff. peak/hole, e Å−3 | 0.58/−0.63 |
Wyckoff positions, atomic coordinates, equivalent isotropic displacement parameters U eq (Å2) and site occupancy factors (S.O.F.) for Ga5Na1–x B12O26–x (OH) x (x = 0.12) in comparison to published data for the homeotypic structure Ga5B12O25(OH). 2 U eq is defined as one third of the trace of the orthogonalized U ij tensor (standard deviations in parentheses).
| Atom | Wyckoff position | x | y | z | U eq (U iso for H3) | S.O.F. |
|---|---|---|---|---|---|---|
| Ga5Na1–x B12O26–x (OH) x (x = 0.12) (present study) | ||||||
|
|
||||||
| Ga1 | 32g | 0.31774(2) | 0.09362(2) | 0.20586(2) | 0.00191(2) | 1 |
| Ga2 | 8b | 0 | 1/4 | 1/8 | 0.00379(3) | 1 |
| B1 | 32g | 0.07346(7) | 0.16230(7) | 0.25065(4) | 0.0023(2) | 1 |
| B2 | 32g | 0.25211(7) | 0.18300(7) | 0.08247(3) | 0.0025(2) | 1 |
| B3 | 32g | 0.40466(7) | 0.00994(7) | 0.08122(3) | 0.0026(2) | 1 |
| O1 | 32g | 0.16059(5) | 0.23023(5) | 0.29120(2) | 0.00238(7) | 1 |
| O2 | 32g | 0.16263(5) | 0.11545(5) | 0.04513(2) | 0.00231(7) | 1 |
| O3 | 32g | 0.18955(5) | 0.26360(5) | 0.12638(2) | 0.00245(7) | 1 |
| O4 | 32g | 0.31404(5) | 0.08062(5) | 0.29302(2) | 0.00261(7) | 1 |
| O5 | 32g | 0.32734(5) | 0.09587(5) | 0.11782(2) | 0.00239(7) | 1 |
| O6 | 32g | 0.33790(5) | 0.25072(5) | 0.04312(2) | 0.00243(7) | 1 |
| O7 | 16d | 0 | 1/4 | 0.03339(3) | 0.0024(2) | 1 |
| Na1 | 8a | 1/2 | 1/4 | 1/8 | 0.0046(2) | 0.876(4) |
|
|
||||||
| Ga 5 B 12 O 25 (OH) 2 | ||||||
|
|
||||||
| Ga1 | 32g | 0.32036(2) | 0.09695(2) | 0.20509(2) | 0.00502(5) | 1 |
| Ga2 | 16f | 0.01270(4) | 0.26270(4) | 1/8 | 0.0094(2) | 0.5 |
| B1 | 32g | 0.0735(2) | 0.1622(2) | 0.25051(6) | 0.0040(2) | 1 |
| B2 | 32g | 0.2540(2) | 0.1824(2) | 0.08195(6) | 0.0040(2) | 1 |
| B3 | 32g | 0.4054(2) | 0.0138(2) | 0.08154(6) | 0.0055(2) | 1 |
| O1 | 32g | 0.16120(8) | 0.22924(8) | 0.29014(4) | 0.0044(2) | 1 |
| O2 | 32g | 0.16229(8) | 0.11458(8) | 0.04561(4) | 0.0044(2) | 1 |
| O3 | 32g | 0.19235(8) | 0.26479(8) | 0.12568(4) | 0.0048(2) | 1 |
| O4 | 32g | 0.31426(8) | 0.08075(8) | 0.29334(4) | 0.0047(2) | 1 |
| O5 | 32g | 0.32661(8) | 0.09573(8) | 0.11742(4) | 0.0042(2) | 1 |
| O6 | 32g | 0.33698(8) | 0.25057(8) | 0.04185(4) | 0.0040(2) | 1 |
| O7 | 16d | 0 | 1/4 | 0.03408(6) | 0.0042(2) | 1 |
| H3 | 32g | 0.38(2) | 0.08(2) | 0.31(2) | 0.2(2) | 0.25 |
Anisotropic displacement parameters U ij (Å2) of Ga5Na1–x B12O26–x (OH) x (x = 0.12) (standard deviations in parentheses).
| Atom | U 11 | U 22 | U 33 | U 12 | U 13 | U 23 |
|---|---|---|---|---|---|---|
| Ga1 | 0.00232(3) | 0.00193(3) | 0.00148(3) | −0.00007(2) | −0.00005(2) | 0.00005(2) |
| Ga2 | 0.00476(4) | 0.00476(4) | 0.00186(5) | 0.00181(5) | 0 | 0 |
| B1 | 0.0022(2) | 0.0023(2) | 0.0024(2) | 0.0002(2) | −0.0002(2) | 0.0000(2) |
| B2 | 0.0029(2) | 0.0021(2) | 0.0024(2) | 0.0001(2) | 0.0001(2) | 0.0000(2) |
| B3 | 0.0024(2) | 0.0029(2) | 0.0025(2) | 0.0001(2) | 0.0002(2) | 0.0003(2) |
| O1 | 0.0027(2) | 0.0018(2) | 0.0027(2) | −0.0001(2) | −0.0011(2) | 0.0003(2) |
| O2 | 0.0023(2) | 0.0019(2) | 0.0027(2) | 0.0001(2) | −0.0012(2) | 0.0002(2) |
| O3 | 0.0023(2) | 0.0023(2) | 0.0028(2) | −0.0000(2) | −0.0001(2) | −0.0012(2) |
| O4 | 0.0035(2) | 0.0021(2) | 0.0022 (2) | 0.0005(2) | 0.0003(2) | −0.0003(2) |
| O5 | 0.0027(2) | 0.0026(2) | 0.0019(2) | 0.0011(2) | −0.0002(2) | −0.0000(2) |
| O6 | 0.0026(2) | 0.0017(2) | 0.0029(2) | −0.0001(2) | 0.0014(2) | −0.0002(2) |
| O7 | 0.0029(2) | 0.0025(2) | 0.0017(2) | 0.0008(2) | 0 | 0 |
| Na | 0.0047(2) | 0.0047(2) | 0.0044(3) | 0 | 0 | 0 |
CSD-2376207 contains the supplementary crystallographic data of Ga5Na1–x B12O26–x (OH) x (x = 0.12) for this paper. The data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif.
2.3 Infrared spectroscopy
The transmission FT-IR spectrum of a single-crystal of Ga5Na1–x B12O26–x (OH) x (x = 0.12) was measured with a Vertex 70 FT-IR spectrometer (spectral resolution 4 cm−1, 15 × IR objective as focus), which was equipped with a KBr beam splitter, a liquid nitrogen cooled mercury cadmium telluride detector and attached to a Hyperion 3,000 microscope (Bruker Corporation, Billerica, Massachusetts, U.S.A.). As a mid-infrared source, a Globar (silicon carbide) rod was applied. During the measurement, the sample was placed on a BaF2 window. 320 scans were recorded in a spectral range of 600–4,000 cm−1. Atmospheric influences were corrected with the software Opus 7.2. 16
2.4 Energy-dispersive X-ray spectroscopy
The chemical composition of the title compound was further investigated with a field emission gun scanning electron microscope (FEG-SEM) Clara Ultra High Resolution (UHR) from TESCAN equipped with an energy-dispersive X-ray spectroscopy (EDX) detector Ultim Max, 65 mm2 from OXFORD. Grinded sample material was prepared on a carbon sticker on an aluminum stub. Imaging and EDX measurements were performed in analysis mode at an acceleration voltage of 20 keV and a beam current of 3 nA at a working distance of 9 mm. For EDX, preferably flat and horizontally aligned surfaces of single crystals or crystalline aggregates were selected.
3 Results and discussion
3.1 Crystal structure
Ga5Na1–x B12O26–x (OH) x (x = 0.12) crystallizes in the tetragonal space group I41/acd (no. 142) with eight formula units (Z = 8) and the lattice parameters a = 11.0884(6), c = 21.730(2) Å, and V = 2,671.8(4) Å3. The structure is homeotypic to M 5B12O25(OH) (M = Al, Cr, Ga, Ga/In, V), 2 , 3 , 4 In5B12O25(OH):Eu3+, 2 Ti5B12O26, 1 Mn5MnB12O22(OH)4, 4 Mn5Mn0.83B12O26, 4 Fe5Fe0.14B12O24.3(OH)1.7 4 and V1.36Co3.64Co0.53[B12O24(OH)2]. 5 Details of the single-crystal data and structure refinement are given in Table 1, the positional and displacement parameters and site occupancy factors in Table 2 and 3. Ga5B12O25(OH) 2 seemed to be most suited for a comparison, which contains the same elements, apart from the vacant cuboctahedral void and a slightly shifted Ga2, which leads to a different site of Ga2.
Ga5Na1–x
B12O26–x
(OH)
x
(x = 0.12) has two different sites for Ga in octahedral, one for Na in cuboctahedral and three different sites for B in tetrahedral coordination (see Table 2). Furthermore, there is a hydrogen site required for charge neutrality, but this could not be exactly assigned in the structure refinement and the situation discussed below. As compared in Table 2, all atoms have the same Wyckoff position as in Ga5B12O25(OH),
2
apart from Na1, which is not present in Ga5B12O25(OH),
2
hydrogen, which could not be refined in Ga5Na1–x
B12O26–x
(OH)
x
(x = 0.12), and Ga2, which is directly located on a special site in the title compound and has therefore a higher site symmetry. In Ga5B12O25(OH),
2
this positional shift at Ga2 is presumably caused in a distortion of the borate network due to a strong hydrogen bond to O3 (see Figure 4).
2
Since there is only a small amount of hydrogen in the title compound, the influence of this hydrogen bond is inferior and did not lead to a shift of the position of Ga2. Figure 2 depicts the arrangement of the metal cation polyhedra in the unit cell. The green and orange edge-sharing units are crystallographically identical, but are differently colored in order to distinguish paired units. Thereby, two [Ga1O6] octahedra are linked with a common edge, forming a [Ga12O10] unit. Along the crystallographic c axis, every second unit is either rotated by 90° or shifted along the b axis relative to the adjacent unit. The Ga2 atoms are surrounded by six O atoms forming single [Ga2O6] octahedra and are located in the holes along the
![Figure 2:
Visualization of the arrangement of [GaO6] octahedra (orange, green or cyan) and [NaO12] cuboctahedra (purple) in Ga5Na1–x
B12O26–x
(OH)
x
(x = 0.12) in viewing direction [100]. To better distinguish the crystallographically identical [Ga2O10] units, they were colored orange and green.](/document/doi/10.1515/znb-2025-0005/asset/graphic/j_znb-2025-0005_fig_002.jpg)
Visualization of the arrangement of [GaO6] octahedra (orange, green or cyan) and [NaO12] cuboctahedra (purple) in Ga5Na1–x B12O26–x (OH) x (x = 0.12) in viewing direction [100]. To better distinguish the crystallographically identical [Ga2O10] units, they were colored orange and green.
![Figure 3:
Formation of the network built of [BO4] tetrahedra in Ga5Na1–x
B12O26–x
(OH)
x
(x = 0.12) in viewing direction [100]. Four tetrahedrally arranged [B3O9] units form a [Na1O12] cuboctahedron, which is repeated eight times per unit cell.](/document/doi/10.1515/znb-2025-0005/asset/graphic/j_znb-2025-0005_fig_003.jpg)
Formation of the network built of [BO4] tetrahedra in Ga5Na1–x B12O26–x (OH) x (x = 0.12) in viewing direction [100]. Four tetrahedrally arranged [B3O9] units form a [Na1O12] cuboctahedron, which is repeated eight times per unit cell.
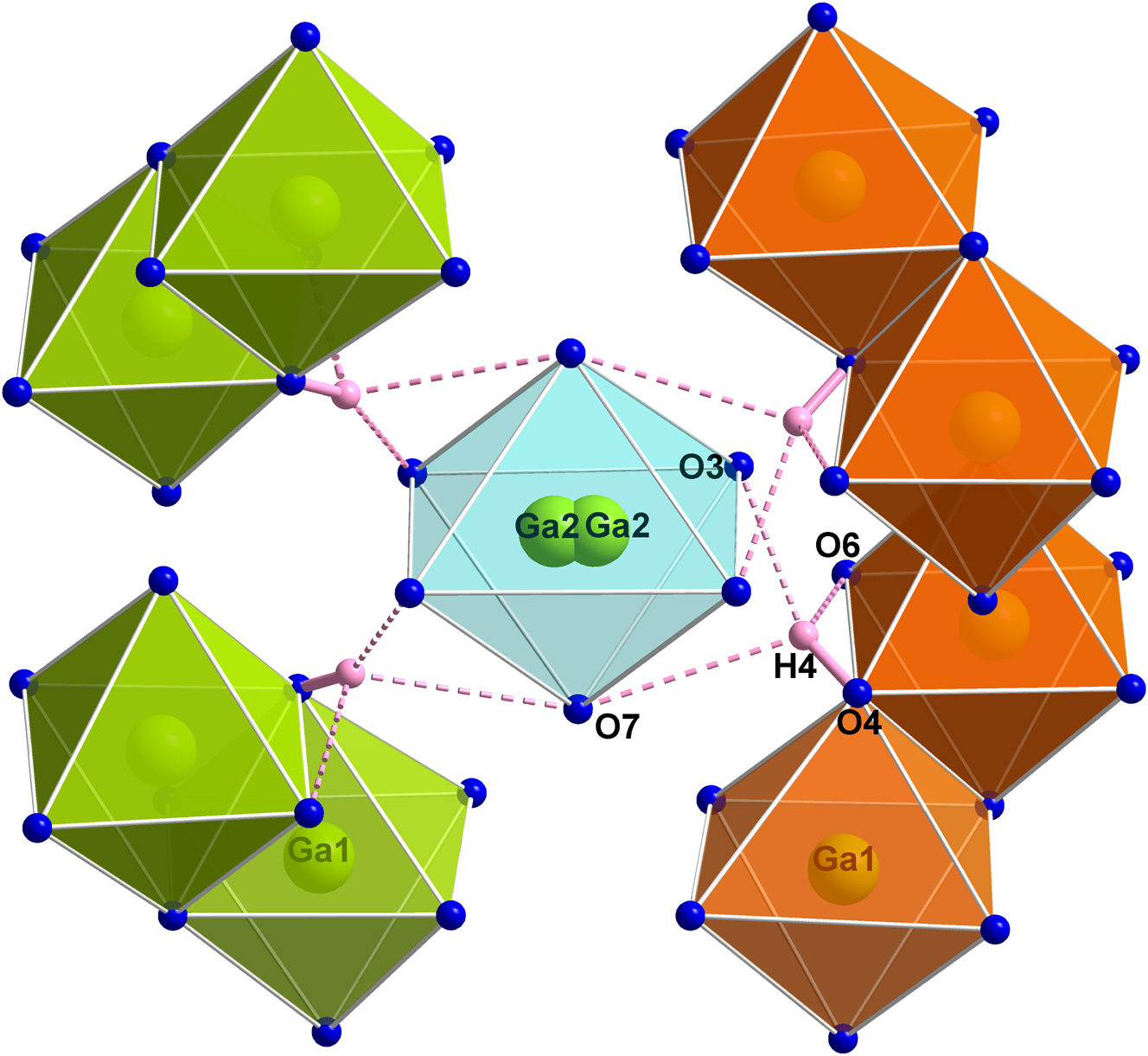
Visualization of the hydrogen bonds in the homeotypic structure Ga5B12O25(OH), 2 which may be similarly arranged in the title compound. O4 represents the donor atom and O3, O6, and O7 the acceptor atoms. Statistically only about 12 % of the four hydrogen bonds and one of the two Ga2 atoms depicted are present at the same time. The same color code as in Figure 2 is used.
The B–O network has a complex arrangement of 96 corner-sharing [BO4] tetrahedra. Four tetrahedrally arranged [B3O9] units form a [B12O30] unit and a cave is formed within three- and six-membered rings containing the Na+ cation in its centre, the assembly thus featuring a [Na1O12] cuboctahedron (see Figure 3).
The Ga1–O distances of the edge-sharing double-octahedra are in the range between 1.9163(5) and 2.0326(6) Å (see Figure 5(a)) with an average value of 1.96 Å, which is in accordance with typical Ga–O distances in borates. 2 , 17 , 18 , 19 However, the Ga2–O bond lengths, which are ranging from 1.9908(8) to 2.1066(7) Å (see Figure 5(b)) with an average value of 2.07 Å, are peculiarly long. Nevertheless, extraordinarily long M2–O distances have been observed in all the other known homeotypic phases 1 , 2 , 3 , 4 , 5 with the exception of Ga4InB12O25(OH), 3 where the larger In atom shows common In–O bond lengths. Furthermore, in Table 4 the comparison of the distances to the values of the previously published homeotypic structure Ga5B12O25(OH) 2 shows that they are in good agreement. The majority of the values as well as the lattice parameters of Ga5Na1–x B12O26–x (OH) x (x = 0.12) are slightly shorter than those of Ga5B12O25(OH), 2 which may be due to the lower measurement temperature (see Table 1). The Na–O distances are in the range between 2.5292(5) and 2.5711(6) Å (see Figure 5(c)) with a mean value of 2.55 Å, which corresponds to values in the literature of 2.65 Å for NaMn7O12 20 and 2.94 Å for NaTi8O13, 21 if one considers that the size of the cuboctahedral void predominantly depends on the surrounding framework. The effective ionic radius for Na+ in 12-fold coordination was estimated at 1.53 Å by Shannon in 1976. 22 Compared to the smallest diameter of the cuboctahedral void of the title compound of about 5.1 Å between two O atoms, there is sufficient space for Na+, giving rise to only in a minimal distortion. The B–O distances are in the range between 1.4624(9) and 1.5099(9) Å with average values of 1.48 Å for B1–O and B2–O, and 1.49 Å for B3–O. These values are in accordance with the mean value of 1.476(35) Å for B–O distances in BO4 tetrahedra 23 and with values commonly found in borates. 24 , 25 , 26
![Figure 5:
Oak Ridge Thermal Ellipsoid Plot Diagram (Ortep)-type representation of [Ga12O10] (a) and [Ga2O6] octahedra (b) and [Na1O12] cuboctahedra (c) in Ga5Na1–x
B12O26–x
(OH)
x
(x = 0.12) with displacement ellipsoids at the 99.99 % probability level. Bond lengths are depicted in Å. The values are shown here without standard deviation, but can be completely found in Table 4.](/document/doi/10.1515/znb-2025-0005/asset/graphic/j_znb-2025-0005_fig_005.jpg)
Oak Ridge Thermal Ellipsoid Plot Diagram (Ortep)-type representation of [Ga12O10] (a) and [Ga2O6] octahedra (b) and [Na1O12] cuboctahedra (c) in Ga5Na1–x B12O26–x (OH) x (x = 0.12) with displacement ellipsoids at the 99.99 % probability level. Bond lengths are depicted in Å. The values are shown here without standard deviation, but can be completely found in Table 4.
Interatomic distances (Å) in Ga5Na1–x B12O26–x (OH) x (x = 0.12; present study) compared to published data of the homeotypic structure Ga5B12O25(OH) 2 (standard deviations in parentheses). O4a and O4b, as well as O3a and O3b, respectively, are crystallographically identical, but are distinguished in this table, if the distances are different.
| Ga5Na1–x B12O26–x (OH) x (x = 0.12) | Ga5B12O25(OH) 2 | Ga5Na1–x B12O26–x (OH) x (x = 0.12) | Ga5B12O25(OH) 2 | |||||
|---|---|---|---|---|---|---|---|---|
| Ga1– | O1 | 1.9689(6) | 1.952(2) | B1– | O1 | 1.5091(9) | 1.503(2) | |
| O2 | 2.0326(6) | 2.065(2) | O2 | 1.4624(9) | 1.475(2) | |||
| O4a | 1.8999(6) | 1.930(2) | O6 | 1.4863(9) | 1.481(2) | |||
| O4b | 1.9326(5) | 1.983(2) | O7 | 1.4684(8) | 1.482(2) | |||
| O5 | 1.9163(5) | 1.909(2) | ØB1–O | 1.48 | 1.49 | |||
| O6 | 2.0140(6) | 2.001(2) | ||||||
| ØGa1–O | 1.96 | 1.97 | B2– | O2 | 1.4845(9) | 1.497(2) | ||
| O3 | 1.4799(9) | 1.490(2) | ||||||
| Ga2– | O3 | 2.1066(7) 4 × | O3a | 2.003(2) 2 × | O5 | 1.4898(9) | 1.478(2) | |
| O3b | 2.307(2) 2 × | O6 | 1.4832(9) | 1.482(2) | ||||
| O7 | 1.9908(8) 2 × | 1.988(2) 2 × | ØB2–O | 1.48 | 1.49 | |||
| ØGa2–O | 2.07 | 2.10 | ||||||
| B3– | O1 | 1.4950(9) | 1.480(2) | |||||
| Na1– | O1 | 2.5564(5) 4 × | O3 | 1.5099(9) | 1.514(2) | |||
| O5 | 2.5711(6) 4 × | O4 | 1.4269(9) | 1.463(2) | ||||
| O6 | 2.5292(5) 4 × | O5 | 1.5084(9) | 1.489(2) | ||||
| ØNa1–O | 2.55 | ØB3–O | 1.49 | 1.49 |
-
Mean values of distances are written in bold.
The angles can be found in Table 5, where they are compared to those in Ga5B12O25(OH), 2 where very similar values have been observed. Furthermore, the mean values for the octahedra show only a minimal deviation from the regular values of 90°, 180°, and those for the Na1 cuboctahedron with 60° and 120°. Individual angle values deviate within acceptable limits.
Interatomic angles (deg) in Ga5Na1–x B12O26–x (OH) x (x = 0.12; present study) compared to reported data of the homeotypic structure Ga5B12O25(OH). 2 Standard deviations are specified in parentheses. Atoms labeled a, b, c, and d are crystallographically identical, but are distinguished in this table, if the angles are different.
| Ga5Na1–x B12O26–x (OH) x (x = 0.12) | Ga5B12O25(OH) 2 | Ga5Na1–x B12O26–x (OH) x (x = 0.12) | Ga5B12O25(OH) 2 | ||
|---|---|---|---|---|---|
| O1–Ga1–O2 | 98.98(2) | 98.92(4) | O2–B2–O3 | 110.11(6) | 109.5(2) |
| O1–Ga1–O4a | 92.61(2) | 92.51(4) | O2–B2–O5 | 109.20(6) | 108.6(2) |
| O1–Ga1–O5 | 90.73(2) | 93.23(4) | O2–B2–O6 | 111.65(6) | 112.0(2) |
| O1–Ga1–O6 | 84.78(2) | 87.04(4) | O3–B2–O5 | 108.77(6) | 108.8(2) |
| O2–Ga1–O4b | 86.84(2) | 85.18(4) | O3–B2–O6 | 111.50(6) | 110.4(2) |
| O2–Ga1–O4a | 89.22(2) | 88.14(4) | O5–B2–O6 | 105.45(6) | 107.5(2) |
| O2–Ga1–O5 | 92.60(2) | 91.64(4) | ØO–B2–O | 109.4 | 109.5 |
| O4a–Ga1–O4b | 84.90(2) | 83.57(4) | |||
| O4b–Ga1–O5 | 91.53(2) | 90.65(4) | O1–B3–O3 | 108.86(6) | 110.1(2) |
| O4b–Ga1–O6 | 89.40(2) | 88.75(4) | O1–B3–O4 | 108.85(6) | 107.9(2) |
| O4a–Ga1–O6 | 90.47(2) | 89.82(4) | O1–B3–O5 | 104.66(5) | 107.5(2) |
| O5–Ga1–O6 | 87.47(2) | 89.80(4) | O3–B3–O4 | 113.78(6) | 111.8(2) |
| ØO–Ga1–O 90 | 90.0 | 89.9 | O3–B3–O5 | 110.24(5) | 110.3(2) |
| O4–B3–O5 | 110.05(6) | 109.1(2) | |||
| O1–Ga1–O4b | 173.66(2) | 174.26(4) | ØO–B3–O | 109.4 | 109.5 |
| O2–Ga1–O6 | 176.24(2) | 173.78(4) | |||
| O4a–Ga1–O5 | 175.90(2) | 174.22(4) | O1–Na1–O1 | 89.15(2) 2 × | |
| ØO–Ga1–O 180 | 175.3 | 174.1 | O1–Na1–O6 | 90.33(2) 4 × | |
| O5–Na1–O5 | 90.211(1) 4 × | ||||
| O3a–Ga2–O3b | 81.80(3) 2 × | 88.67(6) | O6–Na1–O6 | 90.59(2) 2 × | |
| O3c–Ga2–O3d | 74.73(5) | ØO–Na1–O 90 | 90.1 | ||
| O3b–Ga2–O3c | 98.22(3) 2 × | 98.31(5) 2 × | |||
| O3a–Ga2–O7 | 90.81(2) 4 × | 94.55(3) 2 × | O1–Na1–O1 | 120.49(2) 4 × | |
| O3b–Ga2–O7 | 93.71(3) 2 × | O1–Na1–O5a | 118.92(2) 4 × | ||
| O3c–Ga2–O7 | 89.19(2) 4 × | 85.78(3) 2 × | O1–Na1–O5b | 120.33(2) 4 × | |
| O3d–Ga2–O7 | 85.04(3) 2 × | O5–Na1–O6a | 118.97(2) 2 × | ||
| ØO–Ga2–O 90 | 90.0 | 89.8 | O5–Na1–O6b | 118.98(2) 2 × | |
| O5–Na1–O6c | 121.16(2) 4 × | ||||
| O3a–Ga2–O3c | 178.37(3) | 172.99(3) | O6–Na1–O6 | 119.66(2) 4 × | |
| O3b–Ga2–O3d | 178.37(3) | 172.99(3) | |||
| O7–Ga2–O7 | 180.0 | 168.44(4) | ØO–Na1–O 120 | 119.9 | |
| ØO–Ga2–O 180 | 178.9 | 171.5 | |||
| O1–Na1–O5a | 55.24(2) 4 × | ||||
| O1–B1–O2 | 112.59(6) | 111.4(2) | O1–Na1–O5b | 65.26(2) 4 × | |
| O1–B1–O6 | 105.60(6) | 107.2(2) | O1–Na1–O6a | 55.96(2) 4 × | |
| O1–B1–O7 | 108.58(5) | 108.81(9) | O1–Na1–O6b | 63.74(2) 4 × | |
| O2–B1–O6 | 111.31(6) | 110.8(2) | O5–Na1–O6a | 55.26(2) 3 × | |
| O2–B1–O7 | 106.89(6) | 106.6(2) | O5–Na1–O6b | 55.27(2) | |
| O6–B1–O7 | 111.92(5) | 112.0(2) | O5–Na1–O6c | 64.40(2) 4 × | |
| ØO–B1–O | 109.5 | 109.5 | ØO–Na1–O 60 | 60.0 | |
| O1–Na1–O6 | 175.17(2) 4 × | ||||
| O5–Na1–O5 | 173.04(2) 2 × | ||||
| ØO–Na1–O 180 | 174.5 |
-
Angle mean values are written in bold.
To further confirm the structure refinement, calculations of the bond-length/bond-strength values 27 (BLBS, see Table 6), and the charge distribution 28 (CHARDI, see Table 6) were performed. For this purpose, a full occupation of Na1 had to be set, because at a specific position inside the structure there is only the possibility of a full Na atom or none. According to the CHARDI-concept, 28 only minimal deviations (<±1.7 %) of the calculated values compared to the regular values of +3 for Ga and B, +1 for Na, and −2 for O were observed. According to the BLBS-concept, 27 most values also have only a slight deviation from the regular values (≤±8 %), but Ga2 and Na1 do not fit well, because of comparatively long bond lengths. However, compared to the homeotypic compounds, the long Ga2–O distances are indeed to be expected to be characteristic for this structure type 1 , 2 , 3 , 4 , 5 and the Na1–O distances may depend on the surrounding framework. Therefore, the very good values according to the CHARDI 28 concept may be more crucial, due to its consideration of the spatial arrangement of atoms and their electron configuration.
Charge distribution in Ga5Na1–x B12O26–x (OH) x (x = 0.12) according to the bond-length/bond-strength 27 (ΣV) and CHARDI 28 (ΣQ) concept. Values with a deviation from the regular values >±8 % are written in italics.
| Ga1 | Ga2 | B1 | B2 | B3 | O1 | O2 | O3 | O4 | O5 | O6 | O7 | Na1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ΣV | 3.24 | 2.43 | 2.97 | 2.94 | 2.95 | −2.06 | −1.96 | −1.79 | −2.07 | −2.15 | −2.08 | −2.03 | 1.59 |
| ΣQ | 2.97 | 3.00 | 2.97 | 3.05 | 3.01 | −2.01 | −2.00 | −1.99 | −2.00 | −2.00 | −2.00 | −2.00 | 1.00 |
3.2 IR spectroscopy
Figure 6 shows a FT-IR spectrum of a single crystal of Ga5Na1–x B12O26–x (OH) x (x = 0.12) in comparison to published data of the homeotypic phase Ga5B12O25(OH) 2 in the spectral region of 600–4,000 cm−1. In the fingerprint region up to ∼1,400 cm−1, the peaks can be explained by vibrations of M–O, B–O or various overlapping combinations. Specifically, the stretching modes of [BO4] tetrahedra are typically observed between 850 and 1,100 cm−1, 29 the characteristic frequencies of [AlO6] octahedra between 750 and 400 cm−1, 30 which are both present in the experimental spectrum. The position of an O–H stretching vibration was observed in the homeotypic structure Ga5B12O25(OH) 2 at around 3,000 cm−1. If we consider the small amount of O–H in Ga5Na1–x B12O26–x (OH) x (x = 0.12) based on the single-crystal data, only a weak peak is expected, which seems to be present in the FT-IR spectrum.
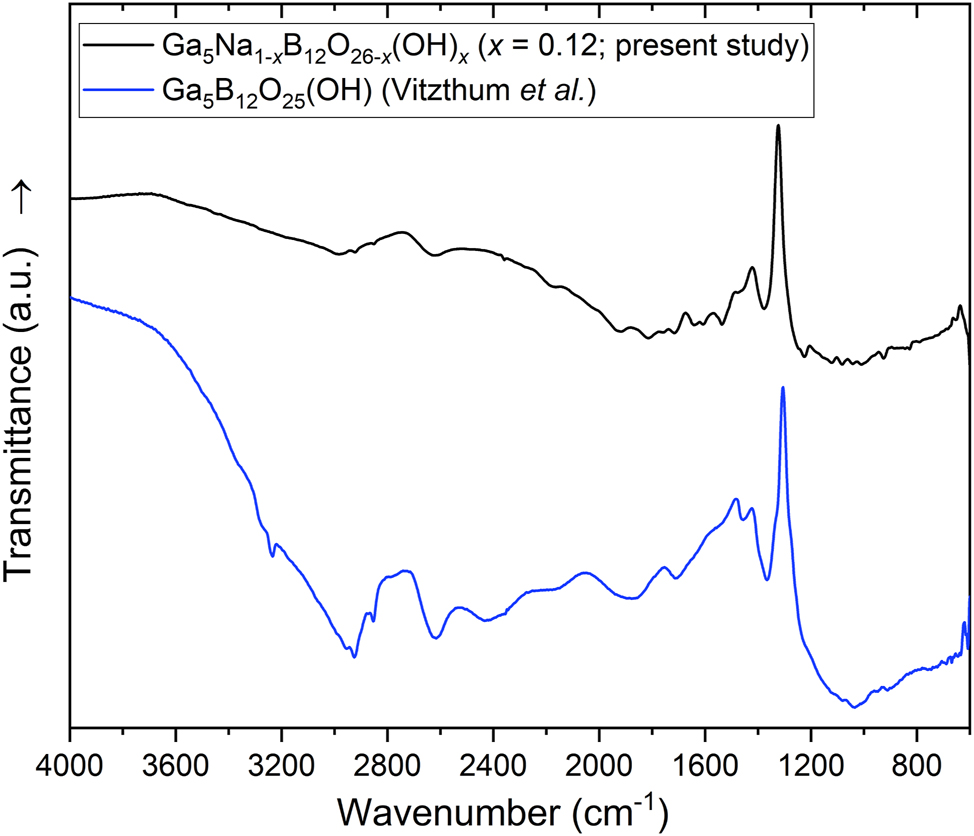
Single-crystal IR spectrum of Ga5Na1–x B12O26–x (OH) x (x = 0.12) (black) compared to published data of the homeotypic structure Ga5B12O25(OH) 2 (blue) in the spectral region of 600–4,000 cm−1 (both experimental data).
3.3 Energy-dispersive X-ray spectroscopy
The results of a semi-quantitative energy-dispersive X-ray (EDX) spectroscopy study confirmed the presence and approximate quantity (within 3 standard deviations or less) of all the elements identified by single-crystal X-ray diffraction with the exception of H, which cannot be detected with this method. Especially the measured sodium content of 1.9(3) at% (averaged from 14 point measurements) is in good agreement with the expected value of 2.0 at%. Figure 7 shows a representative crystal of the title compound, which was obtained by a scanning electron microscope (SEM).
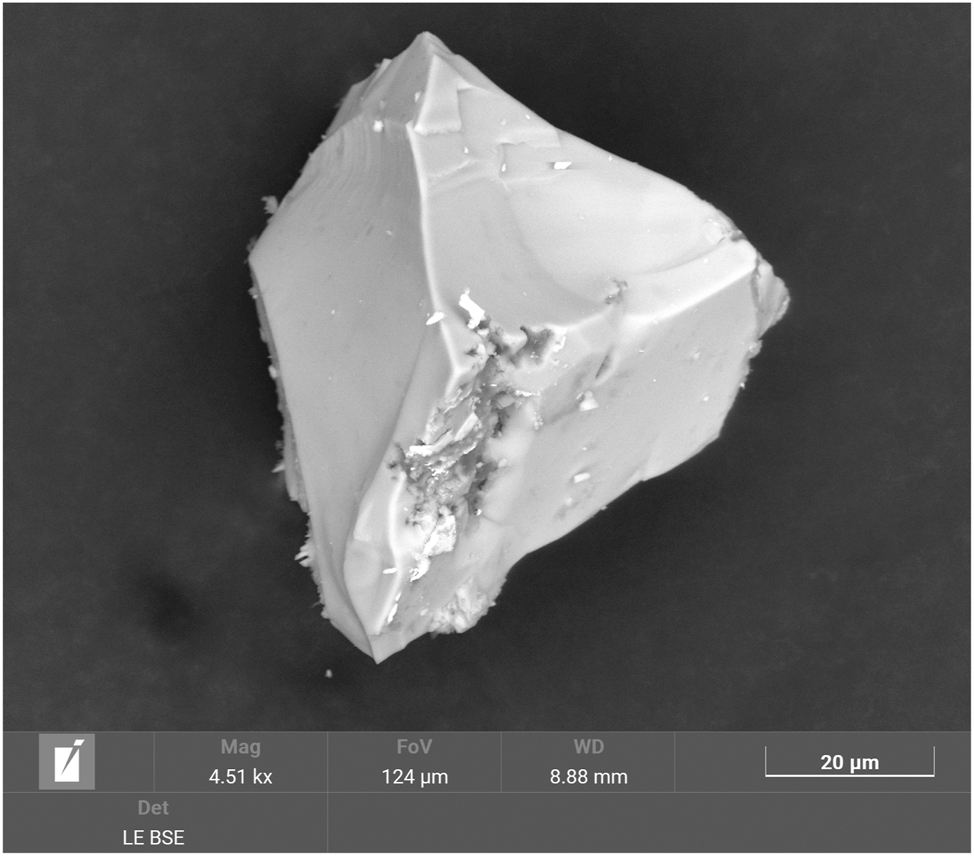
Scanning electron microscope (SEM) image of a representative crystal of Ga5Na1–x B12O26–x (OH) x .
4 Conclusions
Tetragonal Ga5Na1–x B12O26–x (OH) x (x = 0.12) was synthesized in a high-pressure/high-temperature experiment in a multianvil press. Its structure could be determined by single-crystal X-ray diffraction, which revealed single- and double-octahedral and cuboctahedral units centered by the metal cations and a complex network of [BO4] tetrahedra. The presence of hydroxyl groups could be confirmed by IR spectroscopy. EDX investigations confirmed the presence of the elements Ga, B, O, and Na.
Acknowledgements
The authors thank Assoc.-Prof. Dr. Gunter Heymann for recording of the single-crystal data and Dr. Daniela Vitzthum for the IR data of Ga5B12O25(OH). I. W. gratefully acknowledges the Vice Rector for Research for the grant of a doctoral fellowship at the University of Innsbruck.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: ChatGPT and Google Translate were used to improve language when required.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: The raw data can be obtained on request from the corresponding author. CSD-2376207 contains the supplementary crystallographic data for this paper. The data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif.
References
1. Haberer, A.; Huppertz, H. J. Solid State Chem. 2009, 182, 484–490; https://doi.org/10.1016/j.jssc.2008.11.022.Suche in Google Scholar
2. Vitzthum, D.; Wurst, K.; Pann, J. M.; Brüggeller, P.; Seibald, M.; Huppertz, H. Angew. Chem. Int. Ed. 2018, 57, 11451–11455; https://doi.org/10.1002/anie.201804083.Suche in Google Scholar PubMed PubMed Central
3. Vitzthum, D.; Wimmer, D. S.; Widmann, I.; Huppertz, H. Z. Naturforsch. 2020, 75b, 605–613; https://doi.org/10.1515/znb-2020-0075.Suche in Google Scholar
4. Pasqualini, L. C.; Tribus, M.; Huppertz, H. Z. Naturforsch. 2024, 79b, 39–49.10.1515/znb-2023-0082Suche in Google Scholar
5. Somasundaram, J. D.; Pasqualini, L. C.; Krivosudský, L.; Huppertz, H. Z. Naturforsch. 2024, 79b, 497–505.10.1515/znb-2024-0054Suche in Google Scholar
6. Vitzthum, D.; Widmann, I.; Wimmer, D. S.; Wurst, K.; Joachim-Mrosko, B.; Huppertz, H. Eur. J. Inorg. Chem. 2021, 1165–1174; https://doi.org/10.1002/ejic.202001136.Suche in Google Scholar
7. Huppertz, H. Z. Kristallogr. 2004, 219, 330–338; https://doi.org/10.1524/zkri.219.6.330.34633.Suche in Google Scholar
8. Walker, D.; Carpenter, M. A.; Hitch, C. M. Am. Mineral. 1990, 75, 1020–1028.Suche in Google Scholar
9. Walker, D. Am. Mineral. 1991, 76, 1092–1100.10.1007/978-1-4615-3968-1_10Suche in Google Scholar
10. Saint , Area Detector Control and Integration Software, Bruker AXS Inc.: Madison, Wisconsin (USA), 2021.Suche in Google Scholar
11. Apex4 Data Reduction and Frame Integration Program for the CCD Area-Detector System; Bruker AXS Inc.: Madison, Wisconsin (USA), 2021.Suche in Google Scholar
12. Sheldrick, G. M. Sadabs (version 2016). Bruker AXS Inc.: Madison, Wisconsin (USA), 2016.Suche in Google Scholar
13. Sheldrick, G. M. Acta Crystallogr. 2015, A71, 3–8.10.1107/S2053273314026370Suche in Google Scholar PubMed PubMed Central
14. Sheldrick, G. M. Acta Crystallogr. 2015, C71, 3–8.Suche in Google Scholar
15. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
16. Opus , Bruker Corporation: Billerica, Massachusetts (USA), 2012.Suche in Google Scholar
17. Vitzthum, D.; Schauperl, M.; Strabler, C. M.; Brüggeller, P.; Liedl, K. R.; Griesser, U. J.; Huppertz, H. Inorg. Chem. 2016, 55, 676–681; https://doi.org/10.1021/acs.inorgchem.5b02027.Suche in Google Scholar PubMed
18. Vitzthum, D.; Hering, S. A.; Perfler, L.; Huppertz, H. Z. Naturforsch. 2015, 70b, 207–214.10.1515/znb-2015-0015Suche in Google Scholar
19. Gao, W.; Jing, Y.; Yang, J.; Zhou, Z.; Yang, D.; Sun, J.; Lin, J.; Cong, R.; Yang, T. Inorg. Chem. 2014, 53, 2364–2366; https://doi.org/10.1021/ic403175w.Suche in Google Scholar PubMed
20. Marezio, M.; Dernier, P. D.; Chenavas, J.; Joubert, J. C. J. Solid State Chem. 1973, 6, 16–20; https://doi.org/10.1016/0022-4596(73)90200-4.Suche in Google Scholar
21. Akimoto, J.; Takei, H. J. Solid State Chem. 1991, 90, 147–154; https://doi.org/10.1016/0022-4596(91)90180-p.Suche in Google Scholar
22. Shannon, R. Acta Crystallogr. 1976, A32, 751–767.10.1107/S0567739476001551Suche in Google Scholar
23. Zobetz, E. Z. Kristallogr. 1990, 191, 45–57; https://doi.org/10.1524/zkri.1990.191.1-2.45.Suche in Google Scholar
24. Widmann, I.; Kinik, G.; Jähnig, M.; Glaum, R.; Schwarz, M.; Wüstefeld, C.; Johrendt, D.; Tribus, M.; Hejny, C.; Bayarjargal, L.; Dubrovinsky, L.; Heymann, G.; Suta, M.; Huppertz, H. Adv. Funct. Mater. 2024, 34 (9), 2400054; https://doi.org/10.1002/adfm.202400054.Suche in Google Scholar
25. Vitzthum, D.; Widmann, I.; Plank, M.; Joachim-Mrosko, B.; Huppertz, H. Z. Naturforsch. 2020, 75b, 975–981; https://doi.org/10.1515/znb-2020-0151.Suche in Google Scholar
26. Teichtmeister, T. A.; Widmann, I.; Bayarjargal, L.; Tribus, M.; Heymann, G.; Wurst, K.; Huppertz, H. Eur. J. Inorg. Chem. 2024, 27, e202300555; https://doi.org/10.1002/ejic.202300555.Suche in Google Scholar
27. Brown, I. D.; Altermatt, D. Acta Crystallogr. 1985, B41, 244–247.10.1107/S0108768185002063Suche in Google Scholar
28. Hoppe, R. Z. Kristallogr. 1979, 150, 23–52; https://doi.org/10.1524/zkri.1979.150.14.23.Suche in Google Scholar
29. Ross, S. D. Spectrochim. Acta, Part A 1972, 28, 1555–1561; https://doi.org/10.1016/0584-8539(72)80126-0.Suche in Google Scholar
30. Tarte, P. Spectrochim. Acta, Part A 1967, 23, 2127–2143; https://doi.org/10.1016/0584-8539(67)80100-4.Suche in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- In this issue
- Research Articles
- Chemical aspects of the preparation of Vulcanised Fibre using zinc chloride hydrates: from a brief history to a new consideration of the key reactions in ionic liquids
- On the preparation and spectral properties of some hydroxy-substituted triphenylmethane dyes
- High-pressure synthesis and crystal structure of Ga5Na1–x B12O26–x (OH) x (x = 0.12)
- Intermetallic phases with Ho4Ir13Ge9-type structure
- Interplay between oxidative addition and reductive elimination at a diruthenium complex bearing the bis(diphenylphosphanyl)amine ligand
- Anthracene-d- and l-phenylalanine derivatives: synthesis, dual-state emission, mechanochromic luminescence, chiroptical property and enantioselective recognition of free amino acids
- Synthesis, structures and optical properties of 3,3′-disubstituted biphenyl compounds
Artikel in diesem Heft
- Frontmatter
- In this issue
- Research Articles
- Chemical aspects of the preparation of Vulcanised Fibre using zinc chloride hydrates: from a brief history to a new consideration of the key reactions in ionic liquids
- On the preparation and spectral properties of some hydroxy-substituted triphenylmethane dyes
- High-pressure synthesis and crystal structure of Ga5Na1–x B12O26–x (OH) x (x = 0.12)
- Intermetallic phases with Ho4Ir13Ge9-type structure
- Interplay between oxidative addition and reductive elimination at a diruthenium complex bearing the bis(diphenylphosphanyl)amine ligand
- Anthracene-d- and l-phenylalanine derivatives: synthesis, dual-state emission, mechanochromic luminescence, chiroptical property and enantioselective recognition of free amino acids
- Synthesis, structures and optical properties of 3,3′-disubstituted biphenyl compounds

