Abstract
A new polyoxometalate (POM)-based inorganic–organic hybrid compound, [Ag2(bib)3][H2SiW12O40] (1), has been hydrothermally synthesized and characterized by routine methods. Single-crystal X-ray diffraction analysis reveals that inorganic supramolecular chains constructed of POM and [Ag2(bib)3] subunits are fused together via the Ag cations forming a 3D supramolecular structure. The structure possesses the moganite-type topology. The photocatalytic properties have been investigated through the oxidative decomposition of Rhodamine-B dye.
1 Introduction
Supramolecular chemistry investigates intermolecular interactions for determining the packing of molecules in order to design new solid materials with desired physical and chemical properties [1–3]. One of the goals is to recognize and develop synthons that are robust enough to be exchanged from one network to another [4, 5]. Polyoxometalates (POMs), as one kind of well-defined metal oxide clusters with nanosizes, variable topologies and great potential applications [6–9], are widely used as inorganic synthons to construct hybrid compounds with desired properties. Recently POM-based inorganic–organic hybrid compounds have emerged as an intriguing class of hybrid crystalline materials with highly diversified architectures and properties as well as potential applications as, e.g., in catalysis [10–16]. Owing to their reversible redox properties, strong Lewis and Brønsted acidity and high density of catalytically active sites, POM-based materials have been utilized as efficient electron-transfer catalysts and solid-acid catalysts for diverse organic reactions in industry since the late 1970s [17–21]. Among the POM catalysts, the Keggin species [XM12O40]n− (X = B, P, Si, etc.; M = Mo and W) are one of the most important components, which lead to a significantly higher catalytic activity [22]. However, many inherent drawbacks, such as a small specific surface area (<10 m2 g−1), high solubility and low stability [23–27], limit the applications in solid catalysts. In order to overcome these deficiencies, immobilization of POMs in solid substrates is an effective strategy [28–30]. Although this way can avoid the shortcomings of catalysts, it still faces other problems, including ill-defined structures, non-uniform sites, POM leaching and low POM loading. So the design and synthesis of POM-based catalysts with specific catalytic properties has been the core issue of current catalysis chemistry.
We chose Keggin POMs, the N-heterocyclic ligand 1,4-bis(1-imidazole)benzene (hereafter denoted as bib) (Scheme 1) and Ag salt to build a new POM-based supramolecular compound. The POM-based compound, [Ag2(bib)3][H2SiW12O40] (1), which exhibits a supramolecular 3D structure with moganite-type (mog) topology, is presented.
The 1,4-bis(1-imidazole)benzene (bib) ligand used in this work.
2 Results and discussion
As described in the experimental section, the use of NH4VO3 was necessary for the successful isolation of the compound, although it was not contained in the final product. NH4VO3 may play a role as mineralizer. All tungsten atoms are in the +6 oxidation state, as confirmed by charge neutrality, coordination environments and valence sum calculations [31]. Similar to the case of [Ag2(3atrz)2]2[(HPMo12O40)] (3atrz = 3-amino-1,2,4-triazole) [32], to balance the charge of the compound, protons have to be added. The network of the compound was analyzed by using the Topos3.2 program package [33].
2.1 Crystal structure of the title compound
Single crystal X-ray structural analysis reveals that the compound consists of one [SiW12O40]4− (abbreviated to SiW12) polyoxoanion, two Ag cations and three bib molecules (Fig. 1). Similar to other Keggin anions, the SiW12 polyoxoanion includes a SiO4 tetrahedron surrounded by 12 WO6 octahedra, which are grouped into four triads {W3O13} in edge-sharing mode. Oxygen atoms in the polyoxoanion are divided into four groups according to the different coordination environments: Ot (terminal oxygen atoms connecting to one W atom), Ob (oxygen atoms located in the corners shared by two W3O13 units), Oc (oxygen atoms connecting edge-sharing WO6 octahedra in the W3O13 unit) and Oa (oxygen atoms connecting the center Si and W atoms of a W3O13 unit). The Si–O distances and relevant W–O bonds all fall in the normal ranges. The Ag centers adopt a linear coordination mode with two N atoms (N1 and N4) of different bib molecules. The bond lengths are 2.14(1) and 2.15(1) Å for Ag–N1 and Ag–N4, respectively. As a result, an isolated [Ag2(bib)3] subunit is formed (Fig. 1).
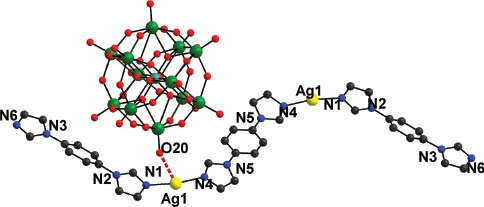
Ball-and-stick representation of the asymmetric unit of the title compound. All H atoms are omitted for clarity.
The Ag centers and POMs link each other forming chains (POM-Ag2)n with much longer and, hence, weaker contacts Ag–O14 3.02 Å and Ag–O20 3.00 Å as shown in Fig. 2. Further, the chains are thus connected via [Ag2(bib)3] subunits forming an inorganic–organic hybrid layer (Fig. 3). Finally, the layers are linked together along the b axis via short interactions between Ag and N4 forming the 3D supramolecular frameworks. From the topology view, if we assign the Ag centers and {SiW12} clusters as 4-connected nodes, and the bib molecules as connectors, the structure can be rationalized as a 4-connected 2-nodal framework with mog topology (Fig. 4).
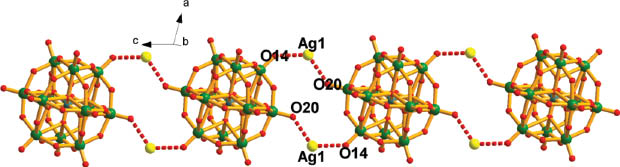
The inorganic chain formed by Ag centers and SiW12 clusters.
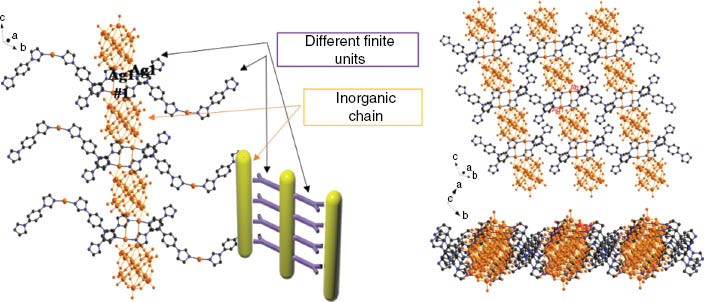
Structure of a layer via Ag ions of inorganic chains from different finite units.
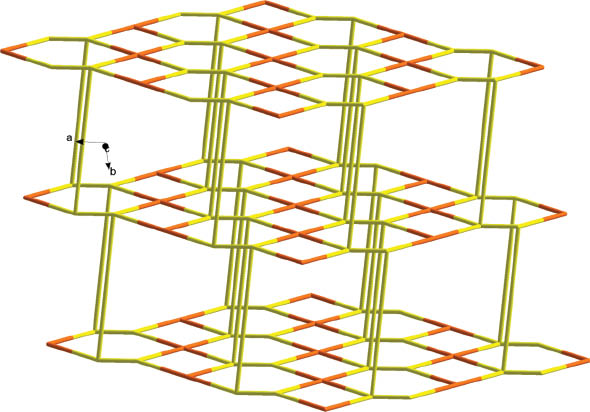
Topology of the 3D supramolecular structure in the title compound (yellow = Ag; orange = POM).
2.2 IR spectrum and TG analysis
The IR spectrum is shown in Fig. 5. Characteristic bands at 968, 922 and 782 cm−1 are attributed to ν(W=O), ν(Si–O) and ν(W–O–W) vibrations, respectively. Bands in the region of 1635–1299 cm−1 are attributed to the organic ligands. The TG curve (Fig. 6) exhibits a weight loss in the temperature range of 40–800 °C. The weight loss of 16.9 % at 40–740 °C corresponds to the loss of bib, agreeing well with the calculated value of 16.9 % for three bib molecules per empirical formula unit. This result further confirms the constitution of compound 1.
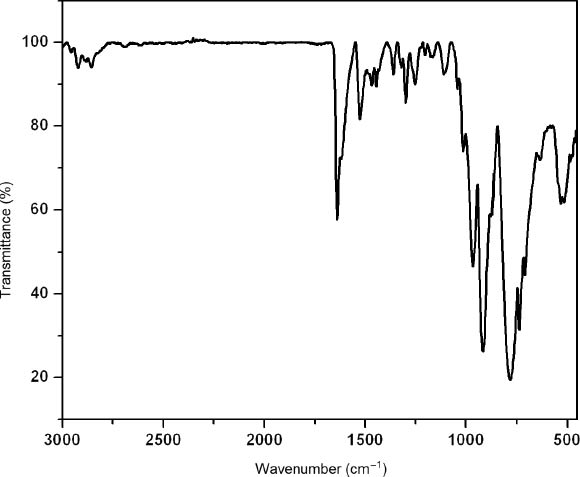
The IR spectrum of the title compound.
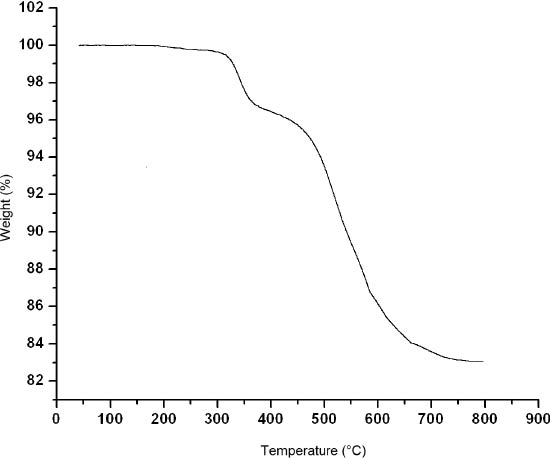
The TG curve of the title compound.
2.3 Photocatalysis properties
The photocatalytic properties of POMs have attracted much attention aiming at potential applications in purifying air and water [34, 35]. The introduction of transition-metal complexes as functional groups into POMs can enrich their potential applications. Herein, we investigated the photocatalytic activities of the new compound as a catalyst. The photodecomposition of rhodamine-B was evaluated under UV light irradiation in a typical process: 50 mg of the powder was distributed in 100 mL of a 2.0 × 10−5 mol L−1 (C0) rhodamine-B solution in a beaker by ultrasonic dispersion for 10 min. The mixture was continuously stirred in the dark for 0.5 h till it reached the surface-adsorption equilibrium on the particles. The suspension was stirred continuously under ultraviolet irradiation from a 250 W high-pressure Hg lamp. After 0, 30, 60, 90, 120, 150 and 180 min, 3 mL samples were taken out and subjected to several centrifugations to remove the catalyst. This way clear solutions were obtained which were subjected to UV/Vis analysis.
As shown in Fig. 7, after irradiating compound 1 for 180 min, the photocatalytic decomposition rate, defined as 1 − C/C0, is 56.9 %. In contrast, the photocatalytic decomposition rate using insoluble (NBu4)4[SiW12O40] as a catalyst is 39.3 % after UV light irradiation of 180 min, which illustrates that the formation of an organic–inorganic hybrid compound based on POMs improves the photocatalytic performance of (NBu4)4[SiW12O40]. The enhanced photocatalytic activity may be due to the interaction between the [Ag2(bib)3] subunit and the SiW12 clusters acting as a photosensitizer and promote the transition of electrons to the POMs. In order to demonstrate that the title compound is stable enough for catalytic cycles, the catalyst was separated from the reaction mixture after the first catalytic run, washed several times with alcohol and water to remove physisorbed molecules, dried and reused in another catalytic cycle. A series of catalytic experiments (56.9 %, 55.8 %, 56.0 %, 55.2 % for 1–4 rounds, respectively) has suggested that the title compound, as a catalyst, is stable and retains its catalytic activity. The result indicates that the title compound presents better degradation activity and may be a potential photocatalyst to decompose organic dyes.
![Fig. 7: Evolution of UV/Vis absorption spectra after 180 min of illumination for the photodegradation of rhodamine-B by (NBu4)4[SiW12O40] (left) and compound 1 (right).](/document/doi/10.1515/znb-2014-0275/asset/graphic/j_znb-2014-0275_fig_007.jpg)
Evolution of UV/Vis absorption spectra after 180 min of illumination for the photodegradation of rhodamine-B by (NBu4)4[SiW12O40] (left) and compound 1 (right).
3 Experimental section
3.1 General procedures
All reagents were purchased commercially and used without further purification. H4SiW12O40, AgNO3, NH4VO3, NaOH (Sinopharm Chemical Reagent Co., Ltd, Shanghai, China), bib (Jinan Henghua Sci. & Technology, Co., Ltd, Shandong, China) were used as supplied. Elemental analyses were performed on a Perkin-Elmer 2400 CHN Elemental Analyzer (C, H and N) and a Leaman inductively coupled plasma spectrometer (Ag). The IR spectra were obtained on an Alpha Centaurt FT/IR spectrometer (Bruker corporation, Germany) with a KBr pellet in the 400–4000 cm−1region. The TG analyses were performed on a Perkin-Elmer TGA7 instrument (PerkinElmer, USA) in flowing N2 with a heating rate of 10 °C min−1. The UV/V is absorption spectra were recorded on a 756 CRT spectrophotometer (Shanghai Youke Instrument CO., Ltd., China).
3.2 Preparation of [Ag2(bib)3][H2SiW12O40] (1)
A mixture of H4SiW12O40 (298 mg, 1 mmol), AgNO3 (150 mg, 1 mmol), bib (30 mg, 1.5 mmol), NH4VO3 (24 mg, 2.0 mmol) and H2O (10 mL) was stirred for 1 h in air. After the pH value was adjusted to about 3.5 with 1 m NaOH, the mixture was sealed into a 20 mL Teflon-lined autoclave and heated to 160 °C in 100 min. The autoclave was kept at 160 °C for 4 days and then slowly cooled to room temperature. Red block-shaped crystals of 1 were filtered, washed with water and dried at room temperature (32 % yield based on W). – Elemental analysis for C36H32N12Ag2SiW12O40 (3722): calcd. C 11.60, H 0.85, N 4.51, Ag 5.80; found C 11.57, H 0.93, N 4.49, Ag 5.79 %.
3.3 X-ray structure determination
Crystal data for the title compound were collected on a Bruker SMART-CCD diffractometer with monochromatized MoKα radiation (λ = 0.71069 Å) at 293 K. The structure was solved by the direct methods and refined by full-matrix least-squares methods on F2 using the Shelxtl crystallographic software package [36, 37]. All non-hydrogen atoms were refined anisotropically. During the refinement, the command ISOR was used to restrain the non-H atoms with anisotropy displacement parameters (ADP) and non-positive definite (NPD) problems, which led to 135 restraints for 1. The command ISOR was used to refined atoms O7, O9, O21 and O22. Additionally, the restraint command DELU was used to average the displacement parameters of atoms with the same environments along the bond. In this way the atoms C1, C2, C3, C16, C17, C18, N1, N2 and N5 were refined. Finally, the restraint command SIMU was used to average the displacement parameters of atoms with the same environments (atoms C1, C2, C3, C16, C17, C18, N1, N2 and N5). The positions of hydrogen atoms on carbon atoms were calculated theoretically. Crystallographic data are given in Table 1.
Crystallographic data for 1.
| Chemical formula | C36H32N12Ag2SiW12O40 |
| Formula weight | 3722.63 |
| Temperature, K | 293 |
| Crystal system | Triclinic |
| Space group | P1̅ (no. 2) |
| a, Å | 10.469(5) |
| b, Å | 12.264(5) |
| c, Å | 12.851(5) |
| α, deg | 104.812(5) |
| β, deg | 105.180(5) |
| γ, deg | 93.664(5) |
| V, Å3 | 1524.3(11) |
| Z | 1 |
| Density, g cm−3 | 4.05 |
| μ(MoKα), mm−1 | 23.2 |
| F(000), e | 1646 |
| Refl. collected/unique/Rint | 10 595/5374/0.0339 |
| Data/restraints/parameters | 5374/135/484 |
| Goodness-of-fit on F2 | 1.067 |
| Final R1/wR2 [I > 2σ(I)] | 0.0375/0.0743 |
| Final R1/wR2 (all data) | 0.0497/0.0802 |
| Δρfin (max/min), e Å−3 | 2.05/−2.42 |
| CCDC no. | 1034377 |
CCDC 1034377 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre viawww.ccdc.cam.ac.uk/data_request/cif.
4 Conclusions
In the paper, we report a new POM-based supramolecular compound by introducing an N-heterocyclic ligand 1,4-bis(1-imidazole)benzene into the POM, which exhibits a 3D supramolecular structure with the mog topology. Furthermore, the new compound exhibits good photocatalytic activities for the degradation of rhodamin-B dyes and may become a green photocatalyst for the reduction of environmental pollution.
Acknowledgments
The authors are grateful for the financial support from Heilongjiang Provincial Natural Science Foundation of China (No. B201422) and National Natural Science Foundation of China (No. 51473046).
References
[1] J. M. Lehn, Angew. Chem. Int. Ed. 1990, 29, 1304.10.1002/anie.199013041Suche in Google Scholar
[2] Z. M. Zhang, Y. G. Li, S. Yao, E. B. Wang, Y. H. Wang, R. Clérac, Angew. Chem., Int. Ed. 2009, 121, 1609.10.1002/ange.200805827Suche in Google Scholar
[3] P. Mal, B. Breiner, K. Rissanen, J. R. Nitschke, Science2009, 324, 1697.10.1126/science.1175313Suche in Google Scholar
[4] I. Dance, M. Scudder, J. Chem. Soc., Dalton Trans. 1996, 3755.10.1039/dt9960003755Suche in Google Scholar
[5] G. R. Desiraju, Chem. Commun. 1997, 1475.10.1039/a607149jSuche in Google Scholar
[6] C. L. Hill, C. M. Prosser-McCartha, Coord. Chem. Rev. 1995, 143, 407.10.1016/0010-8545(95)01141-BSuche in Google Scholar
[7] D. L. Long, E. Burkholder, L. Cronin, Chem. Soc. Rev. 2007, 36, 10.10.1039/B502666KSuche in Google Scholar
[8] Y. F. Song, H. Abbas, C. Ritchie, N. McMillian, D. L. Long, N. Gadegaard, L. Cronin, J. Mater. Chem. 2007, 17, 1903.10.1039/b617830hSuche in Google Scholar
[9] P. Huang, C. Qin, Z. M. Su, Y. Xing, X. L. Wang, K. Z. Shao, Y. Q. Lan, E. B. Wang, J. Am. Chem. Soc. 2012, 134, 14004.10.1021/ja303723uSuche in Google Scholar
[10] A. Dolbecq, E. Dumas, C. R. Mayer, P. Mialane, Chem. Rev. 2010, 110, 6009.10.1021/cr1000578Suche in Google Scholar
[11] N. V. Maksimchuk, K. A. Kovalenko, S. S. Arzumanov, Y. A. Chesalov, M. S. Melgunov, A. G. Stepanov, V. P. Fedin, O. A. Kholdeeva, Inorg. Chem. 2010, 49, 2920.10.1021/ic902459fSuche in Google Scholar
[12] Q. X. Han, C. He, M. Zhao, B. Qi, J. Y. Niu, C. Y. Duan, J. Am. Chem. Soc. 2013, 135, 10186.10.1021/ja401758cSuche in Google Scholar
[13] N. Mizuno, S. Hikichi, K. Yamaguchi, S. Uchida,Y. Nakagawa, K. Uehara, K. Kamata, Catal. Today2006, 117, 32.10.1016/j.cattod.2006.05.002Suche in Google Scholar
[14] C. Y. Sun, S. X. Liu, D. D. Liang, K. Z. Shao, Y. H. Ren, Z. M. Su, J. Am. Chem. Soc. 2009, 131, 1883.10.1021/ja807357rSuche in Google Scholar
[15] S. T. Zheng, J. Zhang, X. X. Li, W. H. Fang, G. Y. Yang, J. Am. Chem. Soc. 2010, 132, 15102.10.1021/ja105986bSuche in Google Scholar
[16] L. Zhou, J. Liu, W. B. Ji, H. Huang, H. L. Hu, Y. Liu, Z. H. Kang, J. Mater. Chem. A2014, 2, 12686.10.1039/C4TA02214ASuche in Google Scholar
[17] I. V. Kozhevnikov, Chem. Rev. 1998, 98, 171.10.1201/NOE0849321702-8Suche in Google Scholar
[18] M. Misono, N. Nojiri, Appl. Catal. 1990, 64, 1.10.1016/S0166-9834(00)81550-XSuche in Google Scholar
[19] Y. Izumi, Catal. Today1997, 33, 371.10.1016/S0920-5861(96)00165-4Suche in Google Scholar
[20] J. Y. Li, S. Z. Luo, J. P. Cheng, J. Org. Chem. 2009, 74, 1747.10.1021/jo802557pSuche in Google Scholar
[21] D. D. Liang, S. X. Liu, F. J. Ma, F. Wei, Y. G. Chen, Adv. Synth. Catal. 2011, 353, 733.10.1002/adsc.201000808Suche in Google Scholar
[22] T. Okuhara, N. Mizuno, M. Misono in Advances in Catalysis, Vol. 41 (Eds.: D. D. Eley, W. O. Haag, B. Gates), Academic Press, New York, 1996, pp. 113.10.1016/S0360-0564(08)60041-3Suche in Google Scholar
[23] Y. Guo, Y. Wang, C. Hu, Y. Wang, E. Wang, Chem. Mater. 2000, 12, 3501.Suche in Google Scholar
[24] G. Peng, Y. Wang, C. Hu, Appl. Catal. A2001, 218, 91.Suche in Google Scholar
[25] H. Salavati, N. Tavakkoli, M. Hosseinpoor, Ultrason. Sonochem. 2012, 19, 546.10.1016/j.ultsonch.2011.09.001Suche in Google Scholar
[26] C. C. Chen, X. Z. Li, W. H. Ma, J. C. Zhao, J. Phys. Chem. B2002, 106, 318.10.1021/jp0119025Suche in Google Scholar
[27] Y. H. Guo, C. W. Hu, J. Mol. Catal. A: Chem. 2007, 262, 136.10.1016/j.molcata.2006.08.039Suche in Google Scholar
[28] F. Lefebvre, Chem. Commun. 1992, 756, 756.10.1055/s-1992-26218Suche in Google Scholar
[29] C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli, J. S. Beck, Nature1992, 359, 710.10.1038/359710a0Suche in Google Scholar
[30] T. Blasco, A. Corma, A. Martinez, P. Martinez-Escolano, J. Catal. 1998, 177, 306.10.1006/jcat.1998.2105Suche in Google Scholar
[31] I. D. Brown, D. Altermatt, Acta Crystallogr. 1985, B41, 244.10.1107/S0108768185002051Suche in Google Scholar
[32] Q. G. Zhai, X. Y. Wu, S. M. Chen, Z. G. Zhao, C. Z. Lu, Inorg. Chem. 2007, 46, 5046.10.1021/ic700415wSuche in Google Scholar
[33] V. A. Blatov, A. P. Shevchenko, V. N. Serezhkin, J. Appl. Crystallogr. 2000, 33, 1193.10.1107/S0021889800007202Suche in Google Scholar
[34] M. Y. Dong, Q. Lin, H. M. Su, D. Chen, T. Zhang, Q. Z. Wu, S. P. Li, Cryst. Growth Des. 2011, 11, 5002.10.1021/cg200904tSuche in Google Scholar
[35] Y. H. Guo, Y. H. Wang, C. W. Hu, Y. H. Wang, E. B. Wang, Y. C. Zhou, S. H. Feng, Chem. Mater. 2000, 12, 3501.10.1021/cm000074+Suche in Google Scholar
[36] G. M. Sheldrick, Shelxtl, Bruker Analytical X-ray Instruments Inc., Madison, WI (USA) 2001.Suche in Google Scholar
[37] G. M. Sheldrick, Acta Crystallogr. 2008, A64, 112.10.1107/S0108767307043930Suche in Google Scholar
©2015 by De Gruyter
Artikel in diesem Heft
- Frontmatter
- In this Issue
- EPR studies on carboxylic esters, 23 [1]. Preparation of new dialkyl azulenedicarboxylates and EPR-spectroscopic study of their radical anions
- Synthesis and crystal structure of a 3D copper(II)–silver(I) coordination polymer assembled through hydrogen bonding, π–π stacking and metal–π interactions, {[Cu(phen)2(CN)][Ag(CN)2] · 3H2O}n (phen = 1,10-phenanthroline)
- A polyoxometalate-based inorganic–organic hybrid material: synthesis, characterization structure and photocatalytic study
- Hydrogen-bonded assemblies of two organically templated borates: syntheses and crystal structures of [(1,10-phen)(H3BO3)2] and [2-EtpyH][(B5O6(OH)4]
- Catalytic activity of the nanoporous MCM-41 surface for the Paal–Knorr pyrrole cyclocondensation
- A new route for the synthesis of 4-arylacetamido-2-aminothiazoles and their biological evaluation
- Structural and spectroscopic characterization of isotypic sodium, rubidium and cesium acesulfamates
- Nd39Ir10.98In36.02 – A complex intergrowth structure with CsCl- and AlB2-related slabs
- Syntheses, structures and magnetic properties of two mononuclear nickel(II) complexes based on bicarboxylate ligands
- Synthesis and characterization of some new fluoroquinolone-barbiturate hybrid systems
- Synthesis of pyrazoles containing benzofuran and trifluoromethyl moieties as possible anti-inflammatory and analgesic agents
Artikel in diesem Heft
- Frontmatter
- In this Issue
- EPR studies on carboxylic esters, 23 [1]. Preparation of new dialkyl azulenedicarboxylates and EPR-spectroscopic study of their radical anions
- Synthesis and crystal structure of a 3D copper(II)–silver(I) coordination polymer assembled through hydrogen bonding, π–π stacking and metal–π interactions, {[Cu(phen)2(CN)][Ag(CN)2] · 3H2O}n (phen = 1,10-phenanthroline)
- A polyoxometalate-based inorganic–organic hybrid material: synthesis, characterization structure and photocatalytic study
- Hydrogen-bonded assemblies of two organically templated borates: syntheses and crystal structures of [(1,10-phen)(H3BO3)2] and [2-EtpyH][(B5O6(OH)4]
- Catalytic activity of the nanoporous MCM-41 surface for the Paal–Knorr pyrrole cyclocondensation
- A new route for the synthesis of 4-arylacetamido-2-aminothiazoles and their biological evaluation
- Structural and spectroscopic characterization of isotypic sodium, rubidium and cesium acesulfamates
- Nd39Ir10.98In36.02 – A complex intergrowth structure with CsCl- and AlB2-related slabs
- Syntheses, structures and magnetic properties of two mononuclear nickel(II) complexes based on bicarboxylate ligands
- Synthesis and characterization of some new fluoroquinolone-barbiturate hybrid systems
- Synthesis of pyrazoles containing benzofuran and trifluoromethyl moieties as possible anti-inflammatory and analgesic agents

