Abstract
An acidic ionic liquid 3-methyl-2-(1-sulfobutyl)-1H-imidazolium hydrogensulfate, [BSO3HMIm]HSO4, was used as an efficient catalyst for the synthesis of a variety of pyrrole derivatives via a one-pot, three-component condensation of amines, nitroolefins, and 1,3-dicarbonyl compounds. Good to excellent yields of 72–96% were obtained under reflux in ethanol. The catalyst could easily be recovered and recycled up to six times, resulting in good yields without prolonging the reaction time. This method was also efficient in large-scale preparation. The procedure could be easily expanded to a one-pot, four-component reaction. This ionic liquid-catalyzed reaction provided an environmentally friendly alternative to the synthesis of pyrrole derivatives.
1 Introduction
Multicomponent reactions (MCRs) offer a rapid and convergent construction of complex molecules without the need of isolation and purification of any intermediates, resulting in substantial minimization of waste, time, and cost [1–5]. Accordingly, MCRs have the advantage of simplicity and synthetic efficiency over conventional chemical reactions [6].
Pyrrole and its derivatives are an important class of heterocyclic compounds, which constitute the key core of various natural products [7–10] as well as pharmaceuticals [11, 12] (Fig. 1). They exhibit a wide range of biological activities such as antitumor [13], antibacterial [14], anticancer [15], antidiabetic [16], and anti-inflammatory properties [17]. Moreover, pyrroles have been extensively used in material science [18, 19]. Thus, many useful strategies have been developed for the synthesis of pyrroles and their derivatives. The most frequently used methods are Hantzsch reactions [20] (reaction of α-haloketones and β-enaminones), Paal–Knorr reactions [21, 22] (the cyclocondensation of primary amines with 1,4-diketones), and Knorr reactions [23–25] (condensation of α-aminoketone and active methylene group of ketones). Additionally, in recent years, three-component reactions of amines, nitroolefins and 1,3-dicarbonyl compounds, and four-component reactions of amines, aldehydes, nitroalkanes, and 1,3-dicarbonyl compounds have been used to prepare functionalized pyrroles. FeCl3 [26, 27], (diacetoxyiodo)benzene (DIB) [28], NiCl2·6H2O [29], CeCl3·7H2O [30], iodine [31], gluconic acid [32], and tungstic acid (STA) [33] were used as catalysts, respectively. Moreover, Guan and co-workers constructed pyrrole rings from enaminones or enamino esters with nitroolefins via Michael-type addition and cyclization under purely thermal conditions [34]. In consideration of the great significance of pyrrole and its derivatives, the development of new methods with environmentally friendly, recoverable, and recyclable catalysts to realize this important transformation is still desirable.
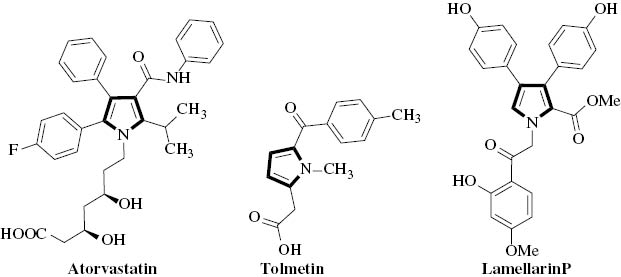
Therapeutically active pyrroles.
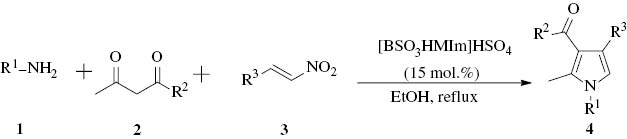
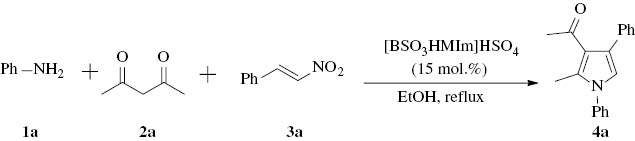
In recent years, ionic liquids have been used as valuable alternatives to the common volatile organic solvents. Besides, some ionic liquids have also been used as acid or base catalysts with several advantages [35], such as more environmentally benign, enhanced rate and activity, ease of catalyst recovery [36]. For instance, a task-specific ionic liquid, 1-butyl-3-methylimidazolium hydroxide, [bmIm]OH, has been used as an efficient catalyst for Michael reactions [37], Knoevenagel condensations [38], and multi-component condensations [39]. Other task-specific proline-tagged ionic liquids, based on 1,2,3-triazolium salts, have also been used for Michael reactions [40]. More recently, a catalyst-free four-component protocol for the synthesis of substituted pyrroles was described using the ionic liquid [Hbim]BF4 as the reaction medium. The reaction conditions were mild; however, a large amount of ionic liquid was employed (5 mL of ionic liquid for 1-mmol scale of reaction) [41]. In this context, we report a simple and efficient method for the synthesis of pyrrole derivatives, using the acidic ionic liquid [BSO3HMIm]HSO4 (3-methyl-2-(1-sulfobutyl)-1H-imidazolium hydrogensulfate) (Fig. 2) (15 mol.%) as an easily available, economical, environmentally benign, and recoverable catalyst. Furthermore, this strategy was efficient in large-scale preparation, and could be expanded to a one-pot, four-component reaction.
![Fig. 2 Chemical structure of the ionic liquid [BSO3HMIm]HSO4.](/document/doi/10.1515/znb-2014-0108/asset/graphic/znb-2014-0108_fig2.jpg)
Chemical structure of the ionic liquid [BSO3HMIm]HSO4.
2 Results and discussion
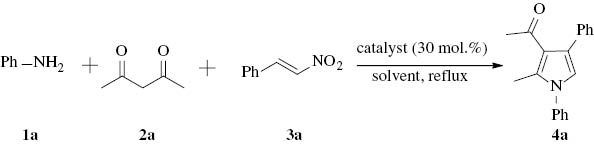
Initially, the one-pot, three-component reaction of aniline, β-nitrostyrene, and acetylacetone was used as a model reaction for the synthesis of a substituted pyrrole. A blank reaction was carried out in the absence of catalyst in ethanol at reflux temperature for 25 h, and the product was only obtained in a low yield of 15% (Table 1, entry 1). Then, several catalysts were evaluated including DBU, l-proline, [BMIm]BF4, [Bpy]BF4, and [BSO3HMIm]HSO4 (Table 1, entries 2–6). The reactions were carried out under reflux in ethanol, and all the tested catalysts showed catalytic effects in different degrees on the model reaction to give the desired product. When [BSO3HMIm]HSO4 was used, the highest yield of 90% was achieved after 10 h (Table 1, entry 6). The reactions with other examined catalysts gave the product in low yields of 32–56% after more than 20 h (Table 1, entries 2–5). Therefore, we chose [BSO3HMIm]HSO4 as the catalyst for this one-pot, three-component reaction. Furthermore, a solvent screening was performed to identify the optimal conditions (Table 1, entries 6–12). The results revealed that the solvent played an important role in the reaction. Among the tested solvents, the highest yield of 90% was obtained in EtOH at reflux temperature (Table 1, entry 6), while the reactions in PEG, toluene, CH3CN, and H2O provided the product in yields of 60–82% (Table 1, entries 7–10), and the reactions in CH2Cl2 and EtOAc gave low yields of 46% and 40 %, respectively (Table 1, entries 11–12). Thus, EtOH was chosen as the optimum solvent for further investigation.
The screening of catalysts and solventsa.
 | ||||
|---|---|---|---|---|
| Entry | Catalyst | Solvent | Time (h) | Yield (%)b |
| 1 | none | EtOH | 25 | 15 |
| 2 | DBU | EtOH | 25 | 32 |
| 3 | l-proline | EtOH | 25 | 35 |
| 4 | [BMIm]BF4 | EtOH | 22 | 54 |
| 5 | [Bpy]BF4 | EtOH | 21 | 56 |
| 6 | [BSO3HMIm]HSO4 | EtOH | 10 | 90 |
| 7c | [BSO3HMIm]HSO4 | PEG | 10 | 82 |
| 8 | [BSO3HMIm]HSO4 | toluene | 10 | 70 |
| 9 | [BSO3HMIm]HSO4 | CH3CN | 10 | 62 |
| 10 | [BSO3HMIm]HSO4 | H2O | 10 | 60 |
| 11 | [BSO3HMIm]HSO4 | CH2Cl2 | 10 | 46 |
| 12 | [BSO3HMIm]HSO4 | EtOAc | 10 | 40 |
aThe reaction conditions were as follows: aniline (0.5 mmol, 1 equiv.), β-nitrostyrene (0.5 mmol, 1 equiv.), acetylacetone (1.0 mmol, 2 equiv.), catalyst (30 mol.%) in solvent (1.0 mL) at reflux temperature. bIsolated yield after silica gel chromatography. cThe reaction was carried out at 120 °C.
Then, a reaction temperature screening was performed from 30°C to reflux temperature in EtOH (Table 2, entries 1–6). The best result was obtained at reflux temperature (Table 2, entry 6), while the reactions at lower temperatures took longer time and gave lower yields (Table 2, entries 1–5). Thus, reflux temperature was selected as the optimum temperature for the reaction. Subsequently, the catalyst loading was investigated, and it was found that 15 mol.% of catalyst was sufficient to give the product in an excellent yield of 94% (Table 2, entry 7). Hence, the catalyst loading was set as 15 mol.% for further investigation.
Effect of temperature on the ionic liquid-catalyzed three-component reactiona.
 | |||
|---|---|---|---|
| Entry | Temp. (°C) | Time (h) | Yield (%)b |
| 1 | 30 | 72 | 78 |
| 2 | 40 | 72 | 81 |
| 3 | 50 | 72 | 82 |
| 4 | 60 | 30 | 85 |
| 5 | 70 | 10 | 89 |
| 6 | reflux | 10 | 92 |
| 7c | reflux | 10 | 94 |
aThe reaction conditions were as follows: aniline (0.5 mmol, 1 equiv.), β-nitrostyrene (0.5 mmol, 1 equiv.), acetylacetone (0.75 mmol, 1.5 equiv.) and [BSO3HMIm]HSO4 (30 mol.%) in EtOH (1.0 mL). bIsolated yield after silica gel chromatography. c[BSO3HMIm]HSO4 (15 mol.%).
In order to explore the scope and generality of this ionic liquid-catalyzed three-component reaction for the synthesis of pyrrole derivatives, various amines, 1,3-dicarbonyl compounds, and nitroolefins were tested under the optimized conditions (Table 3, entries 1–22). A wide range of substrates could effectively participate in the reaction. Aromatic amines bearing either electron-donating (Me, MeO) or electron-withdrawing (F, Cl, Br) groups were tolerated as well (Table 3, entries 1–15). Benzylamine was also applicable to the reaction with various 1,3-dicarbonyl compounds and nitroolefins, giving desired products in good yields (Table 3, entries 16–22). Different 1,3-dicarbonyl compounds including acetylacetone, ethyl acetoacetate, and methyl acetoacetate could be used in the reaction to generate the corresponding products in good to excellent yields. A series of nitrostyrenes containing either electron-donating (MeO, Me) or electron-withdrawing (CN, Cl) substituents in the aromatic ring were examined with different amines and 1,3-dicarbonyl compounds, and the products were obtained in good yields (Table 3, entries 1–21). A heteroaromatic nitroolefin, 2-thienylnitroethylene, was also used in the reaction, giving an acceptable yield of 72% with benzylamine and acetylacetone (Table 3, entry 22).
Investigation of the substrate scope for the ionic liquid-catalyzed three-component reactiona.
 | ||||||
|---|---|---|---|---|---|---|
| Entry | R1 | R2 | R3 | Product | Time (h) | Yield (%)b |
| 1 | Ph | Me | Ph | 4a | 12 | 96 |
| 2 | p-Me-C6H4 | Me | p-OMe-C6H4 | 4b | 12 | 93 |
| 3 | p-Me-C6H4 | Me | p-Me-C6H4 | 4c | 8 | 92 |
| 4 | p-OMe-C6H4 | Me | Ph | 4d | 10 | 89 |
| 5 | p-OMe-C6H4 | Me | p-Cl-C6H4 | 4e | 10 | 88 |
| 6 | p-OMe-C6H4 | OEt | p-Cl-C6H4 | 4f | 14 | 85 |
| 7 | p-OMe-C6H4 | Me | p-OMe-C6H4 | 4g | 12 | 92 |
| 8 | p-F-C6H4 | Me | p-Cl-C6H4 | 4h | 10 | 82 |
| 9 | p-F-C6H4 | Me | p-OMe-C6H4 | 4i | 10 | 83 |
| 10 | p-F-C6H4 | Me | p-Me-C6H4 | 4j | 10 | 84 |
| 11 | p-Cl-C6H4 | Me | p-Me-C6H4 | 4k | 10 | 91 |
| 12 | p-Br-C6H4 | Me | p-OMe-C6H4 | 4l | 12 | 87 |
| 13 | p-Br-C6H4 | Me | p-Me-C6H4 | 4m | 12 | 86 |
| 14 | p-Br-C6H4 | Me | p-Cl-C6H4 | 4n | 10 | 83 |
| 15 | m-Br-C6H4 | Me | p-Me-C6H4 | 4o | 12 | 80 |
| 16 | PhCH2 | OMe | Ph | 4p | 14 | 80 |
| 17 | PhCH2 | OEt | p-Cl-C6H4 | 4q | 12 | 82 |
| 18 | PhCH2 | OMe | p-Cl-C6H4 | 4r | 12 | 83 |
| 19 | PhCH2 | OMe | p-Me-C6H4 | 4s | 12 | 83 |
| 20 | PhCH2 | OMe | p-CN-C6H4 | 4t | 14 | 82 |
| 21 | PhCH2 | OMe | p-OMe-C6H4 | 4u | 12 | 85 |
| 22 | PhCH2 | Me | 2-thienyl | 4v | 14 | 72 |
aReaction conditions: amine (0.5 mmol, 1 equiv.), nitroolefin (0.5 mmol, 1 equiv.), 1,3-dicarbonyl compound (0.75 mmol, 1.5 equiv.) and [BSO3HMIm]HSO4 (15 mol.%) in EtOH (1.0 mL) at reflux temperature. bIsolated yield after silica gel chromatography.
A possible pathway for this ionic liquid-catalyzed one-pot, three-component synthesis of pyrrole derivatives is outlined in Scheme 1 (see also Refs. [20, 21]). The ionic liquid BSO3HMIm]HSO4 as a Brønsted acid catalyzes the reaction sequence.
![Scheme 1 Possible pathway for the [BSO3HMIm]HSO4-catalyzed synthesis of pyrrole derivatives.](/document/doi/10.1515/znb-2014-0108/asset/graphic/znb-2014-0108_scheme1.jpg)
Possible pathway for the [BSO3HMIm]HSO4-catalyzed synthesis of pyrrole derivatives.
To verify that the ionic liquid catalyst [BSO3HMIm]HSO4 could be recovered, a recycling study of the catalyst was performed with the reaction of aniline, β-nitrostyrene, and acetylacetone (Table 4). Upon completing the reaction (monitored by TLC), the solvent was removed. The residue was diluted with ethyl acetate, and water was added. The aqueous phase was washed with ethyl acetate. The organic phase was combined for the isolation of the product. The aqueous phase was evaporated in vacuo. The resulting residue was directly used in the next reaction cycle without adding more catalyst. Although the yield decreased gradually, a good yield was still obtained after 6 cycles without prolonging the reaction time (Table 4).
Recycling and reuse of the catalysta.
 | ||
|---|---|---|
| Cycle | Time (h) | Yield (%)b |
| 1 | 12 | 95 |
| 2 | 12 | 92 |
| 3 | 12 | 90 |
| 4 | 12 | 88 |
| 5 | 12 | 78 |
| 6 | 12 | 75 |
aReaction conditions: aniline (0.5 mmol, 1 equiv.), β-nitrostyrene (0.5 mmol, 1 equiv.), acetylacetone (0.75 mmol, 1.5 equiv.), and [BSO3HMIm]HSO4 (15 mol.%) in EtOH (1.0 mL) at reflux temperature. bIsolated yield after silica gel chromatography.
We further performed a larger-scale reaction with 10 mmol of p-toluidine, 10 mmol of 4-methoxy-nitrostyrene, and 15 mmol of acetylacetone. This experiment could easily be carried out using the same procedure as for the experimental-scale reactions, and a good yield of 90% was obtained after 18 h (Scheme 2).

A large-scale reaction.
Finally, this ionic liquid-catalyzed reaction could be easily expanded to a one-pot, four-component reaction. The reaction of benzaldehyde, aniline, acetylacetone, and nitromethane in the presence of [BSO3HMIm]HSO4 proceeded smoothly at reflux temperature with excess nitromethane as a solvent, and a yield of 74% was obtained after 12 h (Scheme 3).

The ionic liquid-catalyzed one-pot, four-component reaction.
3 Conclusion
An economical and environmentally benign ionic liquid [BSO3HMIm]HSO4-catalyzed one-pot reaction of amines, nitroolefins, and 1,3-dicarbonyl compounds for the preparation of polysubstituted pyrroles was developed. Good to excellent yields (72–96%) were obtained with different types of substrates. The most notable advantages of this catalyst are in its high efficiency for a broad scope of substrates, easy recovery, and the ability to scale up. Moreover, this procedure could easily be expanded to a one-pot, four-component reaction. This ionic liquid-catalyzed reaction provides an environmentally friendly alternative to the synthesis of pyrrole derivatives.
4 Experimental part
4.1 General information
The NMR spectra were recorded with TMS as the internal standard in CDCl3 on a Bruker 300 MHz instrument at room temperature. The chemical shifts (δ) were described in parts per million (ppm), and J values are given in Hertz (Hz). All reactions were monitored by thin-layer chromatography (TLC) with Haiyang GF254 silica gel plates. Flash column chromatography was carried out using 200–300 mesh silica gel at increased pressure. All chemical reagents and solvents were purchased from commercial vendors and used without any further purification. High-resolution mass spectra (Varian QFT-ESI) were obtained using ESI ion-ization sources. The purity of products was determined by HPLC analysis on Chiralpak AD-H.
4.2 General procedure for the ionic liquid-catalyzed three-component reaction
A 10-mL round-bottomed flask was charged with amine (0.5 mmol, 1 equiv.), nitroolefin (0.5 mmol, 1 equiv.), 1,3-dicarbonyl compounds (0.75 mmol, 1.5 equiv.), [BSO3HMIm]HSO4 (15 mol.%), and EtOH (1.0 mL). The mixture was stirred for a specified amount of time at reflux temperature. Upon completing the reaction (monitored by TLC), the solvent was removed under vacuum. The resulting residue was treated with a small amount of CH2Cl2 to dissolve the product, and the ionic liquid was left at the bottom. The CH2Cl2 layer was directly loaded onto the column of silica gel chromatography for purification, eluted with petroleum ether and ethyl acetate to afford the desired product.
4.3 A typical procedure for catalyst recovery
A mixture of aniline (0.5 mmol, 1 equiv.), β-nitrostyrene (0.5 mmol, 1 equiv.), acetylacetone (0.75 mmol, 1.5 equiv.), [BSO3HMIm]HSO4 (15 mol.%), and EtOH (1.0 mL) was stirred at reflux temperature for 12 h. The solvent was evaporated, and the residue was diluted with ethyl acetate (8 mL), and water (3 mL) was added. The aqueous phase was washed with ethyl acetate (2×8 mL). The combined organic layer was concentrated, and purified by silica gel column chromatography to give the product. The aqueous phase was evaporated in vacuo, and the resulting residue was directly used in the next reaction cycle without adding more catalyst.
4.4 A typical procedure for reactions at a larger scale
A 100-mL round-bottomed flask was charged with p-toluidine (10 mmol), 4-methoxy-nitrostyrene (10 mmol), acetylacetone (15 mmol), [BSO3HMIm]HSO4 (15 mol.%), and EtOH (20 mL). The mixture was stirred at reflux temperature for 18 h. The solvent was removed under vacuum. The resulting residue was treated with a small amount of CH2Cl2 to dissolve the product, and the ionic liquid was left at the bottom. The CH2Cl2 layer was directly loaded onto the column of silica gel chromatography for purification, eluted with petroleum ether and ethyl acetate to afford the desired product as a colorless solid (2.87 g, 90% yield).
4.5 A typical procedure for the ionic liquid-catalyzed four-component reaction
To a stirred mixture of benzaldehyde (0.5 mmol), aniline (0.75 mmol), acetylacetone (0.5 mmol), and nitromethane (0.5 mL) was added [BSO3HMIm]HSO4 (15 mol.%). The reaction mixture was heated to reflux for 12 h, and then cooled to r.t. The excess nitromethane was removed under vacuum. The resulting residue was treated with a small amount of CH2Cl2 to dissolve the product, and the ionic liquid was left at the bottom. The CH2Cl2 layer was directly loaded onto the column of silica gel chromatography for purification, eluted with petroleum ether and ethyl acetate to afford the desired product as a colorless solid (103 mg, 74% yield).
4.6 Characterization
All the products are previously described compounds: Refs. [27–30].
1-(2-Methyl-1,4-diphenyl-1H-pyrrol-3-yl)ethanone (4a) Colorless solid; m.p.: 106–108°C (105–107°C [27]). – 1H NMR (300 MHz, CDCl3): δ=7.51–7.42 (m, 3H, Ph), 7.39–7.32 (m, 7H, Ph), 6.67 (s, 1H, CH), 2.41 (s, 3H, COCH3), 2.08 (s, 3H, CH3) ppm. – 13C NMR (75 MHz, CDCl3): δ=197.7(s), 138.7(s), 136.0(s), 135.3(s), 129.4(s), 129.3(s), 128.3(s), 128.1(s), 126.8(s), 126.3(s), 126.2(s), 122.5(s), 120.6(s), 31.1(s), 12.9(s) ppm. – HRMS ((+)-ESI): m/z=298.1205 (calcd. 298.1208 for C19H17NONa, [M+Na]+). – HPLC (Chiralpak AD-H column, 25°C, 254 nm, hexane-i-propanol=80:20): tR=8.73 min.
1-(4-(4-Methoxyphenyl)-2-methyl-1-(p-tolyl)-1H-pyrrol-3-yl)ethanone (4b) Brown liquid. – 1H NMR (300 MHz, CDCl3): δ=7.31–7.26 (m, 4H, Ph), 7.21–7.19 (m, 2H, Ph), 6.93 (d, J=8.7 Hz, 2H, Ph), 6.60 (s, 1H, CH), 3.83 (s, 3H, CH3), 2.41 (s, 3H, COCH3), 2.39 (s, 3H, Ar-CH3), 2.08 (s, 3H, CH3) ppm. – 13C NMR (75 MHz, CDCl3): δ=197.6(s), 158.6(s), 138.1(s), 136.2(s), 135.3(s), 130.4(s), 129.9(s), 128.4(s), 126.0(s), 125.7(s), 122.4(s), 120.5(s), 113.7(s), 55.3(s), 31.1(s), 21.1(s), 12.9(s) ppm. – HRMS ((+)-ESI): m/z=320.1648 (calcd. 320.1651 for C21H22NO2, [M+H]+). – HPLC (Chiralpak AD-H column, 25°C, 254 nm, hexane-i-propanol=80:20): tR=17.56 min.
1-(2-Methyl-1,4-di-p-tolyl-1H-pyrrol-3-yl)ethanone (4c) Colorless solid; m.p.: 95–97°C (94–96°C [30]). – 1H NMR (300 MHz, CDCl3): δ=7.28–7.25 (m, 4H, Ph), 7.21–7.17 (m, 4H, Ph), 6.62 (s, 1H, CH), 2.41 (s, 3H, COCH3), 2.39 (s, 3H, Ar-CH3), 2.38 (s, 3H, Ar-CH3), 2.08 (s, 3H, CH3) ppm. – 13C NMR (75 MHz, CDCl3): δ=197.7(s), 138.0(s), 136.4(s), 136.2(s), 135.3(s), 133.0(s), 129.7(s), 129.2(s), 129.0(s), 126.1(s), 126.0(s), 122.4(s), 120.5(s), 31.1(s), 21.2(s), 21.1(s), 12.9(s) ppm. – HPLC (Chiralpak AD-H column, 25°C, 254 nm, hexane-i-propanol=80:20): tR=14.14 min.
1-(1-(4-Methoxyphenyl)-2-methyl-4-phenyl-1H-pyrrol-3-yl)ethanone (4d) Yellowish solid; m.p.: 93–95°C (90–91°C [27]). – 1H NMR (300 MHz, CDCl3): δ=7.38–7.36 (m, 4H, Ph), 7.32–7.29 (m, 1H, Ph), 7.23 (d, J=8.8 Hz, 2H, Ph), 6.98 (d, J=8.8 Hz, 2H, Ph), 6.62 (s, 1H, CH), 3.84 (s, 3H, CH3), 2.38 (s, 3H, COCH3), 2.07 (s, 3H, CH3) ppm. – 13C NMR (75 MHz, CDCl3): δ=197.6(s), 159.3(s), 136.1(s), 135.7(s), 131.6(s), 129.3(s), 128.3(s), 127.5(s), 126.8(s), 126.0(s), 122.2(s), 120.9(s), 114.5(s), 55.6(s), 31.1(s), 12.9(s) ppm. – HPLC (Chiralpak AD-H column, 25°C, 254 nm, hexane-i-propanol=80:20): tR=14.08 min.
1-(4-(4-Chlorophenyl)-1-(4-methoxyphenyl)-2-methyl-1H-pyrrol-3-yl)ethanone (4e) Colorless solid; m.p.: 118–120°C (117–119°C [27]). – 1H NMR (300 MHz, CDCl3): δ=7.36–7.21 (m, 6H, Ph), 6.99 (d, J=8.7 Hz, 2H, Ph), 6.61 (s, 1H, CH), 3.86 (s, 1H, CH3), 2.37 (s, 3H, COCH3), 2.09 (s, 3H, CH3) ppm. – 13C NMR (75 MHz, CDCl3): δ=197.1(s), 159.3(s), 135.9(s), 134.6(s), 132.6(s), 131.4(s), 130.5(s), 128.4, (s) 127.4(s), 124.7(s), 122.0(s), 121.0(s), 114.4(s), 55.4(s), 31.1(s), 12.8(s) ppm. – HPLC (Chiralpak AD-H column, 25°C, 254 nm, hexane-i-propanol=85:15): tR=30.07 min.
Ethyl 4-(4-chlorophenyl)-1-(4-methoxyphenyl)-2-methyl-1H-pyrrole-3-carboxylate (4f) Orange yellow liquid. – 1H NMR (300 MHz, CDCl3): δ=7.36–7.19 (m, 6H, Ph), 6.99–6.96 (d, J=8.7 Hz, 2H, Ph), 6.64 (s, 1H, CH), 4.19 (q, J=7.1 Hz, 2H, CH2), 3.84 (s, 3H, CH3), 2.41 (s, 3H, CH3), 1.17 (t, J=7.1 Hz, 3H, CH2CH3) ppm. – 13C NMR (75 MHz, CDCl3): δ=165.6(s), 159.3(s), 137.2(s), 134.1(s), 132.0(s), 131.6(s), 130.5(s), 127.6(s), 127.5(s), 125.1(s), 121.2(s), 114.4(s), 111.0(s), 59.5(s), 55.5(s), 14.2(s), 12.6(s) ppm. – HRMS ((+)-ESI): m/z=392.1025 (calcd. 392.1029 for C21H20ClNO3Na, [M+Na]+). – HPLC (Chiralpak AD-H column, 25°C, 254 nm, hexane-i-propanol=85:15): tR=20.28 min.
1-(1,4-Bis(4-methoxyphenyl)-2-methyl-1H-pyrrol-3-yl)ethanone (4g) Colorless solid; m.p.: 134–136°C (133–135°C [27]). – 1H NMR (300 MHz, CDCl3): δ=7.30–7.22 (m, 4H, Ph), 6.99–6.59 (m, 4H, Ph), 3.84 (s, 3H, CH3), 3.82 (s, 3H, CH3), 2.38 (s, 3H, COCH3), 2.08 (s, 3H, CH3) ppm. – 13C NMR (75 MHz, CDCl3): δ=197.5(s), 159.2(s), 158.6(s), 135.5(s), 131.6(s), 130.4(s), 128.4(s), 127.4(s), 126.6(s), 125.6(s), 122.2(s), 120.6(s), 114.4(s), 114.2(s), 113.7(s), 55.5(s), 55.2(s), 31.0(s), 12.8(s) ppm. – HPLC (Chiralpak AD-H column, 25°C, 254 nm, hexane-i-propanol=80:20): tR=20.56 min.
1-(4-(4-Chlorophenyl)-1-(4-fluorophenyl)-2-methyl-1H-pyrrol-3-yl)ethanone (4h) Brown oily liquid. – 1H NMR (300 MHz, CDCl3): δ=7.37–7.27 (m, 6H, Ph), 7.22–7.16 (m, 2H, Ph), 6.63 (s, 1H, CH), 2.37 (s, 3H, COCH3), 2.09 (s, 3H, CH3) ppm. – 13C NMR (75 MHz, CDCl3): δ=197.2(s), 163.7(s), 160.4(s), 135.6(s), 134.3(s), 132.8(s), 130.4(s), 128.5(s), 128.1(s), 127.9(s), 125.0(s), 122.4(s), 120.7(s), 116.5(s), 116.2(s), 31.1(s), 12.8(s) ppm. – HRMS ((+)-ESI): m/z=350.0722 (calcd. 350.0724 for C19H15ClFNONa, [M+Na]+). – HPLC (Chiralpak AD-H column, 25°C, 254 nm, hexane-i-propanol=85:15): tR=18.08 min.
1-(1-(4-Fluorophenyl)-4-(4-methoxyphenyl)-2-methyl-1H-pyrrol-3-yl)ethanone (4i) Brown solid; m.p.: 76–79°C (78–80°C [27]). – 1H NMR (300 MHz, CDCl3): δ=7.31–7.27 (m, 4H, Ph), 7.16 (t, J=8.4 Hz, 2H, Ph), 6.91 (d, J=8.4 Hz, 2H, Ph), 6.58 (s, 1H, CH), 3.82 (s, 3H, CH3), 2.37 (s, 3H, COCH3), 2.06 (s, 3H, CH3) ppm. – 13C NMR (75 MHz, CDCl3): δ=197.6(s), 163.6(s), 160.3(s), 158.7(s), 135.2(s), 130.3(s), 128.1(s), 128.0(s), 127.9(s), 125.9(s), 122.5(s), 120.4(s), 116.4(s), 116.1(s), 113.7(s), 55.2(s), 31.0(s), 12.8(s) ppm. – HPLC (Chiralpak AD-H column, 25°C, 254 nm, hexane-i-propanol=80:20): tR=17.75 min.
1-(1-(4-Fluorophenyl)-2-methyl-4-(p-tolyl)-1H-pyrrol-3-yl)ethanone (4j) Colorless oily liquid. – 1H NMR (300 MHz, CDCl3): δ=7.33–7.24 (m, 4H, Ph), 7.20–7.14 (m, 4H, Ph), 6.61 (s, 1H, CH), 2.38 (s, 6H, COCH3, Ph-CH3), 2.08 (s, 3H, CH3) ppm. – 13C NMR (75 MHz, CDCl3): δ=197.7(s), 163.6(s), 160.3(s), 136.6(s), 135.3(s), 134.8(s), 134.7(s), 132.8(s), 129.1(s), 129.0(s), 128.0(s), 127.9(s), 126.3(s), 122.5(s), 120.5(s), 116.4(s), 116.1(s), 31.1(s), 21.1(s), 12.8(s) ppm. – HPLC (Chiralpak AD-H column, 25°C, 254 nm, hexane-i-propanol=80:20): tR=14.05 min.
1-(1-(4-Chlorophenyl)-2-methyl-4-(p-tolyl)-1H-pyrrol-3-yl)ethanone (4k) Colorless solid; m.p.: 124–126°C (127–129°C [27]). – 1H NMR (300 MHz, CDCl3): δ=7.46–7.43 (m, 2H, Ph), 7.28–7.25 (m, 4H, Ph), 7.20–7.17 (m, 2H, Ph), 6.61 (s, 1H, CH), 2.39 (s, 3H, COCH3), 2.38 (s, 3H, Ph-CH3), 2.08 (s, 3H, CH3) ppm. – 13C NMR (75 MHz, CDCl3): δ=197.6(s), 137.2(s), 136.6(s), 135.0(s), 133.9(s), 132.7(s), 129.5(s), 129.1(s), 127.4(s), 126.5(s), 122.8(s), 120.2(s), 31.1(s), 21.1(s), 12.8(s) ppm. – HRMS ((+)-ESI): m/z=346.0972 (calcd. 346.0975 for C20H18ClNONa, [M+Na]+). – HPLC (Chiralpak AD-H column, 25°C, 254 nm, hexane-i-propanol=85:15): tR=20.09 min.
1-(1-(4-Bromophenyl)-4-(4-methoxyphenyl)-2-methyl-1H-pyrrol-3-yl)ethanone (4l) Colorless solid; m.p.: 123–125°C (119–121°C [27]). – 1H NMR (300 MHz, CDCl3): δ=7.61 (d, J=8.5 Hz, 2H, Ph), 7.27 (d, J=8.5 Hz, 2H, Ph), 7.21 (d, J=8.5 Hz, 2H, Ph), 6.93 (d, J=8.5 Hz, 2H, Ph), 6.60 (s, 1H, CH), 3.83 (s, 3H, CH3), 2.40 (s, 3H, COCH3), 2.07 (s, 3H, CH3) ppm. – 13C NMR (75 MHz, CDCl3): δ=197.6(s), 158.7(s), 137.7(s), 134.9(s), 132.5(s), 130.3(s), 128.0(s), 127.7(s), 126.2(s), 122.9(s), 121.8(s), 120.1(s), 113.7(s), 55.2(s), 31.0(s), 12.9(s) ppm. – HPLC (Chiralpak AD-H column, 25°C, 254 nm, hexane-i-propanol=85:15): tR=27.86 min.
1-(1-(4-Bromophenyl)-2-methyl-4-(p-tolyl)-1H-pyrrol-3-yl)ethanone (4m) Yellow oily liquid. – 1H NMR (300 MHz, CDCl3): δ=7.62–7.59 (d, J=8.5 Hz, 2H, Ph), 7.27–7.18 (m, 6H, Ph), 6.61 (s, 1H, CH), 2.39 (s, 3H, COCH3), 2.38 (s, 3H, Ph-CH3), 2.07 (s, 3H, CH3) ppm. – 13C NMR (75 MHz, CDCl3): δ=197.7(s), 137.7(s), 136.6(s), 134.9(s), 132.7(s), 132.5(s), 129.1(s), 129.0(s), 127.7(s), 126.5(s), 122.9(s), 121.9(s), 120.2(s), 31.1(s), 21.2(s), 12.9(s) ppm. – HPLC (Chiralpak AD-H column, 25°C, 254 nm, hexane-i-propanol=80:20): tR=18.45 min.
1-(1-(4-Bromophenyl)-4-(4-chlorophenyl)-2-methyl-1H-pyrrol-3-yl)ethanone (4n) Yellow liquid. – 1H NMR (300 MHz, CDCl3): δ=7.62–7.60 (d, J=8.4 Hz, 2H, Ph), 7.36–7.27 (m, 4H, Ph), 7.21–7.18 (d, J=8.4 Hz, 2H, Ph), 6.62 (s, 1H, CH), 2.38 (s, 3H, COCH3), 2.07(s, 3H, CH3) ppm. – 13C NMR (75 MHz, CDCl3): δ=197.1(s), 137.5(s), 135.3(s), 134.2(s), 132.9(s), 132.6(s), 130.41(s), 128.5(s), 127.7(s), 125.3(s), 122.7(s), 122.1(s), 120.4(s), 31.1(s), 12.8(s) ppm. – HRMS ((+)-ESI): m/z=411.9902 (calcd. 411.9903 for C19H15BrClNONa, [M+Na]+). – HPLC (Chiralpak AD-H column, 25°C, 254 nm, hexane-i-propanol=80:20): tR=19.52 min.
1-(1-(3-Bromophenyl)-2-methyl-4-(p-tolyl)-1H-pyrrol-3-yl)ethanone (4o) Colorless oily liquid. – 1H NMR (300 MHz, CDCl3): δ=7.57–7.52 (m, 2H, Ph), 7.39–7.33 (m, 1H, Ph), 7.29–7.18 (m, 5H, Ph), 6.62 (s, 1H, CH), 2.41 (s, 3H, COCH3), 2.38 (s, 3H, Ph-CH3), 2.07 (s, 3H, CH3) ppm. – 13C NMR (75 MHz, CDCl3): δ=197.7(s), 139.9(s), 136.6(s), 135.0(s), 132.6(s), 131.2(s), 130.6(s), 129.3(s), 129.1(s), 129.0(s), 126.6(s), 124.9(s), 122.9(s), 122.7(s), 120.2(s), 31.1(s), 21.2(s), 12.9(s) ppm. – HPLC (Chiralpak AD-H column, 25°C, 254 nm, hexane-i-propanol=80:20): tR=9.39 min.
Methyl 1-benzyl-2-methyl-4-phenyl-1H-pyrrole-3-carboxylate (4p) Colorless oily liquid. – 1H NMR (300 MHz, CDCl3): δ=7.39–7.23 (m, 8H, Ph), 7.06 (d, J=6.9 Hz, 2H, Ph), 6.58 (s, 1H, CH), 5.04 (s, 2H, CH2), 3.66 (s, 3H, COOCH3), 2.46 (s, 3H, CH3) ppm. – 13C NMR (75 MHz, CDCl3): δ=166.3(s), 136.7(s), 136.6(s), 135.7(s), 129.0(s), 128.9(s), 128.8(s), 127.8(s), 127.3(s), 126.7(s), 126.5(s), 126.1(s), 120.5(s), 110.7(s), 50.5(s), 11.5(s) ppm. – HRMS ((+)-ESI): m/z=306.1493 (calcd. 306.1494 for C20H19NO2H [M+H]+). – HPLC (Chiralpak AD-H column, 25°C, 254 nm, hexane-i-propanol=85:15): tR=7.89 min.
Ethyl 1-benzyl-4-(4-chlorophenyl)-2-methyl-1H-pyrrole-3-carboxylate (4q) Colorless oily liquid. – 1H NMR (300 MHz, CDCl3): δ=7.31–7.24 (m, 7H, Ph), 7.05 (d, J=6.8 Hz, 2H, Ph), 6.56 (s, 1H, CH), 5.04 (s, 2H, Ph-CH2), 4.15 (q, J=7.1 Hz, 2H, COOCH2), 2.46 (s, 3H, CH3), 1.15 (t, J=7.1 Hz, 3H, CH2CH3) ppm. – 13C NMR (75 MHz, CDCl3): δ=165.6(s), 136.7(s), 136.6(s), 134.3(s), 131.9(s), 130.5(s), 128.9(s), 127.8(s), 127.6(s), 126.5(s), 125.0(s), 120.5(s), 110.8(s), 59.4(s), 50.5(s), 14.0(s), 11.5(s) ppm. – HPLC (Chiralpak AD-H column, 25°C, 254 nm, hexane-i-propanol=80:20): tR=7.97 min.
Methyl 1-benzyl-4-(4-chlorophenyl)-2-methyl-1H-pyrrole-3-carboxylate (4r) Off-white solid; m.p.: 77–79°C (79–80°C [27]). – 1H NMR (300 MHz, CDCl3): δ=7.34–7.25 (m, 7H, Ph), 7.06 (d, J=7.0 Hz, 2H, Ph), 6.57 (s, 1H, CH), 5.05 (s, 2H, Ph-CH2), 3.67 (s, 3H, COOCH3), 2.46 (s, 3H, CH3) ppm. – 13C NMR (75 MHz, CDCl3): δ=166.0(s), 136.8(s), 136.5(s), 134.2(s), 131.9(s), 130.4(s), 128.9(s), 127.8(s), 127.7(s), 126.5(s), 125.0(s), 120.6(s), 111.4(s), 50.6(s), 50.5(s), 11.5(s) ppm. – HPLC (Chiralpak AD-H column, 25°C, 254 nm, hexane-i-propanol=85:15): tR=9.67 min.
Methyl 1-benzyl-2-methyl-4-(p-tolyl)-1H-pyrrole-3-carboxylate (4s) Colorless oil. – 1H NMR (300 MHz, CDCl3): δ=7.24–7.14 (m, 5H, Ph), 7.06–7.03 (m, 2H, Ph), 6.98–6.95 (d, J=6.9 Hz, 2H, Ph), 6.47 (s, 1H, CH), 4.95 (s, 2H, Ph-CH2), 3.59 (s, 3H, COOCH3), 2.36 (s, 3H, Ph-CH3), 2.27 (s, 3H, CH3) ppm. – 13C NMR (75 MHz, CDCl3): δ=166.3(s), 136.7(s), 136.4(s), 135.7(s), 132.7(s), 128.9(s), 128.9(s), 128.8(s), 128.4(s), 127.7(s), 127.3(s), 126.7(s), 126.5(s), 126.1(s), 120.4(s), 110.6(s), 82.7(s), 50.5(s), 50.4(s), 21.2(s), 11.5(s) ppm. – HPLC (Chiralpak AD-H column, 25°C, 254 nm, hexane-i-propanol=85:15): tR=9.58 min.
Methyl 1-benzyl-4-(4-cyanophenyl)-2-methyl-1H-pyrrole-3-carboxylate (4t) Orange yellow liquid. – 1H NMR (300 MHz, CDCl3): δ=7.60–7.57 (m, 2H, Ph), 7.48–7.45 (m, 2H, Ph), 7.35–7.30 (m, 3H, Ph), 7.08–7.05 (m, 2H, Ph), 6.65 (s, 1H, CH), 5.08 (s, 2H, Ph-CH2), 3.69 (s, 3H, COOCH3), 2.47 (s, 3H, CH3) ppm. – 13C NMR (75 MHz, CDCl3): δ=165.7(s), 140.7(s), 137.4(s), 136.2(s), 131.5(s), 131.4(s), 130.5(s), 129.6(s), 129.0(s), 128.3(s), 128.0(s), 127.5(s), 126.6(s), 126.4(s), 121.2(s), 119.4(s), 109.4(s), 50.7(s), 11.6(s) ppm. – HPLC (Chiralpak AD-H column, 25°C, 254 nm, hexane-i-propanol=80:20): tR=14.03 min.
Methyl 1-benzyl-4-(4-methoxyphenyl)-2-methyl-1H-pyrrole-3-carboxylate (4u) Colorless oil. – 1H NMR (300 MHz, CDCl3): δ=7.32–7.26 (m, 4H, Ph), 7.14–7.12 (m, 2H, Ph), 7.06–7.04 (m, 2H, Ph), 6.56 (s, 1H, CH), 5.04 (s, 2H, Ph-CH2), 3.67 (s, 3H, CH3), 2.45 (s, 3H, COOCH3), 2.35 (s, 3H, CH3) ppm. – 13C NMR (75 MHz, CDCl3): δ=166.3(s), 136.7(s), 136.4(s), 135.7(s), 132.7(s), 128.9(s), 128.9(s), 128.8(s), 128.4(s), 127.7(s), 127.3(s), 126.7(s), 126.5(s), 126.1(s), 120.4(s), 110.7(s), 50.5(d), 21.2(s), 11.5(s) ppm. – HRMS ((+)-ESI): m/z=358.1417 (calcd. 358.1419 for C21H21NO3Na [M+Na]+). – HPLC (Chiralpak AD-H column, 25°C, 254 nm, hexane-i-propanol=80:20): tR=8.40 min.
1-(1-Benzyl-2-methyl-4-(thiophen-2-yl)-1H-pyrrol-3-yl)ethanone (4v) Gray solid; m.p.: 137–139°C (136–138°C [27]). – 1H NMR (300 MHz, CDCl3): δ=7.37–7.29 (m, 3H, Ar-H), 7.26–7.25 (m, 1H, Ar-H), 7.10–7.07 (m, 2H, Ar-H), 7.05–7.02 (m, 1H, Ar-H), 6.96 (d, J=2.8 Hz, 1H, Ar-H), 6.63 (s, 1H, CH), 5.05 (s, 2H, Ph-CH2), 2.43 (s, 3H, COCH3), 2.15 (s, 3H, CH3) ppm. – 13C NMR (75 MHz, CDCl3): δ=197.1(s), 137.1(s), 136.2(s), 135.5(s), 129.0(s), 128.9(s), 128.1(s), 127.9(s), 127.1(s), 126.9(s), 126.6(s), 124.8(s), 121.5(s), 117.2(s), 50.3(s), 30.5(s), 11.7(s) ppm. – HPLC (Chiralpak AD-H column, 25°C, 254 nm, hexane-i-propanol=80:20): tR=7.00 min.
5 Supporting information
The spectra and chromatograms of 4a–4v are given as Supporting Information.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (no. 21276211).
References
[1] B. B. Touré, D. G. Hall, Chem. Rev. 2009, 109, 4439–4486.Search in Google Scholar
[2] G. Balme, Angew. Chem. Int. Ed. 2004, 43, 6238–6241.Search in Google Scholar
[3] A. Dömling, I. Ugi, Angew. Chem. Int. Ed. 2000, 39, 3168–3210.Search in Google Scholar
[4] Z. Liu, J. Zhang, S. Chen, E. Shi, Y. Xu, Angew. Chem. Int. Ed. 2012, 51, 3231–3235.10.7312/li--16274-052Search in Google Scholar
[5] X.-Z. Shu, X.-Y. Liu, H.-Q. Xiao, K.-G. Ji, L.-N. Guo, Y.-M. Liang, Adv. Synth. Catal. 2008, 350, 243–248.Search in Google Scholar
[6] G. Balme, E. Bossharth, N. Monteiro, Eur. J. Org. Chem. 2003, 2003, 4101–4111.Search in Google Scholar
[7] D. Boger, L. C. W. Boyce, M. A. Labroli, C. A. Sehon, Q. Jin, J. Am. Chem. Soc. 1998, 121, 54–62.Search in Google Scholar
[8] M. G. Banwell, T. E. Goodwin, S. Ng, J. A. Smith, D. J. Wong, Eur. J. Org. Chem. 2006, 2006, 3043–3060.Search in Google Scholar
[9] J. L. Bullington, R. R. Wolff, P. F. Jackson, J. Org. Chem. 2002, 67, 9439–9442.Search in Google Scholar
[10] H. Fan, J. Peng, M. T. Hamann, J.-F. Hu, Chem. Rev. 2007, 108, 264–287.Search in Google Scholar
[11] F. Bellina, R. Rossi, Tetrahedron 2006, 62, 7213–7256.10.1016/j.tet.2006.05.024Search in Google Scholar
[12] F. A. Davis, K. A. Bowen, H. Xu, V. Velvadapu, Tetrahedron 2008, 64, 4174–4182.10.1016/j.tet.2008.02.102Search in Google Scholar
[13] W. A. Denny, G. W. Rewcastle, B. C. Baguley, J. Med. Chem. 1990, 33, 814–819.Search in Google Scholar
[14] R. W. Bürli, D. McMinn, J. A. Kaizerman, W. Hu, Y. Ge, Q. Pack, V. Jiang, M. Gross, M. Garcia, R. Tanaka, H. E. Moser, Bioorg. Med. Chem. Lett. 2004, 14, 1253–1257.Search in Google Scholar
[15] D. Bandyopadhyay, S. Mukherjee, J. C. Granados, J. D. Short, B. K. Banik, Eur. J. Med. Chem. 2012, 50, 209–215.Search in Google Scholar
[16] A. Goel, N. Agarwal, F. V. Singh, A. Sharon, P. Tiwari, M. Dixit, R. Pratap, A. K. Srivastava, P. R. Maulik, V. J. Ram, Bioorg. Med. Chem. Lett. 2004, 14, 1089–1092.Search in Google Scholar
[17] M. Barceló, E. Raviña, C. F. Masaguer, E. Domínguez, F. M. Areias, J. Brea, M. I. Loza, Bioorg. Med. Chem. Lett. 2007, 17, 4873–4877.Search in Google Scholar
[18] B. Kişkan, A. Akar, N. Kızılcan, B. Ustamehmetoğlu, J. Appl. Polym. Sci. 2005, 96, 1830–1834.Search in Google Scholar
[19] A. Loudet, K. Burgess, Chem. Rev. 2007, 107, 4891–4932.Search in Google Scholar
[20] A. Hantzsch, Ber. Dtsch. Chem. Ges. 1890, 23, 1474–1476.Search in Google Scholar
[21] B. K. Banik, S. Samajdar, I. Banik, J. Org. Chem. 2003, 69, 213–216.Search in Google Scholar
[22] G. Minetto, L. F. Raveglia, A. Sega, M. Taddei, Eur. J. Org. Chem. 2005, 2005, 5277–5288.Search in Google Scholar
[23] G. G. Kleinspehn, J. Am. Chem. Soc. 1955, 77, 1546–1548.Search in Google Scholar
[24] C. M. Shiner, T. D. Lash, Tetrahedron 2005, 61, 11628–11640.10.1016/j.tet.2005.10.019Search in Google Scholar
[25] A. Alberola, A.González Ortega, M. Luisa Sádaba, C. Sañudo, Tetrahedron 1999, 55, 6555–6566.10.1016/S0040-4020(99)00289-6Search in Google Scholar
[26] S. Sarkar, K. Bera, S. Maiti, S. Biswas, U. Jana, Synth. Commun. 2012, 43, 1563–1570.Search in Google Scholar
[27] S. Maiti, S. Biswas, U. Jana, J. Org. Chem. 2010, 75, 1674–1683.Search in Google Scholar
[28] N. C. Jadhav, P. B. Gadhane, H. V. Patile, V. N. Telvekar, Tetrahedron Lett. 2013, 54, 3019–3021.Search in Google Scholar
[29] A. T. Khan, M. Lal, P. Ray Bagdi, R. Sidick Basha, P. Saravanan, S. Patra, Tetrahedron Lett. 2012, 53, 4145–4150.Search in Google Scholar
[30] C. C. Silveira, S. R. Mendes, G. M. Martins, S. C. Schlösser, T. S. Kaufman, Tetrahedron 2013, 69, 9076–9085.10.1016/j.tet.2013.08.035Search in Google Scholar
[31] G. R. Reddy, T. R. Reddy, S. C. Joseph, K. S. Reddy, M. Pal, RSC Adv. 2012, 2, 3387–3395.Search in Google Scholar
[32] B.-L. Li, P.-H. Li, X.-N. Fang, C.-X. Li, J.-L. Sun, L.-P. Mo, Z.-H. Zhang, Tetrahedron 2013, 69, 7011–7018.10.1016/j.tet.2013.06.049Search in Google Scholar
[33] A. B. Atar, Y. T. Jeong, Tetrahedron Lett. 2013, 54, 5624–5628.Search in Google Scholar
[34] Z.-H. Guan, L. Li, Z.-H. Ren, J.-L. Li, M.-N. Zhao, Green Chem. 2011, 13, 1664–1668.Search in Google Scholar
[35] M. M. Heravi, N. Tavakoli-Hoseini, F. F. Bamoharram, Synth. Commun. 2011, 41, 707–714.Search in Google Scholar
[36] N. Isambert, M. d. M. S. Duque, J.-C. Plaquevent, Y. Genisson, J. Rodriguez, T. Constantieux, Chem. Soc. Rev. 2011, 40, 1347–1357.Search in Google Scholar
[37] B. C. Ranu, S. Banerjee, Org. Lett. 2005, 7, 3049–3052.Search in Google Scholar
[38] B. C. Ranu, R. Jana, Eur. J. Org. Chem. 2006, 2006, 3767–3770.Search in Google Scholar
[39] B. C. Ranu, S. Banerjee, S. Roy, Indian J. Chem. 2008, 47B, 1108–1112.Search in Google Scholar
[40] S. Hanelt, J. Liebscher, Synlett 2008, 7, 1058–1060.10.1055/s-2008-1042915Search in Google Scholar
[41] H. M. Meshram, B. Madhu Babu, G. Santosh Kumar, P. B. Thakur, V. M. Bangade, Tetrahedron Lett. 2013, 54, 2296–2302.Search in Google Scholar
Supplemental Material
The online version of this article (DOI: 10.1515/znb-2014-0108) offers supplementary material, available to authorized users.
©2015 by De Gruyter
Articles in the same Issue
- Frontmatter
- In this Issue
- Editorial
- Zeitschrift für Naturforschung B now being published by De Gruyter
- Original Communications
- “Naked” S2O72– ions – the serendipitous formation of the disulfates [HPy]2[S2O7] and [bmim][HPy][S2O7] (HPy = pyridinium; bmim = 1-Butyl-3-methylimidazolium)
- Orthoamide und Iminiumsalze, LXXXVIII. Synthese N,N,N′,N′,N″,N″-persubstituierter Guanidiniumsalze aus N,N′-persubstituierten Harnstoff/Säurechlorid-Addukten**
- The acidic ionic liquid [BSO3HMIm]HSO4: a novel and efficient catalyst for one-pot, three-component syntheses of substituted pyrroles
- The influence of alkali-metal ions on the molecular and supramolecular structure of manganese(II) complexes with tetrachlorophthalate ligands
- New triazolothiadiazole and triazolothiadiazine derivatives as kinesin Eg5 and HIV inhibitors: synthesis, QSAR and modeling studies
- Two copper(I) complexes of bi- (or tri-)pyrazolyl ligands featuring Cu3pz3 or Cu4pz4 motifs
- Synthesis of ferrocenyl aryl ethers via Cu(I)/phosphine catalyst systems
- A prenylated acridone alkaloid and ferulate xanthone from barks of Citrus medica (Rutaceae)
- Synthesis and structural characterization of substituted phenols with a m-terphenyl backbone 2,4,6-R3C6H2OH (R=2,4,6-Me3C6H2, Me5C6)
- 2-Ethyl-1-phenylindazolium hexafluorophosphate. N-heterocyclic carbene formation, rearrangement, ring-cleavage reactions, and rhodium complex formation
Articles in the same Issue
- Frontmatter
- In this Issue
- Editorial
- Zeitschrift für Naturforschung B now being published by De Gruyter
- Original Communications
- “Naked” S2O72– ions – the serendipitous formation of the disulfates [HPy]2[S2O7] and [bmim][HPy][S2O7] (HPy = pyridinium; bmim = 1-Butyl-3-methylimidazolium)
- Orthoamide und Iminiumsalze, LXXXVIII. Synthese N,N,N′,N′,N″,N″-persubstituierter Guanidiniumsalze aus N,N′-persubstituierten Harnstoff/Säurechlorid-Addukten**
- The acidic ionic liquid [BSO3HMIm]HSO4: a novel and efficient catalyst for one-pot, three-component syntheses of substituted pyrroles
- The influence of alkali-metal ions on the molecular and supramolecular structure of manganese(II) complexes with tetrachlorophthalate ligands
- New triazolothiadiazole and triazolothiadiazine derivatives as kinesin Eg5 and HIV inhibitors: synthesis, QSAR and modeling studies
- Two copper(I) complexes of bi- (or tri-)pyrazolyl ligands featuring Cu3pz3 or Cu4pz4 motifs
- Synthesis of ferrocenyl aryl ethers via Cu(I)/phosphine catalyst systems
- A prenylated acridone alkaloid and ferulate xanthone from barks of Citrus medica (Rutaceae)
- Synthesis and structural characterization of substituted phenols with a m-terphenyl backbone 2,4,6-R3C6H2OH (R=2,4,6-Me3C6H2, Me5C6)
- 2-Ethyl-1-phenylindazolium hexafluorophosphate. N-heterocyclic carbene formation, rearrangement, ring-cleavage reactions, and rhodium complex formation

