Abstract
As a part of Alzheimer’s disease (AD) development the mammalian target of rapamycin (mTOR) has been reported to play a crucial role in regulating cognition and can be used as a neuronal marker. Neuro-inflammation is also a cause of the pathophysiological process in AD. Thus, we examined the protein expression levels of mTOR and its downstream pathways as well as pro-inflammatory cytokines (PICs) in the brain of AD rats. We further examined the effects of blocking mTOR on PICs, namely IL-1β, IL-6 and TNF-α. Our results showed that the protein expression of p-mTOR, mTOR-mediated phosphorylation of 4E-binding protein 4 (4E-BP1) and p70 ribosomal S6 protein kinase 1 (S6K1) pathways were amplified in the hippocampus of AD rats compared with controls. Blocking mTOR by using rapamycin selectively enhanced activities of IL-6 and TNF-α signaling pathways, which was accompanied with an increase of Caspase-3, indicating cellular apoptosis and worsened learning performance. In conclusion, our data for the first time revealed specific signaling pathways engaged in the development of AD, including a regulatory role by the activation of mTOR in PIC mechanisms. Stimulation of mTOR is likely to play a beneficial role in modulating neurological deficits in AD.Targeting one or more of these signaling molecules may present with new opportunities for treatment and clinical management of AD
1 Introduction
Alzheimer’s disease (AD) is a chronic neurodegenerative disease that usually starts slowly and gets worse over time [1, 2].The cause of Alzheimer’s disease is poorly understood [1, 2]. Nevertheless, neurodegeneration is the main pathological mechanism of cognitive impairments in AD. Emerging evidence has suggested that the mammalian target of rapamycin (mTOR)-dependent signaling is involved in the neurodegeneration of AD brains [3].
mTOR is a serine threonine protein kinase. There are two distinct mTOR forms of protein complexes, mTOR complex 1 (mTORC1) and mTORC2. In general, mTORC1 is composed of raptor, mLST8 and mTOR, and is known to be involved in the gate translation of most proteins by phosphorylation of specific downstream effectors including, p70 ribosomal S6 protein
kinase (p70 S6Ks) and 4E-BPs [4, 4]. mTOR, S6K1 and 4E-BP1 are expressed in the mammalian nervous system, particularly in the brain [4, 5].
mTORC1 is more sensitive to rapamycin than mTOR and its activation leads to promotion of the phosphorylation of downstream effectors, such p70 S6K1 and this further governs mRNA translation [5]. There is compelling evidence that supports the notion that mTOR plays an important role in the modulation of long-term neuronal plasticity in addition to its well-known roles in protein synthesis regulation and growth [4, 6]. Specifically, mTOR and its downstream effectors have been identified in the brain and their modulation contribute to the cognitive impairments in AD [3].
It should be noted that the role of mTOR activation in neurodegenerative diseases, especially in AD, seems to be very complex [3]. mTOR is mainly a trophic and protective kinase for neurons but could also be activated
sometimes during the progress of neuronal apoptosis. Similarly, an mTOR inhibitor was reported to be a pro-apoptotic agent, but could alleviate the pathological consequences in neurodegenerative diseases. mTOR is also implicated in the molecular mechanisms of learning and memory [7] that are impaired in AD.
Thus, we hypothesized that mTOR and its downstream signals in the hippocampus are upregulated in AD rats. This leads to amplified levels of pro-inflammatory cytokines (PICs) including interleukins IL-1β and IL-6, tumor necrosis factor α (TNF-α) and caspase-3 as indicators of neuronal apoptosis given that cerebral PICs are engaged in the pathophysiological process of AD. We further hypothesized that blocking mTOR signal pathways alters PICs and caspase-3 in the hippocampus of AD rats, thereby leading to changes of memory performance in AD rats.
Materials and methods
Animals
All the animal procedures were approved by the Institutional Animal Care and Use Committee of Jilin University, which were in compliance with the Guideline for the Care and Use of laboratory Animals of the U.S. National Health Institute. A total of fifty-three male Sprague-Dawley rats (3-4 months old weighting 300-350 g) were used in this study.
In order to chronically deliver drugs into the brain tissues via intracerebroventricular (ICV) infusion,the rats were anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneally) and were cannulated with an L-shaped stainless steel cannula aimed at the lateral ventricles (coordinates: 1.2 mm posterior to the bregma, 1.5 mm lateral to the midline, and 3.5 mm under the dura) according to Swanson’s Rat Brain Atlas [8]). The guide cannula was fixed to the skull using dental zinc cement and jewelers’ screw. The cannula was connected to an osmotic minipump (Alzet pump brain infusion kit, DURECT Inc., Cupertino, CA, USA) with polycarbonate tubing. The pumps were placed subcutaneously between the scapulae. This intervention allowed animals to receive continuous ICV infusion via the minipumps before brain tissues were removed.
The Aβ, 1-42 peptides (ANASPEC Inc., Fremont, CA, USA) were dissolved in artificial cerebrospinal fluid (aCSF) at a concentration of 1 mg/ml. Continuous infusion of 10 mg/kg of Aβ1-42 into the right ventricle was maintained for two weeks by attachment of an infusion kit to an osmotic mini-pump (Alzet 1002; Alzet, DURECT Inc., Cupertino, CA, USA) [9]. Infusion of aCSF served as a control. Likewise, a pump implanted into the left ventricle was loaded with rapamycin, an inhibitor of mTOR. Rapamycin was delivered at 0.25 μl per hour (Alzet Model 1002, a total of 500 μg of rapamycin was given for two weeks). Accordingly, the rats were divided into three groups: sham control (group 1 - sham control; n = 15); AD rats (group 2; n = 20) and AD rats with infusion of rapamycin (group 3; n=18).
1 Spontaneous alternation performance
Spatial working memory performance was assessed on the fourth and eighth week after
αβ1-42 infusion, by recording spontaneous alternation performance in a Y-maze.The maze was made of grey-painted vinyl-chloride. Each arm was 50 cm long, 30 cm high and 10 cm wide and converged at an equal angle. Each rat was placed at the center of the maze and allowed to move freely through it during an 8-min period. The numbers of arm entries were recorded for 8 minutes. An alternation was defined as entries into all arms. The percentage of alternation was calculated as (actual alternations/total entered-2)x100.
After completion of the treatments and behavioral test, rats were anesthetized and decapitated. The hippocampal CA1 area was removed for further analysis of the protein expression of mTOR and downstream pathways and the levels of PICs. As previously reported by others [9, 10], in our present study αβ1-42 was infused unilaterally via the ICV. It is noted that we did not observe significant differences in expression of mTOR and its downstream pathways between the right and left sides of the CA1 region in our preliminary experiments. Thus, in the present report we collected all CA1 samples from the right side of CA1 region for measurements.
Western blot analysis
All the tissues from individual rats were sampled for the analysis. In brief, the hippocampus of the rats was removed. Total protein was then extracted by homogenizing the hippocampus sample in ice-cold immunoprecipitation assay buffer with a protease inhibitor cocktail kit. The lysates were centrifuged and the supernatants were collected for measurements of protein concentrations using a bicinchoninic acid assay reagent kit.
After being denatured by heating at 95°C for 5 min in buffer, the supernatant samples containing 20 μg of protein were loaded onto 4-20% Mini-PROTEAN TGX gels and electrically transferred to a polyvinylidene fluoride membrane. The membrane was blocked in 5% nonfat milk in 0.1 % Tween-TBS buffer and was incubated overnight with respective primary antibody. The primary antibodies include: rabbit anti-p-mTOR /p-S6K1/P-4E-BP1 antibodies (1:200); rabbit anti-mTOR/S6K1/4E-BP1 antibodies (1:200-1:500); and rabbit anti-caspase-3 (cleaved) antibody (1:200). Next,the membranes were washed and incubated with an alkaline phosphatase conjugated anti-rabbit secondary antibody (1:500). All these primary and secondary antibodies were purchased from the Abcam Co. (Abcam, Cambridge, UK) and Santa Cruz Biotechology, Inc. (Santa Cruz, CA, USA). The immunoreactive proteins were detected by enhanced chemiluminescence. The bands recognized by the primary antibody were visualized by exposure of the membrane onto an X-ray film. The membrane was stripped and incubated with mouse anti-β-actin to show equal loading of the protein.Then, the film was scanned and the optical density of all protein bands was first analyzed using the Scion Image software, and values for densities of immunoreactive bands/β-actin band densities from the same lane were determined. Each of the values was then normalized to a control sample.
ELISA methods
The levels of PICs were examined using an ELISA assay kit (Promega Co., Madison, Wl, USA) according to the provided description and modification. Briefly, polystyrene 96-well microtiter immunoplates were coated with affinity-purified rabbit anti-IL-1 β, anti-IL-6 and anti-TNF-α antibodies. Parallel wells were coated with purified rabbit IgG for evaluation of nonspecific signals. After overnight incubation, plates were washed. Then, the diluted samples and these PIC standard solutions were distributed in each plate. The plates were washed and incubated with anti- IL-1β, IL-6 and TNF-α galactosidase. Then, the plates were washed and incubated with substrate solution. After incubation, the optical density was measured using an ELISA reader.
1 Statistical analysis
The data to compare control rats and AD rats were analyzed using a student’s t-test. The data to compare control rats, AD rats and AD rats with rapamycin were analyzed using oneway ANOVA with multiple comparisons. Values are presented as means ± standard deviation. For all analyses, differences were considered significant at P < 0.05. All statistical analyses were performed by using SPSS for Windows version 13.0 (SPSS Inc., Chicago, IL, USA).
Results
Expression of mTOR pathways
Figure 1 demonstrates the protein expression of p-mTOR, p-S6K1 and p-4E-BP1 as well as mTOR, S6K1 and 4E-BP1 in control rats and AD rats. The protein levels of p-mTOR and mTOR-mediated p-S6K1 and p-4E-BP1 were significantly increased in the hippocampal tissues of AD rats as compared with control rats (P < 0.05, n = 6-10 in each group). There were no significant differences observed in total protein of mTOR, S6K1 and 4E-BP1 between both groups. Nonetheless, the ratio of p-mTOR, p-S6K1 and p-4E-BP1 levels vs. total protein of mTOR, S6K1 and 4E-BP1 levels was also significantly increased in AD rats.

Averaged data show increases in p-mTOR, p-S6K1 and p-4E-BP1 in the hippocampal tissues of AD rats vs. control rats (n = 6-10). There were no significantly differences in total protein of mTOR, S6K1 and 4E-BP1 between two groups. B. The ratio of p-mTOR, p-S6K1 and p-4E-BP1 levels vs. mTOR, S6K1 and 4E-BP1 levels was significantly increased in AD rats, respectively. *P < 0.05 vs. control rats. C. representative typical bands.
Levels of PICs and caspase-3
Figure 2 shows that IL-1 β, IL-6 and TNF-αwere significantly elevated in the hippocampal tissues of AD rats (n = 20, P < 0.05 vs. control rats, n = 15) as compared with control rats. Also, the protein expression of Caspase-3 was significantly elevated in the hippocampus of AD rats.
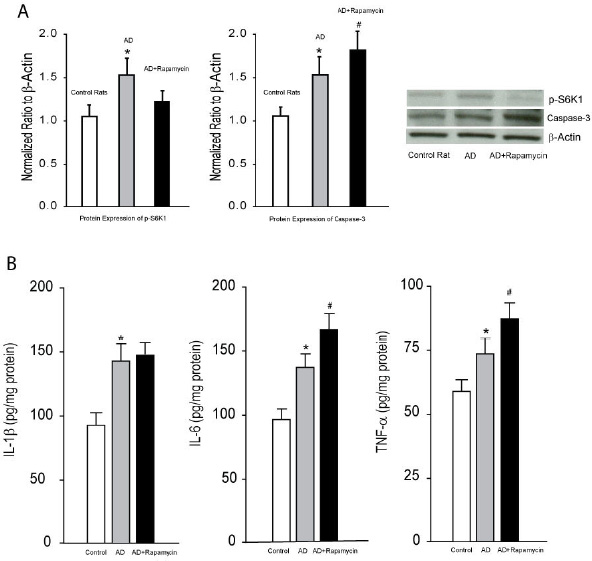
A. Averaged data (left panel) and typical bands (right panel) show that p-S6K and caspase-3 were increased in the hippocampus of AD rats as compared with control rats. Furthermore, infusion of rapamycin attenuated increases of p-S6K and amplified increases of caspase-3 in AD animals. *P < 0.05 vs. control rats and AD rats with rapamycin for p-S6K; and vs. control rats for caspase-3. #P < 0.05 vs. AD rats; n = 6-10 in each group. B. The levels of IL-1 β, IL-6 and TNF-α were amplified in the hippocampal tissues of AD rats. Inhibition of mTOR signal with infusion of rapamycin augmented increases of IL-6 and TNF-α in AD rats, but failed to affect IL-1β. *P < 0.05, AD rats (n = 20) vs. control rats (n = 15); and #P < 0.05, AD rats with rapamycin (n = 18) vs. AD rats (n = 20).
Effects of blocking mTOR on PICs and caspase-3
Figure 2 further demonstrates the effects of blocking mTOR on PICs and caspase-3. First, inhibition of mTOR by ICV infusion of rapamycin effectively attenuated its downstream pathway, indicated by rapamycin significantly decreasing the expression levels of p-S6K1. It is noted that infusion of rapamycin amplified the levels of IL-6 and TNF-α, but not IL-1 β in AD rats [P < 0.05, AD rats with rapamycin (n = 18) vs. AD rats (n = 20)]. Infusion of rapamycin also increased the expression of caspase-3 in AD rats.
Spontaneous alternation performance
The number of arm entries was determined by counting the number of arms each animal entered in the maze during the test as a measure of activity level. No significant differences in the number of arm entries were observed among three groups. The number of arm entries for each group was: control rats, 16.6 ± 3.2; AD rats, 16.8 ± 4.1; AD rats infused with rapamycin, 17.8 ± 3.9, respectively (P > 0.05 among three groups). Then, spatial working memory performance was assessed by recording spontaneous alternation performance. Figure 3 demonstrates that the percentage of spontaneous alternation was impaired in AD rats as compared with control rats. A decrease of spontaneous alternation was greater at the eighth week after Aβ1-42 infusion than at the fourth week after its infusion, i.e., the percentage of spontaneous alternation in control rats with ICV infusion of aCSF was 75 ± 13%; and 63 ± 10% (P < 0.05 vs. control rats) and 53 ± 9% (P < 0.05 vs. control rats) in rats with infusion of Aβ1-42 at the fourth week and at the eighth week, respectively. In addition,blocking mTOR decreased the percentage of spontaneous alternation in AD rats and this appeared to be greater at the fourth week. They were 39 ± 6% at the fourth week (P < 0.05 vs.AD rat without rapamycin) and 43 ± 9% at the eighth week (P < 0.05 vs. AD rat without rapamycin).
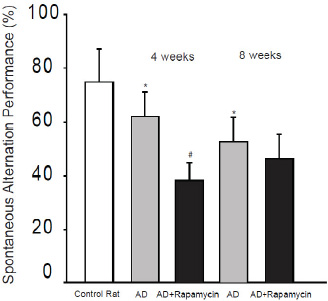
Aβ1-42 was infused intracerebroventricularly to induce AD and its effects on spontaneous alternation performance in rats were determined. The learning performance was examined at fourth week and at eighth week after Aβ1-42. *P < 0.05, AD rats (n = 20) vs. control rats (n = 15); and # P < 0.05, AD rats with rapamycin (n = 18) vs. AD rats (n = 20).
Discussion
A number of animal models have been used to study AD [11]. Among them, administering a solution containing the human form of Aβ 1-42 via ICV route into a rat brain is generally used [9, 12]. Aβ1-42 was chosen because of its superior aggregating properties and because it was thought to constitute the nucleus of any amyloid plaque formation [11]. In addition, oxidative species are generated either from, but not restricted to, neuro-inflammation, mitochondria respiratory chain impairment [13, 14] or from a direct effect of the amyloid peptide [15]. In this animal model, behavioral tests including spontaneous alternation performance and water maze task appeared abnormal [9, 12]. Thus, this rat model was employed in the current study and impairment of the learning performance was observed in rats infused with Aβ1-42. Our data further demonstrated that the protein expression of p-mTOR, mTOR-mediated p- S6K1 and p-4E-BP1 pathways are amplified in the hippocampus of AD rats compared with controls. Interestingly, blocking mTOR using rapamycin selectively enhances activities of IL-6 and TNF-α signaling pathways, which is accompanied with an increase of apoptotic caspase-3.
In general, clinical and experimental studies indicate that inhibition of mTOR can be beneficial for some pathological conditions such as cognitive impairment, whereas direct or indirect activation of mTOR can also be beneficial for other pathologies such as axonal growth and nerve regeneration [16]. Consistent with the prior studies published by Caccamo et al. and by Majumder et al. [17, 18], data of our present study also demonstrated that expression of mTOR and its downstream signal is increased and cognitive function is impaired in AD rats induced by Aβ1-42. In those prior reports [17, 18], blocking mTOR using rapamycin can attenuate the levels of endogenous Aβ and indeed alleviate AD symptoms. In contrast, our data suggest that rapamycin can worsen cognitive function in rats injected with Aβ1-42. Several differences were noticed in our study and those prior reports. First, a rat model of Aβ-induced AD was used in our study; whereas transgenic 3xTg-AD mice were used in their report. Second, rats were 3-4 months old and mice were 6 months old or older. Third, we administered rapamycin via ICV (a total 500 μg for two weeks); whereas they gave rapamycin orally (2.24 mg/kg in a microencapsulated form for ten weeks) [17]. The size of rapamycin dosage can affect neuronal activity and neurological functions differently. It has been reported that acute administration of a low dose of rapamycin adulterates neuronal activity in rats which contribute to the development of neurological behavior such as cognitive functions [19],raising a question of how rapamycin can alter neurological functions. Taken together, the different effects of rapamycin on cognitive functions observed in our study and others’ reports could be due to dosages of rapamycin and their administration pathways, animal species and ages. Moreover, the relationship between Aβ and mTOR is more complex [20], i.e., Aβ itself can increase mTOR activity, whereas a long-term inhibition of mTOR by rapamycin can decrease Aβ and improve cognitive functions [17, 21]. An important difference is that in our rat model exogenous Aβ was given and this is likely to lead to upregulation of mTOR linked to cognitive function. In those rats, PICs were also increased in the hippocampus. It has been reported that there is an interaction between PICs activation and mTOR signal pathway [22]. It has also been reported that mTOR can positively regulate IL-6 and TNF-α and negatively regulate other PICs [23, 24]. In our study, we observed that rapamycin amplified the expression of IL-6 and TNF-α. It should be noted that increases in PICs are also found in the hippocampus of other AD animal models [25]. It is assumed that rapamycin may play a role in regulating the negative feedback loops emanating from the PIC signaling pathway. Although it still needs more mechanistic evidence to support this possibility, this raises an issue of how rapamycin influences neuronal activity and neurological functions [19]. Nonetheless, it needs be acknowledged that there are some study limitations in our present report.Those include that we did not determine the effects of rapamycin on cognitive function in normal rats, and that we did not determine if rapamycin can affect the endogenous levels of Aβ linked to cognitive function since we injected Aβ via ICV. Additional studies need to clarify the remaining issues.
Cell signaling through mTOR is regulated by cellular energy level as well as by mitogens and nutrients [26]. As a main modulator of cell growth and proliferation, mTOR controls the efficiency of protein translation within cells via its downstream targets. These translation regulators include the eukaryotic initiation factor 4E-BP1 and p70-S6K. Although neurons are finally differentiated, the size of the neuronal cell soma in diseased conditions is regulated by mTOR [27]. Additional studies indicate a role for mTOR in the physiological processes of neuronal development as well as in the neuropathological processes of different brain diseases [7, 28].
Also, p-S6K appears especially in neurons that are predicted to develop neurofibrillary tangles
(NFTs) at later stages [29]. By indirect enzymelinked immunosorbent assay, p-S6K studies showed significant increases in homogenates of the medial temporal cortex from AD patients as compared to control brains. In the same set of tissues from AD and control cases, a dramatic increase in p-mTOR and p-4E-BP1 was found in AD brains [30, 31]. The increase of mTOR-dependent signaling was positively and significantly correlated with development of AD. Thus, it is speculated that continuous degenerating neurons are regulated by upregulated mTOR-dependent signaling through the p70S6K and 4E-BP1 pathways [32].
It has been reported that PICs are involved in alterations of cellular damage related to development of AD and significant increases in IL-1 β, IL-6 and TNF-α in the brain were observed following αβ1-42 injection [33]. Consistent with the previous results, our present data further demonstrated that AD induces increasing levels of IL-1β, IL-6 and TNF-α in the hippocampal tissues. Importantly, our data showed that inhibition of mTOR significantly amplified IL-6 and TNF-α. However, the effects of inhibiting mTOR on IL-1 β are not significant. One may consider that IL-6 and TNF-α become transcribed and translated from the very beginning after the activating signal, whereas IL-1β becomes produced first as an inactive pro-form and then is released as its mature and secreted form following the activating signal [34, 36]. We presume that mTOR activated during development of AD affects different signaling pathways in the cellular nucleus and cytoplasm in regulating PICs.
Caspases, a family of thiol proteases, are activated during the neurodegenerative process of AD and play an important regulating role in the apoptotic cascade [37, 38]. A feature of caspases in the cell is that they exist as zymogens, termed pro-caspases, which are inactive until a biochemical change causes their activation [39].The processing of pro-caspase-3 to its active form is considered a key processing point in the death-signaling cascade. As an executioner caspase, the pro-caspase-3 becomes active until it is cleaved by an initiator caspase after apoptotic signaling events have occurred [40]. However, the zymogen feature of caspase-3 is necessary because if unregulated,caspase activity would kill cells indiscriminately [41]. It has been reported that caspase-3 is a predominant target involved in PIC-mediated apoptosis in neuronal cells during αβ1-42-induced memory impairment [42]. Thus, in the current study, we also determined the protein expression levels of a cleaved form caspase-3 in the hippocampal tissues as an indicator of cellular apoptosis. We found that caspase-3 is increased in the hippocampus of AD rats.
Prior studies in humans and animal models have demonstrated that AD is generally associated with an initial diseased tissue insult such as cell loss in the hippocampus during neurodegeneration [43, 44]. It is indicated that apoptosis is engaged in the pathological process of AD-induced tissue loss in the brain, and a greater number of cells that are stained with terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) are found in the hippocampus [44]. Also, sequential activation of caspase-3 plays an important role in the execution-phase of cell apoptosis linked to cell death in AD model. Nevertheless, as an important factor in mediating cell apoptosis, the role played by mTOR in regulating caspase-3 expression in engagement of the pathophysiological process of AD is lacking. Data from our present study provides the first evidence that inhibition of mTOR enhances Caspase-3 observed in the hippocampus of AD rats. Whereas inhibition of the enzymatic activity of Caspase-3 likely provides a mechanism to attenuate the cellular apoptosis, our data suggests a beneficial role played by activation of mTOR. Elevated levels of PICs in the hippocampal tissues are associated with increased disease vulnerability and AD-related pathological changes such as cell death [33]. Our data also showed that inhibition of mTOR increases upregulation of IL-6 and TNF-α in AD rats, but not IL-1β. This also worsens the learning performance observed in AD rats. Overall, we suggest that mTOR activity in the brain is likely to contribute to the tissue damage selectively via IL-6 and TNF-α signaling pathway during development of AD and this leads to neurological deficits.
In conclusion, we have provided evidence that the levels of mTOR as well as IL-1β, IL-6 and TNF-α are amplified in the hippocampus of AD rats.In the process of AD, mTOR has regulatory effects on IL-6 and TNF-α, and caspase-3. Results of our study may offer promising clues for the development of new therapeutic strategies to target specific mTORPICs pathways for managing intractable neurological symptoms observed in patients with AD.
Acknowledgements
Conflict of interest statement: The authors declare that they have no competing interest to disclose. This study was supported by grants from the First Hospital of Jilin University (JDYY52015016), Norman Bethune Program of Jilin University (No. 2015335), Science and Technology Development Program of Jilin Province (20160520160JH), and grants from Health Department of Jilin Province (2012Z005) as well as from the National Natural Science Foundation of China (No. 81471830).
References
[1] Burns A., Iliffe S., Alzheimer’s disease, Brit. Med. J., 2009, 338, b15810.1136/bmj.b158Suche in Google Scholar
[2] Querfurth H.W., LaFerla F.M., Alzheimer’s disease, New Engl. J. Med.,2010, 362, 329-34410.1056/NEJMra0909142Suche in Google Scholar
[3] Pei J.J., Hugon J., mTOR-dependent signalling in Alzheimer’s disease,J. Cell. Mol. Med., 2008, 12, 2525-253210.1111/j.1582-4934.2008.00509.xSuche in Google Scholar
[4] Banko J.L., Poulin F., Hou L., DeMaria C.T., Sonenberg N., Klann E.,The translation repressor 4E-BP2 is critical for elF4F complex formation,synaptic plasticity, and memory in the hippocampus, J. Neurosci.,2005, 25, 9581-959010.1523/JNEUROSCI.2423-05.2005Suche in Google Scholar
[5] Hay N., Sonenberg N., Upstream and downstream of mTOR, Genes Dev.,2004, 18, 1926-194510.1101/gad.1212704Suche in Google Scholar
[6] Costa-Mattioli M., Sossin W.S., Klann E., Sonenberg N., Translational control of long-lasting synaptic plasticity and memory, Neuron,2009, 61, 10-2610.1016/j.neuron.2008.10.055Suche in Google Scholar
[7] Jaworski J., Sheng M., The growing role of mTOR in neuronal development and plasticity, Mol. Neurobiol., 2006, 34, 205-21910.1385/MN:34:3:205Suche in Google Scholar
[8] Swanson L.W. Brain maps: structure of the rat brain, 2nd ed., Elsevier,New York, 1998Suche in Google Scholar
[9] Nakamura S., Murayama N., Noshita T., Annoura H., Ohno T., Progressive brain dysfunction following intracerebroventricular infusion of β1-42-amyloid peptide, Brain Res., 2001, 912, 128-13610.1016/S0006-8993(01)02704-4Suche in Google Scholar
[10] Yun H.M., Kim H.S., Park K.R., Shin J.M., Kang A.R., il Lee K., et al.,Placenta-derived mesenchymal stem cells improve memory dysfunction in an Aβ1-42-infused mouse model of Alzheimer’s disease,Cell Death Dis., 2013, 4, e95810.1038/cddis.2013.490Suche in Google Scholar
[11] Lecanu L., Papadopoulos V., Modeling Alzheimer’s disease with non-transgenic rat models, Alzheimers Res.Ther, 2013, 5, 1710.1186/alzrt171Suche in Google Scholar PubMed PubMed Central
[12] Lecanu L., Greeson J., Papadopoulos V., β-amyloid and oxidative stress jointly induce neuronal death, amyloid deposits, gliosis, and memory impairment in the rat brain, Pharmacology, 2006, 76, 19-3310.1159/000088929Suche in Google Scholar PubMed
[13] Sochocka M., Koutsouraki E.S., Gasiorowski K., Leszek J., Vascular oxidative stress and mitochondrial failure in the pathobiology of Alzheimer’s disease: a new approach to therapy, CNS Neurol. Disord.Drug Targets, 2013, 12, 870-88110.2174/18715273113129990072Suche in Google Scholar PubMed
[14] Yan M.H., Wang X., Zhu X., Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease, Free Radic. Biol. Med., 2013, 62, 90-10110.1016/j.freeradbiomed.2012.11.014Suche in Google Scholar PubMed PubMed Central
[15] Butterfield D.A., Swomley A.M., Sultana R., Amyloid β-peptide (1-42)-induced oxidative stress in Alzheimer disease: importance in disease pathogenesis and progression, Antioxid. Redox Signal., 2013, 19, 823-83510.1089/ars.2012.5027Suche in Google Scholar PubMed PubMed Central
[16] Bockaert J., Marin P., mTOR in brain physiology and pathologies, Physiol. Rev., 2015, 95, 1157-118710.1152/physrev.00038.2014Suche in Google Scholar PubMed
[17] Caccamo A., Majumder S., Richardson A., Strong R., Oddo S., Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-β, and tau: effects on cognitive impairments, J. Biol. Chem., 2010, 285, 13107-1312010.1074/jbc.M110.100420Suche in Google Scholar PubMed PubMed Central
[18] Majumder S., Richardson A., Strong R., Oddo S., Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits, PloS One, 2011, 6, e2541610.1371/journal.pone.0025416Suche in Google Scholar PubMed PubMed Central
[19] Hadamitzky M., Herring A., Keyvani K., Doenlen R., Krugel U., Bosche K., et al., Acute systemic rapamycin induces neurobehavioral alterations in rats, Behav. Brain Res., 2014, 273, 16-2210.1016/j.bbr.2014.06.056Suche in Google Scholar PubMed
[20] Wong M., Mammalian target of rapamycin (mTOR) pathways in neurological diseases, Biomed. J., 2013, 36, 40-5010.4103/2319-4170.110365Suche in Google Scholar PubMed PubMed Central
[21] Spilman P., Podlutskaya N., Hart M.J., Debnath J., Gorostiza O., Bredesen D., et al., Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-β levels in a mouse model of Alzheimer’s disease, PloS One, 2010, 5, e997910.1371/journal.pone.0009979Suche in Google Scholar PubMed PubMed Central
[22] Thomson A.W., Turnquist H.R., Raimondi G., Immunoregulatory functions of mTOR inhibition, Nat. Rev. Immunol., 2009, 9, 324-33710.1038/nri2546Suche in Google Scholar
[23] Paschoal V.A., Amano M.T., Belchior T., Magdalon J., Chimin P., Andrade M.L., et al., mTORC1 inhibition with rapamycin exacerbates adipose tissue inflammation in obese mice and dissociates macrophage phenotype from function, Immunobiology, 2016, 10.1016/j.imbio.2016.09.014 [Epub ahead of print]Suche in Google Scholar
[24] Vangan N., Cao Y., Jia X., Bao W., Wang Y., He Q., et al., mTORCI mediates peptidoglycan induced inflammatory cytokines expression and NF-kappaB activation in macrophages, Microb. Pathog., 2016, 99, 111-11810.1016/j.micpath.2016.08.011Suche in Google Scholar
[25] Sil S., Goswami A.R., Dutta G., Ghosh T., Effects of naproxen on immune responses in a colchicine-induced rat model of Alzheimer’s disease, Neuroimmunomodulation, 2014, 21, 304-32110.1159/000357735Suche in Google Scholar
[26] Tee A.R., Blenis J., Proud C.G., Analysis of mTOR signaling by the small G-proteins, Rheb and RhebL1, FEBS Lett., 2005, 579, 4763-476810.1016/j.febslet.2005.07.054Suche in Google Scholar
[27] Kwon C.H., Zhu X., Zhang J., Baker S.J., mTor is required for hypertrophy of Pten-deficient neuronal soma in vivo, Proc. Natl. Acad. Sci. USA,2003, 100, 12923-1292810.1073/pnas.2132711100Suche in Google Scholar
[28] Swiech L, Perycz M., Malik L., Jaworski J., Role of mTOR in physiology and pathology of the nervous system, Biochim. Biophys. Acta, 2008, 1784, 116-13210.1016/j.bbapap.2007.08.015Suche in Google Scholar
[29] An W.L., Cowburn R.F., Li L., Braak H., Alafuzoff I., Iqbal K., et al.,Up-regulation of phosphorylated/activated p70 S6 kinase and its relationship to neurofibrillary pathology in Alzheimer’s disease,Am.J. Pathol., 2003, 163, 591-60710.1016/S0002-9440(10)63687-5Suche in Google Scholar
[30] Li X., Alafuzoff I., Soininen H., Winblad B., Pei J.J., Levels of mTOR and its downstream targets 4E-BP1, eEF2, and eEF2 kinase in relationships with tau in Alzheimer’s disease brain, FEBS J., 2005, 272, 4211 -422010.1111/j.1742-4658.2005.04833.xSuche in Google Scholar PubMed
[31] Li X., An W.L., Alafuzoff I., Soininen H., Winblad B., Pei J.J., Phosphorylated eukaryotic translation factor 4E is elevated in Alzheimer brain, Neuroreport, 2004, 15, 2237-224010.1097/00001756-200410050-00019Suche in Google Scholar PubMed
[32] Pei J.J., Björkdahl C., Zhang H., Zhou X., Winblad B., p70 S6 kinase and tau in Alzheimer’s disease, J. Alzheimers Dis., 2008, 14, 385-39210.3233/JAD-2008-14405Suche in Google Scholar PubMed
[33] Su F., Bai F., Zhang Z., Inflammatory cytokines and Alzheimer’s disease: a review from the perspective of genetic polymorphisms,Neurosci. Bull., 2016, 32, 469-48010.1007/s12264-016-0055-4Suche in Google Scholar PubMed PubMed Central
[34] Dinarello C.A., Proinflammatory cytokines, Chest, 2000, 118, 503-50810.1378/chest.118.2.503Suche in Google Scholar PubMed
[35] Oppenheim J.J., Cytokines: past, present, and future, Int. J. Hematol.,2001, 74, 3-810.1007/BF02982543Suche in Google Scholar PubMed
[36] Tedgui A., Mallat Z., Cytokines in atherosclerosis: pathogenic and regulatory pathways, Physiol. Rev., 2006, 86, 515-58110.1152/physrev.00024.2005Suche in Google Scholar PubMed
[37] Sinha A., Tamboli R.S., Seth B., Kanhed A.M., Tiwari S.K., Agarwal S., et al., Neuroprotective role of novel triazine derivatives by activating Wnt/β catenin signaling pathway in rodent models of Alzheimer’s disease, Mol. Neurobiol., 2015, 52, 638-65210.1007/s12035-014-8899-ySuche in Google Scholar PubMed
[38] Yun N., Lee Y.M., Kim C., Shibayama H., Tanimura A., Hamanaka Y., et al., Anamorsin, a novel caspase-3 substrate in neurodegeneration, J. Biol. Chem., 2014, 289, 22183-2219510.1074/jbc.M114.552679Suche in Google Scholar PubMed PubMed Central
[39] Salvesen G.S., Caspases: opening the boxes and interpreting the arrows, Cell Death Differ., 2002, 9, 3-510.1038/sj.cdd.4400963Suche in Google Scholar PubMed
[40] Walters J., Pop C., Scott F.L., Drag M., Swartz P., Mattos C., et al., A constitutively active and uninhibitable caspase-3 zymogen efficiently induces apoptosis, Biochem. J., 2009, 424, 335-34510.1042/BJ20090825Suche in Google Scholar PubMed PubMed Central
[41] Boatright K.M., Salvesen G.S., Mechanisms of caspase activation, Curr. Opin. Cell Biol.,, 2003, 15, 725-73110.1016/j.ceb.2003.10.009Suche in Google Scholar PubMed
[42] Lai J., Hu M., Wang H., Hu M., Long Y., Miao M.X., et al., Montelukast targeting the cysteinyl leukotriene receptor 1 ameliorates Aβ1-42-induced memory impairment and neuroinflammatory and apoptotic responses in mice, Neuropharmacology, 2014, 79, 707-71410.1016/j.neuropharm.2014.01.011Suche in Google Scholar PubMed
[43] Day R.J., Mason M.J., Thomas C., Poon W.W., Rohn T.T, Caspase-cleaved tau co-localizes with early tangle markers in the human vascular dementia brain, PloS One, 2015, 10, e013263710.1371/journal.pone.0132637Suche in Google Scholar PubMed PubMed Central
[44] Zhang Q., Li N., Jiao X., Qin X., Kaur R., Lu X., et al., Caspase-3 short hairpin RNAs: a potential therapeutic agent in neurodegeneration of aluminum-exposed animal model, Curr. Alzheimer Res., 2014, 11, 961-97010.2174/1567205011666141107150938Suche in Google Scholar PubMed
© 2016 Xu Wang et al.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Artikel in diesem Heft
- Research Article
- Early predictors and prevention for post-stroke epilepsy: changes in neurotransmitter levels
- Research Article
- The neonatal levels of TSB, NSE and CK-BB in autism spectrum disorder from Southern China
- Research Article
- Role of cerebral blood flow in extreme breath holding
- Review Article
- Teneurins, TCAP, and latrophilins: roles in the etiology of mood disorders
- Review Article
- How air pollution alters brain development: the role of neuroinflammation
- Research Article
- Allelic distribution of BDNF Val66Met polymorphism in healthy Romanian volunteers
- Review Article
- The serotonergic system and cognitive function
- Research Article
- Blocking mammalian target of rapamycin (mTOR) improves neuropathic pain evoked by spinal cord injury
- Research Article
- Emotional conflict occurs at a late stage: evidence from the paired-picture paradigm
- Research Article
- Sleep-wake patterns and sleep quality in urban Georgia
- Research Article
- Presenilin 2 overexpression is associated with apoptosis in Neuro2a cells
- Research Article
- No association of AQP4 polymorphisms with neuromyelitis optica and multiple sclerosis
- Research Article
- Serum C-reactive protein, fibrinogen and D-dimer in patients with progressive cerebral infarction
- Research Article
- Is the left uncinate fasciculus associated with verbal fluency decline in mild Alzheimer's disease?
- Research Article
- High fibrosis indices in cerebrospinal fluid of patients with shunt-dependent post-traumatic chronic hydrocephalus
- Review Article
- Enhancing attention in neurodegenerative diseases: current therapies and future directions
- Research Article
- Prediction of the long-term efficacy of STA-MCA bypass by DSC-PI
- Research Article
- Cortical function in Alzheimer’s disease and frontotemporal dementia
- Research Article
- More than a mere sequence: predictive processing of wh-dependencies in early bilinguals
- Research Article
- Blocking PAR2 alleviates bladder pain and hyperactivity via TRPA1 signal
- Research Article
- Gene expression profiling of the dorsolateral and medial orbitofrontal cortex in schizophrenia
- Research Article
- Cerebral mTOR signal and pro-inflammatory cytokines in Alzheimer’s disease rats
- Research Article
- Effects of ulinastatin on global ischemia via brain pro-inflammation signal
- Research Article
- Comparison of MSC-Neurogenin1 administration modality in MCAO rat model
Artikel in diesem Heft
- Research Article
- Early predictors and prevention for post-stroke epilepsy: changes in neurotransmitter levels
- Research Article
- The neonatal levels of TSB, NSE and CK-BB in autism spectrum disorder from Southern China
- Research Article
- Role of cerebral blood flow in extreme breath holding
- Review Article
- Teneurins, TCAP, and latrophilins: roles in the etiology of mood disorders
- Review Article
- How air pollution alters brain development: the role of neuroinflammation
- Research Article
- Allelic distribution of BDNF Val66Met polymorphism in healthy Romanian volunteers
- Review Article
- The serotonergic system and cognitive function
- Research Article
- Blocking mammalian target of rapamycin (mTOR) improves neuropathic pain evoked by spinal cord injury
- Research Article
- Emotional conflict occurs at a late stage: evidence from the paired-picture paradigm
- Research Article
- Sleep-wake patterns and sleep quality in urban Georgia
- Research Article
- Presenilin 2 overexpression is associated with apoptosis in Neuro2a cells
- Research Article
- No association of AQP4 polymorphisms with neuromyelitis optica and multiple sclerosis
- Research Article
- Serum C-reactive protein, fibrinogen and D-dimer in patients with progressive cerebral infarction
- Research Article
- Is the left uncinate fasciculus associated with verbal fluency decline in mild Alzheimer's disease?
- Research Article
- High fibrosis indices in cerebrospinal fluid of patients with shunt-dependent post-traumatic chronic hydrocephalus
- Review Article
- Enhancing attention in neurodegenerative diseases: current therapies and future directions
- Research Article
- Prediction of the long-term efficacy of STA-MCA bypass by DSC-PI
- Research Article
- Cortical function in Alzheimer’s disease and frontotemporal dementia
- Research Article
- More than a mere sequence: predictive processing of wh-dependencies in early bilinguals
- Research Article
- Blocking PAR2 alleviates bladder pain and hyperactivity via TRPA1 signal
- Research Article
- Gene expression profiling of the dorsolateral and medial orbitofrontal cortex in schizophrenia
- Research Article
- Cerebral mTOR signal and pro-inflammatory cytokines in Alzheimer’s disease rats
- Research Article
- Effects of ulinastatin on global ischemia via brain pro-inflammation signal
- Research Article
- Comparison of MSC-Neurogenin1 administration modality in MCAO rat model

