Abstract
Objectives
In the current study, we synergistically evaluated vascular endothelial growth factor (VEGF) gene expression levels and signal peptide-CUB-EGF domain-containing protein 1 (SCUBE1) gene expression levels in diabetic patients without retinopathy, non-proliferative diabetic retinopathy (NPDR), and proliferative diabetic retinopathy (PDR).
Methods
94 blood samples from 26 healthy controls, 29 non-DR, 22 NPDR, and 17 PDR patients were collected in sterile EDTA tubes. Total RNA was obtained from these samples without waiting and then converted to cDNA. The expression levels of the VEGF and SCUBE1 genes were determined by quantitative real-time polymerase chain reaction (qPCR).
Results
SCUBE1 gene expression levels were 2.15 (p=0.015), 1.75 (p=0.799), 2.37 (p=0.037) times higher, and VEGF gene expression levels were 1.71 (p=0.023), 1.75 (p=0.012), 1.85 (p=0.031) times higher in the non-DR, NPDR, and PDR groups compared to the control group, respectively. VEGF gene expression levels were significantly higher in participants with HbA1c levels ≥5.7% compared to those with <5.7. SCUBE1 and VEGF gene expression levels were significantly higher in participants with fasting plasma glucose (FPG) levels ≥126 mg/dL than those with <126 mg/dL.
Conclusions
As a result, SCUBE1 gene expression levels are higher than VEGF gene expression levels, especially in the PDR group. Therefore, SCUBE1 may contribute to the pathology of DR just like VEGF by generating angiogenesis. However, we believe there is a need for experimental animal model studies with DR examining SCUBE1 gene expression levels in tissue samples.
Introduction
Diabetes mellitus (DM) is a chronic disease that is common in the world and causes morbidity and mortality. As time progresses, the prevalence of DM increases significantly, mainly as a result of the increased incidence of type 2 DM [1]. The increase in oxidative stress and hypercoagulation in the pathophysiology of DM is an important factor in the occurrence of vascular pathologies in diabetes patients [2]. Tissue hypoxia due to microvascular damage plays an important role in forming complications such as diabetic retinopathy (DR) [3]. DR is an important complication of DM that continues to be the cause of preventable and treatable vision loss all over the world. The clinical detection of vascular abnormalities in the retina diagnoses DR. It is divided into two groups: non-proliferative (NPDR) and proliferative diabetic retinopathy (PDR) [4]. Hypoxia caused by microvascular occlusion in retinal tissues leads to the release of vasogenic mediators such as vascular endothelial growth factor (VEGF) and thus to abnormal vascular pathologies [5]. Therefore, VEGF levels in ocular structures and serum have been the subject of research in various studies in the literature [6, 7]. The relationship between VEGF gene expression level and DR has been known for many years [8], [9], [10]. Moreover, patients with DR are cured with treatment strategies that reduce VEGF protein levels. Although successful results are obtained with this treatment, some patients do not experience treatment efficacy because of the side effects of anti-VEGF therapy [11].
Signal peptide-CUB-EGF domain-containing protein 1 (SCUBE1) is a cell surface glycoprotein expressed in platelets and endothelial cells [12]. This protein plays an important role in vascular biology and thrombus development [13]. Studies show that SCUBE1 can be influential in the formation of endothelial dysfunction, hypoxia, and vascular damage [12, 14]. SCUBE1 is highly expressed in platelets and has also been defined as a thrombosis marker today [15]. It is claimed that inhibition of the SCUBE1 protein protects mice from thrombosis, and strategies targeting this protein are promising in the prevention of thromboembolic events [16].
Although some studies have revealed the role of VEGF in the development of DR, data on the effect of SCUBE1 in thrombosis, endothelial dysfunction, and vascular biology in the development of DR are very limited.
In the light of the information above, we evaluated VEGF gene expression levels, a previously proven marker in the literature, and SCUBE1 gene expression levels, which we believe may be related to the pathology of DR in terms of synergistic effects in PDR, NDR, and type 2 DM patients. To our best knowledge, there is no study in which the expression levels of these two proteins are evaluated together in the development of DR. Additionally, we investigated both VEGF and SCUBE1 gene expression levels in terms of HbA1c (<5.7 and ≥5.7%) and FPG (<126 and ≥126 mg/dL).
Materials and methods
This is a prospective controlled study. Patients with diabetes who had been followed up on at the Nigde Training and Research Hospital Endocrinology and Metabolism Diseases Polyclinic for at least 10 years were included in our study. The patients were evaluated for retinopathy by fundus examination in the ophthalmology clinic. Patients diagnosed with DR were divided into NPDR and PDR patients. Patients with retinal microaneurysms, intraretinal microvascular anomalies, hard and soft exudates, and intraretinal haemorrhages were included in the NPDR group. In addition to these findings, patients with neovascularization on the disc or retina, pre-retinal or vitreous haemorrhage, and traction bands were included in the PDR group. Those with any retinal disease other than glaucoma, uveitis, and DR, as well as those with hypertension, diabetic nephropathy, coronary artery disease, carcinoma, any organ failure, polycystic ovary syndrome, ulcerative colitis, inflammatory diseases, pregnancy, any renal disease, and autoimmune systemic diseases such as rheumatoid arthritis, were excluded from the study.
The study group consisted of 29 non-DR, 22 NPDR, 17 PDR patients, and 26 healthy participants. In addition, our study examined VEGF gene and SCUBE1 gene expressions in terms of HbA1c and FPG levels. Patients have had diabetes for over 10 years. Over time, HbA1c values fell below 6.5%, which is the diagnostic criterion for diabetes, because some patients were compliant with treatment. However, even if the treatment has been successful, exposure to high glucose concentrations for a long period of time before treatment may cause the development of diabetic retinopathy in patients [17]. The prediabetes limit value of 5.7% was chosen for HbA1c in order to see the change in SCUBE1 gene expression levels, especially with the decrease in plasma glucose regulation and treatment-related HbA1c value. In addition, a value of <5.7% for HbA1c was used as evidence of the absence of prediabetes and diabetes while selecting participants from healthy volunteers. For this reason, study groups were divided into 2 groups in terms of HbA1c values as <5.7 and ≥5.7% and FPG levels as <126 and ≥126 mg/dL, and gene expression levels between groups were examined.
Biochemical measurements
Our study measured FPG spectrophotometrically in the Roche Cobas C701 (Roche, Germany) device. The within run CV% value (for mean (glucose mg/dL)=59) is 0.5 and the within run CV% value (for mean (glucose mg/dL)=344) is 0.7. The between day CV% are (for mean (glucose mg/dL)=137) is 1.2 and the between day CV% are (for mean (glucose mg/dL)=95.1) is 1.1.
HbA1c was measured by the HPLC method in the Variant II Turbo Biorad device. The within run CV% value (for mean (HbA1c%)=6.2) is 0.82, the within run CV% value (for mean (HbA1c%)=12.5) is 0.54. The between day CVs% are 1.68 and 2.51 respectively.
RNA extraction and cDNA synthesis
A 5 mL blood sample was taken into a sterile EDTA tube to isolate total RNA from all participants in the study groups. Total RNAs were isolated from the whole blood of all the groups by using a FavorPrepTM Blood/Cultured Cell Total RNA Purification Mini Kit (FAVORGEN BIOTECH CORP.), according to the instructions of the manufacturers. The RNA samples’ concentration and quality were determined using a microplate spectrophotometer (Epoch, Biotek, USA). RNA samples of appropriate quality and quantity from each participant were reverse transcribed into cDNA using a Thermo Fisher Scientific RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific Inc., Denmark) according to the manufacturer’s recommendation.
qPCR for SCUBE1 and VEGF
qPCR was performed on a Rotor-Gene 6,000 real-time PCR instrument (Qiagen, Doncaster, Australia) using RealQ Plus 2× Master Mix Green without ROX TM (Amplicon | PCR Enzymes & Reagents, Denmark) according to the manufacturer’s specifications as mentioned in Tables 1 and 2. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as housekeeping genes for accurate quantitative RNA expression in real-time qPCR technique.
Preparation of the reaction mix for qPCR.
| Component | Volume |
|---|---|
| RealQ plus 2× Master mix green | 7.5 μL |
| Specific forward and primers (10 μM) | 0.6 μL |
| Specific reverse primers (10 μM) | 0.6 μL |
| cDNA | 1 μL |
| Nuclease free water | 5.3 |
| Total volume per sample | 15 μL |
Three-step PCR program.
| Cycle | Temperature | Duration of cycle |
|---|---|---|
| 1a | 95 °C | 15 min |
| 40 | 95 °C | 15 s |
| 60 °C | 30 s | |
| 72 °C | 20 s |
-
aFor activation of the TEMPase hot start enzyme.
We used melting curve analysis to confirm the specificity of the PCR products. For the expression analysis, replicates above the 35-cycle threshold (Ct) were excluded from the assay.
Diagnosis of DR
A biomicroscopic fundus examination with a 90° lens was used as the primary method to diagnose DR after pupil dilation. Fundus photography and fundus fluorescein angiography were used when necessary. Fundus fluorescein angiography was not performed in all patients. Background DR is a stage of retinopathy without neovascularization characterized by microhemorrhages, punctate hemorrhages, and exudates. PDR is characterized by neovascularization in the disc or within one disc diameter of the disc and/or neovascularization anywhere in the fundus. The staging was done accordingly.
Statistical analysis
SPSS version 22 (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. In the study, the distribution of the groups was determined with the Kolmogorov-Smirnov test. One-way ANOVA, Kruskal–Wallis assessed differences between groups, and paired comparisons were performed with Tukey t-tests. A p-value of <0.05 was considered statistically significant. The qPCR data were evaluated by the ΔΔCt method, and ANOVA and Student’s t-tests were used. The relative quantification of the gene expression levels was determined by the ΔΔCt method using the RT2 profiler RT-PCR array. The sample size was determined by the G* Power 3.1.9.4 program (α=0.05, actual power=97%, Power (1-β err prob)=0.95 for SCUBE1 and VEGF, (α=0.05, actual power=95%, Power (1-β err prob)=0.95 for HbA1c).
Results
The baseline characteristics of the study groups were given in Table 3.
Baseline characteristics of the study groups.
| Control group (n=26) | Non-DR group (n=29) | NPDR group (n=22) | PDR group (n=17) | p-Value | |
|---|---|---|---|---|---|
| Gender, F/M | 13/13 | 17/12 | 11/11 | 11/6 | 0.736 |
| Age, year | 51.3 ± 9.94 | 58.3 ± 8.72 | 61.7 ± 7.34 | 58.32 ± 9.81 | <0.001a |
| FPG, mg/dL | 83.24 ± 7.20 | 177.11 ± 56.9 | 190.3 ± 75.4 | 156.54 ± 75.71 | <0.001a |
| HbA1c, % | 5.55 ± 0.3 | 8.4 ± 2.14 | 9.42 ± 2.04 | 8.03 ± 2.35 | <0.001a |
-
FPG, fasting plasma glucose. Results are expressed as mean ± SD with 95% confidence intervals. HbA1c, hemoglobin A1c. aStatistically significant (p<0.05). F, female; M, male.
As seen in Table 4, no significant difference was found between the study groups in terms of mean age and gender distribution (p=0.798). It was observed that both FPG and HbA1c levels were significantly higher in the non-DR, NPDR, and PDR groups compared to the control group (p=0.001).
List of primers used for qRT-PCR.
| Gene | Primer sequence (5′ → 3′) | |
|---|---|---|
| SCUBE1 | FW | GTGCCCTATGTCACCTACGAT |
| RV | GAACATCTCCTTGGATTCCTGG | |
| VEGF | FW | CCTCCGAAACCATGAACTTT |
| RV | TTCTTTGGTCTGCATTCACATT | |
| GAPDH | FW | Optimized and spesific PCR primers were used. (Qiagen QuantiTect primer assays; Cat. No: QT00079247 |
| RV |
-
FW, forward primer; RV, reverse primer.
As a result of the analysis, a remarkable increase was observed in the expression levels of both SCUBE1 and VEGF genes in the non-DR, NPDR, and PDR groups compared to the control group. Compared to the control group, SCUBE1 gene expression levels in the non-DR, NPDR, and PDR groups were 2.15 (p=0.015), 1.75 (p=0.799), 2.37 (p=0.038) times higher, and VEGF gene expression levels were 1.71 (p=0.023), 1.75 (p=0.012), 1.85 (p=0.031) times higher, respectively (Figure 1).
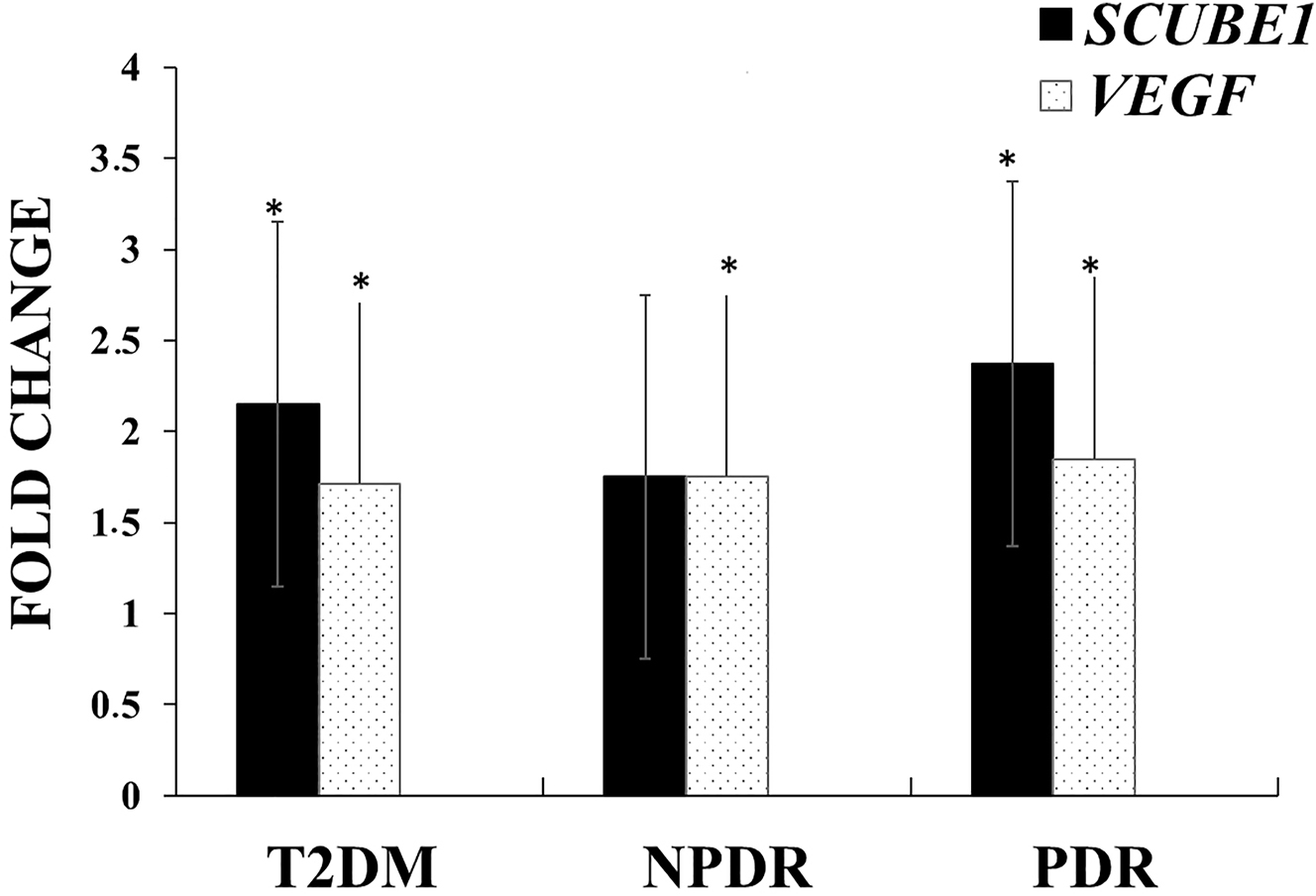
Comparison of SCUBE1 and VEGF gene expression levels in non-DR, NPDR, and PDR groups compared to the control group. *p<0.05.
In addition, VEGF and SCUBE1 gene expressions were examined in terms of HbA1c and FPG levels by forming two groups. Participants with HbA1c levels of ≥5.7% had significantly higher levels of VEGF gene expression than those with <5.7% (p=0.029). In addition, although it was not statistically significant, an increase in SCUBE1 gene expression levels was found in the group with high HbA1c levels (p=0.12). When the study group was divided into 2 groups as those with FPG levels 126 mg/dL and those with ≥126 mg/dL, SCUBE1 gene expression levels were found to be 2.07 times higher in participants with FPG levels ≥126 mg/dL compared to participants with FPG levels <126 mg/dL (p=0.048). Although not statistically significant, a slight increase in VEGF gene expression levels was detected in the group with FPG levels ≥126 mg/dL group, compared to the control group (Figure 2).

Comparison of SCUBE1 and VEGF gene expression levels according to FPG (A) and HbA1c (B) levels. FPG, fasting plasma glucose; HbA1c, hemoglobin A1c. *p<0.05.
Discussion
In the present study, the possible importance of SCUBE1 and VEGF gene expression levels in the pathogenesis of DM and DR in non-DR, NPDR, and PDR patients was discussed. Our first remarkable finding was that VEGF gene expression levels were significantly increased in both the Type 2 DM group without diabetic retinopathy and the NPDR and PDR groups compared to the healthy control group. In addition, the increase in SCUBE1 gene expression level was statistically significant merely in the type 2 DM and PDR groups compared to healthy controls. We believe that our study will be a guide for further genetic studies and the development of different treatment strategies for DR, as it is the first study in which both SCUBE1 and VEGF gene expressions were evaluated together in NPDR and PDR groups.
VEGF plays a role in the pathophysiology of many eye diseases, such as DR, by causing pathological angiogenesis, and anti-VEGF agents are used in the treatment of some eye diseases [18], [19], [20]. These agents are now commonly used as a treatment strategy for DR patients. Despite the fact that anti-VEGF agents have demonstrated significant clinical benefits in DR patients, some patients have not shown the expected visual improvement [21, 22]. The reason for this is unknown, but different responses to anti-VEGF agents in patients with DR may be related to genotypic differences [23]. In addition, the occurrence of various side effects as a result of long-term anti-VEGF treatment reveals the need for different treatment strategies for the inhibition of angiogenesis [24]. Therefore, the application of different combined treatments with different markers may be even more effective [25], [26], [27].
In our study, we examined the gene expression levels of both SCUBE1 and VEGF, which are likely to be effective through similar and different mechanisms in the pathogenesis of DR. As a result, we found that the changes in both VEGF and SCUBE1 gene expression, especially in the PDR group, increased in parallel with each other.
In a recent study in which serum VEGF, apelin, and HO-1 levels were examined in non-DR, NPDR, and PDR groups, it was determined that serum VEGF levels were higher in the NPDR and PDR groups compared to the control group. In the study, serum VEGF levels were reported to be associated with DR progression. In addition, it was suggested that the combined use of VEGF, apelin, and HO-1 levels in both diagnosis and treatment would be beneficial in the continuation of the study [28]. On the other hand, Burgos et al. reported that there was no correlation between VEGF gene expression levels in blood and vitreous fluid in patients with PDR. It has also been claimed that VEGF levels in the vitreous fluid are produced by the tissues surrounding the eye [29]. In another study, it was reported that VEGF gene expression levels in blood and vitreous fluid were correlated with each other [30]. In a study examining plasma VEGF and VEGF receptor levels, it was found that increased VEGF receptor levels and plasma VEGF levels were associated with the severity of DR in patients with type 2 DM [31]. Yang et al. recently published a study in which serum VEGF levels in the DM, NPDR, and PDR groups were examined. It was observed that serum VEGF levels were higher in both the PDR and NPDR groups than in the DM group [32]. In our study, significant increases were observed in all NPDR, PDR, and non-DR groups compared to the control group, and it was found that the increase rates were almost similar in all three groups. The absence of healthy controls in Yang et al.’s study and the investigation of VEGF levels in serum and protein levels may be the reason for the partial difference in the findings obtained from our study. In addition, the fact that VEGF gene expression was higher in patients with FPG ≥126 and higher in patients with HbA1c levels above 5.7% in our study suggests that the expression of this gene is probably caused by high plasma glucose and/or impaired plasma glucose regulation.
Although there are some studies examining VEGF levels in non-DR and DR patients, there is no study examining SCUBE1 gene expression levels, which have important roles in thrombosis, angiogenesis, and vascular biology, in NPDR and PDR subgroups. Studies examining serum SCUBE1 levels have shown that this protein is mostly elevated in acute vascular events [33]. It is also known that SCUBE1 levels are associated with angiogenesis and inflammation [34], [35], [36]. Angiogenesis is a crucial mechanism in the pathogenesis of diabetic retinopathy, and blocking angiogenesis is extremely important for treatment [32, 37]. In a study examining the serum SCUBE1 level in microvascular complications of diabetes such as DR and diabetic nephropathy, it was reported that although SCUBE1 levels were increased, they were not statistically significant. In addition to this, it has been indicated that there was a positive correlation between serum SCUBE1 and FPG levels [38]. In our study, we determined that it was 2.07 times higher in patients with high FPG (≥126 mg/dL) than in the group with low FPG (<126 mg/dL) (p<0.05). Although it was not significant, we also found that SCUBE1 expression was higher (1.89 times) in patients with HbA1c levels ≥5.7% compared to patients with HbA1c levels <5.7%.
In another recent study, serum SCUBE1 levels were found to be significantly higher in patients who developed DR compared to those who did not, and it was suggested that SCUBE1 could be used as an early diagnostic marker in patients who developed DR. In addition, there was an opinion to investigate the role of this marker as a predictable factor in the progression of NPDR to PDR [39]. It has been suggested that inhibition of the SCUBE1 protein protects mice from thrombosis and that treatments including SCUBE1 protein inhibition may be effective in preventing thromboembolic events [15]. We observed a significant increase in SCUBE1 levels in the non-DR and PDR groups. Although there is no obvious difference between these increases, we found that the highest increase was in the PDR group. It seems not surprising that SCUBE1 levels, which are also involved in angiogenesis, increase more in PDR, where neovascularization is dominant in its pathophysiology.
As a result of our study, while VEGF and SCUBE1 gene expression levels were significantly increased in non-DR, NPDR, and PDR patients compared to the control group, no significant difference could be detected among the non-DR, NPDR, and PDR groups. Herein, the fact that SCUBE1 expression levels are higher than VEGF gene expression levels, especially in the PDR group, can be considered an important finding due to being an advanced level DR of PDR. Based on these results, we can report that, apart from VEGF, the SCUBE1 molecule may contribute to the pathology of DR. For this reason, treatment strategies that reduce SCUBE1 levels or combine treatments can be developed for patients who do not benefit from treatment strategies that reduce VEGF levels. However, in this regard, we believe that there is a need for studies of experimental animal models with DR that examine the relevant genes in tissue samples.
Limitations of the study
Although our study was well planned, it had some limitations. The number of patients can be increased. In addition, examining the expression levels at the tissue level is very important for understanding the activities of SCUBE1 and VEGF genes on tissue and their contribution to the pathology of diabetic retinopathy. In our study, only circulating expression levels were examined. For this reason, tissue expression levels could be examined.
-
Research funding: None declared.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: Authors state no conflict of interest.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Ethical approval: This study was approved by the Local Ethics Committee of Niğde Ömer Halisdemir University Faculty of Medicine (date: 08/09/2022; approval number: 2022-89) and conducted in accordance with the Helsinki Declaration.
References
1. Lovic, D, Piperidou, A, Zografou, I, Grassos, H, Pittaras, A, Manolis, A. The growing epidemic of diabetes mellitus. Curr Vasc Pharmacol 2020;18:104–9. https://doi.org/10.2174/1570161117666190405165911.Search in Google Scholar PubMed
2. Strain, WD, Paldánius, PM. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc Diabetol 2018;17:1–10. https://doi.org/10.1186/s12933-018-0703-2.Search in Google Scholar PubMed PubMed Central
3. Curtis, TM, Gardiner, TA, Stitt, AW. Microvascular lesions of diabetic retinopathy: clues towards understanding pathogenesis? Eye 2009;23:1496–508. https://doi.org/10.1038/eye.2009.108.Search in Google Scholar PubMed
4. Wang, W, Lo, AC. Diabetic retinopathy: pathophysiology and treatments. Int J Mol Sci 2018;19:1816. https://doi.org/10.3390/ijms19061816.Search in Google Scholar PubMed PubMed Central
5. Fu, X, Gens, JS, Glazier, JA, Burns, SA, Gast, TJ. Progression of diabetic capillary occlusion: a model. PLoS Comput Biol 2016;12:e1004932. https://doi.org/10.1371/journal.pcbi.1004932.Search in Google Scholar PubMed PubMed Central
6. Bozkurt, E, Çakır, B, Çelik, E, Doğan, E, Uçak, T, Alagöz, G. Correlation of the aqueous humor total antioxidant capacity, total oxidant status, and levels of IL-6 and VEGF with diabetic retinopathy status. Arq Bras Oftalmol 2019;82:136–40. https://doi.org/10.5935/0004-2749.20190021.Search in Google Scholar PubMed
7. Niranjan, G, Srinivasan, AR, Srikanth, K, Pruthu, G, Reeta, R, Ramesh, R, et al.. Evaluation of circulating plasma VEGF-A, ET-1 and magnesium levels as the predictive markers for proliferative diabetic retinopathy. Indian J Clin Biochem 2019;34:352–6. https://doi.org/10.1007/s12291-018-0753-y.Search in Google Scholar PubMed PubMed Central
8. Ahuja, S, Saxena, S, Akduman, L, Meyer, CH, Kruzliak, P, Khanna, VK. Serum vascular endothelial growth factor is a biomolecular biomarker of severity of diabetic retinopathy. Int J Retin Vitr 2019;5:29. https://doi.org/10.1186/s40942-019-0179-6.Search in Google Scholar PubMed PubMed Central
9. Zhang, D, Lv, FL, Wang, GH. Effects of HIF-1α on diabetic retinopathy angiogenesis and VEGF expression. Eur Rev Med Pharmacol Sci 2018;22:5071–6. https://doi.org/10.26355/eurrev_201808_15699.Search in Google Scholar PubMed
10. Gupta, N, Mansoor, S, Sharma, A, Sapkal, A, Sheth, J, Falatoonzadeh, P, et al.. Diabetic retinopathy and VEGF. Open Ophthalmol J 2013;7:4–10. https://doi.org/10.2174/1874364101307010004.Search in Google Scholar PubMed PubMed Central
11. Zhao, Y, Singh, RP. The role of anti-vascular endothelial growth factor (anti-VEGF) in the management of proliferative diabetic retinopathy. Drugs Context 2018;7:212532. https://doi.org/10.7573/dic.212532.Search in Google Scholar PubMed PubMed Central
12. Erdogan, M, Findikli, HA, Teran, IO. A novel biomarker for predicting sepsis mortality: SCUBE1. Medicine 2021;100:e24671. https://doi.org/10.1097/md.0000000000024671.Search in Google Scholar
13. Sun, W, Tang, Y, Tai, YY, Handen, A, Zhao, J, Speyer, G, et al.. SCUBE1 controls BMPR2-relevant pulmonary endothelial function: implications for diagnostic marker development in pulmonary arterial hypertension. Basic Transl Sci 2020;5:1073–92. https://doi.org/10.1016/j.jacbts.2020.08.010.Search in Google Scholar PubMed PubMed Central
14. Dai, DF, Thajeb, P, Tu, CF, Chiang, FT, Chen, CH, Yang, RB, et al.. Plasma concentration of SCUBE1, a novel platelet protein, is elevated in patients with acute coronary syndrome and ischemic stroke. J Am Coll Cardiol 2008;51:2173–80. https://doi.org/10.1016/j.jacc.2008.01.060.Search in Google Scholar PubMed
15. Tekin, YB, Erin, KB, Yilmaz, A. Evaluation of SCUBE1 levels as a placental dysfunction marker at gestational diabetes mellitus. Gynecol Endocrinol 2020;36:417–20. https://doi.org/10.1080/09513590.2019.1683537.Search in Google Scholar PubMed
16. Wu, MY, Lin, YC, Liao, WJ, Tu, CF, Chen, MH, Roffler, SR, et al.. Inhibition of the plasma SCUBE1, a novel platelet adhesive protein, protects mice against thrombosis. Arterioscler Thromb Vasc Biol 2014;34:1390–8. https://doi.org/10.1161/atvbaha.114.303779.Search in Google Scholar PubMed
17. Xianjin, C, Qianna, S, Yansa, W. Correlation between serum VEGF and glycosylated hemoglobin levels with the efficacy and prognosis of laser photocoagulation in patients with diabetic retinopathy. Int J Clin Exp Med 2018;11:12405–12.Search in Google Scholar
18. Bahrami, B, Hong, T, Gilles, MC, Chang, A. Anti-VEGF therapy for diabetic eye diseases. Asia-Pac J Ophthalmol 2017;6:535–45. https://doi.org/10.22608/APO.2017350.Search in Google Scholar PubMed
19. Sultan, MB, Zhou, D, Loftus, J, Dombi, T, Ice, KS, Macugen 1013 study group. A phase 2/3, multicenter, randomized, double-masked, 2-year trial of pegaptanib sodium for the treatment of diabetic macular edema. Ophthalmology 2011;118:1107–18. https://doi.org/10.1016/j.ophtha.2011.02.045.Search in Google Scholar PubMed
20. Adamis, AP, Altaweel, M, Bressler, NM, Cunningham, ET, JrDavis, MD, Goldbaum, M, et al.. Changes in retinal neovascularization after pegaptanib (Macugen) therapy in diabetic individuals. Ophthalmology 2006;113:23–8. https://doi.org/10.1016/j.ophtha.2005.10.012.Search in Google Scholar PubMed
21. Gross, JG, Glassman, AR, Klein, MJ, Jampol, LM, Ferris, FL, Bressler, NM, et al.. Interim safety data comparing ranibizumab with panretinal photocoagulation among participants with proliferative diabetic retinopathy. JAMA Ophthalmol 2017;135:672–3. https://doi.org/10.1001/jamaophthalmol.2017.0969.Search in Google Scholar PubMed PubMed Central
22. Palmer, JM, Amoaku, WM, Kamali, F. Quality of bevacizumab compounded for intravitreal administration. Eye 2013;27:1090–7. https://doi.org/10.1038/eye.2013.139.Search in Google Scholar PubMed PubMed Central
23. Abdelghany, AA, Toraih, EA, Mohamed, AA, Lashine, RM, Mohammad, MH, Nafie, MS, et al.. Association of VEGF gene family variants with central macular thickness and visual acuity after Aflibercept short-term treatment in diabetic patients: a pilot study. Ophthalmic Res 2021;64:261–72. https://doi.org/10.1159/000511087.Search in Google Scholar PubMed
24. Nakamura, S, Hara, H. Prospects and challenges of anti-VEGF drug treatment for pathological angiogenesis of the retina. Yakugaku Zasshi 2021;141:1307–17. https://doi.org/10.1248/yakushi.21-00158-1.Search in Google Scholar PubMed
25. İnan, ÜÜ, Sibel, İ. Management and treatment protocols in retinal vein occlusions. RetVit 2015;23:107–18.Search in Google Scholar
26. Hussain, RM, Ciulla, TA. Treatment strategies for refractory diabetic macular edema: switching anti-VEGF treatments, adopting corticosteroid-based treatments, and combination therapy. Expert Opin Biol 2016;16:365–74. https://doi.org/10.1517/14712598.2016.1131265.Search in Google Scholar PubMed
27. Maturi, RK, Bleau, L, Saunders, J, Mubasher, M, Stewart, MW. A 12-month, single-masked, randomized controlled study of eyes with persistent diabetic macular edema after multiple anti-VEGF injections to assess the efficacy of the dexamethasone-delayed delivery system as an adjunct to bevacizumab compared with continued bevacizumab monotherapy. Retina 2015;35:1604–14. https://doi.org/10.1097/iae.0000000000000533.Search in Google Scholar PubMed
28. Wu, R, Zhu, Z, Zhou, D. VEGF, apelin and HO-1 in diabetic patients with retinopathy: a correlation analysis. BMC Ophthalmol 2020;20:1–6. https://doi.org/10.1186/s12886-020-01593-9.Search in Google Scholar PubMed PubMed Central
29. Burgos, R, Simo, R, Audi, L, Mateo, C, Mesa, J, Garcia-Ramirez, M, et al.. Vitreous levels of vascular endothelial growth factor are not influenced by its serum concentrations in diabetic retinopathy. Diabetologia 1997;40:1107–9. https://doi.org/10.1007/s001250050794.Search in Google Scholar PubMed
30. Baharivand, N, Zarghami, N, Panahi, F, Ghafari, MYD, Fard, AM, Mohajeri, A. Relationship between vitreous and serum vascular endothelial growth factor levels, control of diabetes and microalbuminuria in proliferative diabetic retinopathy. Clin Ophthalmol 2012;6:185. https://doi.org/10.2147/opth.s27423.Search in Google Scholar PubMed PubMed Central
31. Paine, SK, Mondal, LK, Borah, PK, Bhattacharya, CK, Mahanta, J. Pro-and antiangiogenic VEGF and its receptor status for the severity of diabetic retinopathy. Mol Vis 2017;23:356.Search in Google Scholar
32. Yang, Y, Liu, Y, Li, Y, Chen, Z, Xiong, Y, Zhou, T, et al.. MicroRNA-15b targets VEGF and inhibits angiogenesis in proliferative diabetic retinopathy. J Clin Endocrinol Metab 2020;105:3404–15. https://doi.org/10.1210/clinem/dgaa538.Search in Google Scholar PubMed PubMed Central
33. Dai, DF, Thajeb, P, Tu, CF, Chiang, FT, Chen, CH, Yang, RB, et al.. Plasma concentration of SCUBE1, a novel platelet protein, is elevated in patients with acute coronary syndrome and ischemic stroke. J Am Coll Cardiol 2008;51:2173–80. https://doi.org/10.1016/j.jacc.2008.01.060.Search in Google Scholar PubMed
34. Yang, M, Guo, M, Hu, Y, Jiang, Y. Scube regulates synovial angiogenesis-related signaling. Med Hypotheses 2013;81:948–53. https://doi.org/10.1016/j.mehy.2013.09.001.Search in Google Scholar PubMed
35. Capkin, AA, Demir, S, Mentese, A, Bulut, Ç, Ayar, A. Can signal peptide-CUB-EGF domain-containing protein (SCUBE) levels be a marker of angiogenesis in patients with psoriasis? Arch Dermatol Res 2017;309:203–7. https://doi.org/10.1007/s00403-017-1722-7.Search in Google Scholar PubMed
36. Lin, YC, Chao, TY, Yeh, CT, Roffler, SR, Kannagi, R, Yang, RB. Endothelial SCUBE2 interacts with VEGFR2 and regulates VEGF-induced angiogenesis. Arterioscler Thromb Vasc Biol 2017;37:144–55. https://doi.org/10.1161/atvbaha.116.308546.Search in Google Scholar PubMed
37. Melincovici, CS, Boşca, AB, Şuşman, S, Mărginean, M, Mihu, C, Istrate, M, et al.. Vascular endothelial growth factor (VEGF)-key factor in normal and pathological angiogenesis. Rom J Morphol Embryol 2018;59:455–67.Search in Google Scholar
38. Bingol, U, Kevser, DA, Kutluturk, F, Cansel, OZ, Demir, O. Investigation of serum SCUBE1 level in relation to microvascular complications in patients with type 2 diabetes mellitus. Endocr Abstr 2018;56:438.10.1530/endoabs.56.P438Search in Google Scholar
39. Icel, E, Icel, A, Mertoglu, C, Tasli, NG, Karakurt, Y, Ucak, T, et al.. Serum SCUBE1 levels in patients with diabetic retinopathy. Int Ophthalmol 2020;40:859–65. https://doi.org/10.1007/s10792-019-01249-8.Search in Google Scholar PubMed
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/tjb-2023-0008).
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Opinion Paper
- Ischemia – modified albumin by albumin cobalt binding test: a false myth or reality
- Research Articles
- Machine learning models can predict the presence of variants in hemoglobin: artificial neural network-based recognition of human hemoglobin variants by HPLC
- Investigation of the preanalytical process practices in primary care in Istanbul regarding the newborn screening tests
- Cell counting chamber vs. Sysmex XN-1000 for determining white blood cell count and differentiation for body fluids
- Synthesis of PEITC-loaded gold nanoparticles and evaluation of the hepatoprotective effect on CCl4-induced damage through Nrf2 pathway
- Concentrations of B cell-activating factor, aquaporin-4 antibody and brain-derived neurotrophic factor in neuromyelitis optica spectrum disorder
- Macula, choroid and retinal nerve fiber layer optical coherence tomography biomarkers in liver dysfunction
- Direct-acting antiviral therapy may help restore HCV-induced impaired redox balance and liver fibrosis process
- Are VEGF and SCUBE1 gene expressions increased in diabetic retinopathy?
- Biochemical analysis of microbiotas obtained from healthy, prediabetic, type 2 diabetes, and obese individuals
- Inflammation parameters, monocyte subgroups and toll-like receptor expression before and after dialysis in patients with chronic kidney disease
- Matrix metalloproteinase 9 gene-MMP9-DNA methylation status in Turkish schizophrenia patients
- HLA DRB1 alleles, IFN-γ and TGF-β Gene Variants in childhood ALL patients
- Epithelial-mesenchymal transition as a potential route for DAPT resistance in breast cancer cells
- Comparison with molecular effects of ukrain, tamoxifen, and docetaxel on human breast cancer cell lines
- In vitro evaluation of 2-pyrazoline derivatives as DPP-4 inhibitors
- Effects of polyphenolic-rich extracts from Citrus hystrix on proliferation and oxidative stress in breast and colorectal cancer
- The effects of sodium benzoate exposure on learning and neurobehavior during the prepubertal period in rats
- The association between βeta 2-microglobulin and bronchopulmonary dysplasia
Articles in the same Issue
- Frontmatter
- Opinion Paper
- Ischemia – modified albumin by albumin cobalt binding test: a false myth or reality
- Research Articles
- Machine learning models can predict the presence of variants in hemoglobin: artificial neural network-based recognition of human hemoglobin variants by HPLC
- Investigation of the preanalytical process practices in primary care in Istanbul regarding the newborn screening tests
- Cell counting chamber vs. Sysmex XN-1000 for determining white blood cell count and differentiation for body fluids
- Synthesis of PEITC-loaded gold nanoparticles and evaluation of the hepatoprotective effect on CCl4-induced damage through Nrf2 pathway
- Concentrations of B cell-activating factor, aquaporin-4 antibody and brain-derived neurotrophic factor in neuromyelitis optica spectrum disorder
- Macula, choroid and retinal nerve fiber layer optical coherence tomography biomarkers in liver dysfunction
- Direct-acting antiviral therapy may help restore HCV-induced impaired redox balance and liver fibrosis process
- Are VEGF and SCUBE1 gene expressions increased in diabetic retinopathy?
- Biochemical analysis of microbiotas obtained from healthy, prediabetic, type 2 diabetes, and obese individuals
- Inflammation parameters, monocyte subgroups and toll-like receptor expression before and after dialysis in patients with chronic kidney disease
- Matrix metalloproteinase 9 gene-MMP9-DNA methylation status in Turkish schizophrenia patients
- HLA DRB1 alleles, IFN-γ and TGF-β Gene Variants in childhood ALL patients
- Epithelial-mesenchymal transition as a potential route for DAPT resistance in breast cancer cells
- Comparison with molecular effects of ukrain, tamoxifen, and docetaxel on human breast cancer cell lines
- In vitro evaluation of 2-pyrazoline derivatives as DPP-4 inhibitors
- Effects of polyphenolic-rich extracts from Citrus hystrix on proliferation and oxidative stress in breast and colorectal cancer
- The effects of sodium benzoate exposure on learning and neurobehavior during the prepubertal period in rats
- The association between βeta 2-microglobulin and bronchopulmonary dysplasia

