Abstract
Objectives
The data of the monocyte subgroups and expressed toll like receptors (TLR) in the innate immune system response, which develop against chronic inflammation in patients with predialysis chronic kidney disease (CKD) and in patients who undergo dialysis treatment, are limited. We aimed to investigate the effect of the dialysis procedure on the current chronic inflammatory condition and which role of monocyte subgroups ratios, the expressions of TLR2/4 and serum Tumor necrosis factor alpha (TNF-α) levels involved in the innate immune response process.
Methods
We investigated monocyte subgroups, TLR2/TLR4 expressions and serum TNF-α levels in 30 predialysis CKD patients, 90 CKD patients undergoing dialysis and 30 healthy control subjects. Monocyte subgroup percentages and TLR2/TLR4 expressions were determined using the flow cytometry, serum TNF-α levels were investigated using the enzyme-linked immunosorbent assay (ELISA).
Results
In the dialysis patients, the percentages of classical (p=0.0001) and non-classical (p=0.078) monocytes were found to be higher when compared with the predialysis CKD patients. The percentages of TLR4 expression on non-classical monocytes was higher in dialysis and predialysis patients compared with the healthy controls (p<0.0001, p=0.796). Serum TNF-α level was significantly higher in dialysis and predialysis patients compared with the healthy controls (p=0.013, p=0.022) and a positive correlation between the classical monocyte subgroup and TNF-α was observed (r=0.285, p=0.006).
Conclusions
Increased percentages of non-classical monocytes, TLR4 expressions and serum TNF-α levels observed in the predialysis CKD patients and dialysis patients might be related to inflammation.
Introduction
Chronic kidney disease (CKD) is an important problem; Currently, more than 2 million individuals in the world and 70.000 patients in Turkey undergo dialysis or are transplant recipients [1]. The morbidity and mortality rates in CKD patients are substantially high because of malnutrition, inflammation and accelerated atherosclerosis [2, 3]. Particularly in dialysis patients, various infections, blood-membrane interaction during hemodialysis procedures and increased oxidative stress are attributed to this syndrome, which can be detected by increased proinflammatory cytokine and C-reactive protein (CRP) levels [4, 5].
In healthy individuals, the cells of the innate immunity system (monocytes, macrophages, neutrophils, dendritic cells, natural killer cells, mast cells, eosinophils and basophils) are primarily activated against infectious agents. These cells release proinflammatory markers including Tumor necrosis factor alpha (TNF-α) by means of ‘Toll Like Receptor (TLR) signalization or complement activation; and activate the cells of the acquired immune system (T and B lymphocytes) and strengthen inflammatory response [4], [5], [6]. Monocytes are predominantly involved in immune response in the inflammatory process which occurs during renal replacement therapies [7]. Monocytes have subgroups showing different phenotypes and functions according to CD14 and CD16 antigen expression on their surface. The CD14++CD16− monocytes are the highest rate in the peripheral blood and are named classical monocytes. CD16 positive, further subdivided into CD14++CD16+ and CD14+CD16++ cells, intermediate and nonclassical monocytes, respectively. CD16 positive monocytes are also called proinflammatory monocytes because of their properties of expressing higher levels of major histocompatibility complex (MHC) class II antigens, adhesion molecules, chemokine receptors, and proinflammatory cytokines such as TNF-α, but lower levels of the anti-inflammatory cytokine (IL-10) compared to the CD16 negative monocytes [7, 8].
In some studies, it has been reported that the CD14++CD16+ and CD14+CD16++ monocyte subgroup cells constitute approximately 10% of all monocytes in healthy individuals under normal conditions, while they markedly increase in different infectious or inflammatory diseases, including AIDS and asthma; these monocytes also show more than the 10-fold increase during septicemia and become the predominant type of monocytes in some septic patients [9], [10], [11], [12]. Increased expression of both TLR2 and TLR4 in monocytes is associated with the presence of inflammation [13].
In our study, monocyte subgroups, TLR2/TLR4 expressions, serum TNF-α levels and their inflammatory functions were investigated in predialysis, dialysis CKD patients and also in healthy control subjects it was aimed to elucidate the predominant mechanisms involved in the pathogenesis of inflammation in each group by comparing the data obtained.
Materials and methods
Study population
This prospective study approved by the Istanbul University Hospital Clinical Research Committee was conducted at the Istanbul University, Istanbul Faculty of Medicine, Department of Medical Biology between October 2014- November 2016. Ninety CKD patients receiving dialysis treatment, 30 pre-dialysis CKD patients and 30 healthy controls were included in this study. Sample size was calculated with a two-sided confidence interval for the evaluation of results from hemodialysis, hemodiofiltration, predialysis, and control groups. It was calculated that at least 30 patients from each group were needed for a 20% difference based on the 0.80 power 0.05 error and the TNF averages included in the studies. Considering that there may be a loss of 15% during data collection, it was decided to include 120 patients and 30 controls in the study. Hemodialysis and hemodiafiltration treatments had been applied for at least 3 months (4 h, three times weekly) in a dialysis center. Dialysis procedures were performed by using 5008 S Fresenius CorDiax machines. Dialysers FX40 was used in two, FX50 in four, FX600 in forty nine and FX800 in thirty five patients. Fistula was used in 85.5% (n=77) of patients, indwelling vascular catheter in 11.1% (n=10), and graft in 3.3% (n=3) of patients as a vascular access route. The dialysate used in the dialysis process complied with AAMI criteria. In the predialysis CKD group, the mean GFR level was ≤15 mL/min/1.73 m2; however these patients were not begun dialysis at the time of the study. Suffering patients with acute infection in the last 3 months and receiving antibiotics or immunosuppressive treatments were excluded from the study. Volunteers older than 18 years of age, without any chronic disease, and without any acute infection in the last 3 months were included in the healthy control group. Volunteers with a history of cancer, under any medication, and any acute infection in the last 3 months were excluded from the study.
Collection of samples
Blood samples of dialysis patients were obtained just before the dialysis procedure and at the end of the same dialysis session. The results of the before dialysis session samples were taken into account when calculating all the results except for the comparison of the values before and after the dialysis session. Blood samples of the predialysis CKD patients were obtained during outpatient follow-up visits. Informed consent forms were obtained from all patients and healthy controls before obtaining blood samples and the study was conducted in accordance with the Declaration of Helsinki. The Scientific Committee of the Istanbul University Hospital clinical research committee (IRB approval number: 819) approved the study.
Test procedures
Serum TNF-α level was measured using the ELISA method according to the BD OptEIA™ kit (catalog no: 550610) protocol. Flow cytometry method was used to determine monocyte subgroup percentages and TLR2, TLR4 expression rates. 100 µL blood anticoagulated with EDTA was stained with flourochrome-conjugated monoclonal antibodies (mAb). FITC-labeled mAb (catalog no: 555397) was used for CD14 and PERCP-labeled mAb (catalog no: 560717) was used for CD16, HU CD282 Alexa 647 MAB (catalog no: 558319) was used for TLR2 and HU TLR4 BIOTIN MAB (catalog no: 551975) was used for TLR4. Reading was realized in flow cytometry device (Beckton Dickinson, Facs Calibur). 150,000 cells were analyzed in each sample and general monocytes were primarily gated. Afterward, the monocyte subgroups expressing CD14+ and CD16+ antigens were specified among the gated monocytes and their percentages were compared. Finally, the rates of TLR2 and TLR4 expressed in monocyte subgroups were compared.
Statistical analysis
Power analysis was used to determine the sample size. Descriptive statistical methods were used to evaluate the basic demographic data of the control group and the patients. In the analysis and assessment of the numerical data of the study, the mean, standard deviation, median and (IQR) values of the data addressed were specified. The compatibility of all data with the normal distribution was tested using Kolmogorov-Smirnov test. The paired t-test among parametric tests was used for matched data that were compatible with the normal distribution. Student’s t-test was used among parametric tests in comparisons of two groups which were compatible with the normal distribution. Whereas the Mann-Whitney U test was used in comparisons of two groups that were not compatible with the normal distribution. Kruskal Wallis test was used for the data that were incompatible with the normal distribution and in comparisons of more than two groups. After the Kruskal Wallis test significance, an intergroup comparison test was also applied as a post hoc test. Bonferroni adjustment made for the p-value. Categorical data were compared by frequency and percentages and the results were given as percentage values. The Chi-square test was used to determine the differences between the groups. Correlation analysis was performed to determine the correlations between the variables and to demonstrate the correlations as mathematical relations. Spearman’s rho test was used in the correlation analysis of the data with non-normal distribution. Statistical analyses of the data were performed using Statistic Package of Social Science (SPSS 21.0) software. A p-value of <0.05 was considered statistically significant.
Results
Demographic data
The mean age of all dialysis patients was 60.1 ± 15.8 (range 21–90 years). The mean age was 51.7 ± 17.3 (range 24–84 years) in the predialysis CKD patients and 35.5 ± 15.2 (range 18–75 years) in the healthy controls (Table 1). Forty two percent (n=38/90), 63% (n=19/30) and 43% (n=13/30) of the patients were male, on the other hand 58% (n=52/90), 37% (n=11/30) and 57% (n=17/30) were female in dialysis, predialysis and control groups respectively (Table 1).
The demographic characteristics and statistical comparisons of patient groups and healthy controls.
| Groups | n | Mean ± SD/% | p-Value | Pc | |
|---|---|---|---|---|---|
| Age, years | Healthy control | 30 | 35.5 ± 15.2 | <0.0001a | 0.045a |
| Predialysis CKD | 30 | 51.7 ± 17.3 | |||
| Healthy control | 30 | 35.5 ± 15.2 | <0.0001a | ||
| Dialysis (HD-HDF) | 90 | 60.1 ± 15.8 | |||
| Predialysis CKD | 30 | 51.7 ± 17.3 | 0.001a | ||
| Dialysis (HD-HDF) | 90 | 60.1 ± 15.8 | |||
| Gender (M/F), n (%) | Healthy control (%) | 13/17 | (43.3%)/(56.7) | 0.123 | – |
| Predialysis CKD (%) | 19/11 | (63.3%)/(36.7) | |||
| Dialysis (HD-HDF) (%) | 38/52 | (42.2%)/(57.8) | |||
| DM (+/−), n (%) | Predialysis CKD | 2/28 | (6.7%/93.3%) | 0.013a | – |
| Dialysis (HD-HDF) | 23/67 | (25.6%/74.4%) |
-
CKD, chronic kidney disease; HD, hemodialysis; HDF, hemodiafiltration; M, male; F, female; DM, diabetes mellitus; PC, post hoc test (Bonferroni). ap<0.05.
The mean period of renal replacement therapy was 103.6 (3–312) months in the dialysis patients. Twenty six percent of the dialysis patients and 7% of the predialysis patients were diabetic. The rate of diabetes was significantly higher in the patients receiving dialysis treatment compared to the predialysis CKD patients (p=0.013) (Table 1).
Laboratory data
The leukocyte count, blood urea nitrogen level and glomerular filtration rate value (eGFR) were significantly higher in the predialysis CKD patients compared to the patients who were receiving dialysis treatment. Creatinine levels were significantly higher in the patients who were receiving dialysis treatment than in the predialysis CKD patients (p<0.001). A significant difference was not found between the dialysis group and the predialysis group regarding hemoglobin and albumin values and peripheral blood monocyte count (Table 2). CRP levels were 0.62 (0.03–9.28) in dialysis patients and 0.56 (0.07–6.91) in predialysis CKD patients. The CRP level was above the reference values both in the dialysis patients and predialysis CKD patients; however, the difference between the two groups was not found to be statistically significant (p=0.484) (Table 2). The correlation between CRP and monocyte subgroups was not statistically significant.
Laboratory findings of dialysis and predialysis CKD patients.
| Parameters (mean ± SD)/median (IQR) | Dialysis patients (n=90) | Predialysis CKD patients (n=30) | p-Value |
|---|---|---|---|
| Hemoglobin, g/dL | 11.44 ± 1.56 | 10.93 ± 1.75 | 0.072 |
| Leukocyte, 103/mm3 | 6.56 ± 1.75 | 8.98 ± 3.17 | 0.006a |
| Monocyte, 103/mm3 | 0.64 ± 0.21 | 0.66 ± 0.36 | 0.719 |
| Creatinine, mg/dL | 7.00 (2.15) | 4.15 (1.70) | <0.0001a |
| GFR, mL/min/1.73 m2 | 7.30 (2.10) | 14.80 (5.23) | <0.0001a |
| Urea, mg/dL | 109.71 ± 27.27 | 136.99 ± 37.01 | 0.002a |
| Albumin, g/dL | 3.89 ± 0.41 | 3.80 ± 0.60 | 0.272 |
| CRP, mg/dL (median) (IQR) |
0.62 (1) | 0.56 (1) | 0.484 |
-
CKD, chronic kidney disease; CRP, C, reaktif protein; IQR, interquartile range; GFR, glomerüler filtration rate. ap<0.05.
Comparison of monocyte subgroups
The classical monocyte subgroup was significantly higher in the patients who were receiving dialysis treatment compared to the healthy controls and the predialysis CKD patients. The non-classical monocyte subgroup percentages were also higher in the patients receiving dialysis treatment compared to the healthy controls and the predialysis patients; however, this difference did not reach statistical significance (Figure 1).

Classic and non-classic monocyte subgroup ratios in the patient groups and controls.
Comparison of TLR expressions
The rates of TLR2 expression on classical monocytes was higher in the dialysis patients compared with the predialysis patients (p=0.001) and healthy control (p=0.206). And also the rates of TLR2 expression on non-classical monocytes were higher in the dialysis patients compared with the healthy control (p=0.011) (Table 3). The rates of TLR4 expression on non-classical monocytes were significantly higher in the dialysis patients when compared with the predialysis patients (p=0.001) and healthy control (p<0.0001). There was no significant difference between the groups for TLR4 expression rates on classical monocytes (Table 4).
TLR2 expression rates on classical and non-classical monocytes.
| Parameters | Groups | n | Median (IQR) | p-Value | Pc |
|---|---|---|---|---|---|
| CD14+, % CM | Healthy control | 30 | 73.34 (32.64) | <0.0001a | 0.302 |
| Predialysis KD | 30 | 64.22 (25.80) | |||
| CD14+, % CM | Healthy control | 30 | 73.34 (32.64) | 0.206 | |
| Dialysis | 90 | 83.02 (31.64) | |||
| CD14+, % CM | Predialysis CKD | 30 | 64.22 (25.80) | 0.001a | |
| Dialysis | 90 | 83.02 (31.64) | |||
| CD14+CD16+, % NCM | Healthy control | 30 | 99.14 (0.99) | 0.001a | 0.060 |
| Predialysis CKD | 30 | 98.49 (1.85) | |||
| CD14+CD16+, % NCM | Healthy control | 30 | 99.14 (0.99) | 0.011a | |
| Dialysis | 90 | 96.59 (23.01) | |||
| CD14+CD16+, % NCM | Predialysis CKD | 30 | 98.49 (1.85) | 0.054a | |
| Dialysis | 90 | 96.59 (23.01) |
-
CKD, chronic kidney disease; min, minimum; max, maximum; CM, classical monocyte; NCM, non-classical monocyte; IQR, interquartile range; PC, post hoc test (Bonferroni). ap<0.05.
TLR4 expression rates on classical and non-classical monocytes.
| Parameters | Groups | n | Median (IQR) | p-Value | Pc |
|---|---|---|---|---|---|
| CD14+, % CM | Healthy control | 30 | 4.48 (2.85) | 0.053 | 0.787 |
| Predialysis CKD | 30 | 5.60 (3.89) | |||
| CD14+, % CM | Healthy control | 30 | 4.48 (2.85) | 0.237 | |
| Dialysis | 90 | 2.67 (7.61) | |||
| CD14+, % CM | Predialysis CKD | 30 | 5.60 (3.89) | 0.534 | |
| Dialysis | 90 | 2.67 (7.61) | |||
| CD14+CD16+, % NCM | Healthy control | 30 | 0.02 (0.06) | <0.0001a | 0.796 |
| Predialysis CKD | 30 | 0.03 (0.05) | |||
| CD14+CD16+, % NCM | Healthy control | 30 | 0.02 (0.06) | <0.0001a | |
| Dialysis | 90 | 0.11 (4.30) | |||
| CD14+CD16+, % NCM | Predialysis CKD | 30 | 0.03 (0.05) | 0.001a | |
| Dialysis | 90 | 0.11 (4.30) |
-
CKD, chronic kidney disease; IQR, interquartile range; PC, post hoc test (bonferroni); CM, classical monocyte; NCM, non-classical monocyte. ap<0.05.
Serum TNF-α levels
It was found that the TNF-α level was significantly higher in the patients who were receiving dialysis treatment compared to the healthy controls and the predialysis CKD patients (Figure 2). And also the positive correlation between the classical monocyte subgroup and TNF-α was observed in our study (r=0.224, p=0.034) (Figure 3). Additionally, we detected a positively correlated between TLR4 expression rates on non-classical monocytes and serum TNF-α levels (r=0.201, p=0.028).

Serum TNF-α levels in dialysis patients, predialysis patients and healthy controls.
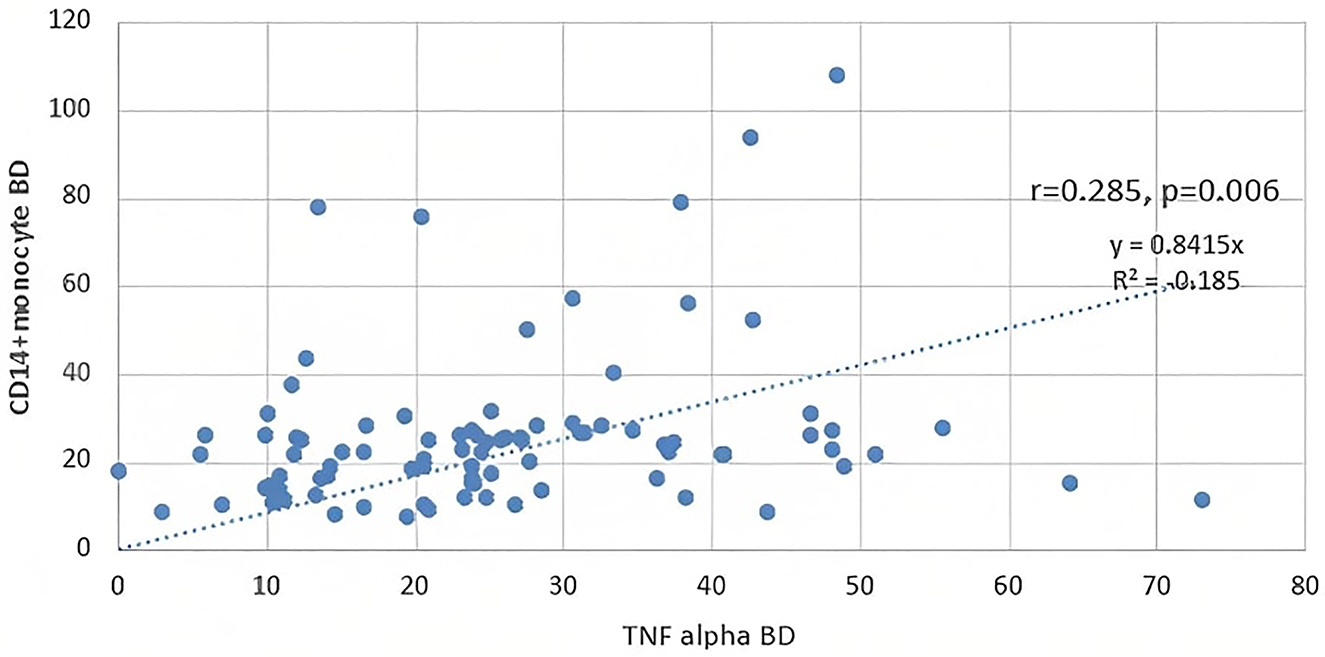
The correlation between serum TNF-α levels and classical monocyte subgroup.
Comparison of the findings before and after the dialysis session
The classical monocyte percentages and serum TNF-α levels did not show the significant difference before and after dialysis. On the other hand, the non-classical monocyte percentages were significantly lower at the end of the dialysis session (p<0.0001) (Table 5).
Monocyte subgroup ratios and serum TNF-α levels in before and post dialysis sessions.
| Parameters | n | Median (IQR) | p-Value | |
|---|---|---|---|---|
| CD14+, % CM | B.D. | 90 | 24.0 (21.09) | 0.103 |
| A.D. | 90 | 19.4 (19.38) | ||
| CD14+CD16+, % NCM | B.D. | 90 | 17.2 (15.44) | <0.0001a |
| A.D. | 90 | 7.1 (11.22) | ||
| TNF-α, pg/mL | B.D. | 90 | 22.3 (12.14) | 0.744 |
| A.D. | 90 | 22.1 (11.83) | ||
-
Data are presented as median (IQR) and SD, standard deviation; IQR, interquartile range; B.D, before dialysis; A.D, after dialysis; TNF, tumor necrosis factor; CM, classical monocyte; NCM, non-classical monocyte. ap<0.05.
Discussion
In chronic kidney patients presence of chronic infections and dialysis-associated factors cause an increase in inflammatory status which is an important contributor to morbidity and mortality. During inflammation, the cells of the innate immune system are primarily activated and induce the release of proinflammatory markers including TNF-α; TLR signalization or complement activation plays an important role in this procedure [5].
In our study, monocyte subgroups, TLR2, TLR4 expressions, TNF-α levels and their inflammatory functions were investigated in dialysis patients, predialysis CKD patients and healthy controls and it was aimed to elucidate the predominant mechanisms involved in the pathogenesis of inflammation in each group by comparing the data obtained.
As reported in the literature the leukocyte count was normal in our chronic renal patient group as well. On the other hand, impaired leukocyte function might have contributed to the increased inflammatory status in chronic kidney patients [4].
CRP is an acute-phase protein; it may be involved in the pathogenesis of inflammation per se; as well as being a marker of inflammation [14]. Persistently increased levels of CRP indicate chronic inflammation [15]. In our study, a statistically significant difference was not found between the CRP values of the predialysis CKD patients and dialysis patients, but the CRP values were above the reference values in both groups. This may be explained by the induction of inflammation during chronic kidney disease by acute bacterial infections [16], latent chronic infections [17] and dialysis-associated factors [18].
It is known that monocytes are activated and monocytosis occurs as a result of the induction of the immune system by the factors to which the patients are exposed during the dialysis procedure [19]. In our study, the classical monocyte percentages were significantly higher in the dialysis patients compared to the healthy controls and predialysis CKD patients in parallel to the studies in the literature [19], [20], [21].
The CD14++CD16+ and CD14+CD16++ cell subgroups of monocytes are named proinflammatory monocytes because of more efficient antigen presentation and a higher level of secretion of proinflammatory cytokines including mainly TNF-α, IL-1β and IL-6 [22]. Proinflammatory cytokines are also induced by many other triggering factors; these triggering factors include microorganisms, microbial products, antigens, inflammatory agents, plant lectins, lymphokines and some chemical substances. Thus, the synthesis of acute phase proteins including CRP, serum amyloid A, fibrinogen, complement and alpha 1-antitrypsin increases [15]. In some studies, it has been reported that the non-classical monocyte subgroup cells constitute approximately 5–10% of all monocytes, but their percentages may increase up to 40% in some acute and chronic conditions of inflammation including HIV infection, autoimmune diseases, sepsis and hemodialysis [7, 10, 19, 22]. In addition, the cells of this monocyte subgroup have a high endothelial affinity and express a high number of adhesion molecules which enable them to adhere to endothelial cells and result in injury [23]. This condition may be involved in the pathogenesis of atherosclerosis which is observed in the inflammation process.
In our study, the percentage of the non-classical monocyte subgroup was found to be higher in the patients who were receiving dialysis treatment and in the predialysis CKD patients compared to the healthy controls in parallel to the other studies in the literature [19], [20], [21]. This finding supports the information that the non-classical monocytes are primarily involved in the immune response and chronic inflammation developing against the pathogenesis which occurs in the course of chronic renal failure and dialysis treatment [19], [20], [21].
In addition, the percentage of the non-classical monocyte subgroup was higher in the patients receiving dialysis treatment compared to the predialysis CKD patients in our study. This finding shows that the condition of chronic inflammation which is already present in predialysis CKD patients [2] becomes even more prominent with the contribution of factors including catheters, fistulas or graft infections [4], dialyzate contamination [24, 25] or blood-membrane interaction [25].
It has been reported that the non-classical monocytes are depleted during dialysis sessions [26]. In our study, blood samples were obtained just before and after the dialysis session and it was observed that the non-classical monocyte percentages were statistically significantly reduced after the dialysis session similar to this study. This reduction might be related to the sequestration of cells in the dialyzer and complement activation because of oxidative stress occuring during the procedure [27]. In another study in the literature, the blood samples obtained at the 300th min and 24th h after the dialysis procedure were evaluated and it was found that the non-classical monocyte percentages were reduced in the early stage after a dialysis session, but reached basal values in later stages [13].
Caren C Grabulosa et al. showed that in monocytes, TLR4 expression was significantly higher in CKD 3 and 4 groups than in the control and HD groups and positively and negatively correlated with IL-6 and MCP-1 and cathelicidin, respectively and the researchers reported that these results suggest that a uremic environment induces high TLR4, cathelicidin and cytokine expression and may increase inflammation [28]. And also, Verzola D et al. reported overexpression of TLR4 and upregulation of TNF-α in uremic muscle in CKD stage 5 and researchers suggested that the progressive decline in renal function mediated TLR4 activation and increased inflammation [29]. In parallel to these studies, TLR4 expression rates on non-classical monocytes were higher in the patients who were receiving dialysis treatment and predialysis CKD patients compared to the healthy control group in our study. Additionally, we detected a positively correlated between TLR4 expression rates on non-classical monocytes and serum TNF-α levels.
Koç et al. found that the ratio of TLR2 expressed on CD14 + monocytes was higher in patients with stage 3–4 CKD and HD than controls and reported that higher MFI values of TLR2 in these patients may be associated with chronic low-grade monocyte activation [13]. Kuroki et al. investigated TLR2 expression in peripheral blood monocytes in their studies and found TLR2 expression in peripheral blood monocytes of HD patients to be slightly stronger than in healthy controls [21]. In our study, we found that the rates of TLR2 expressed on non-classical monocytes were higher in dialysis patients than in healthy controls and predialysis patients.
An increase in pro-inflammatory cytokines have also been reported in dialysis patients’ correlation with non-classical monocytes, compared to healthy controls. Using the intracellular staining method, it has been shown that the non-classical monocytes produce TNF-α to a large extent [20].
In parallel to these studies, serum levels of TNF-α were significantly higher in dialysis patients compared to the predialysis CKD patients and healthy controls in our study. The positive correlation between the non-classical monocyte subgroup and TNF-α was observed in our study in accordance with other studies [30], but this correlation did not reach statistical significance. However the positive correlation between the classical monocyte subgroup and TNF-α was observed in our study.
In conclusion, this study was shown that increased percentages of non-classical monocytes and increased serum TNF-α levels observed in the predialysis CKD patients and dialysis patients might be related to inflammation. In addition, we showed that inflammation is exacerbated by the contribution of factors that are exposed during the dialysis procedure in patients with CKD. The understanding of the mechanisms of chronic inflammation occurring during the CKD process and dialysis contributes to the development of treatment strategies in CKD patients and this may positively affect the unfavorable prognosis of CKD patients. Prospective studies including high numbers of patients are needed to prove all these hypotheses
Limitation of Study
Predialysis CKD patient group population included the study is small due to the difficulty of finding patients with dialysis indications who have not yet received dialysis treatment may be a limitation of our study. In addition, although the CRP values of the patients in the dialysis and predialysis patient groups were above the reference values but CRP was not evaluated in healthy controls may be another limitation of our study.
Acknowledgments
We thank the staff of the Fresenius Medical Care Bahçelievler Dialysis Unit for their help with conducting this study.
-
Research funding: This work was supported by the Scientific Research Projects Coordination Unit of Istanbul University. Project Numbers: 45394/53062/54889.
-
Author contributions: All authors contributed to the study conception and design. Material preparation data collection and analysis were performed by Demet Kivanc and Hayriye Senturk Ciftci, Lab work was performed Demet Kivanc, Literature search was performed by Demet Kivanc, The first draft of the manuscript was written by Demet Kivanc and Fatma Savran Oguz Critical Reviewed and edited by Fatma Savran Oguz, Mehmet Sukru Sever and Filiz Aydin All authors read and approved the final manuscript.
-
Conflict of interest statement: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
-
Informed consent: Informed consent was obtained from all individual participants included in the study.
-
Ethical approval: All procedures performed in studies involving human participants will be in accordance with the ethical standards of the Istanbul University Hospital clinical research committee (IRB approval number: 819) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
1. Pippias, M, Kramer, A, Noordzij, M, Afentakis, N, de la Torre, RA, Ambühl, PM, et al.. The European renal association – European dialysis and transplant association registry annual report 2014: a summary. Clin Kidney J 2017;10:154–69. https://doi.org/10.1093/ckj/sfw135.Search in Google Scholar PubMed PubMed Central
2. Tucker, PS, Scanlan, AT, Dalbo, VJ. Chronic kidney disease influences multiple systems: describing the relationship between oxidative stress, inflammation, kidney damage, and concomitant disease. Oxid Med Cell Longev 2015;2015:806358. https://doi.org/10.1155/2015/806358.Search in Google Scholar PubMed PubMed Central
3. Zyga, S, Christopoulou, G, Malliarou, M. Malnutrition-inflammation-atherosclerosis syndrome in patients with end-stage renal disease. J Ren Care 2011;37:12–5. https://doi.org/10.1111/j.1755-6686.2011.00201.x.Search in Google Scholar PubMed
4. Sharif, MR, Chitsazian, Z, Moosavian, M, Raygan, F, Nikoueinejad, H, Sharif, AR, et al.. Immune disorders in hemodialysis patients. Iran J Kidney Dis 2015;9:84–96.Search in Google Scholar
5. Kato, S, Chmielewski, M, Honda, H, Pecoits-Filho, R, Matsuo, S, Yuzawa, Y, et al.. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol 2008;3:1526–33. https://doi.org/10.2215/cjn.00950208.Search in Google Scholar
6. Kim, HW, Woo, YS, Yang, HN, Choi, HY, Jo, SK, Cho, WY, et al.. Primed monocytes: putative culprits of chronic low-grade inflammation and impaired innate immune responses in patients on hemodialysis. Clin Exp Nephrol 2011;15:258–63. https://doi.org/10.1007/s10157-010-0379-8.Search in Google Scholar PubMed
7. Ziegler-Heitbrock, L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol 2007;81:584–92. https://doi.org/10.1189/jlb.0806510.Search in Google Scholar PubMed
8. Ziegler-Heitbrock, L, Ancuta, P, Crowe, S, Dalod, M, Grau, V, Hart, DN, et al.. Nomenclature of monocytes and dendritic cells in blood. Blood 2010;116:e74–80. https://doi.org/10.1182/blood-2010-02-258558.Search in Google Scholar PubMed
9. Wong, KL, Yeap, WH, Tai, JJ, Ong, SM, Dang, TM, Wong, SC, et al.. The three human monocyte subsets: implications for health and disease. Immunol Res 2012;53:41–57. https://doi.org/10.1007/s12026-012-8297-3.Search in Google Scholar PubMed
10. Thieblemont, N, Weiss, L, Sadeghi, HM, Estcourt, C, Haeffner-Cavaillon, N. CD14lowCD16high: a cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. Eur J Immunol 1995;25:3418–24. https://doi.org/10.1002/eji.1830251232.Search in Google Scholar PubMed
11. Nockher, WA, Bergmann, L, Scherberich, JE. Increased soluble CD14 serum levels and altered CD14 expression of peripheral blood monocytes in HIV-infected patients. Clin Exp Immunol 1994;98:369–74. https://doi.org/10.1111/j.1365-2249.1994.tb05499.x.Search in Google Scholar PubMed PubMed Central
12. Fingerle, G, Pforte, A, Passlick, B, Blumenstein, M, Ströbel, M, Ziegler-Heitbrock, HW. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood 1993;15:3170–6. https://doi.org/10.1182/blood.v82.10.3170.3170.Search in Google Scholar
13. Koc, M, Toprak, A, Arikan, H, Odabasi, Z, Elbir, Y, Tulunay, A, et al.. Toll-like receptor expression in monocytes in patients with chronic kidney disease and haemodialysis: relation with inflammation. Nephrol Dial Transplant 2011;26:955–63. https://doi.org/10.1093/ndt/gfq500.Search in Google Scholar PubMed
14. Haag-Weber, M, Horl, WH. Dysfunction of polymorphonuclear leukocytes in uremia. Semin Nephrol 1996;16:192–201.Search in Google Scholar
15. Grover, HS, Saini, R, Bhardwaj, P, Bhardwaj, A. Acute-phase reactants. J Oral Res Rev 2016;8:32.10.4103/2249-4987.182491Search in Google Scholar
16. Cazzavillan, S, Ratanarat, R, Segala, C, Corradi, V, de Cal, M, Cruz, D, et al.. Inflammation and subclinical infection in chronic kidney disease: a molecular approach. Blood Purif 2007;25:69–76. https://doi.org/10.1159/000096401.Search in Google Scholar PubMed
17. Eleftheriadis, T, Liakopoulos, V, Leivaditis, K, Antoniadi, G, Stefanidis, I. Infections in hemodialysis: a concise review - Part 1: bacteremia and respiratory infections. Hippokratia 2011;15:12–7.Search in Google Scholar
18. Jofre, R, Rodriguez-Benitez, P, Lopez-Gomez, JM, Perez-Garcia, R. Inflammatory syndrome in patients on hemodialysis. J Am Soc Nephrol 2006;17(12 Suppl 3):S274–80. https://doi.org/10.1681/ASN.2006080926.Search in Google Scholar PubMed
19. Nockher, WA, Scherberich, JE. Expanded CD14+ CD16+ monocyte subpopulation in patients with acute and chronic infections undergoing hemodialysis. Infect Immun 1998;66:2782–90. https://doi.org/10.1128/iai.66.6.2782-2790.1998.Search in Google Scholar PubMed PubMed Central
20. Kim, HW, Yang, HN, Kim, MG, Choi, HM, Jo, SK, Cho, WY, et al.. Microinflammation in hemodialysis patients is associated with increased CD14CD16(+) pro-inflammatory monocytes: possible modification by on-line hemodiafiltration. Blood Purif 2011;31:281–8. https://doi.org/10.1159/000321889.Search in Google Scholar PubMed
21. Kuroki, Y, Tsuchida, K, Go, I, Aoyama, M, Naganuma, T, Takemoto, Y, et al.. A study of innate immunity in patients with end-stage renal disease: special reference to toll-like receptor-2 and -4 expression in peripheral blood monocytes of hemodialysis patients. Int J Mol Med 2007;19:783–90. https://doi.org/10.3892/ijmm.19.5.783.Search in Google Scholar
22. Ramirez, R, Carracedo, J, Berdud, I, Carretero, D, Merino, A, Rodríguez, M, et al.. Microinflammation in hemodialysis is related to a preactivated subset of monocytes. Hemodial Int 2006;10(Suppl 1):S24–7. https://doi.org/10.1111/j.1542-4758.2006.01186.x.Search in Google Scholar PubMed
23. Ramirez, R, Carracedo, J, Merino, A, Soriano, S, Ojeda, R, Alvarez-Lara, MA, et al.. CD14+CD16+ monocytes from chronic kidney disease patients exhibit increased adhesion ability to endothelial cells. Contrib Nephrol 2011;171:57–61. https://doi.org/10.1159/000327134.Search in Google Scholar PubMed
24. Coulliette, AD, Arduino, MJ. Hemodialysis and water quality. Semin Dial 2013;26:427–38. https://doi.org/10.1111/sdi.12113.Search in Google Scholar PubMed PubMed Central
25. Huang, Z, Gao, D, Letteri, JJ, Clark, WR. Blood-membrane interactions during dialysis. Semin Dial 2009;22:623–8. https://doi.org/10.1111/j.1525-139x.2009.00658.x.Search in Google Scholar
26. Sester, U, Sester, M, Heine, G, Kaul, H, Girndt, M, Köhler, H. Strong depletion of CD14(+)CD16(+) monocytes during haemodialysis treatment. Nephrol Dial Transplant 2001;16:1402–8. https://doi.org/10.1093/ndt/16.7.1402.Search in Google Scholar PubMed
27. Rogacev, KS, Ziegelin, M, Ulrich, C, Seiler, S, Girndt, M, Fliser, D, et al.. Haemodialysis-induced transient CD16+ monocytopenia and cardiovascular outcome. Nephrol Dial Transplant 2009;24:3480–6. https://doi.org/10.1093/ndt/gfp287.Search in Google Scholar PubMed
28. Grabulosa, CC, Manfredi, SR, Canziani, ME, Quinto, BMR, Barbosa, RB, Rebello, JF, et al.. Chronic kidney disease induces inflammation by increasing Toll-like receptor-4, cytokine and cathelicidin expression in neutrophils and monocytes. Exp Cell Res 2018;365:157–62. https://doi.org/10.1016/j.yexcr.2018.02.022.Search in Google Scholar PubMed
29. Verzola, D, Bonanni, A, Sofia, A, Montecucco, F, D’Amato, E, Cademartori, V, et al.. Toll-like receptor 4 signalling mediates inflammation in skeletal muscle of patients with chronic kidney disease. J Cachexia Sarcopenia Muscle 2017;8:131–44. https://doi.org/10.1002/jcsm.12129.Search in Google Scholar PubMed PubMed Central
30. Belge, KU, Dayyani, F, Horelt, A, Siedlar, M, Frankenberger, M, Frankenberger, B, et al.. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol 2002;168:3536–42. https://doi.org/10.4049/jimmunol.168.7.3536.Search in Google Scholar PubMed
© 2022 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Opinion Paper
- Ischemia – modified albumin by albumin cobalt binding test: a false myth or reality
- Research Articles
- Machine learning models can predict the presence of variants in hemoglobin: artificial neural network-based recognition of human hemoglobin variants by HPLC
- Investigation of the preanalytical process practices in primary care in Istanbul regarding the newborn screening tests
- Cell counting chamber vs. Sysmex XN-1000 for determining white blood cell count and differentiation for body fluids
- Synthesis of PEITC-loaded gold nanoparticles and evaluation of the hepatoprotective effect on CCl4-induced damage through Nrf2 pathway
- Concentrations of B cell-activating factor, aquaporin-4 antibody and brain-derived neurotrophic factor in neuromyelitis optica spectrum disorder
- Macula, choroid and retinal nerve fiber layer optical coherence tomography biomarkers in liver dysfunction
- Direct-acting antiviral therapy may help restore HCV-induced impaired redox balance and liver fibrosis process
- Are VEGF and SCUBE1 gene expressions increased in diabetic retinopathy?
- Biochemical analysis of microbiotas obtained from healthy, prediabetic, type 2 diabetes, and obese individuals
- Inflammation parameters, monocyte subgroups and toll-like receptor expression before and after dialysis in patients with chronic kidney disease
- Matrix metalloproteinase 9 gene-MMP9-DNA methylation status in Turkish schizophrenia patients
- HLA DRB1 alleles, IFN-γ and TGF-β Gene Variants in childhood ALL patients
- Epithelial-mesenchymal transition as a potential route for DAPT resistance in breast cancer cells
- Comparison with molecular effects of ukrain, tamoxifen, and docetaxel on human breast cancer cell lines
- In vitro evaluation of 2-pyrazoline derivatives as DPP-4 inhibitors
- Effects of polyphenolic-rich extracts from Citrus hystrix on proliferation and oxidative stress in breast and colorectal cancer
- The effects of sodium benzoate exposure on learning and neurobehavior during the prepubertal period in rats
- The association between βeta 2-microglobulin and bronchopulmonary dysplasia
Articles in the same Issue
- Frontmatter
- Opinion Paper
- Ischemia – modified albumin by albumin cobalt binding test: a false myth or reality
- Research Articles
- Machine learning models can predict the presence of variants in hemoglobin: artificial neural network-based recognition of human hemoglobin variants by HPLC
- Investigation of the preanalytical process practices in primary care in Istanbul regarding the newborn screening tests
- Cell counting chamber vs. Sysmex XN-1000 for determining white blood cell count and differentiation for body fluids
- Synthesis of PEITC-loaded gold nanoparticles and evaluation of the hepatoprotective effect on CCl4-induced damage through Nrf2 pathway
- Concentrations of B cell-activating factor, aquaporin-4 antibody and brain-derived neurotrophic factor in neuromyelitis optica spectrum disorder
- Macula, choroid and retinal nerve fiber layer optical coherence tomography biomarkers in liver dysfunction
- Direct-acting antiviral therapy may help restore HCV-induced impaired redox balance and liver fibrosis process
- Are VEGF and SCUBE1 gene expressions increased in diabetic retinopathy?
- Biochemical analysis of microbiotas obtained from healthy, prediabetic, type 2 diabetes, and obese individuals
- Inflammation parameters, monocyte subgroups and toll-like receptor expression before and after dialysis in patients with chronic kidney disease
- Matrix metalloproteinase 9 gene-MMP9-DNA methylation status in Turkish schizophrenia patients
- HLA DRB1 alleles, IFN-γ and TGF-β Gene Variants in childhood ALL patients
- Epithelial-mesenchymal transition as a potential route for DAPT resistance in breast cancer cells
- Comparison with molecular effects of ukrain, tamoxifen, and docetaxel on human breast cancer cell lines
- In vitro evaluation of 2-pyrazoline derivatives as DPP-4 inhibitors
- Effects of polyphenolic-rich extracts from Citrus hystrix on proliferation and oxidative stress in breast and colorectal cancer
- The effects of sodium benzoate exposure on learning and neurobehavior during the prepubertal period in rats
- The association between βeta 2-microglobulin and bronchopulmonary dysplasia

