Abstract
Objectives
Temozolomide (TMZ) is an effective drug for glioblastoma multiforme (GBM), but the mechanism underlying TMZ resistance is poorly understood. New evidence has revealed that the release of heat shock proteins (Hsps) derived from extracellular vesicles (EVs) play an important role in cancer progression by modulating tumor microenvironment and cellular cross-talk. This study aims to evaluate the effects of TMZ on the expression of EV-derived and cellular Hsps and cell motility in U87MG human glioblastoma cell line.
Methods
Glial-EVs were isolated from the culture medium and characterized by SEM and immunoblotting. The effect of TMZ treatments (25, 200 and 750 µM) on cell proliferation (MTT assay), migration (scratch assay), and Hsp60 and Hsp70 levels (immunoblotting) were evaluated.
Results
TMZ treatments led to an increase in intracellular Hsp70 while decreasing EV-derived Hsp70. Cellular Hsp60 level was elevated at the low dose of TMZ, but it reduced at higher TMZ concentrations. Hsp60 was also decreased in EVs secreted from TMZ-treated cells. Besides, TMZ treatment reduced the proliferation and migration of glioma cells in a dose-dependent manner.
Conclusions
Our results suggest that TMZ has the potential to target both EV-derived and cellular Hsps for GBM treatment, thus it may reduce cell motility.
Öz
Amaç
Temozolomid (TMZ), glioblastoma multiforme (GBM) için etkili bir ilaçtır, ancak TMZ direncinin altında yatan mekanizma tam olarak anlaşılamamıştır. Yeni kanıtlar, hücre dışı veziküllerden (“extracellular vesicles” EV) kökenlenen ısı şoku proteinlerinin (“heat shock proteins” Hsp) salınımının, tümör mikroçevresi ve hücresel cross-talk’ı modüle ederek kanserin ilerlemesinde önemli bir rol oynadığını ortaya koymuştur. Bu çalışmanın amacı TMZ’nin U87MG insan glioblastoma hücre hattında EV’den kökenlenmiş ve hücresel Hsp ekspresyonu ile hücre motilitesi üzerindeki etkilerini değerlendirmektir.
Gereç ve Yöntem
Glia kökenli EV’ler hücre kültürü ortamından izole edildi, SEM ve immunoblotlama ile karakterize edildi. TMZ uygulamalarının (25, 200 ve 750 μM) hücre proliferasyonu (MTT testi), migrasyon (scratch testi) ve Hsp60 ve Hsp70 seviyeleri (immünoblotlama) üzerindeki etkisi değerlendirildi.
Bulgular
TMZ uygulamaları, EV kökenli Hsp70’i azaltırken hücre içi Hsp70’de bir artışa yol açtı. Hücresel Hsp60 seviyesi, düşük TMZ dozunda yükseldi, ancak daha yüksek TMZ konsantrasyonlarında azaldı. Hsp60, TMZ uygulanan hücrelerden salgılanan EV’lerde de azaldı. Ayrıca, TMZ uygulması doza bağlı bir şekilde glioma hücrelerinin çoğalmasını ve göçünü azalttı.
Sonuç
Sonuçlarımız, TMZ’nin GBM tedavisi için hem EV’den kökenlenen hem de hücresel Hsps’yi hedefleme potansiyeline sahip olduğunu, dolayısıyla hücre motilitesini azaltabileceğini göstermektedir.
Introduction
Glioblastoma multiforme (GBM) is one of the most common primary malignant brain tumors that is highly aggressive and has a poor prognosis. In the standard care of GBM, multidisciplinary strategies are followed. After surgical resection is performed, it is necessary to be coupled with a drug and/or radiation therapy. Currently, acceptable penetration through the blood-brain barrier (BBB), temozolomide (TMZ) is a primarily preferential chemotherapeutic agent, which FDA approved for glioblastoma. TMZ is an alkylating prodrug that breaks the DNA double-strand, thus leading to cell cycle arrest and resulted in cell death [1, 2].
Extracellular vesicles (EVs) are submicron structures and are released from many cell types into the extracellular space after the fusion of multivesicular bodies (MVBs) with the cell membrane. Cancer cells secrete EVs at high levels, and their cargos are particularly to promote cancer progression. Once uptake by recipient cells, the tumor-derived EVs can alter the functions of the cells by affecting both surrounding and distal cells to provide a suitable microenvironment for cancer cell proliferation, metastasis, angiogenesis, and development of drug resistance. EVs are widely investigated for their involvement in regulating tumor pathobiology in GBM as well as their use as potential biomarkers [3, 4].
In GBM progression, cell migration and metastasis are considered key events. EVs secreted by GBM cells are enriched in many mRNAs, miRNAs, and proteins (particularly heat shock proteins, Hsps) associated with tumor cell proliferation and metastasis. EVs are considered as a potential contribution to cancer prognosis and a novel approach [5, 6]. Various studies have shown that the increased stress levels in cancer cells are associated with differentiation under lethal conditions, invasion, metastasis, tumor cell proliferation and survival, and drug resistance. In this context, Hsps have become a good target in cancer therapy. Inhibition of Hsp expression is used as a new approach in the treatment of cancerous cells in order to increase cell apoptosis and reduce resistance to chemotherapeutic drugs [7].
EV-associated Hsp60 is a key player in intercellular communication and has the potential as a biomarker useful for diagnosing and assessing the prognosis of a variety of cancers. There is cumulative evidence supporting the notion that Hsp60 works not only intracellularly but also extracellularly and is involved in intercellular cross-talk. The level of Hsp60 increases in many tumor types and Hsp60 overexpression has a significant role in cancer progression [8], [9], [10].
Hsp70 is significantly effective in tumor development and is generally overexpressed in cancer cells. High Hsp70 levels play a key role in malignancy, cell proliferation, resistance to therapies and poor prognosis. The secretory mechanisms of Hsp70 from tumor cells are complex and incompletely understood. Importantly, Hsp70 is known to be actively secreted by cancer cells via EV-mediated pathways. EV-derived Hsp70 can induce metastasis and cytolytic activity of tumor cells [11].
Although TMZ is an effective drug for GBM, the mechanism underlying TMZ resistance is poorly understood. Given the potential role of Hsps in drug resistance, we tested the hypothesis that glioma cells would increase intracellular Hsp levels while decreasing EV-derived Hsps against TMZ treatment. And if so, increased Hsp in the cell may protect the cell against the drug, while decreased levels of Hsps in EV content may be slowing GBM progression. In the present study, we aimed to investigate how TMZ affects the expression levels of cellular and EV-derived Hsp60 and Hsp70 and to understand the relationship between Hsp levels and migration in the U87MG human glioblastoma cell line. Our findings demonstrated that TMZ treatment altered the levels of cellular and EV-derived Hsp60 and Hsp70, reduced proliferation and migration ability of glioma cells. Thus far, there is no data available regarding that the levels of both Hsp60 and Hsp70 secreted by the GBM-associated EVs are reduced with treatment with different doses of TMZ and consequently decrease the migration. These findings may help bring a new perspective to the treatment of highly aggressive GBM and the potential use of TMZ.
Materials and methods
Reagents and antibodies
Temozolomide was purchased from Sigma-Aldrich (T2577, St Louis, MO, USA) dissolved in dimethyl sulfoxide (DMSO, Merck, Darmstadt, Germany). Standard cell culture reagents were obtained from Gibco (Carlsbad, CA, USA). ExoEasy Maxi Kit was from Qiagen (South Korean). EDTA-free protease inhibitor cocktail was purchased from Roche (Darmstadt, Germany). SMART™ BCA Protein Assay Kit was obtained from iNtRON Biotechnology (Seongnam, Korea). Polyvinylidene fluoride (PVDF) membrane for Western blotting, the Amicon® 50 K centrifugal ultrafiltration tubes, and 0.45 μm diameter pore filters were from Millipore (Bedford, MA, USA). SuperSignal™ West Pico PLUS Chemiluminescent Substrate was from Thermo Fisher Scientific (Kwartsweg, Holland). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA). The antibodies used in the study were given in Table 1.
Primary and Secondary antibodies were used in the immunological analysis.
| Antibody | Clonality | Manufacturer company | Code | Source | Dilution |
|---|---|---|---|---|---|
| Anti-Hsp70 | Monoclonal | Enzo Life Sciences | ADI-SPA-810-F | Mouse | 1:1,000 |
| Anti-Hsp60 | Monoclonal | Boster | M01280-1 | Rabbit | 1:1,000 |
| Anti-Alix | Monoclonal | Santa Cruz | sc-53540 | Mouse | 1:200 |
| Anti-TSG101 | Monoclonal | Novus | NB200-112 | Mouse | 1:500 |
| Anti-GAPDH | Monoclonal | Thermo Scientific | MA5-15738 | Mouse | 1:2000 |
| Anti-CD81 | Monoclonal | Novus | NB100-65805 | Mouse | 1:500 |
| Anti-β-catenin | Polyclonal | Thermo Scientific | PA5-19469 | Rabbit | 1:1,000 |
| Anti-mouse IgG secondary antibody, HRP | Polyclonal | Enzo Life Sciences | ADI-SAB-100-J | Goat | 1:2,500 |
| Anti-rabbit IgG secondary antibody, HRP | Polyclonal | Thermo Scientific | 31,460 | Goat | 1:3,000 |
Cell line and cell culture
The human glioblastoma cell line U87MG was obtained from Istanbul University Cell Culture Collections. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM with 4.5 g/L glucose) supplemented with 10% fetal bovine serum (FBS), 1% antibiotics-antimitotic solution (100 U/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B) and 1% non-essential amino acids (NEAA) at 37 °C with 5% CO2 in a humidified incubator. Experiments were performed using cells from passages 3–10.
Cell viability assay
MTT (3 -(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was performed to determine cell viability as previously described [12]. Briefly, U87MG cells (1.5 × 104 per well) were seeded in 96-well plates and incubated at 37 °C for 24 h. Different concentrations of TMZ (1, 10, 50, 100, 250, 500, 750, 1,000, and 1,500 μM) were tested on the cells for 24, 48 and 72 h. After treatments, the cells were incubated with MTT at a final concentration of 0.5 mg/mL at 37 °C for 4 h, followed by the addition of DMSO to dissolve the formazan crystals. The absorbance of each well was measured at 570 nm using a microplate reader (EON, BioTek Instruments Inc., Winooski, VT, USA). Half-maximal inhibitory concentrations (IC50) of TMZ were calculated by fitting the data to a sigmoidal dose-response curve using nonlinear regression analysis. All treatment groups were measured in triplicate.
Temozolomide treatment
U87MG cells (2 × 106) were seeded in T-175 culture dishes and incubated at 37 °C for 24 h prior to the TMZ treatment. Then, the old medium was removed and replaced with a fresh medium containing EVs depleted serum. The cells were treated for 48 h with three different doses of TMZ which were selected according to MTT results: 25 µM (non-toxic dose), 200 µM (20% toxic dose, IC20 value), and 750 µM (IC50 value). Experiments were performed 48 h after treatments. Each experiment was performed at least three times.
Morphological assessment
U87MG cells were seeded in 24 well cell culture plates, followed by treatment with 25, 200, and 750 µM TMZ or DMSO. Following treatment for 48 h, morphological alterations were analyzed by observation under an inverted phase-contrast microscope (Nikon Eclipse Ti-E, NY, USA) at ×20 magnification.
Isolation of EVs
U87MG derived EVs from culture medium supplemented with exosome-depleted serum were purified after temozolomide treatment at different concentrations. Briefly, the supernatants of U87MG cells were collected, subsequently were filtrated via a 0.45 μm diameter pore filter and centrifuged at 4 °C at 5,000×g for 5 min to remove cells and debris (Eppendorf Centrifuge 5810 R, Hamburg, Germany). The supernatants were concentrated by ultrafiltration using a 50 K centrifugal ultrafiltration tube (50 kDa membrane filter) at 5,000×g for 15 min. EVs were isolated from remaining supernatants by using the ExoEasy Maxi isolation kit according to the manufacturer’s instructions.
Scanning electron microscopy
To confirm the success of the EVs isolation, a scanning electron microscope (SEM, FEI, Versa 3D LoVac) was used through the following standard protocol [13]. Briefly, the EVs isolated from cell culture supernatant of U87MG cells were lyophilized (Christ Freeze Dryer Alpha 1–4 LD plus) overnight. After the samples were dried, they were transferred onto carbon-coated electron microscopy grids. EVs sizes were analyzed at 10.0 kV.
Protein extraction and western blot analysis
U87MG human glioblastoma cells treated with TMZ were harvested and centrifuged at 3,000×g for 5 min. The proteins were extracted from cell pellet and EVs samples in RIPA buffer. Then the extracts were centrifuged at 20,000×g for 20 min at 4 °C (Sigma 3–30 K).
In order to concentrate EV proteins, the methanol/chloroform protein precipitation method was used [14]. Samples were analyzed by immunoblotting using standard procedure [15]. Equal quantities of protein samples (30 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by transferring onto PVDF by using Wet/Blotting Tank System (Bio-Rad) at 100 V. The membranes were blocked with Tris-buffered saline with 0.1% Tween-20 (TBST) buffer containing 5% skim milk for 1 h at RT followed by incubation with primary antibodies (Table 1) at overnight 4 °C. Then, the membranes were washed with TBST for 15 min (five times), incubate with HRP-conjugated secondary antibody (Table 1) for 2 h at RT, washed with TBST again, and then protein bands were visualized by using an enhanced chemiluminescent (ECL) substrate kit. GAPDH was used for internal control. The protein expressions were quantitated by using the Image Lab 5.2.1 software (Bio-Rad).
In vitro scratch assay
Scratch assay was used to evaluate the cell migration in U87MG cells [16]. Briefly, the cells (1.5 × 104/well) were cultured in 24-well plates and incubated at 37 °C for 24 h. Then, the cell monolayer was gently and slowly scratched a straight line with a sterile 200 μL pipette tip. Detached cells were removed by gently washing with phosphate-buffered saline (PBS; pH 7.4). Control group cells were treated with the only medium with EVs-depleted serum; the experimental group cells were treated TMZ at 25, 200, and 750 μM concentrations. Cells were then allowed to grow for 24 and 48 h. The cells were observed, and images were captured at ×40 magnification under an inverted microscope (Nikon Eclipse Ti-E, Nikon Instruments Inc., NY, U.S.A.). The relative migration of the cells was quantitatively assessed based on the gap at the 0 h point was evaluated using Image J software.
Statistical analysis
All the quantitative data were expressed as the mean ± standard deviation and each experiment was performed triplicate. Arithmetic means and standard deviation (SD) were calculated by using GraphPad Prism® 7.01 (GraphPad Software Inc., La Jolla, CA, USA). The statistically significant differences between the groups were determined using one-way ANOVA (analysis of variance)-followed by Tukey’s post-hoc test for multiple-group comparison. The statistically significant difference was accepted p<0.05.
Results
The effects of temozolomide (TMZ) on U87MG cell viability
TMZ treatments reduced the cell viability in a dose-dependent manner (Figure 1A). According to MTT data, both for 24, 48, and 72 h at low doses of TMZ (1 and 10 μM) were showed proliferative effects, and at high doses of TMZ (1,000 and 1,500 μM) inhibited cell viability by less than 50%. After 48 h treatment, a proliferative or cytotoxic effect for 25 μM was not observed. The results showed that TMZ blocked U87MG cell proliferation in a dose-dependent manner (Figure 1B). The IC50 value of TMZ was determined as 748.27 μM for 48 h (Figure 1A, B). The value of IC50 was calculated by fitting the data to a sigmoidal dose-response curve using GraphPad Prism 7.00 software.
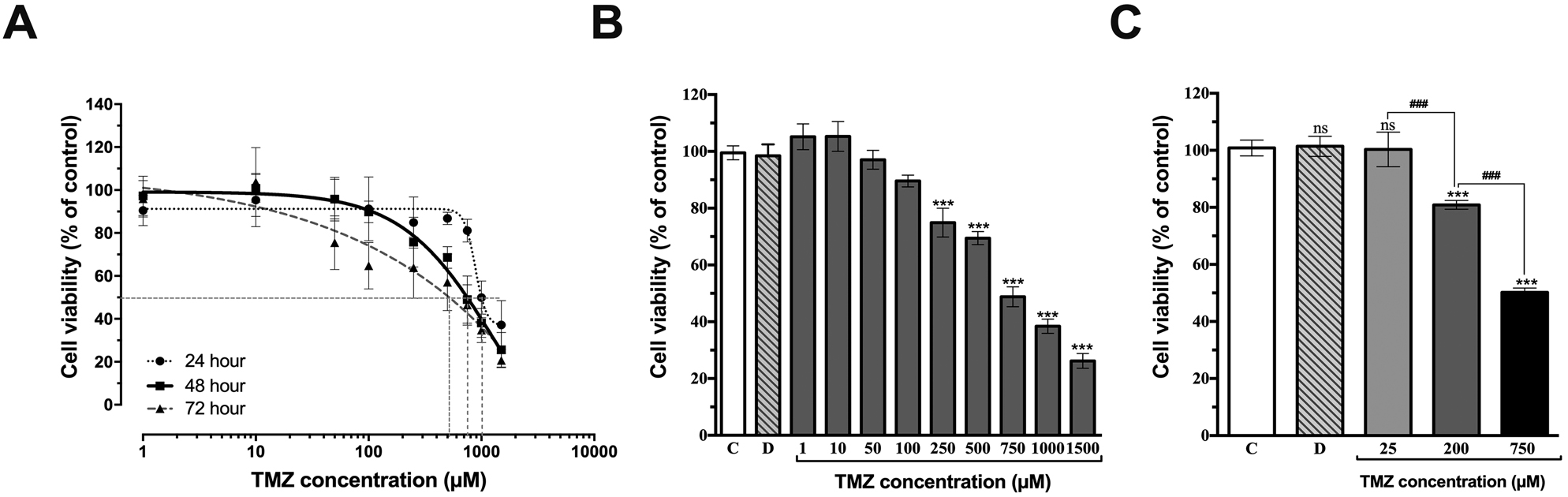
Effect of temozolomide (TMZ) on the viability of U87MG cells. A) Cell viability was determined by MTT assay. The dose-dependent curve of TMZ on U87MG cell viability for 24, 48, and 72 h. IC50 value for 48 h is 748.27 μM (R 2 = 0.872) B) TMZ affects the cell viability at different concentrations C) Selected concentrations of TMZ on cell viability by 100, ≥80, ≥50%. The value of IC50 was calculated by fitting the data to a sigmoidal dose-response curve using nonlinear regression analysis. The control (C), treated with 0.75% DMSO (D, the final concentration in the culture medium did not exceed 0.75% and not effect on cell viability), ns not significant. ***p<0.001 vs. untreated group (C) determined by one-way ANOVA using Dunnett’s multiple comparison test. ###p<0.001 indicates statistical difference between different groups. Vertical bars indicate standard deviation values.
TMZ reduced U87MG proliferation (cell viability) and induced morphological alterations in dose-dependent manner (Figure 2). According to results, distinct cell death can be observed in U87MG cells following treatment with 25, 200, and 750 µM of TMZ. We selected three doses of TMZ to be used in experiments based on the cell viability=100, ≥80, ≥50% (Figure 1C). After 48 h TMZ treatment on glioblastoma cells, significant disruptions and detachments were observed in the cell monolayer by 750 µM concentration. Cells became disconnecting and lost their original shapes. TMZ led to cell death compared to control group (Figure 2).

Cells were imaged using an inverted phase contrast microscope 48 h post TMZ exposure. Distinct cell death observed in U87MG cells following treatment with 25, 200, and 750 µM TMZ. Magnification, ×20, Scale bar, 200 μm.
Isolation and characterization of U87MG cell derived EVs
EVs isolation ad protein extractions were performed as described in “Materials and Methods”. The EVs marker proteins were evaluated (Figure 3A). Characterization of EVs by western blot analysis confirmed the presence of CD63, CD81 Alix, and TSG101. Alix and TSG101 were also detected in the cell lysate, but their expressions in EVs were higher than in cells. Also, none of these four marker proteins were detected in the EV-free supernatant (SN) cell medium. Moreover, the morphology of EVs was evaluated by scanning electron microscopy (SEM); this analysis indicated that EVs were round nanovesicles about 50–100 nm in diameter (Figure 3B).
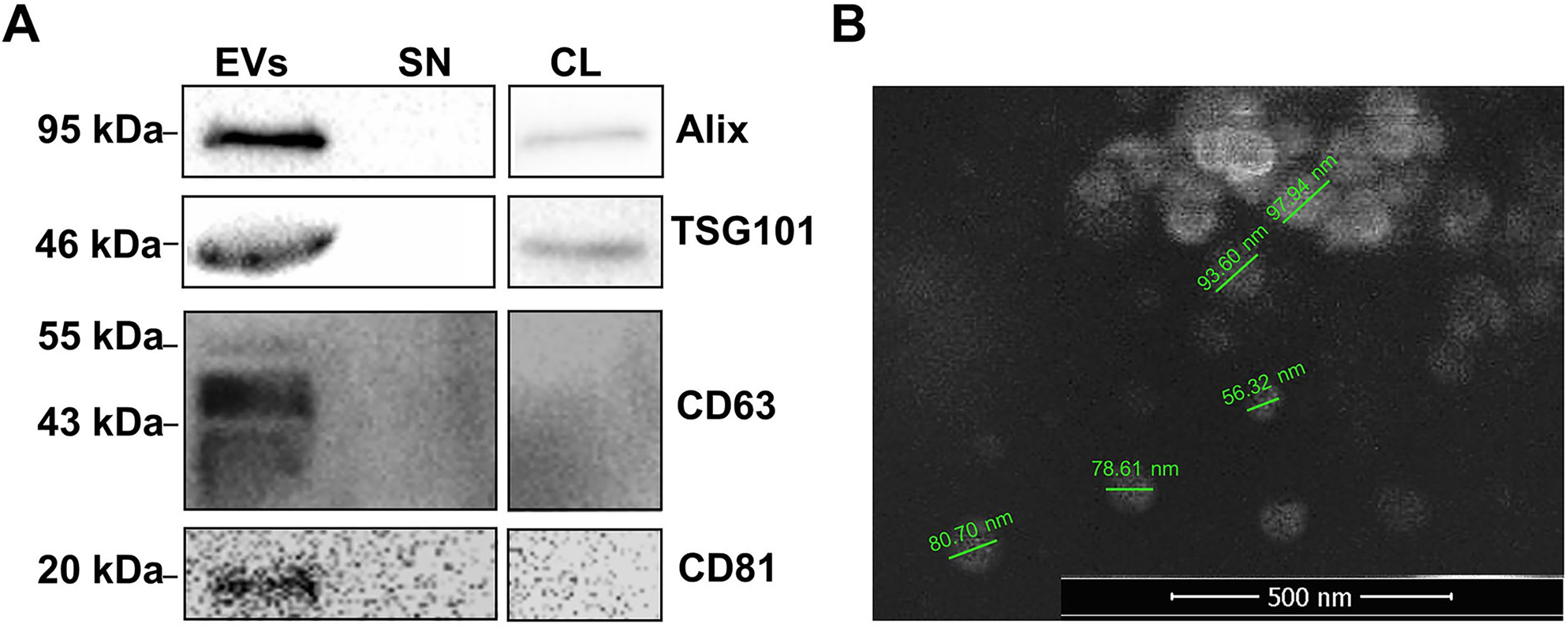
Identification of EVs released from U87MG by Western blotting and SEM imaging. A) Western blot analysis demonstrated expressions of EV marker proteins in extracellular vesicles released from glioblastoma cells. B) The characterization of EVs were performed using Scanning electron microscope (SEM). Original magnification ×50,000. The bar 500 nm. Extracellular vesicles (EVs), Supernatant (SN), Cell Lysate (CL).
The effects of TMZ treatment on the expression of Hsp70 and Hsp60 in U87MG cells and EVs
Depending on the various concentrations of TMZ treatment, which were selected based upon MTT assay results, the expression level of both Hsp70 and 60 was determined by Western blotting analysis (Figure 4). TMZ treatments except for 750 μM (25 and 200 μM, respectively) increased cellular Hsp70 expression by 230 and 154%, whereas EV-derived Hsp70 levels reduced (68, 74, and 94%) by TMZ treatments (25, 200, and 750 μM, respectively) in a dose-dependent manner. Hsp70 was depleted in cells treated with a toxic dose TMZ (750 μM). The content of EVs secreted from U87MG cells was decreased in terms of Hsp70 due to TMZ treatment (Figure 4A, C). Cellular Hsp60 expression showed the highest level at a non-toxic dose of TMZ and then kept diminishing as TMZ concentration raised. On the other hand, while Hsp60 was decreased in EVs secreted from TMZ-treated cells, no significant dose-related changes were observed (Figure 4B, C).

The effects of temozolomide (TMZ) treatment on expression of Hsp70 and Hsp60. A) The expression levels of cellular, EV-derived, and extracellular (Ex) Hsp70 in dose dependent manner. B) The expression levels of cellular, EV-derived, and extracellular (Ex) Hsp60 in dose dependent manner. C) Western blotting analysis of Hsp70 and Hsp60 expression in cell lysate (CL), extracellular vesicles (EVs), and supernatant-cell media (SN). While using GAPDH as a loading control for CL, Ponceau staining was performed for SN and EVs. p-Values were determined using one-way ANOVA followed by Tukey’s post hoc test. *p<0.05, **p<0.01, and ***p<0.001 vs. control (C) group. #p<0.05, ##p<0.01, and ###p<0.001 indicate statistical difference between different groups. Vertical bars indicate standard deviation values.
In addition to intracellular and EV-derived Hsps, it was also demonstrated that at least Hsp70 and Hsp60 were shed from glioma cells into the cell culture media (supernatant, SN). In the extracellular milieu, Hsp70 level was elevated in a toxic dose of TMZ (750 μM), which might explain the depleted Hsp70 in both cells and EVs. The level of extracellular Hsp60 secreted from TMZ-treated cells significantly decreased, but did not change depending on TMZ doses (Figure 4C).
TMZ suppresses the cell migration ability in U87MG cells
The impact of TMZ treatments (25, 200, and 750 μM) on the migration capacity of U87MG cells was investigated for 24 and 48 h (Figure 5A, B). Cell migration activity was assessed by conducting in vitro scratch assay. Closure of scratches was imaged and calculated as a percentage of migration. The toxic dose of TMZ (750 μM) reduced the migration rate of glioma cells higher than control and other doses (25 and 200 μM). In addition, the diminishing in the migration of cells was supported by Western blotting analysis. The reduction in β-catenin expression indicated that TMZ exerted an influence on the cell migration ability (Figure 5C).

The effect of temozolomide (TMZ) on the cell migration ability. A) Cell migration rate (%) was determined by in vitro scratch assay. Vertical bars indicate standard deviation values. *p<0.05 and ***p<0.001 vs. control group for 24 h, ###p<0.001 vs. control group for 48 h, &&p<0.01 and &&&p<0.001 indicates statistical difference between different groups B) Images of wounded monolayer of U87MG taken at times 0, 24 and 48 h after treatment with TMZ. C) The expression of β-catenin of TMZ treated cells. **p<0.01 vs. control group, #p<0.05 indicate statistical difference between different groups.
Discussion
Studies conducted on brain tumor cell lines have shown the importance of EVs released from glioma cells in cancer progression, as well as the importance of EV content. In particular, EV-derived Hsp60 and Hsp70 have essential roles in malignant glioma cells [17, 18]. Therefore, we examined the levels of Hsp60 and Hsp70 in EVs released from GBM and how their expression levels were affected by the treatment with the TMZ drug. We also investigated the effect of TMZ on intracellular and EV-derived Hsps as well as the extracellular environment. To our best knowledge to date, only one study by Panzarini et al. [19] demonstrated the reducing effect of TMZ on Hsp70 in EVs released from glioma cells. However, only the effect of a 200 µM dose of TMZ was reported. Therefore, this is the first research that revealing the effect of TMZ on EV-derived Hsps in glioma cells and cell migration rate. Additionally, the same study [19] showed that the EV concentration release from GBM in culture medium was the highest in U87MG cells compared to other glioma cell lines U373MG, T98G, and U251MG. Therefore, we chose the U87MG cell line in our study.
Hsp70 plays a key role in the regulation of malignancy, cell proliferation, resistance to therapies and poor prognosis [10, 20]. It can exist in four forms: (a) intracellular Hsp70, (b) extracellular Hsp70, (c) exosomal Hsp70, and (d) membrane-bound form. Also, the level of Hsp60 increases in many tumors, and its overexpression has a significant role in cancer progression. Hsp60 works not only intracellularly but also extracellularly and is involved in intercellular cross-talk [7], [8], [9]. Anticancer drugs used in therapy cause changes in Hsp levels and increase cell death. Herein we showed that intracellular, extracellular, and EV-derived forms of Hsp60 and Hsp70 levels after TMZ treatments. A decrease in Hsp60 level was observed after TMZ treatments (200 and 750 µM) compared to the control group, but 25 µM TMZ increased Hsp60 expression. Unlike, extracellular and EV-derived Hsp60 levels were higher in the control group than in the treatment groups. Besides, intracellular Hsp70 expression increased by treatment with 25 and 200 µM TMZ compared to the control group. It is well known that high cytosolic levels of Hsp70 predominantly mediate resistance to apoptosis, so also in our study, it was observed that 25 and 200 µM TMZ treatment did not cause a decrease in cell number. However, Hsp70 is nearly depleted in both intracellular and EVs at a toxic dose (750 µM). Remarkably, when the supernatant was analyzed separately, we observed that Hsp70 elevated in the glioma cells treated with 750 µM of TMZ. Free Hsp70 is generally assumed to originate from dying tumor cells [21], [22], [23], [24]. We showed the expression levels of Hsps under TMZ treatment in the extracellular environment comparatively. Our study contributes new data; TMZ treatment caused increased extracellular Hsp70 level while cellular and EV-derived Hsp70 almost depleted at 750 µM. These findings suggested that Hsp70 either translocated or released to the extracellular milieu via a toxic dose of TMZ. In addition to intracellular and EV-derived Hsps, it was also demonstrated that at least Hsp70 and Hsp60 were shed from glioma cells into the extracellular milieu. Hsp70 level was elevated in the toxic dose of TMZ, which explains the depleted Hsp70 in both cells and EVs. However, the extracellular Hsp60 level was reduced due to TMZ treatment. It was documented that in vivo tumors grown from glioma cell lines express at high levels Hsp60 and 70, which is expressed in glioma cells is released as EVs under the stress conditions [25]. Consequently, new data emerged on the effects of these two Hsps on glioma progression and their response to TMZ therapy.
Cell migration and metastasis are considered key events in glioma progression. EVs actively participate in the metastasis process in different ways. Particularly, GBM EVs have mRNA and proteins associated with metastasis and promote endothelial cell proliferation. Moreover, tumor-derived EVs carry Hsp70, so they trigger and activate the migratory and cytolytic activity of natural killer (NK) cells and macrophages. Also, Hsp60 stimulates cancer progression by migration, invasion, and drug resistance in cancer cells [25], [26], [27], [28], [29]. For developing effective and novel glioma therapeutics targeting Hsps, it is necessary to downregulate expressions of Hsp. The effect of TMZ on the inhibition of cell growth and migration of both U251 and U87MG cells was reported in several studies. One of the previous studies on the U87MG cell line treated with TMZ and afatinib has accomplished effective silencing tumorigenicity [30, 31]. In the present study, we investigated the effect of different concentrations of TMZ on the cell migration and motility of U87MG cells by in vitro scratch assay. With comparable initial scratch sizes, cells showed limited growth under TMZ, thereby resulting in significantly decreased cell migration. This migration assay revealed that TMZ affects slowing cell migration in a dose-dependent manner. Our findings suggested that the decrease in migration rate may be related to the reduced levels of EV-derived Hsp60 and Hsp70. However, the reduced cell migration rate may also be related to the growth inhibitory effect of TMZ on the cells. One of the major limitations of our study is the lack of direct evidence for the relationship between reduced EV-induced Hsp levels and decreased migration. This phenomenon requires further analysis in the presence of Hsp-silenced cells.
A study based on proteomic analysis of extracellular vesicles that originated from different tumors showed some proteins like SERPINA1, SERRPINF2, and MMP are released from EVs that function in EMC remodeling vascular leakiness, and invasiveness. Another MS-based study of EVs revealed that after TMZ treatment, glioblastoma stem-like cells (GSC4) release EVs which contain some proteins involved in the modulation of cell adhesion [32, 33]. Luga et al. [34] showed that fibroblasts secrete EVs, and these could promote breast cancer cells’ protrusive activity, motility, and metastasis by activating autocrine Wnt-planar cell polarity (PCP) signaling. All the studies conducted up to now showed that EVs could affect invasion, motility, and metastasis activity by their specific protein content. In our study, TMZ treatments resulted in a decrease in glioma migration ability in addition to a reduction in the amount of Hsp60 and Hsp70 in the EV content. Further experiments should be performed to determine the potential relationship between EV-derived Hsp levels and migration ability in glioblastoma cells. Besides, it is well known that EVs carry abundant levels of cell adhesion-related proteins after TMZ treatment, so in further experiments, we consider a proteomic approach to determine the levels of cell adhesion-related proteins after TMZ treatment.
Another study has recently suggested that the Wnt/β-catenin signaling pathway can regulate the growth of gliomas and is closely related to the survival of glioma cells. Our data suggested that the treatment of U87MG cells with TMZ reduced the proliferation of glioma cells. Similar to our findings, it has been reported that treating U87MG cells with both ICG-001 and IWR-1-endo could diminish the migration rates of gliomas [35, 36]. Herein, this has been tried to confirm by our Western Blotting results. β-catenin expression decreased in glioma cells only at the toxic dose of TMZ (750 µM). Similarly, a decrease in migration rate was determined as a result of 48 h of TMZ treatment at this toxic dose (another limitation of our study is the lack of detailed evaluation of the Wnt/β-catenin signaling pathway).
Many researchers have been conducting combined therapy trials to improve the effectiveness of the treatment of gliomas. Among these, promising results targeting Hsp inhibition are included in the literature. The common result of these studies is that siRNA and antioxidant/drug combination therapy applications lead to an increase of apoptosis levels in glioma cells than single applications [15, 37, 38]. In our future studies, we are planning to focus on siRNA + drug combination therapies to confirm the relationship between Hsp silencing and the migration-reducing effect of TMZ and further enhance treatment efficacy. In conclusion, all the studies performed to reduce the extracellular pathway expression level of stress proteins in glioma cells can help develop new therapeutic approaches to treat notoriously poor glioma.
Funding source: Istanbul University Research Foundation, Turkey
Award Identifier / Grant number: 29087
-
Research funding: This work was supported by the Istanbul University Research Foundation, Turkey (Grant number 29087), Turkey.
-
Author contributions: MP designed the study and supervised the work. CK performed the experiments. CK and MP analyzed the data and wrote the manuscript.
-
Competing interests: Authors state no conflict of interest.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Ethical approval: The local Institutional Review Board deemed the study exempt from review.
-
Availability of data and materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
1. Daniel, P, Sabri, S, Chaddad, A, Meehan, B, Jean-Claude, B, Rak, J, et al.. Temozolomide induced hypermutation in glioma: evolutionary mechanisms and therapeutic opportunities. Front Oncol 2019;9:41. https://doi.org/10.3389/fonc.2019.00041.Search in Google Scholar PubMed PubMed Central
2. Vardanyan, R, Hruby, V. Antineoplastic agents. In: Vardanyan, R, Hruby, V, editors. Synthesis of best-seller drugs. London: Elsevier; 2016:495–547 pp.10.1016/B978-0-12-411492-0.00028-6Search in Google Scholar
3. Li, X, Corbett, AL, Taatizadeh, E, Tasnim, N, Litlle, JP, Garnis, C, et al.. Challenges and opportunities in exosome research perspectives from biology, engineering, and cancer therapy. APL Bioeng 2019;3:011503. https://doi.org/10.1063/1.5087122.Search in Google Scholar PubMed PubMed Central
4. Zhang, Y, Wang, XF. A niche role for cancer exosomes in metastasis. Nat Cell Biol 2015;17:709–11. https://doi.org/10.1038/ncb3181.Search in Google Scholar PubMed
5. Graner, MW, Cumming, RI, Bigner, DD. The heat shock response and chaperones/heat shock proteins in brain tumors: surface expression, release, and possible immune consequences. Neuroscience 2007;27:11214–27. https://doi.org/10.1523/jneurosci.3588-07.2007.Search in Google Scholar
6. Whitehead, CA, Luwor, RB, Morokoff, AP, Kaye, AH, Stylli, SS. Cancer exosomes in cerebrospinal fluid. Transl Cancer Res 2017;6:1352–70. https://doi.org/10.21037/tcr.2017.08.31.Search in Google Scholar
7. Yun, CW, Kim, HJ, Lim, JH, Lee, SH. Heat shock proteins: agents of cancer development and therapeutic targets in anti-cancer therapy. Cells 2019;9:60. https://doi.org/10.3390/cells9010060.Search in Google Scholar PubMed PubMed Central
8. Maio, AD, Vazquez, D. Extracellular heat shock proteins. Shock 2013;40:239–46. https://doi.org/10.1097/shk.0b013e3182a185ab.Search in Google Scholar
9. Önay-Uçar, E. Heat shock proteins and cancer: plant based therapy. In: Asea, AAA, Calderwood, SK, editors. Heat shock protein-based therapies. New York: Springer; 2015:27–48 pp.10.1007/978-3-319-17211-8_3Search in Google Scholar
10. Bavisotto, CC, Cappello, F, Macario, AJ, Macario, ECD, Logozzi, M, Fais, S, et al.. Exosomal HSP60: a potentially useful biomarker for diagnosis, assessing prognosis, and monitoring response to treatment. Expert Rev Mol Diagn 2017;17:815–22. https://doi.org/10.1080/14737159.2017.1356230.Search in Google Scholar PubMed
11. Lancaster, GI, Febbraio, MA. Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J Biol Chem 2005;280:23349–55. https://doi.org/10.1074/jbc.m502017200.Search in Google Scholar
12. Pekmez, M, Önay-Uçar, E, Arda, N. Effect of α-tocopheryl succinate on the molecular damage induced by indomethacin in C6 glioma cells. Exp Ther Med 2015;9:585–90. https://doi.org/10.3892/etm.2014.2101.Search in Google Scholar
13. Wu, Y, Deng, W, Klinke, DJ2nd. Exosomes: improved methods to characterize their morphology, RNA content, and surface protein biomarkers. Analyst 2015;140:6631–42. https://doi.org/10.1039/c5an00688k.Search in Google Scholar
14. Wessel, D, Flügge, UI. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem 1984;138:141–3. https://doi.org/10.1016/0003-2697(84)90782-6.Search in Google Scholar
15. Önay Uçar, E, Şengelen, A. Resveratrol and siRNA in combination reduces Hsp27 expression and induces caspase-3 activity in human glioblastoma cells. Cell Stress Chaperones 2019;24:763–75. https://doi.org/10.1007/s12192-019-01004-z.Search in Google Scholar PubMed PubMed Central
16. Liang, CC, Park, AY, Guan, JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2007;2:329–33. https://doi.org/10.1038/nprot.2007.30.Search in Google Scholar PubMed
17. Rappa, F, Farina, F, Zummo, G, David, S, Campanella, C, Carini, F, et al.. HSP-molecular chaperones in cancer biogenesis and tumor therapy: an overview. Anticancer Res 2012;32:5139–50.Search in Google Scholar
18. Graner, MW, Alzate, O, Dechkovskaia, AM, Keene, JD, Sampson, JH, Mitchell, DA, et al.. Proteomic and immunologic analyses of brain tumor exosomes. Faseb J 2009;23:1541–57. https://doi.org/10.1096/fj.08-122184.Search in Google Scholar PubMed PubMed Central
19. Panzarini, E, Tacconi, S, Carata, E, Mariano, S, Tata, MA, Dini, L. Molecular characterization of temozolomide-treated and non temozolomide-treated glioblastoma cells released extracellular vesicles and their role in the macrophage response. Int J Mol Sci 2020;21:8353. https://doi.org/10.3390/ijms21218353.Search in Google Scholar PubMed PubMed Central
20. Edkins, AL, Price, JT, Pockley, AG, Blatch, G. Heat shock proteins as modulators and therapeutic targets of chronic disease: an integrated perspective. Philos Trans R Soc Lond B Biol Sci 2018;373:20160521. https://doi.org/10.1098/rstb.2016.0521.Search in Google Scholar PubMed PubMed Central
21. Calderwood, SK, Gong, J, Murshid, A. Extracellular HSPs: the complicated roles of extracellular HSPs in immunity. Front Immunol 2016;7:159. https://doi.org/10.3389/fimmu.2016.00159.Search in Google Scholar PubMed PubMed Central
22. Anand, PK, Anand, E, Bleck, CKE, Anes, E, Griffiths, G. Exosomal Hsp70 induces a pro-inflammatory response to foreign particles including mycobacteria. PloS One 2010;5:e10136. https://doi.org/10.1371/journal.pone.0010136.Search in Google Scholar PubMed PubMed Central
23. Pockley, AG, Henderson, B. Extracellular cell stress (heat shock) proteins—immune responses and disease: an overview. Philos Trans R Soc Lond B Biol Sci 2018;373:20160522. https://doi.org/10.1098/rstb.2016.0522.Search in Google Scholar PubMed PubMed Central
24. Shevtsov, M, Huile, G, Multhoff, G. Membrane heat shock protein 70: a theranostic target for cancer therapy. Philos Trans R Soc Lond B Biol Sci 2018;373:20160526. https://doi.org/10.1098/rstb.2016.0526.Search in Google Scholar PubMed PubMed Central
25. Caruso Bavisotto, C, Graziano, F, Rappa, F, Gammazza, AM, Logozzi, M, Fais, S, et al.. Exosomal chaperones and miRNAs in gliomagenesis: state-of-art and theranostics perspectives. Int J Mol Sci 2018;19:2626. https://doi.org/10.3390/ijms19092626.Search in Google Scholar PubMed PubMed Central
26. Lang, H-L, Hu, G-W, Zhang, B, Kuang, W, Chen, Y, Wu, L, et al.. Glioma cells enhance angiogenesis and inhibit endothelial cell apoptosis through the release of exosomes that contain long non-coding RNA CCAT2. Oncol Rep 2017;38:785–98. https://doi.org/10.3892/or.2017.5742.Search in Google Scholar PubMed PubMed Central
27. Towner, RA, Smith, N, Saunders, D, Brown, CA, Cai, X, Ziegler, J, et al.. OKN-007 increases temozolomide (TMZ) sensitivity and suppresses TMZ-resistant glioblastoma (GBM) tumor growth. Transl Oncol 2019;12:320–35. https://doi.org/10.1016/j.tranon.2018.10.002.Search in Google Scholar PubMed PubMed Central
28. Gastpar, R, Gehrmann, M, Bausero, MA, Asea, A, Gross, C, Schroeder, JA, et al.. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Canc Res 2005;65:5238–47. https://doi.org/10.1158/0008-5472.can-04-3804.Search in Google Scholar
29. Taha, EA, Ono, K, Eguchi, T. Roles of extracellular HSPs as biomarkers in immune surveillance and immune evasion. Int J Mol Sci 2019;20:4588. https://doi.org/10.3390/ijms20184588.Search in Google Scholar PubMed PubMed Central
30. Skog, J, Würdinger, T, Rijn, SV, Meijer, DH, Gainche, L, Curry, WTJr, et al.. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 2008;10:1470–6. https://doi.org/10.1038/ncb1800.Search in Google Scholar PubMed PubMed Central
31. Vengoji, R, Macha, MA, Nimmakayala, RK, Rachagani, S, Siddiqui, JA, Mallya, K, et al.. Afatinib and temozolomide combination inhibits tumorigenesis by targeting EGFRvIII-cMet signaling in glioblastoma cells. J Exp Clin Canc Res 2019;38:266. https://doi.org/10.1186/s13046-019-1264-2.Search in Google Scholar PubMed PubMed Central
32. Maacha, S, Bhat, AA, Jimenez, L, Raza, A, Haris, M, Uddin, S, et al.. Extracellular vesicles-mediated intercellular communication: roles in the tumor microenvironment and anti-cancer drug resistance. Mol Canc 2019;18:55. https://doi.org/10.1186/s12943-019-0965-7.Search in Google Scholar PubMed PubMed Central
33. André-Grégoire, G, Bidère, N, Gavard, J. Temozolomide affects extracellular vesicles released by glioblastoma cells. Biochimie 2018;155:11–5. https://doi.org/10.1016/j.biochi.2018.02.007.Search in Google Scholar PubMed
34. Luga, V, Zhang, L, Viloria-Petit, AM, Ogunjimi, AA, Inanlou, MR, Chiu, E, et al.. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 2012;151:1542–56. https://doi.org/10.1016/j.cell.2012.11.024.Search in Google Scholar PubMed
35. Duan, R, Han, L, Wang, Q, Wei, J, Chen, L, Zhang, J, et al.. HOXA13 is a potential GBM diagnostic marker and promotes glioma invasion by activating the Wnt and TGF-β pathways. Oncotarget 2015;6:27778–93. https://doi.org/10.18632/oncotarget.4813.Search in Google Scholar PubMed PubMed Central
36. Gao, L, Chen, B, Li, J, Yang, F, Cen, X, Liao, Z, et al.. Wnt/β-catenin signaling pathway inhibits the proliferation and apoptosis of U87 glioma cells via different mechanisms. PloS One 2017;12:e0181346. https://doi.org/10.1371/journal.pone.0181346.Search in Google Scholar PubMed PubMed Central
37. Jakubowicz-Gil, J, Langner, E, Bądziul, D, Wertel, I, Rzeski, W. Silencing of Hsp27 and Hsp72 in glioma cells as a tool for programmed cell death induction upon temozolomide and quercetin treatment. Toxicol Appl Pharmacol 2013;273:580–9. https://doi.org/10.1016/j.taap.2013.10.003.Search in Google Scholar PubMed
38. Şengelen, A, Önay-Uçar, E. Rosmarinic acid and siRNA combined therapy represses Hsp27 (HSPB1) expression and induces apoptosis in human glioma cells. Cell Stress Chaperones 2018;23:885–96. https://doi.org/10.1007/s12192-018-0896-z.Search in Google Scholar PubMed PubMed Central
© 2021 Murat Pekmez and Cansu Kılcı, published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review Article
- Is carnosine effective to alleviate lung injury: a systematic review
- Research Article
- Neutralizing antibody response and associated factors in Coronavirus-19 disease (COVID-19) up to one month
- Technical Note
- Comparison of enzyme-linked fluorescent assay and electrochemiluminescence immune assay in procalcitonin measurement
- Research Articles
- Pretreatment of diabetic aged rats with combination of ginsenoside-Mc1 and silibinin protects liver from ischemia-reperfusion injury through an AMPK-dependent mechanism
- Effects of exercise and quercetin on muscle energy charge in metabolic syndrome model of rats
- Effects of peroxisome proliferator activated receptor gamma (PPARγ) agonist on fasting model applied neuron cultures
- Combined effect of midostaurin and sphingosine kinase-1 inhibitor on FMS-like tyrosine kinase 3 (FLT3) wild type acute myeloid leukemia cells
- Association between systemic immune inflammation index and newly diagnosed adult celiac disease
- Total cholesterol/HDL cholesterol ratio and monocyte/HDL cholesterol ratio are related with subclinical hypothyroidism in polycystic ovary syndrome
- Effects of different exercise loads on serum betatrophin (ANGPTL-8/lipasin) and cartonectin (CTRP-3) levels in metabolic syndrome
- Impact of serum 25 hydroxyvitamin D deficiency on lipid biomarkers in established coronary artery disease
- The effect of temozolomide on Hsp60 and Hsp70 expression in extracellular vesicles derived from U87MG glioma cells
- Investigation of RASSF4 gene in head and neck cancers
- The role of A268V exon-7 polymorphism of PPARA in development of axial spondyloarthritis
- Analysis of beta globin gene mutations in Diyarbakir
- Education Section
- Mobile learning in a flipped classroom: findings from a “5-lecture-5” blended learning design for large classes
- Flipped learning in faculty development programs: opportunities for greater faculty engagement, self-learning, collaboration and discussion
- Medical interns’ attitudes towards One Health approach
- Coexisting of interprofessional education and organizational culture
- A snapshot of the coaching practices in undergraduate nursing education: evaluation of stakeholders’ perceptions and program costs
Articles in the same Issue
- Frontmatter
- Review Article
- Is carnosine effective to alleviate lung injury: a systematic review
- Research Article
- Neutralizing antibody response and associated factors in Coronavirus-19 disease (COVID-19) up to one month
- Technical Note
- Comparison of enzyme-linked fluorescent assay and electrochemiluminescence immune assay in procalcitonin measurement
- Research Articles
- Pretreatment of diabetic aged rats with combination of ginsenoside-Mc1 and silibinin protects liver from ischemia-reperfusion injury through an AMPK-dependent mechanism
- Effects of exercise and quercetin on muscle energy charge in metabolic syndrome model of rats
- Effects of peroxisome proliferator activated receptor gamma (PPARγ) agonist on fasting model applied neuron cultures
- Combined effect of midostaurin and sphingosine kinase-1 inhibitor on FMS-like tyrosine kinase 3 (FLT3) wild type acute myeloid leukemia cells
- Association between systemic immune inflammation index and newly diagnosed adult celiac disease
- Total cholesterol/HDL cholesterol ratio and monocyte/HDL cholesterol ratio are related with subclinical hypothyroidism in polycystic ovary syndrome
- Effects of different exercise loads on serum betatrophin (ANGPTL-8/lipasin) and cartonectin (CTRP-3) levels in metabolic syndrome
- Impact of serum 25 hydroxyvitamin D deficiency on lipid biomarkers in established coronary artery disease
- The effect of temozolomide on Hsp60 and Hsp70 expression in extracellular vesicles derived from U87MG glioma cells
- Investigation of RASSF4 gene in head and neck cancers
- The role of A268V exon-7 polymorphism of PPARA in development of axial spondyloarthritis
- Analysis of beta globin gene mutations in Diyarbakir
- Education Section
- Mobile learning in a flipped classroom: findings from a “5-lecture-5” blended learning design for large classes
- Flipped learning in faculty development programs: opportunities for greater faculty engagement, self-learning, collaboration and discussion
- Medical interns’ attitudes towards One Health approach
- Coexisting of interprofessional education and organizational culture
- A snapshot of the coaching practices in undergraduate nursing education: evaluation of stakeholders’ perceptions and program costs

