Abstract
Background/Objective
Ginsenosides, the major active components of the ginseng, are known to have various effects on nervous systems. The present study aimed to clarify the inhibition potentials of ginsenosides Rb1, Rc, Re and Rg1 on acetylcholinesterase (AChE) and butrylcholinesterase (BChE) activities, and to evaluate the underlying mechanisms of inhibitions provided by protein-ligand interactions considering their probable candidates of prodrug.
Materials and methods
The inhibitory mechanisms of ginsenosides related with their structural diversity were analyzed kinetically and protein-ligand interactions for both enzymes were evaluated with most potent ginsenosides, by molecular docking studies.
Results
Ginsenosides Re and Rg1, with sugar moieties attached to the C-6 and C-20 positions of core structure were found to possess the most powerful inhibitory effect on AChE and BChE activities. Molecular docking studies have been confirmed by kinetic studies. Ginsenosides having a direct interaction with amino acid residues belonging to the catalytic triad revealed the most powerful inhibition with lowest enzyme-inhibitor dissociation constant (Ki) values.
Conclusions
Ginsenosides Re and Rg1, either alone or in a specific combination, may provide beneficial effects on neurodegenerative pathologies in therapeutic terms.
Öz
Genel Bilgiler/Amaç
Ginsenositler, ginsengin temel aktif bileşenleri olup, ginsenositlerin sinir sistemi üzerinde çeşitli etkileri olduğu bilinmektedir. Çalışmada, ginsenosit Rb1, Rc, Re ve Rg1’in asetilkolinesteraz (AChE) ve bütirilkolinesteraz (BChE) aktiviteleri üzerindeki inhibitör etkilerininin belirlenmesi ve bu inhibisyonlara neden olan mekanizmaların protein-ligand etkileşimleri de göz önüne alınarak açıklanması amaçlanmıştır.
Gereç ve Yöntemler
Ginsenositlerin inhibe edici etkileri kinetik çalışmalarla belirlenmiş ve en etkin inhibitör özelliğe sahip olanların detaylı mekanizmaları protein-ligand etkileşimlerini incelenmesi ile doğrulanmıştır.
Bulgular
Ginsenositlerden C6 ve C20 pozisyonlarında şeker kalıntıları içeren Re ve Rg1’in AChE ve BChE aktiviteleri üzerinde en etkin inhibitör olduğu belirlenmiştir. Moleküler modelleme sonuçları kinetik analizlerle doğrulanmıştır. Katalitik triadda bulunan amino asitlerle doğrudan etkileşimi olan ginsenositlerin en düşük Ki (Enzim-inhibitör dissosiyasyon sabiti) değerlerine ve en yüksek inhibitör etkiye sahip olduğu belirlenmiştir.
Sonuç
Ginsenosit Re ve Rg1’in nörodejeneratif patolojilerde yalnız ya da birlikte terapötik amaçlı kullanımının yararlı olabileceği düşünülmektedir.
Abbreviations: AChE, acetylcholinesterase; BChE, butrylcholinesterase; ACh, acetylcholine; MOPS, N-morpholinopropane sulfonic acid; ATCh, acetylthiocholine; BTCh, butrylthiocholine; DTNB, 5,5′-dithiobis(2-nitrobenzoic acid); DMSO, dimethyl sulfoxide; Ki, enzyme-inhibitor dissociation constant; PAS, peripheral anionic site.
Introduction
Acetylcholinesterase (AChE; E.C.3.1.1.7) and butyrylcholinestrase (BChE; E.C.3.1.1.8) are structurally and functionally related serine hydrolases present in various tissues including central nervous system. Their primary function is directly related with the cholinergic neurotransmission. Attenuation of cholinergic neurotransmission is achieved by the catalytic activities of cholinesterases, hydrolysis of esters of choline, mainly acetylcholine. Besides their regulator/co-regulator effect(s) on cholinergic neurotransmission, they probably contribute to the development of the nervous system and also overall brain functions [1], [2], [3], [4]. Besides this, BChE is an excellent biomarker of exposure to nerve agents and organophosphorus pesticides. Potential therapeutic uses of human BChE for protection from nerve agent toxicity and also for cocaine intoxication and cocaine addiction have been reported recently [2], [3], [5].
Evidences have revealed that their activities are altered in neurodegenerative pathologies such as Alzheimer’s disease. In these pathologies the deficiency of acetylcholine may be overcomed by the inhibition of AChE and BChE activities. Some cholinesterase inhibitors are currently used for therapeutic and prophylactic purposes. Nowadays, extensive studies are focused on the identification/development of more effective and selective natural or synthetic therapeutic agents as cholinesterase inhibitors [6], [7], [8], [9], [10], [11], [12], [13].
Ginseng, a traditional medicinal plant belonging to genus Panax, family Araliaceae, has been used for thousands of years in East Asia countries either alone or in combination with other medicinal ingredients. Recently, it has also gained significant importance all over the world as herbal medicine or natural health product, nutritional supplement, due to its defined physiological and therapeutic effects [7], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20].
Ginsenosides, the major active component present in all parts of plant, are triterpene saponins. Most of them have a common four-ring hydrophobic steroid-like core structure with various sugar moieties which may be attached mainly to the hydroxyl groups present at carbon-3 or carbon-6 and carbon-20 positions (Figure 1) [16].

Structure of the selected protopanaxdiols (Ginsenoside Rb1 and Rc) and protopanaxtriols (Ginsenoside Re and Rg1).
Experiments have indicated that ginsenosides have various effects on different systems including central and peripheral nervous systems [10], [11], [16], [19], [20], [21], [22], [23], [24]. In vivo and in vitro studies have suggested that ginseng extracts and also some ginsenosides, including Rb1, Re and Rg1, improve cognitive impairments and also neuroinflammatory responses in various experimental models with impaired memory. In these studies, the significant enhancement of cholinergic functions, through an increase in acetylcholine (ACh) and some ionotropic and ACh receptors, and also reduced AChE activity, were reported [7], [9], [10], [11], 24], [25], [26], [27], [28], [29], [30], [31]. On the other hand, no comparative kinetic studies including the protein-ligand interactions have been performed. Our presented data will be the first dealing with this concept. To provide specificity for the diverse therapeutic effects of variable ginsenosides, the present study aimed (a) to clarify the inhibition potentials of interested ginsenosides against AChE and BChE, (b) to evaluate the underlying mechanisms of inhibitions provided by protein-ligand interactions considering their probable candidates of prodrug.
Materials and methods
Human recombinant AChE, equine serum BChE (more than 90% identical with human enzyme) [32] and ginsenosides Rb1, Rc, Re and Rg1 were purchased from Sigma–Aldrich (MO, USA). All other chemicals and biochemicals were obtained from Sigma–Aldrich (MO, USA) or Merck (Darmstadt, Germany).
All biochemical studies were performed in triplicate and data were expressed as mean±SEM.
Activity determinations
AChE and BChE activities were assayed at 25°C, in 100 mM MOPS, pH 8.0, containing 0.05–0.5 mM ATCh or BTCh as a substrate and 0.125 mM DTNB. Reactions were initiated by the addition of enzyme(s) (0.02 μg/mL) and monitored through the increase in absorbance at 412 nm against sample blank (Shimadzu-1601, Japan). Initial rate was calculated by using milimolar extinction coefficient of thionitrobenzoic acid at 412 nm, i.e. 14.2 mM−1 cm−1 [33].
Reactions with ginsenosides
As reported previously, the structural diversity of ginsenosides were provided by differences in sugar types, quantities and attachment positions [16]. These mentioned differences were directly related with their solubility differences in water. To overcome this situation, all ginsenosides were dissolved in 10% DMSO. In the assay medium, the final concentration of DMSO was less than 0.1% (v/v) and found to have no effect on enzyme activities [34].
In our study, 10–100 μM ginsenoside solutions were used in the same assay conditions. In the absence of inhibitors (ginsenosides), enzymes were stable during the period of observations. Reactions were initiated by the addition of enzyme and activity differences were followed for a minute. Quantitation was carried out by using milimolar extinction coefficients of thionitrobenzoic acid at 412 nm, i.e. 14.2 mM−1 cm−1 [33].
Molecular modeling studies
Docking studies were performed using Molecular Operating Environment software, version 2015.1001 (MOE, Canada). The crystalized structures of human AChE [35] and BChE [36] (Protein Data Bank IDs: 4EY7 and 1P01, respectively) were used as the receptors. All non-protein atoms and water molecules were removed from the enzymes. The structural errors in the enzymes were corrected by the “Structure Preparation” application and binding sites were determined by using the “site finder” tool in MOE. The ligands were built using the MOE builder tool and energy was minimized using the Merck Molecular Force Field (MMFF94x, gradient: 0.05 kcal mol−1 Å−1). Docking studies were performed using the default Triangle Matcher method. In the process of docking, the enzymes were kept rigid while the ligands were flexible. The results were ranked with London dG scoring function and rescored with GBVI/WSA dG scoring function. The poses with the lowest S score were selected for the enzymes.
Results
The effect of ginsenosides Rb1, Rc, Re and Rg1 on AChE activity
All four selected ginsenosides reversibly inhibited AChE activity. The inhibition mechanisms for each ginsenosides were analyzed by using Lineweaver-Burk plots including their secondary plots and verifications were achieved by Dixon plots. In the presence and absence of inhibitor, all Lineweaver-Burk plots of activity vs. substrate concentration were linear. The same situation was valid for Dixon plots and data confirmed that all of the inhibitions were linear. The type of inhibitions and also the values of enzyme-inhibitor dissociation constants (Ki) for each studied ginsenosides were given in Table 1. As shown in Table 1, Ki values were in the range of 17–115 μM. Protopanaxdiols, Rb1 and Rc, inhibited both cholinesterases noncompetitively whereas, protopanaxtriols, Re and Rg1, caused to uncompetitive inhibition on AChE activity.
Kinetic data for the inhibition of cholinesterases by selected ginsenosides.
| Ginsenoside | Enzyme | Type of inhibition | Ki (μM) | α |
|---|---|---|---|---|
| Rb1 | AChE | Noncompetitive | 79±7.0 | – |
| BChE | Noncompetitive | 64±6.5 | – | |
| Rc | AChE | Noncompetitive | 115±4.5 | – |
| BChE | Noncompetitive | 119±6.5 | – | |
| Re | AChE | Uncompetitive | 17±2.4 | – |
| BChE | Noncompetitive (0–50 μM) Mixed type (75, 100 μM) |
188±18.0 80±3.0 |
– 2.4±0.12 |
|
| Rg1 | AChE | Uncompetitive | 30±2.0 | – |
| BChE | Mixed type | 53±3.0 | 2.5±0.22 |
-
Values are expressed as mean±SEM of three independent experiments.
Among these ginsenosides, Re revealed the most powerful inhibitory activity on AChE and caused uncompetitive inhibition with a Ki value of 17±2.4 μM (Figure 2).
![Figure 2:
The inhibition of AChE by Ginsenoside Re (A). The Lineweaver-Burk plot (B). Secondary plot of Lineweaver-Burk plot, i.e. v−1-axis intercept vs. [Re] replot (C). The Dixon plot. Each point is the average of at least three determinations.](/document/doi/10.1515/tjb-2018-0534/asset/graphic/j_tjb-2018-0534_fig_002.jpg)
The inhibition of AChE by Ginsenoside Re (A). The Lineweaver-Burk plot (B). Secondary plot of Lineweaver-Burk plot, i.e. v−1-axis intercept vs. [Re] replot (C). The Dixon plot. Each point is the average of at least three determinations.
The effect of ginsenosides Rb1, Rc, Re and Rg1 on BChE activity
All studied ginsenosides reversibly inhibited BChE activity. For the evaluation of the type of inhibitions and also to determine the Ki values for each of them, Lineweaver-Burk and Dixon plots were used. Data indicated linear inhibitions. The type of inhibitions and Ki values were given in Table 1. Among ginsenosides, Re inhibited BChE activity in a dose-related manner. At lower concentrations, below 50 μM, Re inhibited BChE activity in a noncompetitive manner with a Ki value of 188±18 μM, whereas, above 50 μM, mixed type inhibition with a Ki value of 80±3.0 μM (α=2.4 and β=0) was obtained. As shown in Table 1, Rg1 generated the most potent inhibitory activity on BChE, exerted mixed type inhibition with a Ki value of 53±3.0 μM (α=2.5 and β=0) (Figure 3).
![Figure 3:
The inhibition of BChE by Ginsenoside Rg1 (A). The Lineweaver-Burk plot (B). Determination of Ki value, slope of reciprocal plot vs. corresponding [Re] replot (C). Estimation of α value, v−1-axis intercept of reciprocal plot vs. [Rg1] replot (D). The Dixon plot. Each point is the average of at least three determinations.](/document/doi/10.1515/tjb-2018-0534/asset/graphic/j_tjb-2018-0534_fig_003.jpg)
The inhibition of BChE by Ginsenoside Rg1 (A). The Lineweaver-Burk plot (B). Determination of Ki value, slope of reciprocal plot vs. corresponding [Re] replot (C). Estimation of α value, v−1-axis intercept of reciprocal plot vs. [Rg1] replot (D). The Dixon plot. Each point is the average of at least three determinations.
Identification of protein-ligand interactions
To clarify the main interactions between selected ginsenosides and cholinesterases, molecular docking studies were performed. Ginsenosides having the most potent inhibitory activity on both cholinesterases were taken into consideration. As described in Table 1, ginsenoside Re revealed the most effective inhibitory activity on AChE and ginsenoside Rg1 exhibited most powerful inhibitory activity on BChE. That is why, Re and Rg1 were used for docking studies.
Interactions between AChE and ginsenosides Re and Rg1
Protein-ligand interactions between ginsenoside Re and AChE were given in Figure 4. The active site cavity was not completely occupied by ginsenoside Re (Figure 4A). As seen in and 4B, the hydroxyl group present in the core structure, at the C-3 position, and hydroxyl groups belonging to the rhamnose moieties were located above the cavity. At the active site gorge, the hydroxyl groups of glucose moieties present at the C-6 and C-20 of ginsenoside Re formed three hydrogen bonds. Two of them were generated between the same hydroxyl group of glucose moiety at the C-20 position of ginsenoside Re and the residues of catalytic triad Ser203 and His447. The other hydrogen bond was formed between the hydroxyl group of glucose moiety present at the C-6 and Ser293. Also hydrophobic interactions between residues of Trp86, Trp286, Val294, Leu289, Phe296, Phe338, Phe297 and Re were found to be crucial for protein-ligand interactions.
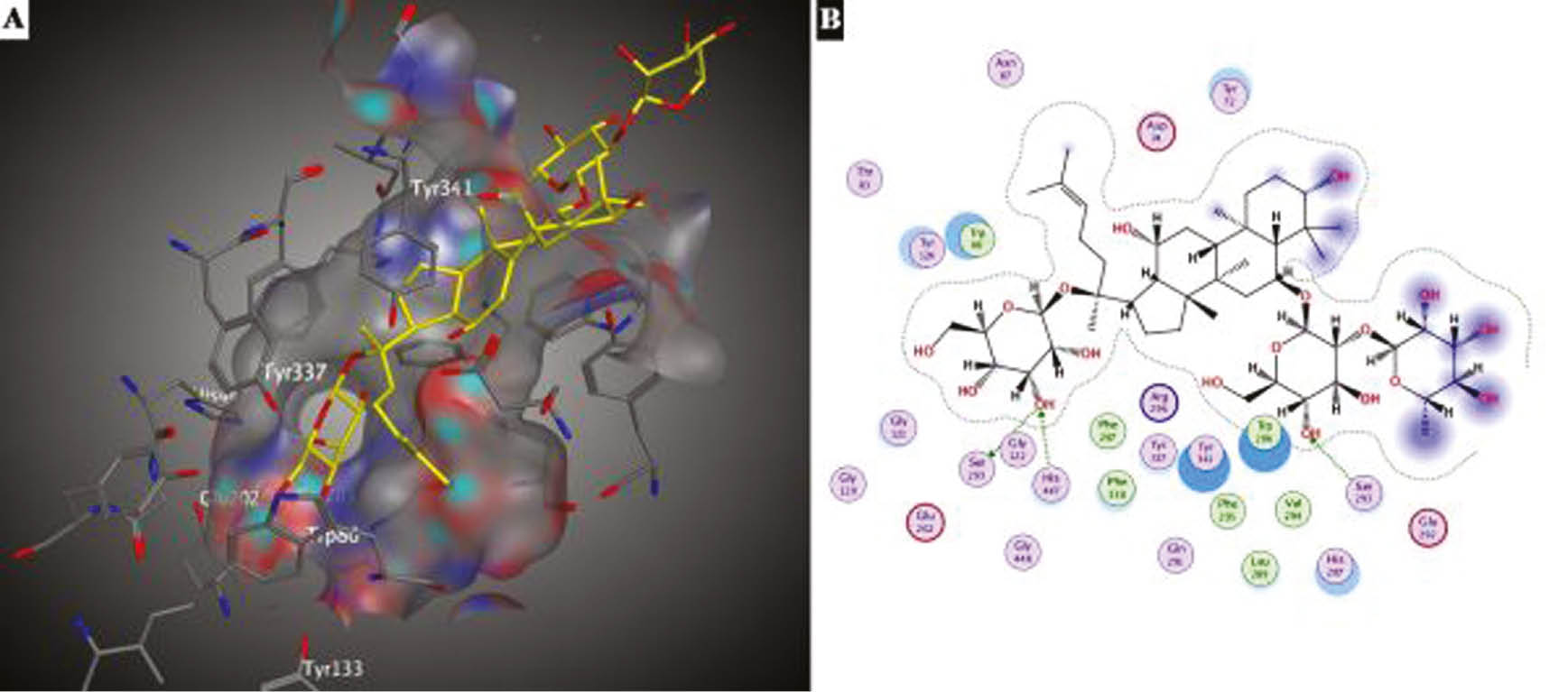
Docking interactions of ginsenoside Re on AChE (A) 3D (B) 2D representation.
Docking studies revealed that the ginsenoside Rg1 did not pass through the rim of the active site gorge. Instead, it was located at that place, the mid gorge region (Figure 5). At the same region, a hydrogen bond was formed between oxygen atom of hydroxyl group belonging to the core structure of Rg1, at the C-20 position with a glucose moiety, and Tyr124 residue of AChE. As seen in Figure 5B, there was a σ-π interaction between Phe338 residue and methyl group located at the C-20 position of ginsenoside Rg1. Also, some of the defined hydrophobic interactions between Rg1 and AChE (Leu289, Trp86, Phe295, Phe297, Trp286 and Val294) contributed to protein-ligand interactions.

Docking interactions of ginsenoside Rg1 on AChE (A) 3D (B) 2D representation.
Interactions between BChE and ginsenosides Re and Rg1
As seen in Figure 6, ginsenoside Re occupied the active site gorge of BChE. Docking studies revealed the existence of three hydrogen bonds between BChE and ginsenoside Re. One of them was generated between Asp70 and the hydroxyl group of glucose moiety present at the C-20 position of ginsenoside Re. The other hydrogen bonds were formed between hydroxyl groups of rhamnose residues located at the C-6 position of ginsenoside Re and BChE residue of Asn68 and NH backbone of Leu274 (Figure 6B). The hydrophobic residues of Phe329, Trp82, Trp430, Ala328, Leu273, Leu274, Phe279, Ala277, Ile69, Trp231, Leu286, and Val288 contributed to the protein-ligand interactions.

Docking interactions of ginsenoside Re on BChE (A) 3D (B) 2D representation.
Interactions between BChE and ginsenoside Rg1 were given in Figure 7. As seen in figure, Rg1 occupied almost all of the active site cavity of BChE. Results indicated the existence of one hydrogen bond between the hydroxyl group of glucose moiety present at the C-20 position of Rg1 and one of the members of catalytic triad, His438. As seen in Figure 7B, Pro285, Trp430, Ala328, Met437, Trp82, Ala277, Ile69 residues were also participated in the protein-ligand interactions.

Docking interactions of ginsenoside Rg1 on BChE (A) 3D (B) 2D representation.
Discussion
In the present study, all studied ginsenosides were found to cause reversible linear inhibition of AChE and BChE activities which revealed the existence of reversible protein-ligand interactions.
It has been reported that physiological and therapeutic effects of ginsenosides are directly related with their structural diversity [9], [10], [11], [12], [29], [30], [31]. Our kinetic studies supported this situation. In the present study, our data have indicated that structural differences of ginsenosides have a crucial importance for the establishment of their inhibitory activities on cholinesterases and also types of inhibitions. Ginsenosides with sugar moieties attached to the C-6 and C-20 positions of steroid-like core structure, protopanaxtriols, were found to have the most powerful inhibitory effect on AChE and BChE activities compared to protopanaxdiols, Rb1 and Rc.
Besides the differences in the attachment positions of sugar moieties to the core structure of ginsenosides, the types and the quantities of sugar molecules also contribute to the inhibition potentials of ginsenosides on cholinesterases. The hydroxyl groups in the core structure and/or the sugar moieties are responsible for the hydrophilic properties of ginsenosides. In other words, enhancement of free hydroxyl groups increased the hydrophilic character of ginsenoside [10], [15], [16].
On the other hand, high aromatic amino acid content in the active site cavity creates various specific hydrophobic areas in both cholinesterases [4], [37], [38]. The peripheral anionic (PAS) and the central anionic sites are the very well defined hydrophobic regions that can bind to several types of ligands and are important for the substrate activation and inhibition. Various amino acid residues present in these regions have crucial importance in the regulation of active site conformation and so, catalysis [1], [32], [37], [38], [39]. In our study, ginsenosides having more hydrophobic characters, i.e. protopanaxtriols, exerted more effectively inhibited cholinesterase activities (Table 1).
In structural point of view, the only difference between ginsenosides Re and Rg1 is the existence of one more different type of sugar moiety in 6C-position of ginsenoside Re that is rhamnose as the first sugar residue. However, only glucose moiety is present in Rg1 at the same position (Figure 1).
While both ginsenosides caused the same type of inhibition, i.e. uncompetitive, Re was the most effective/potent inhibitor for AChE since Ki value of Re was found as half of the Ki value of Rg1. On the other hand, our kinetic studies have indicated that the inhibition of BChE by ginsenoside Re follows a special pattern, which may indicate the existence of more than one binding site. A dose-related change in the type of inhibition, i.e. from noncompetitive to mixed, has suggested that occupation/interaction of extra binding site by Re on BChE probably alters the conformation of the active center at higher Re concentrations, i.e. 75–100 μM.
The Asp70 residue, a member of Ω loop revealing a key role in the catalytic behaviors of cholinesterases, facilitates the sliding of the second substrate molecule down the gorge. This loop in BChE, one of the common characteristics of cholinesterases, is a flexible segment, formed by residues Cys65 to Cys92 [38]. As proposed previously, binding of the second ligand (in our case ginsenoside Re) to the PAS modulates the catalytic activity through conformational changes involving the Ω loop. Also, as predicted before, the binding of a second substrate to the PAS affects the rates of substrate/ligand binding and product release [40]. Molecular docking studies demonstrated the interaction of Re with the members of Ω loop, Asp70 and Asn68 (Figure 6). These interactions may probably be directly or indirectly responsible for the conformational changes, resulting from the extra binding of Re to the protein with high concentrations of Re which was reflected as a change of type of inhibition, from noncompetitive to mixed.
Molecular docking studies have confirmed by kinetic studies. Ginsenosides having a direct interaction with amino acid residues belonging to the catalytic triad considered as the most powerful/potent inhibitors. The most powerful inhibition, with lowest Ki values than other interested ginsenoside, was achieved by the studies of ginsenoside Re which was directly interacted with catalytic triad of AChE, i.e. His447 and Ser203 hydrogen bonds. Comparable results were obtained from the inhibition studies of BChE with Rg1, in which again there was a direct interaction with one of the residues of catalytic triad, i.e. His438.
Conclusion
Our data clarified that ginsenoside Re and Rg1 had effective inhibitory effects on AChE and BChE activities, respectively. These ginseng components, either alone or in a specific combination, may provide beneficial effects on neurodegenerative pathologies in terms of therapeutic points of view.
Acknowledgements
This study was funded by Başkent University after the approval of Medical Research Committee (Project no: DA16/06). Each author equally contributed to this study.
References
1. Dvir H, Silman I, Harel M, Rosenberry TL, Sussman JL. Acetylcholinesterase: from 3D structure and function. Chem Bio Interact 2010;187:10–22.10.1016/j.cbi.2010.01.042Search in Google Scholar PubMed PubMed Central
2. Masson P, Lockridge O. Butyrylcholinesterase for protection from organophosphorus poisons: catalytic complexities and hysteretic behavior. Arch Biochem Biophys 2010;494:107–20.10.1016/j.abb.2009.12.005Search in Google Scholar PubMed PubMed Central
3. Patocka J, Kuca K, Jun D. Acetylcholinesterase and butyrylcholinesterase-important enzymes of human body. Acta Medica 2004;47:215–28.10.14712/18059694.2018.95Search in Google Scholar
4. Darvesh S, Hopkins DA, Geula C. Neurobiology of butyrylcholinesterase. Nat Rev Neurosci 2003;4:131–7.10.1038/nrn1035Search in Google Scholar PubMed
5. Lockridge O. Review of human butyrylcholinesterase structure, function, genetic variants, history of use in the clinic, and potential therapeutic uses. Pharmacol Ther 2015;148:34–46.10.1016/j.pharmthera.2014.11.011Search in Google Scholar PubMed
6. Unzeta M, Esteban G, Bolea I, Fogel WA, Ramsay RR, Youdim MB, et al. Multi-target directed donepezil-like ligands for Alzheimer’s disease. Front Neurosci 2016;10:125.10.3389/fnins.2016.00205Search in Google Scholar PubMed PubMed Central
7. Baradaran A, Rabiei Z, Rafieian M, Shirzad H. A review study on medicinal plants affecting amnesia through cholinergic system. J HerbMed Pharmacol 2012;1:3–9.Search in Google Scholar
8. Oboh G, Ademiluyi AO, Akinyemi AJ. Inhibition of acetylcholinesterase activities and some pro-oxidant induced lipid peroxidation in rat brain by two varieties of ginger (Zingiber officinale). Exp Toxicol Pathol 2012;64:315–9.10.1016/j.etp.2010.09.004Search in Google Scholar PubMed
9. Nah SY, Kim DH, Rhim H. Ginsenosides: are any of them candidates for drugs acting on the central nervous system? CNS Drug Rev 2007;13:381–404.10.1111/j.1527-3458.2007.00023.xSearch in Google Scholar PubMed PubMed Central
10. Choi RJ, Roy A, Jung HJ, Ali MY, Min BS, Park CH, et al. BACE1 molecular docking and anti-Alzheimer’s disease activities of ginsenosides. J Ethnopharmacol 2016;190:219–30.10.1016/j.jep.2016.06.013Search in Google Scholar PubMed
11. Kim HJ, Jung SW, Kim SY, Cho IH, Kim HC, Rhim H, et al. Panax ginseng as an adjuvant treatment for Alzheimer’s disease. J Ginseng Res 2018;42:401–11.10.1016/j.jgr.2017.12.008Search in Google Scholar PubMed PubMed Central
12. Jiang Y, Gao H, Turdu G. Traditional Chinese medicinal herbs as potential AChE inhibitors for anti-Alzheimer’s disease: a review. Bioorg Chem 2017;75:50–61.10.1016/j.bioorg.2017.09.004Search in Google Scholar PubMed
13. Yang Y, Liang X, Jin P, Li N, Zhang Q, Yan W, et al. Screening and determination for potential acetylcholinesterase inhibitory constituents from ginseng stem-leaf saponins using ultrafiltration (UF)-LC-ESI-MS. Phytochem Anal 2018;30:26–33.10.1002/pca.2787Search in Google Scholar PubMed
14. Mancuso C, Santangelo R. Panax ginseng and Panax quinquefolius: from pharmacology to toxicology. Food Chem Toxicol 2017;107:362–72.10.1016/j.fct.2017.07.019Search in Google Scholar PubMed PubMed Central
15. Patel S, Rauf A. Adaptogenic herb ginseng (Panax) as medical food: status quo and future prospects. Biomed Pharmacother 2017;85:120–7.10.1016/j.biopha.2016.11.112Search in Google Scholar PubMed
16. Lü JM, Yao Q, Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol 2009;7:293–302.10.2174/157016109788340767Search in Google Scholar PubMed PubMed Central
17. Ong WY, Farooqui T, Koh HL, Farooqui AA, Ling EA. Protective effect of ginseng on neurological disorders. Front Aging Neurosci 2015;7:129.10.3389/fnagi.2015.00129Search in Google Scholar PubMed PubMed Central
18. Sun A, Xu X, Lin J, Cui X, Xu R. Neuroprotection by saponins. Phytother Res 2015;29:187–200.10.1002/ptr.5246Search in Google Scholar PubMed
19. Qi LW, Wang CZ, Yuan CS. Ginsenosides from American ginseng: chemical and pharmacological diversity. Phytochemistry 2011;72:689–99.10.1016/j.phytochem.2011.02.012Search in Google Scholar PubMed PubMed Central
20. Mohanan P, Subramaniyam S, Mathiyalagan R. Molecular signaling of ginsenosides Rb1, Rg1, and Rg3 and their mode of actions. J Ginseng Res 2018;42:123–32.10.1016/j.jgr.2017.01.008Search in Google Scholar PubMed PubMed Central
21. Shi DD, Huang YH, Lai CS, Dong CM, Ho LC, Li XY, et al. Ginsenoside Rg1 prevents chemotherapy-induced cognitive impairment: associations with microglia-mediated cytokines, neuroinflammation, and neuroplasticity. Mol Neurobiol 2019;56:5626–42.10.1007/s12035-019-1474-9Search in Google Scholar PubMed
22. Zhang Y, Hu W, Zhang B, Yin Y, Zhang J, Huang D, et al. Ginsenoside Rg1 protects against neuronal degeneration induced by chronic dexamethasone treatment by inhibiting NLRP-1 inflammasomes in mice. Int J Mol Med 2017;40:1134–42.10.3892/ijmm.2017.3092Search in Google Scholar PubMed PubMed Central
23. Zheng M, Xin Y, Li Y, Xu F, Xi X, Guo H, et al. Ginsenosides: a potential neuroprotective agent. Biomed Res Int 2018;2018:8174345.10.1155/2018/8174345Search in Google Scholar PubMed PubMed Central
24. Lu C, Dong L, Lv J, Wang Y, Fan B, Wang F, et al. 20(S)-protopanaxadiol (PPD) alleviates scopolamine-induced memory impairment via regulation of cholinergic and antioxidant systems, and expression of Egr-1, c-Fos and c-Jun in mice. Chem Biol Interact 2018;279:64–72.10.1016/j.cbi.2017.11.008Search in Google Scholar PubMed
25. Kim EJ, Jung IH, Le TK, Jeong JJ, Kim NJ, Kim DH. Ginsenosides Rg5 and Rh3 protect scopolamine-induced memory deficits in mice. J Ethnopharmacol 2013;146:294–9.10.1016/j.jep.2012.12.047Search in Google Scholar PubMed
26. Kim JM, Park CH, Park SK, Seung TW, Kang JY, Ha JS, et al. Ginsenoside Re ameliorates brain insulin resistance and cognitive dysfunction in high fat diet-induced C57BL/6 mice. J Agric Food Chem 2017;65:2719–29.10.1021/acs.jafc.7b00297Search in Google Scholar PubMed
27. Shin K, Gua H, Cha Y, Ban YH, Seo da W, Choi Y, et al. Cereboost, an American ginseng extract, improves cognitive function up-regulation of choline acetyltransferase expression and neuroprotection. Regul Toxicol Pharmacol 2016;78:53–8.10.1016/j.yrtph.2016.04.006Search in Google Scholar PubMed
28. Jin Y, Peng J, Wang X, Zhang D, Wang T. Ameliorative effect of ginsenoside Rg1 on lipopolysaccaride-induced cognitive impairment: role of cholinergic system. Neurochem Res 2017;42:1299–307.10.1007/s11064-016-2171-ySearch in Google Scholar PubMed
29. Kim JH, Yi YS, Kim MY, Cho JY. Role of ginsenosides, the main active componenets of Panax ginseng, in inflammatory responses and diseases. J Ginseng Res 2017;41:435–43.10.1016/j.jgr.2016.08.004Search in Google Scholar PubMed PubMed Central
30. Scholey A, Ossoukhova A, Owen L, Ibarra A, Pipingas A, He K, et al. Effects of American ginseng (Panax quinquefolius) on neuro-cognitive function; an acute, randomized, double-blind, placebo-controlled, crossover study. Psychopharmacol (Berl) 2010;212:345–56.10.1007/s00213-010-1964-ySearch in Google Scholar PubMed PubMed Central
31. Lee MR, Yun BS, Sung CK. Comparative study of white and steamed black Panax ginseng, P. quinquefolium, and P. notoginseng on cholinesterase inhibitory and antioxidative activity. J Ginseng Res 2012;36:93–101.10.5142/jgr.2012.36.1.93Search in Google Scholar PubMed PubMed Central
32. Golicnik M, Sinko G, Simeo-Rudolf V, Grubic Z, Stojan J. Kinetic model of ethopropazine interaction with horse serum butyrylcholinesterase and its docking into the active site. Arch Biochem Biophys 2002;398:23–31.10.1006/abbi.2001.2697Search in Google Scholar
33. Eyer P, Worek F, Kiderlen D, Sinko G, Stuglin A, Simeon-Rudolf V, et al. Molar absorption coefficients for the reduced Ellman reagent: reassessment. Anal Biochem 2003;312:224–7.10.1016/S0003-2697(02)00506-7Search in Google Scholar
34. Feng X, Wang X, Liu Y, Di X. Linarin inhibits the acetylcholinesterase activity in-vitro and ex-vivo. Iran J Pharm Res 2015;14: 949–54.Search in Google Scholar
35. Cheung J, Rudolph MJ, Burshteyn F, Cassidy MS, Gary EN, Love J, et al. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J Med Chem 2012;55:10282–6.10.1021/jm300871xSearch in Google Scholar
36. Nicolet Y, Lockridge O, Masson P, Fontecilla-Camps JC, Nachon F. Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J Biol Chem 2003;278:41141–7.10.1074/jbc.M210241200Search in Google Scholar
37. Park B, Nam JH, Kim JH, Kim HJ, Onnis V, Balboni G, et al. 3,4-Dihydro quinazoline derivatives inhibit the activities of cholinesterase enzymes. Biorg Med Chem Lett 2017;27:1179–85.10.1016/j.bmcl.2017.01.068Search in Google Scholar
38. Masson P, Xie W, Froment MT, Levitsky V, Fortier PL, Albaret C, et al. Interaction between the peripheral site residues of human butyrylcholinesterase, D70 and Y332, in binding and hydrolysis of substrates. BBA 1999;1433:281–93.10.1016/S0167-4838(99)00115-6Search in Google Scholar
39. Lushchekina S, Nemukhin A, Varfolomeev S, Masson P. Understanding the non-catalytic behavior of human butyrylcholinesterase silent variants: comparision of wild type enzyme, catalytically active Ala328Cys mutant, and silent Ala328Asp. Chem Biol Interact 2016;259:223–32.10.1016/j.cbi.2016.04.007Search in Google Scholar
40. Masson P, Xie W, Froment MT, Lockridge O. Effects of mutations of active site residues and amino acids interacting with the Ω loop on substrate activation of butyrylcholinesterase. BBA 2001;1544:166–76.10.1016/S0167-4838(00)00217-XSearch in Google Scholar
©2019 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Review Article
- Lipidomics and cognitive dysfunction – A Narrative review
- Research Articles
- Effects of topiramate on adipocyte differentiation and gene expression of certain carbonic anhydrase isoenzymes
- Heterologous expression of a plant WRKY protein confers multiple stress tolerance in E. coli Bir bitkinin heterolog ifadesi WRKY proteini çoklu stres yaratır E. coli’de tolerans
- Cytogenetic impact of sodium chloride stress on root cells of Vigna radiata L. seedlings
- Understanding the impacts of self-shuffling approach on structure and function of shuffled endoglucanase enzyme via MD simulations
- Evaluation ofTrichoderma atroviride and Trichoderma citrinoviride growth profiles and their potentials as biocontrol agent and biofertilizer
- Physio-biochemical analyses in seedlings of sorghum-sudangrass hybrids that are grown under salt stress under in vitro conditions
- The structural diversity of ginsenosides affects their cholinesterase inhibitory potential
- Extracellular acidity and oxygen availability conjointly control eukaryotic cell growth via modulation of cytoplasmic translation
- Genome-wide identification, phylogeny and expression analysis of G6PC gene family in common carp, Cyprinus carpio
- The effect of Diplotaenia turcica root extract in streptozotocin-induced diabetic rats
- Letter to the Editor
- Metabolomics analysis of medicinal insect Protaetia brevitarsis after Bacillus subtilis fermentation
- Opinion Paper
- Identifying and solving scientific problems in the medicine: key to become a competent scientist
Articles in the same Issue
- Frontmatter
- Review Article
- Lipidomics and cognitive dysfunction – A Narrative review
- Research Articles
- Effects of topiramate on adipocyte differentiation and gene expression of certain carbonic anhydrase isoenzymes
- Heterologous expression of a plant WRKY protein confers multiple stress tolerance in E. coli Bir bitkinin heterolog ifadesi WRKY proteini çoklu stres yaratır E. coli’de tolerans
- Cytogenetic impact of sodium chloride stress on root cells of Vigna radiata L. seedlings
- Understanding the impacts of self-shuffling approach on structure and function of shuffled endoglucanase enzyme via MD simulations
- Evaluation ofTrichoderma atroviride and Trichoderma citrinoviride growth profiles and their potentials as biocontrol agent and biofertilizer
- Physio-biochemical analyses in seedlings of sorghum-sudangrass hybrids that are grown under salt stress under in vitro conditions
- The structural diversity of ginsenosides affects their cholinesterase inhibitory potential
- Extracellular acidity and oxygen availability conjointly control eukaryotic cell growth via modulation of cytoplasmic translation
- Genome-wide identification, phylogeny and expression analysis of G6PC gene family in common carp, Cyprinus carpio
- The effect of Diplotaenia turcica root extract in streptozotocin-induced diabetic rats
- Letter to the Editor
- Metabolomics analysis of medicinal insect Protaetia brevitarsis after Bacillus subtilis fermentation
- Opinion Paper
- Identifying and solving scientific problems in the medicine: key to become a competent scientist

