Abstract
Sodium calcium silicate (Na2CaSiO4) is a bioactive silicate with Na2O, CaO and SiO2 as its basic components, which is similar to that of the composition of bioactive glasses. In the present study, pure sodium calcium silicate was synthesized by rapid combustion technique, and the synthesized sample was characterized by powder X-ray diffraction to check the phase purity. The scaffolds were prepared by varying the ratio of sodium calcium silicate and polyvinyl alcohol, and the apatite-formation ability of the scaffolds was examined by soaking them in a simulated body fluid. The results revealed the formation of hydroxyapatite on the surface of the scaffold after 5 days, which is found to be rapid when compared with the bioactivity of the calcium silicates and calcium magnesium silicates. The scaffolds were also loaded with ciprofloxacin as a model drug and analyzed for its drug release profile using UV spectrophotometer. The release profile did not vary with the change in bioceramic-to-biopolymer ratio, and 60% of the drug was released in 10 days, which is within the appreciable range for a targeted drug delivery system. Moreover, the experimental and simulated values of the release kinetics were compared by applying the existing mathematical model.
1 Introduction
The human bone is a composite of inorganic and organic materials, 70% of which consists of inorganic materials with hydroxyapatite as a major constituent and the remaining 30% constitutes the organic materials in which collagen is the chief component. Bioceramics can be prepared with the same chemical composition similar to that of the human bone [1, 2]. Bioceramics are commonly used in the medical fields as dental and bone implants because they have the ability to form a direct bond with the living bone in the presence of human physiological environment and because they have the potency to produce biomimetic hydroxyapatite apatite layer on the bone surface [3]. Joint replacements are commonly coated with bioceramic materials to reduce wear and inflammatory response [4–10].
Two types of ceramic implants have been designed based on their biological activity for bone tissue regeneration technologies. Materials such as alumina, zirconia, and carbon fall in the category of bioinert materials because they do not influence any biological response on their surfaces. In contrast, materials categorized as bioactive ceramics will interact with the physiological environment, leading to their integration into the living tissues of organisms [11]. Different bioceramics have been developed for various biomedical applications, among which silicate ceramics and phosphate ceramics are the most significant materials owing to their biocompatibility and bioactivity. Between these two materials, silicate ceramics have a higher rate of apatite deposition on their surface than phosphate ceramics [12].
Silicate-based bioactive glasses and glass ceramics and alkali and alkaline earth-containing silicate ceramics, such as akermanite (Ca2MgSi2O7), merwinite (Ca3MgSi2O8), bredigite (Ca7MgSi4O16), diopside (CaMgSi2O6), and combeite (Na2Ca2Si3O9), have attracted much attention in recent years because of their biocompatibility and bioactivity. Bioactive ceramics have attractive features for bone repair, such as direct bone-bonding ability with existing hard tissues [13].
Rapid combustion synthesis (RCS) is a rapid and energy-saving technique used by many researchers for the preparation of various metal oxides. During combustion reaction, the nitrate ions behave like conventional oxidants and the organic compounds function as a fuel. It is an exothermic redox reaction associated with nitrate decomposition and fuel oxidation, which releases an enormous amount of heat energy. The nature of the fuel and the ratio of oxidizer to fuel control the exothermic nature of the combustion. Organic substances used as fuel in RCS play a dual role in the synthesis because they function not only as a chelating agent, but also avoid the precipitation of ions by forming a complex with metal ions. The vigorous redox reaction between fuel and oxidant mixture gives rise to a very high local temperature with the evolution of large volume of gases. A large amount of heat is released during the combustion, which produces an intense local heating that induces the phase formation [14, 15].
Drug delivery is the process of administering a pharmaceutical compound to achieve a therapeutic effect in humans or animals. Owing to the biological properties of ceramic materials, bioceramics and its composites with synthetic biopolymers are suitable candidates for antibiotic drug delivery in cases of bone infection and its prevention, enabling the provision of a necessary supply of a large amount of antibiotics to reach the adequate therapeutic level in the affected region [16, 17]. Staphylococcus aureus, a gram-positive bacterium, is one of the main causes of bone infection in both the adults and children. Other bacteria, such as Groups A and B Streptococcus, Hemophilus influenzae, Enterobacteriaceae, Escherichia coli and Salmonellae, also cause bone infection [18]. In this study, the drug ciprofloxacin, which belongs to the fluoroquinolone class of antibiotics used to treat bacterial infections, was used as the model drug.
In the present study, sodium calcium silicate was synthesized by the combustion method using citric acid as fuel. This is the first study performed to test the bioactivity and drug delivery applications of sodium calcium silicate. The results showed that sodium calcium silicate can be used as a ceramic drug delivery system.
2 Materials and methods
2.1 Preparation of sodium calcium silicate
One molar stock solution of calcium nitrate [Ca(NO3)2·4H2O; 99%, AR, Rankem, India], sodium silicate (Na2SiO3·xH2O; 99%, AR, Merck, India) and citric acid (C6H8O7; 99.7%, AR, SRL, India) was prepared by dissolving the salts in demineralised water at room temperature. Equal volumes of sodium silicate, calcium nitrate and citric acid solutions were pipetted into a glass beaker, and the pH of the solution was adjusted to 1 by adding concentrated nitric acid. The clear solution was kept in the pre-heated muffle furnace at 300°C. Within few minutes, the solution underwent combustion by self-propagating auto-ignition and formed a black, fine precursor. Given that the reaction is highly exothermic, the reaction was performed inside a closed furnace with a suitable exhaust system to remove the evolved gases. The precursor was kept for heat treatment at 900°C for 6 h.
2.2 Formulation and drug loading
To mimic the composition of the human bone, the scaffolds were prepared by replacing hydroxyapatite with sodium calcium silicate and collagen with polyvinyl alcohol. Scaffolds with different ratios of sodium calcium silicate and PVA were made to optimize the ratio of bioceramics and biopolymers. Ciprofloxacin was used as the model drug to investigate the drug release kinetics of sodium calcium silicate.
A total of 100 mg of ciprofloxacin was dissolved in 100 ml of water, and 1 g of PVA was dissolved in hot water. The resultant solution was allowed to cool and then made up to 100 ml for use as a stock solution for further experiments. To achieve thorough distributions of the drug in both the ceramic and polymer matrix, the volume of the solution containing 5 mg of the drug solution was added to the sodium calcium silicate powder and made into slurry. Different ratios of the polymer solution were added to the slurry and thoroughly mixed using mortar and pestle. The resultant slurry was dried at 60°C for 6 h in a hot-air oven. Four slurries with 5%, 10%, 15% and 20% weight ratio of the polymer and 5 mg of the drug content were prepared and pelletized in a hydraulic press. By following this procedure, 5 mg of ciprofloxacin was loaded uniformly throughout the matrix in all four scaffolds.
2.3 Drug delivery studies
Scaffolds with different concentrations of the polymer were placed in 50 ml of the simulated body fluid (SBF) taken in four different conical flasks and incubated at 37.5°C for 10 days. Every 24 h, 2 ml of the sample was collected from the flask and replaced with the fresh SBF of the same volume. The collected sample was analyzed using a UV-visible spectrometer at 278 nm to determine the release kinetics of the drug from the scaffolds. Using the absorbance values, the amount of drug released was determined for 10 days [19–23].
3 Results and discussion
3.1 Synthesis of bioceramic
Sodium calcium silicate was synthesized by the rapid combustion method using citric acid as fuel. Citric acid plays a dual role in the synthesis because it does not only function as a reducing agent, but also avoids the precipitation of ions by complexing with metal ions. The vigorous redox reaction between fuel and oxidant mixture at the time of combustion gives rise to a very high local temperature with the evolution of large volume of gases. In the present study, although the furnace temperature was maintained at 300°C, the particles could have attained higher local temperatures (approximately 1500°C) during auto-ignition. A large amount of heat was released during the combustion, which produced an intense local heating that enhanced the phase formation. The volume of gas generated and the increase in the local temperature due to combustion depend on various factors, such as the nature of the fuel, temperature, water content and the ratio of fuel to oxidizer, because the thermochemistry of combustion is different for different fuels. This was evident from the sudden increase in temperature inside the furnace from the set temperature of 300 to 500°C during combustion.
The precursor formed after combustion was calcined at 500°C to eliminate the remaining organic constituents present in the product, which results in the formation of sodium oxide, calcium carbonate and amorphous silica. Subsequently, the sample was calcined at 900°C for 6 h to aid the phase formation of sodium calcium silicate from the products of intermittent calcination.
3.2 Characterization of the bioceramic
The phase purity of the synthesized sodium calcium silicate was confirmed by the powder X-ray diffraction (XRD) patterns (Figure 1). The XRD pattern of the sodium calcium silicate was matched with that of the standard JCPDS file (24-1069). The major phase formed was sodium calcium silicate, and trace amount of larnite was present as an impure phase.

XRD pattern of the sodium calcium silicate synthesized by rapid combustion method.
3.3 Bioactivity of the scaffolds
The scaffolds were made with different concentrations of polymer and sodium calcium silicate (Table 1). In all the scaffolds, the concentration of ciprofloxacin was uniform and only the concentration of polyvinyl alcohol was varied. The scaffolds were placed separately in 50 ml SBF, and the samples’ absorbance was analyzed at 278 nm every 24 h. The concentration was calculated by plotting a graph between time vs.% release.
Formulations of sodium calcium silicate.
| Scaffold | Weight of Na2CaSiO4 (mg) | PVA (mg) | Ciprofloxacin (mg) |
|---|---|---|---|
| BCP20 | 160 | 40 | 5 |
| BCP15 | 170 | 30 | 5 |
| BCP10 | 180 | 20 | 5 |
| BCP5 | 190 | 10 | 5 |
Hydroxylapatite was the major constituent deposited on the scaffold in the physiological environment. Consequently, the deposition of hydroxyapatite on the surface of the sodium calcium silicate/PVA scaffolds was analyzed periodically. After incubation in SBF for about 10 days, the scaffolds were analyzed by XRD to check for the formation of hydroxyapatite on their surfaces. Formation of apatite on the surface of bioceramics in SBF depends on various factors, such as super-saturation of the solution, surface energy of the substrate and interfacial energy of the substrate. An important issue that should be mentioned here is the formation of calcite accompanying the formation of apatite. Interaction between bioactive materials and physiological solutions in vitro is known to occur in two steps: dissolution and back precipitation. In the dissolution step, ions are released from the material into the surrounding solution, which will change the local pH and ionic concentration at the material solution interface and then promote the back precipitation of a bone-like apatite layer onto the surface of bioactive materials. The composite BCP20 has a ratio of 80:20 of sodium calcium silicate and polymer, which is close to that of the human bone [70% hydroxyapatite (bioceramic phase) and 30% collagen (organic phase)].
The XRD pattern shows the formation of calcium apatite phases on the surface of all the scaffolds, confirming its bioactivity (Figure 2). However, the relative intensity of sodium calcium silicate peaks compared with that of hydroxyapatite peaks indicates the effectiveness of the scaffold as its composition varies. Compared with the other formulations, BCP20 shows more calcium apatite deposition as the composition comes closer to that of the natural bone. BCP15 exhibits less calcium apatite deposition, indicating that it has lesser bioactivity, which may be due to the deviation in concentration of the polymer from that of the natural bone. BCP10 has a moderate calcium apatite formation on its surface, whereas BCP5 shows broad peak in the XRD pattern. This trend observed in these two scaffolds might probably be similar to that found in BCP15.
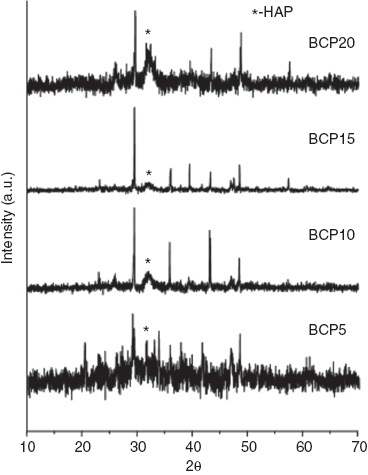
XRD pattern showing the bioactivity of different formulations of sodium calcium silicate.
3.4 Drug delivery kinetics of the scaffolds
The release of drug from the ceramic matrix will follow the dissolution pathway, whereas that from the polymer matrix will follow the diffusion pathway. In the present system, the matrix contains both the constituents so the release kinetics may get altered depending on the ratio of ceramic to polymer. The drug release profile of all the compositions shows almost a similar kind of release, but the scaffold BCP20 shows the least release compared with the other scaffolds (Figure 3). This observation may be attributed to the high polymer concentration in the scaffold, which led to the diffusion of the drug from the matrix, resulting in the sustained release of the drug. The scaffold BCP5 shows the highest release because its polymer concentration was low. Consequently, the dissolution pathway predominates, resulting in maximum drug release. Moreover, there was a burst in the release of ciproflaxocin from all the compositions at the onset of the experiment, as 18% of the drug was released within 24 h in all compositions. The release pattern of the drug from the different scaffolds shows similar trend with very small variation. Therefore, the drug release kinetics is independent of composition.

Drug release kinetics of different formulations of sodium calcium silicate.
3.5 Simulation studies of drug delivery kinetics
A mathematical model is a description of a system using mathematical language, and the process of developing a mathematical model is termed mathematical modeling. Mathematical modeling of drug delivery and predictability of drug release is a field of steadily increasing academic and industrial importance with an enormous future potential. Due to the significant advances in information technology, the in-silicon optimization of novel drug delivery systems can be expected to significantly improve its accuracy and easiness of application. Analogous to other scientific disciplines (e.g., aviation and aerospace), computer simulations are likely to become an integral part of future research and development in pharmaceutical technology. It is only a question of time when mathematical programs will be routinely used to help in optimizing the design of novel dosage forms. Considering the desired type of administration, drug dose to be incorporated and targeted drug release profile, mathematical predictions will allow for good estimates of the required composition.
There is no mathematical model available that considers the nature of two different (polymer and ceramic) constituents of the composite, porosity of the matrix, particle size of bioceramics and diffusion of drug in composite matrix. The major problem in designing a model for the delivery system of ceramic drug arises because of the bioactivity of the system: as the deposition of apatite layer takes place on the surface it will affect the diffusion of drug and porosity of the system, hence the model cannot be employed for the data obtained for the entire period.
The mathematical modeling employed here is based on the hypothesis proposed for thin films [24–26], and most of the criteria defined for this model are satisfied by the present system. Accordingly, the model expression is given by
where Mt is the amount of drug released until the time t, A is the release area, D is the drug diffusion coefficient, C0 is the initial drug concentration in the matrix, and Cs is the drug solubility.
This model expression is semi-empirical as it uses both the theoretical and practical data in predicting the values using this model. A particularly fruitful, but also very challenging aspect is to combine the mathematical theories with models quantifying drug transport in the living organism, including drug distribution in the various organs and even within the different cell compartments. Ideally, theoretical calculations should allow for a quantitative prediction of the effects of formulation and processing parameters not only on the resulting drug release kinetics, but also on the resulting drug concentration time profiles at the site of action in the human body and on the pharmaco-dynamic effects in the patients under the disease conditions. This type of mathematical modeling is much more complex, but in the very long run it could help to allow for customized drug delivery to the patient.
In this study, mathematical modeling was performed for all the formulations (Figure 4). The model shows that the real-time and the theoretical values are well correlated for all the samples, proving that the model is valid for this system.
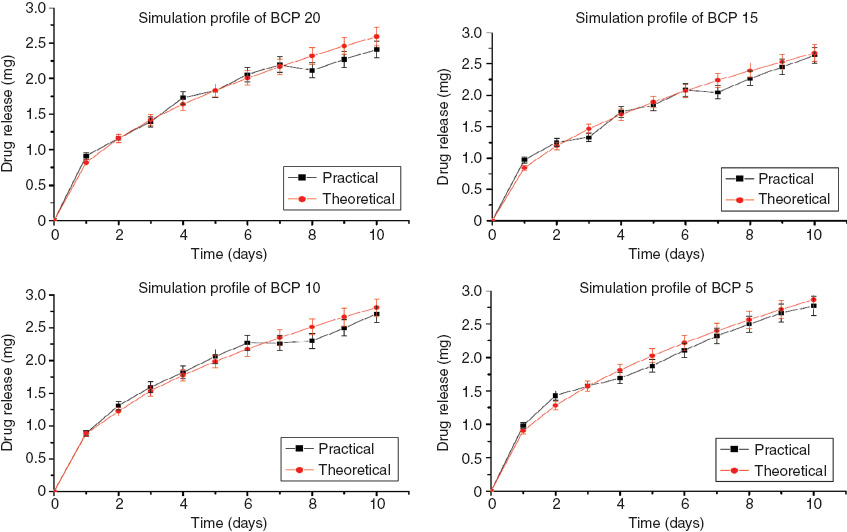
Simulated drug release profile of different formulations of sodium calcium silicate.
4 Conclusion
Sodium calcium silicate was successfully synthesized by a rapid combustion method, an energy-saving technique. Sodium calcium silicate has a high apatite-inducing ability, which is a promising candidate material for bone tissue engineering. The drug release ability of the scaffolds was investigated for various formulations in the SBF medium with ciprofloxacin as the model drug. Based on the release profile, the bioceramic composite demonstrated sustained drug release ability. The entire drug delivery kinetics was mathematically modeled using the Higuchi model. Mathematical modeling of drug delivery shows significant potential in facilitating product development in the future and in understanding complex pharmaceutical dosage forms. Modeling of drug delivery systems provides the opportunity to correlate the experimental and theoretical data, thereby validating the studies.
Acknowledgments
The authors thank the administration of VIT University for providing financial support. The authors express their sincerest gratitude to Prof. R. Vijayaraghavan, Assistant Director, Centre for Excellence in Nanomaterials, VIT University, for his continuous support and motivation.
References
[1] Sasikumar S, Vijayaraghavan R. J. Mater. Sci. Technol. 2010, 26, 1114.10.1016/S1005-0302(11)60010-8Search in Google Scholar
[2] Kashiwazaki H, Harada N, Akazawa T, Kabir MA, Minamida Y, Murata M. J. Hard Tissue Biol. 2013, 22, 337–342.10.2485/jhtb.22.337Search in Google Scholar
[3] Yumoto A, Yamamoto T, Hiroki F, Shiota I, Niwa N. Sci. Eng. Compos. Mater. 2011, 18, 265–269.10.1515/SECM.2011.051Search in Google Scholar
[4] Mendez FB, Bocardo JCE, Hernandez DAC, Robles JMA, Ramos EMM. Ceram. Int. 2011, 37, 2445–2451.10.1016/j.ceramint.2011.03.035Search in Google Scholar
[5] Haiyan Li, Chang J. J. Control. Release 2005, 107, 463–473.10.1016/j.jconrel.2005.05.019Search in Google Scholar PubMed
[6] Perdomo MM, Pena P, Aza PND, Carrodeguas RG, Rodriguez MA, Turrillas X, Aza SD, Aza AHD. Acta. Biomater. 2009, 5, 3057–3066.10.1016/j.actbio.2009.04.026Search in Google Scholar PubMed
[7] Tanga H, Guo J, Sun Y, Chang B, Ren Q, Yang W. Int. J. Pharm. 2011, 421, 388–396.10.1016/j.ijpharm.2011.10.013Search in Google Scholar PubMed
[8] Zhu M, Wang H, Liu J, He H, Hua X, He Q, Zhang L. Biomater. 2011, 32, 1986–1995.10.1016/j.biomaterials.2010.11.025Search in Google Scholar PubMed
[9] Xina R, Lenga Y, Chen J, Zhang Q. Biomater. 2005, 26, 6477–6486.10.1016/j.biomaterials.2005.04.028Search in Google Scholar PubMed
[10] Mastrogiacomo M, Scaglionec S, Martinettie R, Dolcinie L, Beltramec F, Cancedda R, Quartod R. Biomater. 2006, 27, 3230–3237.10.1016/j.biomaterials.2006.01.031Search in Google Scholar PubMed
[11] Huang XH, Chang. J. Mater. Chem. Phys. 2009, 115, 1–4.10.1016/j.matchemphys.2008.11.066Search in Google Scholar
[12] Liu X, Ding C, Chu P. Biomater. 2004, 25, 1755–1761.10.1016/j.biomaterials.2003.08.024Search in Google Scholar PubMed
[13] Colilla M, Manzano M, Regi MV. Int. J. Nanomed. 2008, 3, 403–414.10.2147/IJN.S3548Search in Google Scholar PubMed PubMed Central
[14] Zhao Y, Ning C, Chang J. J. Sol-Gel. Sci. Technol. 2009, 52, 170.10.1007/s10971-009-2038-7Search in Google Scholar
[15] Singh V, Rao TKG, Zhu JJ. J. Lumin. 2008, 128, 583–588.10.1016/j.jlumin.2007.08.014Search in Google Scholar
[16] Shukla AK, Sharma V, Arul Dhas N, Patil KC. Mater. Sci. Eng. B 1996, 40, 153–157.10.1016/0921-5107(96)01651-0Search in Google Scholar
[17] Enesu D, Olteanu CE. Chem. Eng. Commun. 2008, 195, 1269–1291.10.1080/00986440801958808Search in Google Scholar
[18] Wang JL, Tang HJ, Hsieh PH, Chiu F-Y, Chen YH, Chang MC, Huang CT, Liu CP, Lau YJ, Hwang KP, Ko WC, Wang CT, Liu CY, Liu CL, Hsueh PR. Int. J. Antimicrob. Agents 2012, 40, 103–107.10.1016/j.ijantimicag.2012.03.010Search in Google Scholar PubMed
[19] Sivakumar M, Panduranga Rao K. Biomater. 2002, 23, 3175–3181.10.1016/S0142-9612(02)00066-2Search in Google Scholar PubMed
[20] Li H, Chang J. J. Control. Release 2005, 107, 463–473.10.1016/j.jconrel.2005.05.019Search in Google Scholar PubMed
[21] Husseini GA, Christensen DA, Rapoport DA, Pitt GWW. J. Control. Release 2002, 83, 303–305.10.1016/S0168-3659(02)00203-1Search in Google Scholar PubMed
[22] Barry BW, Meyer MC. Int. J. Pharm. 1979, 2, 1–25.10.1016/0378-5173(79)90025-5Search in Google Scholar
[23] Husseini GA, Myrup GD, Pitt WG, Christensen DA, Natalya Y. J. Control. Release 2000, 69, 43–52.10.1016/S0168-3659(00)00278-9Search in Google Scholar PubMed
[24] Grassi M, Grassi G. Curr. Drug. Delivery 2005, 2, 97–116.10.2174/1567201052772906Search in Google Scholar PubMed
[25] Siepmann J, Siepmann F. Int. J. Pharm. 2008, 364, 328–343.10.1016/j.ijpharm.2008.09.004Search in Google Scholar PubMed
[26] Lopez T, Ortiz E, Meza D, Basaldella E, Bokhimif X, Magana C, Sepulveda A, Rodriguez F, Ruizg J. Mater. Chem. Phys. 2011, 126, 922–929.10.1016/j.matchemphys.2010.12.011Search in Google Scholar
©2016 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Original articles
- Influence of bis(triethoxysilylpropyl) tetrasulfide amount on the properties of silica-filled epoxidized natural rubber-based composites
- Microstructure-based modeling of the dynamic mechanical properties of SiCp/Al composites
- Effect of moisture content of jute fabric and hybridization structure on the impact properties of jute and jute/glass hybrid composites
- Synthesis, characterization and formulation of sodium calcium silicate bioceramic for drug delivery applications
- Synthesis of iron nanocomposite reinforced by TiC particles via mechanical activation from ilmenite concentrate and carbon black
- The effect of an excessive amount of carbon nanotubes on the properties of zinc oxide-carbon nanotube nanocomposites
- Microstructure and erosion characteristics of Ni-AlN thin films prepared by electrodeposition
- Study on the preparation and characterization of high-dispersibility nanosilica
- Comparing the influence of different kinds of zirconia on properties and microstructure of Al2O3 ceramics
- Structure of intercalated organic montmorillonite and its pyrolysis properties analyzed using the Agrawal integral equation
- Prediction, modeling and characterization of surface texturing by sulfuric etchant on non-toxic titanium bio-material using artificial neural networks and fuzzy logic systems
- Investigation of the WEDM of Al/B4C/Gr reinforced hybrid composites using the Taguchi method and response surface methodology
- A low-cost fiberglass polymer resin dielectric material-based microstrip patch antenna for multiband applications
- Free vibration analysis of axially layered functionally graded short beams using experimental and finite element methods
- Physical properties and microstructures of a BN-NiCoCrAlY laser amorphous-nanocrystal reinforced composite coating
Articles in the same Issue
- Frontmatter
- Original articles
- Influence of bis(triethoxysilylpropyl) tetrasulfide amount on the properties of silica-filled epoxidized natural rubber-based composites
- Microstructure-based modeling of the dynamic mechanical properties of SiCp/Al composites
- Effect of moisture content of jute fabric and hybridization structure on the impact properties of jute and jute/glass hybrid composites
- Synthesis, characterization and formulation of sodium calcium silicate bioceramic for drug delivery applications
- Synthesis of iron nanocomposite reinforced by TiC particles via mechanical activation from ilmenite concentrate and carbon black
- The effect of an excessive amount of carbon nanotubes on the properties of zinc oxide-carbon nanotube nanocomposites
- Microstructure and erosion characteristics of Ni-AlN thin films prepared by electrodeposition
- Study on the preparation and characterization of high-dispersibility nanosilica
- Comparing the influence of different kinds of zirconia on properties and microstructure of Al2O3 ceramics
- Structure of intercalated organic montmorillonite and its pyrolysis properties analyzed using the Agrawal integral equation
- Prediction, modeling and characterization of surface texturing by sulfuric etchant on non-toxic titanium bio-material using artificial neural networks and fuzzy logic systems
- Investigation of the WEDM of Al/B4C/Gr reinforced hybrid composites using the Taguchi method and response surface methodology
- A low-cost fiberglass polymer resin dielectric material-based microstrip patch antenna for multiband applications
- Free vibration analysis of axially layered functionally graded short beams using experimental and finite element methods
- Physical properties and microstructures of a BN-NiCoCrAlY laser amorphous-nanocrystal reinforced composite coating

