Abstract
This article reports on experiments aimed at modifying nanosilica powder by adding a silane coupling agent, KH570, to achieve high dispersibility and strengthen the compatibility and interface bonding between it and the organic phases. Experiments were first done to prepare the common nanosilica powder, dried at 120°C for 4 h in a drying oven; second, an ethanol/water solution (volume ratio 11:1, total volume not more than two-thirds of the capacity of the flask) was blended in a three-necked flask with a reflux condenser and magnetic stirring; third, appropriate amount of dried nanosilica powder and appropriate mass fraction ratio of the silane coupling agent KH570 were added into the ethanol/water solution, and the pH of the mixture was adjusted to about 4–5 by adding acetic acid solution; fourth, the three-necked flask was placed in a water bath, and the modification reaction of the above mixing solution was sustained for an appropriate time in the appropriate temperature with magnetic stirring; fifth, the above mixing solution was separated by using a centrifuge (10,000 rpm) for 3 min, and the precipitate at the bottom of the flask was obtained after the supernatant was poured out; sixth, the precipitate was dried at 120°C for 48 h in a drying oven after it was washed with acetone several times, finally yielding the high-dispersibility nanosilica powder. The orthogonal test method was adopted to optimize three key test parameters: mass fraction ratio of the silane coupling agent KH570, modification reaction temperature, and modification reaction time. The dispersion effect of the high-dispersibility nanosilica powder was characterized by using infrared, X-ray diffraction, and scanning electron microscopic analyses from different views. The results revealed that the best dispersibility effect was achieved when the mass fraction ratio of the silane coupling agent KH570 was 3%, the modification reaction temperature was 80°C, and the modification reaction time was 2 h. Furthermore, the modification reaction resulted in chemical bonding, but not simple physical adsorption, owing to the presence of organic bond groups in the nanosilica modified by the silane coupling agent. The crystal structure of the nanosilica powder remained amorphous after the modification reaction.
1 Introduction
Being called the “industrial monosodium glutamate”, nanosilica is widely used in catalyst carriers, polymer composite materials, electronic packaging materials, and cosmetics and other industries. However, because of the high specific surface area, high surface energy, high ratio of surface atomic number, and the total number of atoms, agglomeration of nanosilica powder is unavoidable, and it is difficult to eliminate it with the ordinary methods of vibration and dissolution. Besides the interior structure of nanosilica being polysiloxane, active Si-OH groups exist on the outside surface of nanosilica and the surface is surrounded by many empty chemical bonds showing hydrophilicity; moreover, it is also difficult to disperse in the organic resin phase. In an attempt to achieve high dispersibility and strengthen the compatibility and interface bonding between the nanosilica and the organic phases, thus improving its effectiveness and expanding its application areas, domestic and foreign scholars have done many studies [1, 2]. Fu et al. [3] prepared nanosilica by using the carbonization method, and enhanced its absorption value and specific surface area with a variety of surfactants. By using the supercritical fluid technique, Tang et al. [4] prepared nanosilica powder with greatly improved hydrophobicity. Wang et al. [5] used the gas-phase hexamethyl disilylamine (HMDS) to modify nanosilica, and their experimental results showed that the ethoxy group on the surface of the aerogel was oxidized to hydroxy, which easily reacts with HMDS to form silicon methyl; thus, the hydrophobicity of nanosilica was strengthened considerably. However, all the above studies have limitations. In the modification using a surfactant, the positive charge and the negative charge were absorbed on the surface of nanosilica, thus reducing its binding affinity to the other phases. Nanosilica modification by using the supercritical fluid technique is difficult to carry out in a large-scale production because this process is complex and requires much equipment. The dry method modification also has some disadvantages, such as the difficulty of surface treatment for homogenization, need for more modifier, high equipment requirements, and high product cost. In the experiments reported in this article, the wet method of modifying nanosilica powder by adding a silane coupling agent, KH570, has universal applicability owing to the simple process and good modification effect. The basic mechanisms of the modification method were as follows: first, the silane coupling agent was hydrolyzed in silicon alcohol (type 1 reaction). Second, the alcohol-based silicon was reacted with silicon hydroxyl groups on the surface of the nanosilica, and they were linked by chemical bonds [6, 7] (type 2 reaction).
 (1)
(1)
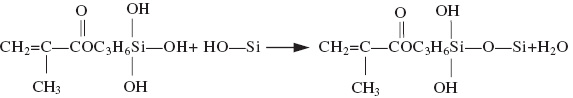 (2)
(2)
2 Materials and methods
2.1 Experimental materials
The relevant parameters of nanosilica and the silane coupling agent KH570 in the experiments are shown in Tables 1 and 2. Other additional experimental materials included absolute ethyl alcohol, acetic acid, sodium hydroxide, and hydrochloric acid. All the above experimental materials were available in the market, and they were all in accordance with green environmental protection requirements.
Performance parameters of nanosilica.
| Type | SG-200 |
|---|---|
| Appearance | White loose powder |
| Specific surface area (m2 g-1) | 190–220 |
| Residue/325 sieve | 0.02% |
| Combustion loss/1000°C | 2% |
| pH | 3.7–4.3 |
| Effective substance content | 99.8% |
| Water | <1.5% |
| Apparent density (g l-1) | 40–60 |
Performance parameters of the silane coupling agent KH570.
| Appearance | Colorless or light yellow liquid |
| Boiling point (°C) | 255 |
| Density (20°C, g cm-3) | 1.043–1.053 |
| Refraction (ND25) | 1.4285–1.4310 |
| Flash point (°C) | 88 |
| Content of effective substances | ≥95% |
2.2 Experimental methods
The experiments were carried out according to the following steps. First, the common nanosilica powder was dried at 120°C for 4 h in a drying oven; second, an ethanol/water solution (volume ratio 11:1, total volume not more than two-thirds of the capacity of the flask) was blended in a three-necked flask with a reflux condenser and magnetic stirring; third, appropriate amounts of the dried nanosilica powder and the silane coupling agent KH570 (mass fraction ratio, 3% of nanosilica powder) were added into the ethanol/water solution, and the pH of the mixture was adjusted to about 4–5 by adding acetic acid solution; fourth, the three-necked flask was placed in a water bath, and the modification reaction of the above mixing solution sustained for 2 h at a temperature of 80°C with magnetic stirring; fifth, separation of the above mixing solution was conducted on a centrifuge (10,000 rpm) for 3 min, and the precipitate at the bottom of the flask was obtained after the supernatant was poured out; sixth, the precipitate was dried at 120°C for 48 h in a drying oven after it was washed with acetone several times, finally yielding the high-dispersibility nanosilica powder.
The three key experimental parameters of step four above were optimized and chosen with the orthogonal test method, and the experimental factors and levels are shown in Table 3.
Experimental factors and levels.
| Levels | Factor A | Factor B | Factor C |
|---|---|---|---|
| Mass fraction ratio of KH570 | Modification reaction temperature (°C) | Modification reaction time (h) | |
| 1 | 2% | 70 | 1.5 |
| 2 | 3% | 80 | 2.0 |
| 3 | 4% | 90 | 2.5 |
The activation grade and the surface hydroxyl number were tested for each high-dispersibility nanosilica powder obtained from each set of the test conditions. A high activation degree means excellent dispersibility, whereas a low surface hydroxyl number means a strong bonding affinity between it and the organic phases. Repeated confirmatory tests for each of the above tests were carried out, and the activation grade and the surface hydroxyl number values were averaged. These values comprehensively characterized the modification effect of the nanosilica powder.
The dispersibility effect of the high-dispersibility nanosilica powder prepared with the test conditions optimized and chosen with the orthogonal test method was characterized by infrared spectroscopy (IR), X-ray diffraction (XRD), and scanning electron microscope (SEM) analyses from different views. IR analysis was conducted with a TENSOR27 Fourier transformation infrared spectrometer (measurement range: 400–4000 cm-1; Bruker Corporation, Germany), with the tablet specimen being diluted with KBr. The crystal structure of the dispersed nanosilica powder was analyzed by XRD analysis using the D8 Advance X-ray diffractometer produced by Bruker Corporation, with CuKα being the radiation source, the tube current being 20 mA, and tube voltage being 30 kV. SEM observation was conducted using an S-3000N scanner produced by Hitachi Japan.
The activation grade, expressed as η, is the ratio of the weight of the floating part in water and the total weight of the sample. The test methods were as the follows: first, 50 ml deionized water was placed into a 100 ml separatory funnel, and m1 grams of dispersed nanosilica powder was added into the separatory funnel; second, the mixture was vibrated for 5 min and then allowed to stand for 30 min; third, the powder sinking in water was placed into a fixed weight crucible; fourth, the powder sinking in water was dried in a drying oven, and then the weight of the powder sinking in water was measured (m2 grams); finally, the activation grade was derived according to Formula (3)
The surface hydroxyl number, expressed as N, was determined and calculated as follows: first, 25 ml of absolute ethyl alcohol and 75 ml of 20% NaCl solution were placed in a 200 ml beaker, and m grams of dispersed nanosilica powder was added into the beaker; second, vibration through magnetic stirring was conducted and the pH of the mixture was adjusted to 4 by adding 0.1 mol/l HCl solution; third, the pH of the mixture was adjusted to 9 by adding 0.1 mol/l NaOH solution, and the volume of NaOH solution needed was recorded as V milliliters; finally, the surface hydroxyl number was derived according to Formula (4)
where C=concentration of NaOH solution, 0.1 mol/l; V=volume of NaOH solution need, ml; NA=Avogadro’s number; S=specific surface area of nanosilica, nm2 g-1; m=weight of nanosilica, g.
3 Results and discussion
3.1 Results of the orthogonal test design and the corresponding analyses
The scheme of the orthogonal test design and the corresponding test results of the activation grade and the surface hydroxyl number are shown in Table 4.
Orthogonal test design and test results.
| Sample number | Factor A | Factor B | Factor C | Results | |
|---|---|---|---|---|---|
| Mass fraction ratio of KH570 | Modification reaction temperature (°C) | Modification reaction time (h) | Activation grade (%) | Surface hydroxyl number (1 nm-2) | |
| 1 | 1 | 1 | 1 | 61 | 0.7 |
| 2 | 1 | 2 | 2 | 76 | 0.35 |
| 3 | 1 | 3 | 3 | 73 | 0.4 |
| 4 | 2 | 1 | 3 | 76.5 | 0.45 |
| 5 | 2 | 2 | 1 | 92 | 0.32 |
| 6 | 2 | 3 | 2 | 90 | 0.35 |
| 7 | 3 | 1 | 2 | 80 | 0.45 |
| 8 | 3 | 2 | 3 | 89 | 0.36 |
| 9 | 3 | 3 | 1 | 86.5 | 0.35 |
On the basis of the calculation of the sum of the experimental results (T), the average value (t), and the range (R), the superior levels, and the primary- and secondary-influence experimental factors were measured, as shown in Table 5. The optimized combination of experimental parameters for the activation grade was A2B2C2, whereas that for the surface hydroxyl number was B2A2C2.
Analysis of orthogonal test results.
| Activation grade (%) | Surface hydroxyl number (1 nm-2) | |||||
|---|---|---|---|---|---|---|
| Factor A | Factor B | Factor C | Factor A | Factor B | Factor C | |
| Sum of the experimental results, T | ||||||
| T1 | 210 | 217.5 | 240.5 | 1.45 | 1.6 | 1.37 |
| T2 | 258.5 | 258 | 245 | 1.12 | 1.03 | 1.15 |
| T3 | 255.5 | 248.5 | 238.5 | 1.16 | 1.1 | 1.21 |
| Average value, t | ||||||
| t1 | 70 | 73 | 80 | 0.48 | 0.53 | 0.46 |
| t2 | 86 | 86 | 82 | 0.37 | 0.34 | 0.38 |
| t3 | 85 | 83 | 80 | 0.39 | 0.37 | 0.40 |
| Range, R | 16 | 13 | 2 | 0.11 | 0.19 | 0.08 |
| Superior levels | 2 | 2 | 2 | 2 | 2 | 2 |
| Primary- and secondary-influence experimental factors | A2B2C2 | B2A2C2 | ||||
Thus, the superior levels for the activation grade and the surface hydroxyl number were all level 2, which means that the optimized combination of experimental parameters was as follows: mass fraction ratio of the silane coupling agent KH570, 3%; modification reaction temperature, 80°C; and modification reaction time, 2 h. However, the optimized combination of experimental parameters above was not included in the scheme of the orthogonal test design. Therefore, repeated confirmatory parallel experiments with the above-mentioned optimized combination of experimental parameters were conducted. The results of the activation grade and the surface hydroxyl number are shown in Table 6. The IR, XRD, and SEM patterns are shown in the Figures 1–3.
Optimized parallel experiments results.
| Sample no. | Activation grade (%) | Surface hydroxyl number (1 nm-2) |
|---|---|---|
| 1 | 93.26 | 0.33 |
| 2 | 92.66 | 0.32 |
| 3 | 93.25 | 0.31 |
| 4 | 91.68 | 0.34 |
| 5 | 92.31 | 0.31 |
| Average | 92.63 | 0.32 |
| 0 (Unmodified) | 10.12 | 1.03 |

Infrared spectroscopy of nanosilica.
(A) Unmodified. (B) Modified with KH570.
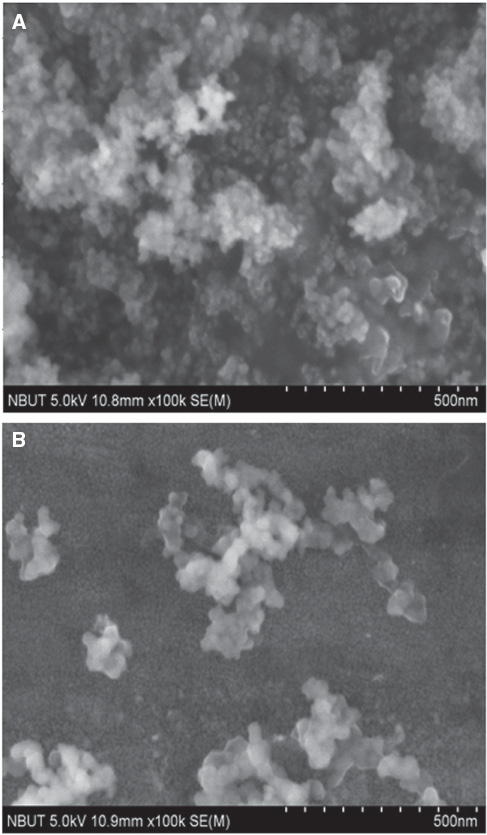
SEM of nanosilica.
(A) Unmodified. (B) Modified with KH570.

XRD pattern of nanosilica.
(A) Unmodified. (B) Modified with KH570.
The optimized combination of experimental parameters meant that all factors were in the middle level. Only a single molecular coating layer around the nanosilica powder was needed; >3% silane coupling agent KH570 would result in the deterioration of the modification effect because of the self-aggregation of the silane coupling agent KH570. However, <3% of KH570 could not eliminate the self-aggregation of the nanosilica powder because only a small quantity of the surface of the nanosilica powder was covered by KH570. Not enough energy could be afforded to the modification reaction at a low modification temperature. With the increase of modification temperature, the intermolecular thermal motion became intense, the probability of intermolecular collision increased, the percentage of grafting between KH570 and the nanosilica powder increased, and the modification effect became superior [8]. In contrast, the self-aggregation, decomposition of KH570, or even the reverse reaction between KH570 and the nanosilica powder would be inevitable because of the intense intermolecular thermal motion in the higher modification temperature of >80°C; therefore, the modification effect would become negative. To consider the reaction time, it was found that regardless of the activation grade or the surface hydroxyl number, the results from different levels of experiments showed a narrow difference according to their ranges, R. Thus, it was concluded that the reaction time had a limited impact on the modification reaction [9].
3.2 IR analysis of the nanosilica
The IR spectrum of unmodified and modified nanosilica is shown in Figure 1A and B, respectively. Normal absorption peaks existed in the IR spectrum of the unmodified nanosilica, including the stretching vibration absorption peak of Si-O at 808 and 1106 cm-1, bending vibration absorption peak of O-H at 1640 cm-1, stretching vibration absorption peak of Si-O-H and stretching vibration absorption peak of O-H at 3412 cm-1, as Figure 1A shows. However, there were new characteristic absorption peaks existing in the IR spectrum of nanosilica modified by the silane coupling agent KH570. There were vibration absorption peaks of C-C at 1505 cm-1 and of -CH3 and -CH2 at 2928 cm-1, which fully proved the presence of organic bond groups in the nanosilica modified by KH570, as Figure 1B shows. At the same time, owing to the obvious increase of peak intensity and peak area at 3460 cm-1, the increase of Si-O-H bonds in the modified nanosilica by KH570 was the evidence that Formula (1) was completed. Furthermore, owing to the much stronger stretching vibration absorption peak of Si-O at 808 and 1106 cm-1, the increase of Si-O bonds in the nanosilica modified by KH570 proved that Formula (2) was completed. From the chemistry viewpoint, the modification was successful and the high dispersibility and hydrophobicity of the modified nanosilica was assured [10].
3.3 SEM analysis
Of course, the most apparent and intuitive evidence of the excellent dispersion effect was taken from the SEM observation. The different SEM morphologies of the particle distribution between the unmodified and the modified nanosilica are shown in Figure 2A and B.
3.4 XRD analysis of the nanosilica
Moreover, no apparent changes occurred in the XRD patterns of the modified nanosilica powder, proving that the crystal structure of nanosilica powder remained amorphous after the modification by the silane coupling agent KH570, as shown in Figure 3A and B.
4 Conclusions
Adding the silane coupling agent KH570 with a mass fraction ratio of 3% to the nanosilica powder provided the nanosilica with the best dispersion ability after the modification reaction at 80°C for 2 h. The highest activation grade and the lowest surface hydroxyl number, which indicate the best dispersibility effect, were achieved with the optimized combination of experimental parameters mentioned above.
IR analysis verified that there were new bonds, Si-O-H and Si-O, that eliminated the empty bonds of the surface of the modified nanosilica; thus, the modification was not just a physical absorption but also a chemical reaction. SEM analysis showed the good dispersibility of the nanosilica particles, whereas XRD analysis showed that the crystal structure of the nanosilica powder remained amorphous after modification by the silane coupling agent KH570.
Acknowledgments
This work was supported by the Fundamental Research Funds for the Central Universities (2012QNB05), China Postdoctoral Science Foundation funded project (2013M531422). We gratefully acknowledge the Jiangsu Province Post-doctorate Scientific Research Program, State Key Laboratory of Tribology of Tsing Hua University, and general manager You-liang Wu from Xuzhou Baoding Supporting Technology Co., Ltd., for fruitful discussion and experimental assistance.
References
[1] Zheng L-h, Liu Q-f, Cheng H-f. Chin. Non-Metal. Min. Ind. Herald 2008, 1, 12–15.Search in Google Scholar
[2] Zhang Q, Yang Y-f, Hu G-s. New Chem. Mater. 2009, 37, 103–105.Search in Google Scholar
[3] Fu J-k, Qu Y-x, Guo K, Zhao G-l. J. Beijing Univ. Chem. Technol. 2008, 35, 13–17.Search in Google Scholar
[4] Tang H-b, Zhang X-m, Ma B-j, Zhong Y-h. J. Shenyang Univ. Technol. 2007, 299, 663–666.Search in Google Scholar
[5] Wang Y-q, Wang Z-m, Shen C-j. Sci. Eng. Compos. Mater. 2012, 19, 423–429.10.1515/secm-2011-0141Search in Google Scholar
[6] Yan H-x, Zhang Y, Zhang Y-x, Sun K. Chin. Rubber Ind. 2004, 51, 376–379.Search in Google Scholar
[7] Wu W, Chen J-f, Qu Y-x. J. Chin. Ceram. Soc. 2004, 32, 570–575.Search in Google Scholar
[8] Lombardi M, Fino P, Montanaro L. Sci. Eng. Compos. Mater. 2014, 21, 23–28.10.1515/secm-2012-0139Search in Google Scholar
[9] Silva L-J, Campos Rubio J-C, Panzera T-H. Sci. Eng. Compos. Mater. 2013, 20, 203–208.10.1515/secm-2012-0088Search in Google Scholar
[10] Hao S-f, Zheng Z-x, Fan W-q, Xu G-q, Lu J, Wu Y-c. Bull. Chin. Ceram. Soc. 2011, 30, 529–533.Search in Google Scholar
©2016 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Original articles
- Influence of bis(triethoxysilylpropyl) tetrasulfide amount on the properties of silica-filled epoxidized natural rubber-based composites
- Microstructure-based modeling of the dynamic mechanical properties of SiCp/Al composites
- Effect of moisture content of jute fabric and hybridization structure on the impact properties of jute and jute/glass hybrid composites
- Synthesis, characterization and formulation of sodium calcium silicate bioceramic for drug delivery applications
- Synthesis of iron nanocomposite reinforced by TiC particles via mechanical activation from ilmenite concentrate and carbon black
- The effect of an excessive amount of carbon nanotubes on the properties of zinc oxide-carbon nanotube nanocomposites
- Microstructure and erosion characteristics of Ni-AlN thin films prepared by electrodeposition
- Study on the preparation and characterization of high-dispersibility nanosilica
- Comparing the influence of different kinds of zirconia on properties and microstructure of Al2O3 ceramics
- Structure of intercalated organic montmorillonite and its pyrolysis properties analyzed using the Agrawal integral equation
- Prediction, modeling and characterization of surface texturing by sulfuric etchant on non-toxic titanium bio-material using artificial neural networks and fuzzy logic systems
- Investigation of the WEDM of Al/B4C/Gr reinforced hybrid composites using the Taguchi method and response surface methodology
- A low-cost fiberglass polymer resin dielectric material-based microstrip patch antenna for multiband applications
- Free vibration analysis of axially layered functionally graded short beams using experimental and finite element methods
- Physical properties and microstructures of a BN-NiCoCrAlY laser amorphous-nanocrystal reinforced composite coating
Articles in the same Issue
- Frontmatter
- Original articles
- Influence of bis(triethoxysilylpropyl) tetrasulfide amount on the properties of silica-filled epoxidized natural rubber-based composites
- Microstructure-based modeling of the dynamic mechanical properties of SiCp/Al composites
- Effect of moisture content of jute fabric and hybridization structure on the impact properties of jute and jute/glass hybrid composites
- Synthesis, characterization and formulation of sodium calcium silicate bioceramic for drug delivery applications
- Synthesis of iron nanocomposite reinforced by TiC particles via mechanical activation from ilmenite concentrate and carbon black
- The effect of an excessive amount of carbon nanotubes on the properties of zinc oxide-carbon nanotube nanocomposites
- Microstructure and erosion characteristics of Ni-AlN thin films prepared by electrodeposition
- Study on the preparation and characterization of high-dispersibility nanosilica
- Comparing the influence of different kinds of zirconia on properties and microstructure of Al2O3 ceramics
- Structure of intercalated organic montmorillonite and its pyrolysis properties analyzed using the Agrawal integral equation
- Prediction, modeling and characterization of surface texturing by sulfuric etchant on non-toxic titanium bio-material using artificial neural networks and fuzzy logic systems
- Investigation of the WEDM of Al/B4C/Gr reinforced hybrid composites using the Taguchi method and response surface methodology
- A low-cost fiberglass polymer resin dielectric material-based microstrip patch antenna for multiband applications
- Free vibration analysis of axially layered functionally graded short beams using experimental and finite element methods
- Physical properties and microstructures of a BN-NiCoCrAlY laser amorphous-nanocrystal reinforced composite coating

