Dear Editor,
A 35-year-old woman was admitted to the hospital one week before delivery due to sudden severe headache accompanied by blurred vision, subsequently diagnosed as severe preeclampsia. During her follow-up visit six months post-delivery, physical examination revealed bilateral carotid arteries, the right upper limb artery, and both lower limb arteries were found to be pulseless. Vascular murmurs were detected in bilateral carotid arteries and subclavian arteries. Ultrasonography disclosed severe stenosis of the bilateral common carotid arteries and the external carotid arteries, as well as uneven thickness and localized dilation of the bilateral iliac arteries. Besides, laboratory tests indicated persistently elevated serum creatine kinase (CK) levels at rest, with significant fluctuations during monitoring (ranging from 1209 U/L to 4419 U/L), despite the absence of notable patient discomfort.
The patient was diagnosed with Takayasu’s arteritis (TAK) and underwent surgery for carotid artery intimal dissection. Following the procedure, the patient experienced a transient loss of consciousness and headache. The computed tomography scan showed subarachnoid hemorrhage in the right frontal lobe (Figure 1). Additionally, the patient suffered an epileptic seizure. Upon discharge, the patient was prescribed aspirin, levetiracetam, prednisolone and methotrexate.
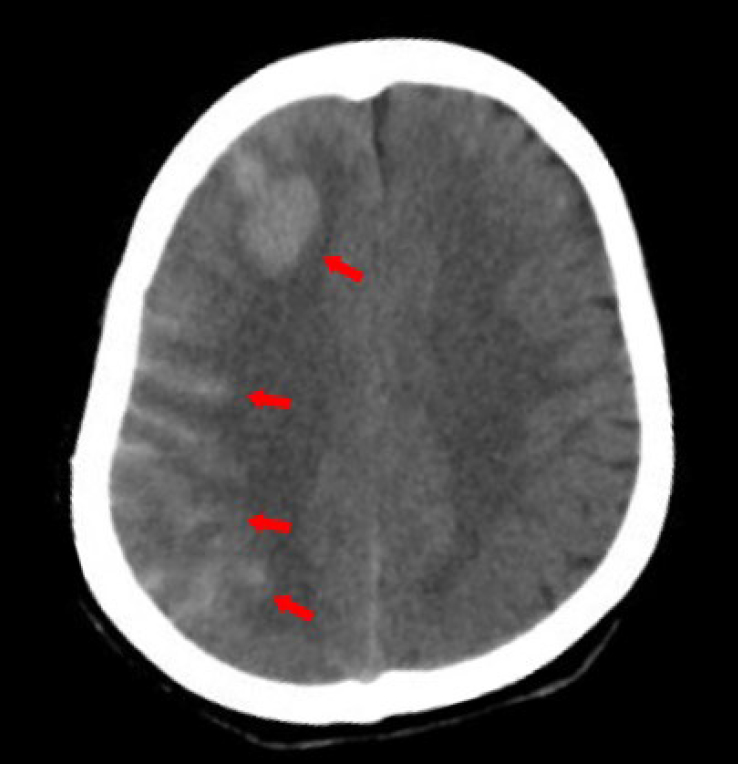
Computed tomography evidence of intracranial hemorrhage after surgery.
During the follow-up, cardiac ultrasound disclosed aortic valve thickening and mild aortic regurgitation. Subsequent routine ultrasound examinations that were performed after 18 months identified a true aneurysm of the distal abdominal aorta. Besides, the CK level remained consistently elevated and showed no response to immunosuppressive therapy. During the consultation with a neurologist, the patient recalled relatively poor endurance in sports since childhood alongside unrestricted daily activities. Furthermore, she experienced lower limb weakness and cramps when climbing and running. No similar manifestations were noted in her family members. The molecular genetic testing of peripheral blood showed the c. 148C > T mutation in the PYGM gene, leading to a diagnosis of McArdle’s disease. The patient was advised to abstain from intense exercise to reduce the symptoms of myalgia and the risk of rhabdomyolysis.
McArdle’s disease, known as glycogen storage disease V, arises from mutations in the PYGM gene encoding muscle glycogen phosphorylase (myophosphorylase), resulting in exercise intolerance including fatigue, muscle soreness, and cramps during the initial stages of exercise.[1] The onset of symptoms typically occurs during childhood, with approximately 60% in the first decade and 28% in the second decade of life. However, the condition is often diagnosed late in adulthood and frequently misdiagnosed.[2,3] TAK is characterized by granulomatous inflammation of the aorta and its major branches, and manifested as systemic symptoms, vascular lesions including wall thickening, luminal stenosis or occlusion, aneurysm formation and ischemic manifestations within affected organs. TAK primarily impacts females, typically occurring between the ages of 10 and 40 years.[4] Generally, McArdle’s disease primarily affects skeletal muscle with infrequent involvement of cardiac and vascular smooth muscles. Conversely, TAK typically leads to vascular smooth muscle dysfunction. This report describes a rare case of a 35-year-old woman diagnosed with McArdle’s disease combined with Takayasu’s arteritis who presented with muti-vascular lesions. The involvement of cardiovascular system in McArdle’s disease is quite rare, and the coexistence of McArdle’s disease and Takayasu’s arteritis seems to jointly exacerbate the muscle damage.
Intracranial hemorrhage is quite rare in patients with TAK. Besides, this is also the first report in the literature that illustrates McArdle’s disease combined with aneurysm and intracranial hemorrhage. The involvement of cardiovascular system in McArdle’s disease is notably rare. Previous studies have documented hypertrophic cardiomyopathy and electro-cardiogram changes in McArdle’s disease [5,6] Pompe’s disease, attributed to a deficiency in acid α-glucosidase, is referred as type II of glycogen storage disease, and manifests as hypertrophic cardiomyopathy, hypotonia and motor delay. Existing reports suggest a prevalence of intracranial artery abnormalities in Pompe’s disease, with cerebrovascular disease posing a potential risk factor for mortality in late-onset Pompe’s disease patients.[7] These data demonstrate that although not common, the degenerative vascular disorders may exist in glycogen storage diseases. McArdle’s disease seems to increase the potential risk of cerebral hemorrhage which may pose surgical challenges in the future. Due to weakened or degenerated status of blood vessel walls, this patient was more prone to developing aneurysm and valve damage.
Another noteworthy point is that the patient developed an aneurysm, indicating the dysfunction of vascular smooth muscle cells. The prevailing hypothesis in TAK is that vascular smooth muscle cells may function as local enhancers of the inflammatory processes within vascular lesions. A substantial number of patients with TAK exhibited aneurysmal diseases.[8] This evidence supports the relationship between TAK and aneurysm in our case. McArdle’s disease has a specific preference for skeletal muscle. Although no prior reports documented the involvement of vascular smooth muscle in McArdle’s disease, the biopsy of a Pompe’s disease patient with thoracic aortic aneurysm showed lysosomal glycogen deposits within smooth muscle tissues, and disruption of the aortic wall architecture, characterized by excessive fragmentation of elastic fibers within the media.[9] We speculate that the aneurysm in our case may be caused or aggravated by McArdle’s disease leading to glycogen accumulation and functional degradation in the vascular smooth muscle cells, which requires confirmation of myophosphorylase activity in vascular smooth muscle via biopsy. Regardless of the cause of the aneurysm, aneurysms carry a risk of rupture and are associated with a higher rate of relapse in TAK, which requires clinicians to pay closer attention to subsequent progression.
Funding statement: This study was supported by CAMS Innovation Fund for Medical Sciences (CIFMS) (2021-I2M-1-005), National High Level Hospital Clinical Research Funding (2022-PUMCH-A-006, 2022-PUMCH-B-013 and 2022-PUMCH-C-068).
Acknowledgements
None.
-
Author contributions
Guo S and Li J wrote the main manuscript text. Wei Y, Wu D and Zeng X prepared Figures 1. All authors reviewed and approved the final manuscript.
-
Informed consent
Written informed consent was obtained from the patient.
-
Ethical approval
The study of human sample was approved by the Ethics Committee of Peking Union Medical College Hospital (S-478) and complied with all relevant ethical guidelines.
-
Conflict of interest
None declared.
-
Data availability statement
The datasets analyzed for this study are available from the corresponding author Dr. Jing Li (lijing6515@pumch.cn) upon reasonable request.
References
[1] Hannah WB, Derks TGJ, Drumm ML, et al. Glycogen storage diseases. Nat Rev Dis Primers. 2023;9:46.10.1038/s41572-023-00456-zSuche in Google Scholar PubMed
[2] Vieitez I, Teijeira S, Fernandez JM, et al. Molecular and clinical study of McArdle’s disease in a cohort of 123 European patients. Identification of 20 novel mutations. Neuromuscul Disord. 2011;21:817–823.10.1016/j.nmd.2011.07.002Suche in Google Scholar PubMed
[3] Lucia A, Ruiz JR, Santalla A, et al. Genotypic and phenotypic features of McArdle disease: insights from the Spanish national registry. J Neurol Neurosurg Psychiatry. 2012;83:322–328.10.1136/jnnp-2011-301593Suche in Google Scholar PubMed
[4] Pugh D, Karabayas M, Basu N, et al. Large-vessel vasculitis, Nat Rev Dis Primers. 2022;7:93.10.1038/s41572-021-00327-5Suche in Google Scholar PubMed PubMed Central
[5] Nicholls DP, Campbell NP, Stevenson HP, et al. Angina in McArdle’s disease. Heart. 1996;76:372–373.10.1136/hrt.76.4.372Suche in Google Scholar PubMed PubMed Central
[6] Moustafa S, Patton DJ, Connelly MS. Unforeseen cardiac involvement in McArdle’s disease. Heart Lung Circ. 2013;22:769–771.10.1016/j.hlc.2012.12.004Suche in Google Scholar PubMed
[7] Garibaldi M, Sacconi S, Antonini G, et al. Long term follow-up of cerebrovascular abnormalities in late onset Pompe disease (LOPD). J Neurol. 2017;264:589–590.10.1007/s00415-017-8396-0Suche in Google Scholar PubMed
[8] Lefebvre F, Ross C, Soowamber M, et al. Aneurysmal Disease in Patients With Takayasu Arteritis. J Rheumatol. 2024;51:277–284.10.3899/jrheum.2023-0629Suche in Google Scholar PubMed
[9] Goeber V, Banz Y, Kaeberich A, et al. Huge aneurysm of the ascending aorta in a patient with adult-type Pompe’s disease: histological findings mimicking fibrillinopathy. Eur J Cardiothorac Surg. 2013;43:193–195.10.1093/ejcts/ezs489Suche in Google Scholar PubMed
© 2024 Shuning Guo, Yanping Wei, Wu Di, Jing Li, published by De Gruyter on behalf of NCRC-DID
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Editorial
- Enhancing standardized diagnosis and treatment to improve the prognosis of patients with rheumatoid arthritis in China
- The potential for biosimilars to address unmet clinical need — Is the developed world missing out?
- Guideline
- Chinese guidelines for the diagnosis and treatment of rheumatoid arthritis: 2024 update
- Review
- Therapeutic potential of CD20/CD3 bispecific antibodies in the treatment of autoimmune diseases
- Original Article
- 14-3-3 Eta protein as a novel biomarker in early detection of uveitis in Egyptian juvenile idiopathic arthritis and rheumatoid arthritis patients: Diagnostic and prognostic value
- Immunomodulatory effects of novel nano micelle based curcumin in rheumatoid arthritis patients: A double blind randomized clinical trial
- Letter to the Editor
- McArdle’s disease presents with multiple large vessel lesions
- Images
- “Bouncing temporomandibular joint”: Multiple temporomandibular lesions in a patient with rheumatoid arthritis
Artikel in diesem Heft
- Editorial
- Enhancing standardized diagnosis and treatment to improve the prognosis of patients with rheumatoid arthritis in China
- The potential for biosimilars to address unmet clinical need — Is the developed world missing out?
- Guideline
- Chinese guidelines for the diagnosis and treatment of rheumatoid arthritis: 2024 update
- Review
- Therapeutic potential of CD20/CD3 bispecific antibodies in the treatment of autoimmune diseases
- Original Article
- 14-3-3 Eta protein as a novel biomarker in early detection of uveitis in Egyptian juvenile idiopathic arthritis and rheumatoid arthritis patients: Diagnostic and prognostic value
- Immunomodulatory effects of novel nano micelle based curcumin in rheumatoid arthritis patients: A double blind randomized clinical trial
- Letter to the Editor
- McArdle’s disease presents with multiple large vessel lesions
- Images
- “Bouncing temporomandibular joint”: Multiple temporomandibular lesions in a patient with rheumatoid arthritis

