Chinese guidelines for the diagnosis and treatment of rheumatoid arthritis: 2024 update
-
Xinping Tian
und Xiaofeng Zeng
Abstract
Rheumatoid arthritis (RA) is an autoimmune disease with destructive arthritis as its main clinical manifestation, which is a major cause of disability. It is very important to formulate and update a guideline for the diagnosis and treatment of RA that adhere to international guideline development standards and can be applied to clinical practice in China. This guideline is endorsed and developed by the National Clinical Research Center for Dermatologic and Immunologic Diseases, collaborated with Rheumatologists Branch of Chinese Medical Doctor Association, Rheumatology Rehabilitation Branch of Chinese Association of Rehabilitation Medicine, Rheumatology Branch of Chinese Research Hospital Association, and Rheumatology Branch of Beijing Association of Holistic Integrative Medicine, based on grading of recommendations assessment, development and evaluation (GRADE) and reporting items for practice guidelines in healthcare (RIGHT). Evidence-based recommendation were developed for 10 clinical scenario that are most relevant to Chinese rheumatologists, aiming to improve and standardize the diagnosis and treatment of RA in China, which may finally improve the quality of life and prognosis of patients.
Introduction
Rheumatoid arthritis (RA) is an auto-immune disease with destructive arthritis as its main clinical manifestation, which has a peak age of onset of 45 to 60 years but may occur at any age.[1] Epidemiological surveys showed that RA has a global incidence of 0.5%–1% [1] and an incidence of 0.42% in mainland China. Accordingly, there are currently more than 5 million RA patients in China,[2] with a male-to-female ratio of approximately 1: 4.[3,4] Although the etiology and pathogenesis of RA have not yet been fully elucidated, it has been clarified that the basic pathological changes of RA are synovitis and pannus formation, which gradually cause destruction of joint cartilages and bones, eventually leading to joint deformity and function loss.[5] RA is a highly disabling disease and an important cause of disability in Chinese population, and its disability rate increases as the course of disease extends.[6,7] In addition, RA may be complicated by pulmonary disorders, cardiovascular/cerebrovascular disorders, osteoporosis, and malignancies,8,9,10] which not only causes a decline in the patients’ physical function, quality of life, and social participation, but also imposes a huge economic burden on the patient families and the society.[11,12]
In recent years, multiple international rheumatology academic organizations including the American College of Rheumatology (ACR), European Alliance of Associations for Rheumatology (EULAR), and Asia Pacific League of Associations for Rheumatology (APLAR) have updated their guidelines or recommendations for the diagnosis and treatment of RA,[13,14,15] and the Chinese Rheumatology Association has also updated the guideline for the diagnosis and treatment of RA in 2018.[16] However, with the continuous update of therapeutic drugs for RA, more and more new drugs have been approved for treatment of RA in China, while epidemiological and clinical research evidence of RA in the Chinese population is rarely included in international RA guidelines, the clinical diagnosis and medication prescription habits of foreign rheumatologists are different from those of Chinese rheumatologists. In addition, the specialty setting of rheumatology in China and reimbursement policy are also significantly different from those of foreign hospitals and countries. Therefore, revising and updating the Chinese guideline for the clinical diagnosis and treatment of RA is very important in improving the ability of clinicians engaged in RA-related diagnosis and treatment in rheumatology, internal medicine, and orthopedics departments, especially physicians in primary healthcare institutions, to correctly diagnose and treat RA, strengthening patient education, and improving the level of RA diagnosis and treatment in China. Under this background, the National Clinical Research Center for Dermatologic and Immunologic Diseases (NCRC-DID) (Peking Union Medical College Hospital), as the organizer and initiator, in conjunction with the Rheumatologists Branch of Chinese Medical Doctor Association, Rheumatology Rehabilitation Branch of Chinese Association of Rehabilitation Medicine, Rheumatology Branch of Chinese Research Hospital Association, and Rheumatology Branch of Beijing Association of Holistic Integrative Medicine, in accordance with the methods and steps formulated by evidence-based clinical practice guidelines, based on the current best evidence, combined with the experience of clinicians and the preferences and values of Chinese patients, and balancing the benefits and risks of intervention measures, updated and revised the “2018 Chinese guideline for the diagnosis and treatment of rheumatoid arthritis”, forming the “2024 Guideline for the diagnosis and treatment of rheumatoid arthritis”.
Method
Guidelines sponsors: This guideline is endorsed and developed by the NCRC-DID (Peking Union Medical College Hospital). The development of this guideline was launched on 09 Jun 2023 and finalized on 07 Mar 2024.
Guideline development group: A collaborative and multidisciplinary team was meticulously assembled for the purpose of developing this guideline and experts in the panel are mainly from the Department of Rheumatology and Immunology and Evidence-Based Medicine. According to the work they contributed to, they are divided into the guideline expert panel and evidence evaluation group. The guideline expert panel participates in Delphi consensus, is mainly responsible for giving suggestions and comments and reviewing the drafts. The role of the evidence evaluation group includes conducting comprehensive evidence retrieval, evaluation, grading, summarizing recommendations. The drafts are composited by some of the expert panel jointly worked with evidence evaluation group. All the members of the development group completed a mandatory Conflict of Interest Disclosure form, thereby affirming the absence of conflicts of interest pertaining to this guideline.
Guidelines registration and plan writing: This guideline was registered on the Practice guideline REgistration for transPAREncy (PREPARE-2023CN490) on 12 Jul 2023. This guideline is an update of 2018 Chinese Guidelines[17] for the Diagnosis and Treatment of Rheumatoid Arthritis. The updates of this guideline strictly follows the guidance for updating clinical practice guidelines,[16,18] the World Health Organization (WHO) handbook for guideline development in 2014[19] and Guiding Principles for Formulating/Revising Clinical Diagnosis and Treatment Guideline in China (2022 Edition),[20] and refers to the items from Reporting Items for Practice Guideline in Healthcare (RIGHT)[21] and Reporting Items for Updated Clinical Guidelines.[22]
Guideline users and target population: The intended users of this guideline are rheumatologists, orthopaedic surgeons, general practitioners, clinical pharmacists, radiologists and healthcare professionals that may engage in the treatment and management of RA. The guideline primarily targets patients with RA.
Selection and determination of clinical questions: Based on the clinical questions in the 2018 Chinese guidelines for the diagnosis and treatment of rheumatoid arthritis,[16] 67 experts were involved to collect and expand the questions again. After discussion by the expert panel, a total of 10 key clinical questions were selected for this guideline.
Evidence retrieval: The Evidence Evaluation Group meticulously deconstructed the 10 identified clinical questions into their respective Population, Intervention, Comparison, and Outcome (PICO) components before embarking on an extensive search process. (1) Multiple comprehensive databases were searched, including Pubmed, Cochrane Library, China National Knowledge Infrastructure (CNKI) and Sinomed. The research type mainly included systematic review or meta-analysis, network meta-analysis, randomized controlled trial (RCT), cohort study, case-control study, case series, epidemiological surveys and other original studies, which were retrieved from January 1, 2018 to December 31, 2023. (2) search was performed on RA-related guidelines and consensuses via British National Institute for Health and Clinical Excellence (NICE), ACR, EULAR and APLAR and other official websites, as well as MEDLINE and China National Knowledge Infrastructure database. (3) Google Scholar and other websites were searched for supplementation.
Evaluation and grading of evidence: The evidence evaluation group adopted a measurement tool to assess systematic reviews (AMSTAR)[23] for the risk of bias assessment of included systematic reviews, meta-analyses and network meta-analyses., Cochrane tool risk of bias (ROB, for RCT),[24] quality assessment of diagnostic accuracy studies (QUADAS-2, for diagnostic accuracy studies),[25] Newcastle-Ottawa Scale (NOS, for observational studies) were adopted for assessing methodological quality of original studies of corresponding types,[26] respectively. Two investigators independently performed the assessments, and any discrepancies were resolved through discussion or by consulting a third investigator. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was utilized to grade the evidence and formulaterecommendations (Table 1).[27,28,29,30]
Table 1Grading of Recommendations Assessment, Development and Evaluation (GRADE)
GRADE rating Description Quality of evidence High (A) The authors have a lot of confidence that the true effect is similar to the estimated effect Medium (B) The authors believe that the true effect is probably close to the estimated effect Low (C) The true effect might be markedly different from the estimated effect Very Low (D) The true effect is probably markedly different from the estimated effect Strength of recommendation Strong (1) The desirable effects of an intervention clearly outweigh the undesirable effects, or clearly do not Weak (2) The trade-offs are less certain—either because of low quality evidence or because evidence suggests that desirable and undesirable effects are closely balanced Formation of recommendations: The recommendations were formulated by the Expert Panel based on the evidence summarized by the evidence evaluation group. The preferences of Chinese patients, as well as the costs and benefits of the interventions, were taken into consideration. Two rounds of Delphi recommendations surveys were conducted on Jan 19, 2024 and Jan 29, 2024. A total of 116 feedback suggestions were received. Then consensus was reached and further modification to the draft was made based on the expert panel discussions. The draft of the guidelines was finalized after the final meeting for discussion held on 07 Mar 2024.
Update of guidelines: A plan for proactive approach to guideline updates is made with a timeframe of 5 years for revision. The updates will adhere to international guideline update requirements and guideline.
Recommendations
Recommendation 1: Early diagnosis has a significant impact on the treatment and prognosis of RA, so clinicians need to make the diagnosis promptly based on patient’s clinical presentations, laboratory tests results, and imaging examinations (1A). The 1987 ACR and 2010 ACR/ EULAR classification criteria for RA are recommended as the reference for RA diagnosis (2B)
Early diagnosis should be based on patient’s clinical presentations, results of laboratory tests and imaging examinations. Emerging evidence have shown that early diagnosis and treatment can reduce the incidence of joint damage and disability and ultimately improve prognosis.[31,32,33,34] Currently, there are 2 international classification criteria that can be used to help diagnosing RA. There are some limitations of the 1987 ACR classification criteria for identifying early RA.[35,36,37] The 2010 ACR/EULAR RA classification criteria can identify early RA patients among inflammatory arthritis patients with synovitis. 2010 ACR/EULAR RA classification criteria can assure patients to be diagnosed, so can be treated with disease-modifying anti-rheumatic drugs (DMARDs) at early stage, which may delay disease progression. Numerous studies have shown that the 2010 ACR/EULAR classification criteria have a higher diagnostic sensitivity for early RA compared with the 1987 ACR classification criteria (72.3% vs. 39.1%) especially for elderly patients.[34,38,39] However, 2010 ACR/ EULAR classification criteria has been shown to have a lower diagnostic sensitivity for seronegative RA patients,[40,41] who are negative for both rheumatoid factor (RF) and anti-citrullinated peptide antibodies (ACPA) while imaging examinations such as musculoskeletal ultrasound or magnetic resonance imaging (MRI) can assist in the diagnosis of sero-negative RA. The specificity of the 2010 classification criteria was lower than that of the 1987 criteria (83.2% compared to 92.4% in the 1987 criteria), especially in elderly patients.[39] If the 2010 classification criteria are applied to all patients with arthralgia, some patients with nonspecific arthritis may be misdiagnosed as RA, while the 1987 criteria have a better predictive value for bone erosion.[34]
In summary, the diagnosis of RA should be based on the combination of the clinical manifestations, laboratory and imaging examinations. The classification criteria in 1987 and 2010 have their own advantages in the diagnosis of RA. Clinicians can refer to both criteria as references to make accurate diagnosis of RA based on specific characteristics of Chinese RA patients. Early diagnosis of RA facilitates early treatment and delays disease progression.
Recommendation 2: We recommend that clinicians select the most suitable imaging modalities such as X-rays, ultrasound, computed tomography (CT), and MRI (2B) when conditions permit, based on the signs and symptoms of the patient (2B)
Imaging examinations are effective tools to assist clinicians to make the diagnosis of RA. The value for diagnosis and disease monitoring as well as the advantages of various imaging modalities are listed in Table 2. Evidence-based recommendations for selecting imaging modalities for RA diagnosis were issued in both 2013 EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis and 2018 Chinese guidelines for the diagnosis and treatment of rheumatoid arthritis.[16,42] In addition, there are considerable differences in the availability of imaging equipment and technology in different regions of the country, therefore, clinicians should choose the most suitable diagnostic imaging modalities available locally to assist in making the diagnosis.[42,43,44,45,46,47]
The value of imaging modalities in the diagnostics, follow up, and monitoring of RA
| Image modalities | Applied situations | Advantages | Disadvantages |
|---|---|---|---|
| X-Ray |
|
|
|
| Ultrasound |
|
|
|
| CT | CT is valuable particularly for large joint lesions and lung disease, but CT cannot detect active inflammation in synovitis, tenosynovitis, etc.[42,45] |
|
|
| MRI |
|
|
|
| Special imaging modalities | It can not only assess inflammation of multiple joints throughout the body, but can also semi-quantify the severity of inflammation. |
|
RA, rheumatoid arthritis; FDG, fluoro-2-deoxy-D-glucose; PET, positron emission tomography; CT, computed tomography; MRI, magnetic resonance imaging.
Recommendation 3: The principles of management of RA are early, standardized treatment, regular monitoring and follow up (1A); the goal of RA treatment is to achieve disease remission or low disease activity, that is, to treat the disease to the target. The overall goal of treatment is to control the disease activity, reduce disability, and improve patients’ quality of life (1B)
The joint lesions of RA are caused by synovial inflammation caused by inflammatory cell infiltration and release of inflammatory factors.[48] To inhibit the production of cytokines and their effects as early as possible can effectively prevent or minimize the destructions in joint synovium and cartilage.[49] Therefore, timely treatment should be carried out as soon as the diagnosis is made. Indeed, studies have shown that irregular use of DMARDs is one of the independent risk factors for joint function limitation in RA patients.[6]
Although RA is not curable, the treat-to-target strategy is effective in alleviating symptoms and controlling the disease progression. Treat-to-target is referred to treat to achieve clinical remission or low disease activity.[50] Clinical remission is currently defined as follows: 28 joint disease activity (DAS28) ≤ 2.6, or clinical disease activity index (CDAI) ≤ 2.8, or simplified disease activity index (SDAI) ≤ 3.3. Low disease activity can be the alternative treatment target, i.e., DAS28 ≤ 3.2, CDAI ≤ 10 or SDAI ≤ 11. However, it should be noted that there are limitations in the disease activity evaluation tools and studies have shown that RA patients with swollen joints can still suffer further joint damage even when their DAS28 score is less than 2.6.[51] In 2011, the ACR and EULAR proposed Boolean remission criteria, which included tender joint count, swollen joint count, C-reactive protein (CRP) level, and patient global assessment were all ≤ 1.[52] Due to its high specificity and the ease of implementation, the criteria has been gradually adopted in clinical practice. Nevertheless, remission rate based on Boolean 1.0 criterion is low.[53] Studies have shown that the severity of the disease and synovitis may be overestimated when using Boolean 1.0 criteria.[54,55] Therefore, the ACR and EULAR updated Boolean response criteria (Boolean 2.0 criteria) in 2023 with a change in patient global assessment (PtGA) threshold from ≤ 1 to ≤ 2.[56] In addition, it should be noted that the efficacy of biological DMARDs (bDMARDs) or targeted synthetic DMARDs (tsDMARDs) may be overestimated when evaluating with composite measures (e.g., DAS28 or SDAI) including acute phase reactants.[57] Clinicians should choose the appropriate evaluation criteria according to their real practice situation.
Recommendation 4: For patients who are treatment naïveor who do not achieve the treatment target, we recommend to assess their disease activity once every 1–3 months (2B); For patients who have reached the treatment target, we recommend to monitor their disease activity once every 3–6 months (2B)
A systematic review in 2019 comprehensively analyzed 22 guidelines on the treatment of RA, of which 18 recommend regular assessments using a variety of clinical assessments.[58] A cohort study have found that every-3-month assessment of disease activity and following a treat-to-target strategy lead to higher rates of remission in patients with RA.[59] In a RCT, monitoring and adjusting the medication regimen monthly resulted in better treatment response compared to the regimen done once every 3 months.[60] For RA patients on initial therapy, we recommend to assess their disease activity monthly, taking into account the onset time of effect of DMARDs and the adverse reactions; the monitoring frequency for patients who have reached the therapeutic target can be adjusted to be once every 3–6 months.
Recommendation 5: The choice of treatment should be based on a comprehensive consideration to the disease activity and poor prognostic factors, as well as extra-articular manifestations and comorbidities (1B)
Evaluation of disease activity and poor prognostic factors can provide important clues to clinicians to adjust treatment plans and select appropriate medications. As mentioned above, DAS28, SDAI and CDAI, which are composite criteria composed of swollen joint count, tender joint count, erythrocyte sedimentation rate (ESR), and CRP level, can reflect the disease activity accurately so to provide basis for setting treatment targets and adjustment of treatment regimen. In addition, multiple prognostic studies and predictive models have shown that RF and ACPA are predictive factors for joint damage progression in addition to disease activity[61,62,63]; however, it should be noted that these autoantibodies do not directly correlate with disease activity, and the reduction in RF and ACPA titer shall not be chosen as a treat target. Disease activity and poor prognostic factors can help physicians to select the optimal treatment regimen.
Furthermore, RA patients, especially patients who have a long-term disease course and are not well controlled, may be at the risk of extra-articular manifestations, including rheumatoid nodules, interstitial lung disease, pleurisy, pericarditis, vasculitis, peripheral neuropathy, keratitis, scleritis, and Felty’s syndrome.[8] RA patients with extra-articular manifestations will develop more complications and carry poorer prognosis, especially when complicated with severe interstitial lung disease.[8,64,65,66]
Studies have shown that compared with the general population, RA patients have increased risks of cardiovascular/ cerebrovascular disorders,[67,68,69] osteoporosis and fragility fracture,[70] sarcopenia,[71,72] malignancy,[73] and tuberculosis. [74] These comorbidities will also cause an adverse impact on disease activity, joint damage progression, and treatment regimen of RA patients.[8,75,76]
Therefore, clinicians should evaluate every patient’s condition comprehensively and monitor the disease activity, poor prognostic factors, extra-articular manifestations, and comorbidities regularly, in order to develop the most appropriate treatment regimen and adjust the treatment plans accordingly.
Recommendation 6: Once RA is diagnosed, conventional synthetic DMARD therapy should be initiated as early as possible (1A); we recommend methotrexate (MTX) monotherapy as the first-line therapy, but other conventional synthetic DMARDs should be considered when MTX is contraindicated or not tolerated (1B)
Once RA is diagnosed, conventional synthetic DMARD therapy should be initiated as early as possible, which will help alleviate clinical symptoms, delay radiographic progression, and improve prognosis. At present, MTX monotherapy is recommended as the first-line therapy by international guidelines.[13,14,15] MTX is usually given at an oral dosage of 7.5 to 20 mg/week and the dose should be adjusted timely according to disease activity, treatment response, and adverse reactions.[77,78]. Folic acid supplementation at 5 mg/week is recommended for patients receiving MTX to alleviate adverse reactions.[79,80] Sulfasalazine or leflunomide is recommended to use in the patients who are contraindicated or intolerant to MTX.[14,81,82,83,84] The recommended doses for sulfasalazine is 3 g per day, and the dosage of leflunomide is 20 mg per day. The mechanism of action, dosage, and adverse reactions of conventional synthetic DMARDs (csDMARDs) for the treatment of RA can be referred to Table 3.
csDMARDs in the treatment of RA
| Drug | Mechanism of Action | Route of administration | Usual dose | Common adverse reactions |
|---|---|---|---|---|
| Methotrexate | Inhibition of folate metabolism | Oral, intramuscular, and intravenous | 7.5–20 mg/ week | Gastrointestinal discomfort, liver function damage (elevated liver enzymes), stomatitis, alopecia, skin rash, bone marrow suppression can happen occasionally, drug-induced pneumonitis was reported but very rare |
| Sulfasalazine | The metabolite 5-aminosalicylic acid inhibits prostaglandin, leukotriene synthesis and neutrophil function | Oral | 2 ~ 6 g/day, divided into 2 ~ 4 times | Allergic reactions (should not be used in patients allergic to sulfonamides), and gastrointestinal reactions happen occssionally; bone marrow suppression was reported |
| Leflunomide | Inhibition of pyrimidine synthesis | Oral | 10–20 mg/ day as a single dose | Liver function abnormality as elevated liver enzymes, gastrointestinal discomfort, alopecia, skin rash can happen occasionally, drug-induced pneumonitis are rare |
| Hydroxychloroquine | Stabilizes lysosomal membranes, inhibits multiple enzyme activities, inhibits prostaglandin and interleukin 1 synthesis, and inhibits neutrophils function | Oral | 0.2–0.4 g/ day 1–3 times | Allergic reaction, dye fundus lesion |
| Tripterygium wilfordii Hook II | Not completely eludicated. But may be related with multiple immunosuppressive and antiinflammatory effects | Oral | 30–60 mg/ day in 2–3 divided doses | Gonadotoxicity, abnormal liver function tests, gastrointestinal discomfort, skin rash, bone marrow suppression occur occasionally |
| Iguratimod | Inhibits nuclear factor kappa B (NF-κB) activity, inhibits immunoglobulin synthesis and cyclooxygenase-2 (COX-2) | Oral | 50 mg/day in 2 divided doses | Abnormal liver function tests, gastrointestinal discomfort, skin rash happen occasionally |
RA, rheumatoid arthritis; NF-κB, nuclear factor kappa B.
There is currently insufficient evidence to support the use of bD-MARDs or tsDMARDs as the first-line therapy. According to existing evidence, the combination of bDMARDs/tsDMARDs are mostly prescribed to patients with poor response or intolerance to csDMARDs. Studies have shown that the combination of MTX with biologics is more effective than MTX alone in MTX - naive RA patients. However, it is lack of evidence that biologic monotherapy is superior to MTX monotherapy.[85,86] We still recommend MTX as the preferred csDMARD for the first-line therapy of treatment-naive RA patients in China taking efficacy, adverse reactions, economy, and accessibility of the medications as well as physicians’ practice experience into consideration.
Recommendation 7: Short-term glucocorticoid (GCs) should be considered based on disease activity when initiating or changing csDMARDs (2B). Adverse reactions should be closely monitored during GCs treatment. Monotherapy or long-term, high-dose glucocorticoid use is strongly not recommended (1A)
GCs has strong anti-inflammatory effects and can be used to inhibit acute inflammation in RA. Numerous studies have suggested that the combination of a csDMARD with short-term, low-dose GCs can relieve pain, shorten the duration of morning stiffness, reduce SJC and TJC and improve physical function, quality of life, patient global assessment (PGA) and PtGA in patients with active RA.[87,88,89] GCs could not prevent or delay joint erosion in RA and thus, should not be used alone. Moreover, long-term or high-dose GC therapy is not recommended due to increased risks of various complications such as infections, cardiovascular/cerebrovascular disorders and osteoporosis.[90,91,92] The dose of GCs should not be higher than prednisone 10 mg/day or equivalent. GCs should be tapered and discontinued as early as possible, usually within 6 months. There is no need to continue GCs use in patients on bMDARDs/tsDMARDs. EULAR recommends that GCs should be discontinued as quickly as possible after initiation of bDMARDs/tsDMARDs.[14] Non-steroidal anti-inflammatory drugs (NSAIDs) can be used to relieve pain in RA patients but NSAIDs should be used with caution due to the risk of cardiovascular and gastrointestinal adverse reactions,[90,93] especially in elderly patients and patients with related underlying diseases.
Recommendation 8: When a single conventional synthetic DMARD (csDMARDs) treatment can’t reach clinical improvement within 3 months or can’t achieve treatment target within 6 months, we recommend to switch to another csDAMRD or combine with another DMARDs (2B); or combine one DMARD with one of the biological/ tsDMARDs (2B);
After being treated with methotrexate, or leflunomide or sulfasalazine monotherapy, the patients can’t reach the treatment target, the current therapy should be adjusted timely. Inadequate response is generally defined as failure to achieve remission or low disease activity within 3 months with less than 50% improvement in composite disease activity criteria, or failure to achieve remission or low disease activity after 6-month treatment. For second-line therapy, there is very limited evidence to clarify the advantages and disadvantages between switching to another csDMARD, combination of csDMARDs and combining one csDMARD with one of the bDMARDs or one of the small molecule tsDMARDs. Limited RCT evidence showed non-significant difference between these treatment regimens.[94] Although EULAR and ACR guidelines conditionally recommend the combination a bDMARD or tsDMARD prior to csDMARD combination, this recommendation is made mainly based on considerations of quicker efficacy onset and drug retention rate, however, the evidence level is low.[13,14] We do not differentiate the priority of these two regimens due to the low evidence level and consideration of China’s national economic conditions and comorbidities, such as viral hepatitis and tuberculosis infection. In addition, this guideline does not specially distinguish the treatment regimens according to the presence of poor prognostic factors or not, though the factors should be considered by clinicians when they formulating treatment regimens. However, the existing evidence suggests no precedence over each other for adjusting csD-MARDs or combining bDMARDs/tsDMARDs in second-line treatment selection of RA based on the poor prognostic factors only.[13,95]
If csDMARDs combination therapy is adopted, either two of MTX, sulfasalazine, and leflunomide can be combined; however, if MTX is used in combination with leflunomide, attention should be paid to liver function impairment[96,97] and hematological adverse reactions.[98] Hydroxychloroquine is commonly used in combination regimens due to lower structural efficacy but can also be used alone in patients with low disease activity at the early stage of the disease.[13,14,99] In addition, hydroxychloroquine can improve patients’ glucose and fat metabolism, making itself suitable for patients with concomitant cardiovascular diseases.[100]
The efficacy of the herbal medicine Tripterygium wilfordii Hook II in the treatment of RA has been recognized. Studies have shown that the efficacy of Tripterygium wilfordii Hook II monotherapy is not inferior to MTX monotherapy in the treatment of RA, and Tripterygium wilfordii Hook II in combination with MTX or tumor necrosis factor α (TNFα) inhibitor also has good efficacy and safety.[101,102] Tripterygium wilfordii Hook II can be used as csDMARD in addition to MTX, sulfasalazine, and leflunomide, but they are contraindicated in patients who are preparing for pregnancy, in pregnancy, in lactation, or with fertility requirements because of their genital toxicity. Although a few reports have shown better efficacy of total glucosides of paeony in combination with csDMARD,[103,104] its efficacy in RA treatment needs further investigation.
Iguratimod, an anti-rheumatic drug developed in China with characteristics of csDMARDs, has been used in the treatment of RA in China and some Asian countries. There is evidence that iguratimod plus MTX is superior to MTX alone and has a good safety profile,[105,106,107] so it can be used as a second-line therapy for RA.
Tumor necrosis factor-alpha (TNF-a) inhibitors are the most widely used biological DMARDs in treating RA with abundant evidence. Several TNFα inhibitors have been launched in China including the monoclonal antibody drugs adalimumab, infliximab, golimumab and certolizumab, as well as the receptor fusion protein drug etanercept, all of which have been demonstrated to be efficacious and safe in the treatment of RA with sufficient evidence.[108,109,110,111,112,113,114,115,116,117,118,119] We recommend combining TNFα inhibitors with csDMARD.[120,121,122,123] For patients receiving treatment with TNFα inhibitors, special attention should be paid to the risk of hepatitis virus and Mycobacterium tuberculosis infections or reactivation of pre-existing infections. Patients should be screened prior to the treatment with TNFα inhibitors and be monitored regularly during treatment.[124,125,126] Pre-treatment screening should include hepatitis B virus (HBV) and hepatitis C virus (HCV) serology tests (including HBV antigen and anti-HBV antibodies, anti-HCV antibody, and viral load test should be considered if necessary); Purified protein derivative (PPD) skin test and/or interferon (IFN)-γ release assay (T-SPOT. TB or QuantiFERON-TB GOLD) should be selected according to local accessibility, chest radiograph (X-ray or CT should be chosen according to local medical technological availability and patient’s conditions).[126] For patients with hepatitis virus infection and latent mycobacterium tuberculosis infection, prophylaxis treatment should be administered. The prophylaxis treatment should follow the recommendations from infectious disease experts and be adjusted based on the patient’s situation.[15,126]
Evidence have proven the efficacy and safety of tocilizumab, a recombinant humanized IgG1 monoclonal antibody that targeted interleukin-6 (IL-6) receptors, in the treatment of RA. Recent research have shown that tocilizumab monotherapy can also achieve good clinical efficacy.[127,128,129,130,131] For RA patients who are intolerant to conventional synthetic DMARDs, tocilizumab monotherapy should be considered.
Abatacept is a T-cell co-stimulatory inhibitor that inhibits T cell activation by specifically interfering with CD28 interaction with CD80/86.[132] Many evidence have supported the efficacy and safety of abatacept in treating RA[133,134,135] and it can be used as one of the bDMARD medications.
JAK inhibitors are a class of tsDMARDs targeted at the JAK-STAT signaling pathway. At present, JAK inhibitors approved in China include tofacitinib, baricitinib, and upadacitinib. Current research evidence have shown that JAK inhibitors have good efficacy and safety in RA,[136,137,138,139,140,141,142,143,144] but it should be noted that such drugs may increase the risk of major cardiovascular events (MACEs), malignancies and venous thrombosis.[145,146,147,148] The following risk factors for MACE and malignancies must be taken into consideration when prescribing a JAK-inhibitor: age over 65 years, history of current or past smoking, other cardiovascular risk factors (such as diabetes, obesity, hypertension), other risk factors for malignancy (current or previous history of malignancy), risk factors for thromboembolic events (history of myocardial infarction or heart failure, cancer, inherited coagulation disorders or a history of thrombosis, as well as patients taking combined female hormonal contraceptives or hormone replacement therapy, undergoing major surgery or immobile).[14,149] These risk factors should be adequately assessed prior to treatment with JAK inhibitors and be monitored regularly.
Rituximab, an anti-CD20 monoclonal antibody, has been shown to be effective in the treatment of RA.[150,151,152] It may serve as an alternative for patients with RA who show inadequate response or intolerance to biologics and JAK inhibitors.
Based on the existing evidence, no precedence over each other for TNF-a inhibitors, tocilizumab and tofacitinib in the treatment of RA.[153] If a patient failed bDMARD or tsDMARD, treatment with another bDMARD or tsDMARD should be considered. Switching to another TNFα inhibitor, tocilizumab, rituximab, or JAK inhibitor have been proved to be effective after failure of one of the TNFα inhibitors.[154,155,156,157,158] However, the efficacy of switching to another JAK inhibitor after failure of one JAK inhibitor is uncertain. For patients who failed tocilizumab or one JAK inhibitor may be treated with an agent with different mode of action. bDMARDs and tsDMARDs are associated with increased risk of infections compared with csDMARDs.[159,160,161] Therefore, for all patients receiving bD-MARDs or tsDMARDs, special attention should be paid not only to the aforementioned adverse events related to TNFα inhibitors and JAK inhibitors, but also to the risk of various infections, especially respiratory tract infections (including influenza virus, Streptococcus pneumoniae, etc.) and herpes zoster. Vaccination should be considered for patients without contraindications.[126,162,163]
Biosimilars have the same mechanism as original biologics with lower cost, so with increased accessibility to biologics for patients.[164] A variety of biosimilars have got approved in China. According to the results of a meta-analysis including 27 RCTs, the efficacy and safety of the approved biosimilars are not significantly different from those of the originals in the treatment of RA.[165] The efficacy and safety of biosimilars have been endorsed by 2021 American College of Rheumatology guideline for the Treatment of RA[13] and EULAR recommendations for the management of RA with synthetic and biological DMARDs: 2022 update.[14]
Intra-articular injection of GCs or etanercept can improve symptoms in patients with single affected joints,[166] however, overuse of this procedure should be avoided. Intra-articular injection should be applied with caution to the risk of secondary infections related to joint cavity puncture. Studies have demonstrated that technetium(99Tc) methylenediphosphonate (99Tc-MDP) may be beneficial in the treatment of RA,[167,168] but further research is needed.
Most RA patients can reach disease remission or low disease activity through standard care. However, some patients still have active disease even after being treated with standard treatment. These patients are defined as “refractory or difficult-to-treat (D2T) RA”, accounting for 5%-20% of total RA patients.[169,170] The following three criteria were agreed by all Task Force members of EULAR as mandatory elements of the definition of difficult-to-treat RA: (1) Treatment according to European League Against Rheumatism (EULAR) recommendation and failure of ≥2 biological disease-modifying antirheumatic drugs (DMARDs) /targeted synthetic DMARDs (with different mechanisms of action) after failing conventional synthetic DMARD therapy (unless contraindicated); (2) presence of at least one of the following: a, at least moderate disease activity (e.g., DAS28-ESR > 3.2 or CADI > 10); b, Signs (including acute phase reactants and imaging) and/or symptoms suggestive of active disease (joint related or other); c, inability to taper GCs treatment ( < 7.5 mg/day prednisone or equivalent); d, rapid radiographic progression (with or without signs of active disease); e, Well-controlled disease according to above standards, but still having RA symptoms that are causing a reduction in quality of life; and (3) the management of signs and/or symptoms is perceived as problematic by the rheumatologist and/or the patient.[169,171] The factors contributing to D2T include drug ineffectiveness or presence of factors for adverse drug reactions (e.g., smoking, obesity, patient’s genetic and immunologic background), as well as comorbidities and other factors affecting the clinical outcome (e.g., interstitial pneumonia and fibromyalgia).[172] Multivariate analysis identified high RF levels, DAS28-ESR, and coexisting pulmonary disease as predictive risk factors of D2T RA.[170] For such patients, the factors contributing to D2T should be fully evaluated, and individualized treatment regimens should be formulated.[173,174]
Recommendation 9: Tapering of DMARDs (bDMARDs/ tsDMARDs or csDMARDs) could be considered if the patient reached disease remission for at least 6 months. Close monitoring of disease activity during tapering is warranted given the potential for disease flare. (2C). For patients treated with DMARDs combination therapy, if the patient is in sustained remission after tapering of one DMARD, then this drug could be discontinuation (2C)
Based on the current evidence, dose reduction of DMARDs can be considered for RA patients in sustained remission, but this is only considered as an option rather than a recommendation, and patients with dose reduction should be closely monitored.[13,14,175,176] Currently the specific duration of “sustained remission” is still inconclusive, and a systemic review shows that a 6-month remission period may be appropriate.[175] The 2021 ACR guidelines also recommend that tapering of DMARDs should only be started if a patient is in persistent stringent remission for at least 6 months to ensure stable disease control.[13] For patients receiving csDMARD + b/tsDMARD combination therapy, whether csDMARDs or b/ tsDMARDs should be tapered first is still under debate.[177,178,179] Considering that discontinuation of all DMARDs is associated with moderate to high risk of RA flare and potentially irreversible damages in most patients, we recommend that patients maintain at least 1 DMARD rather than discontinue all DMARDs.[180,181] It is still controversial whether DMARDs can be tapered in RA patients who achieve low disease activity without remission.
Recommendation 10: RA patients should be educated on the nature, course, treatment, and self-management of the disease and psychological support should also be provided (1A). Patients with RA should be advised to lifestyle modification, including smoking cessation, weight control, healthy diet and exercise (1A)
Patient education is essential for disease management, because it helps to improve the effectiveness of RA treatment. Clinicians should help patients to fully understand the nature and prognosis of RA, to help them to set up the confidence to receive standard treatment, and to remind them to be monitored and followed-up regularly and facilitate patients to take appropriate self-management measures.[182,183,184,185] Health education can also provide guidance on smoking cessation, weight control, healthy diet, and appropriate exercise, helping patients improve their lifestyle. Compared with the general population, RA patients have increased incidences of anxiety and depression, and RA patients with anxiety and/ or depression tend to have worse clinical outcomes.[186,187,188] Studies have shown that positive, effective cognitive intervention and psychological support are significantly helpful for relieving pain and improving physical function, mental health, and disease activity in RA patients.[189,190] Smoking is closely related to the onset and progression of RA, the efficacy of drug therapy, and risk of interstitial lung disease, cardiovascular diseases, osteoporosis, and tumors.[191,192,193] Thus all RA patients should quit smoking. Obese people have an increased risk of RA [194,195] and obesity have an adverse impact on the disease activity and adverse drug reactions of RA.[196,197] Weight control can help RA patients improve disease activity and prognosis.[198,199,200] A healthy diet helps reduce inflammation and alleviate symptoms in RA patients.[201,202,203,204,205] Appropriate exercise and physical therapy (e.g., aerobic exercise, resistance exercise, and functional exercise) can enhance the flexibility and stability of the joint and improve the patients’ symptoms, physical function, and quality of life.[206,207,208,209,210,211,212]
This guideline provides recommendations for important clinical issues on the diagnosis, evaluation, treatment, and follow-up of RA based on the existing evidence published domestic and abroad, as well as the disease characteristics and medical conditions of RA and the practical experience of rheumatologists in China. Rheumatologists and physicians in other medical specialty engaged in the diagnosis and treatment of RA should carry out standardized diagnosis and treatment referring to this guideline, so as to ensure the quality of treatment, improve the diagnosis and treatment of RA in China, and improve the prognosis of patients. However, in view of the individual differences in RA, it is necessary to fully consider patients’ specific situation and develop individualized diagnosis and treatment regimens through doctor-patient joint decision-making in clinical practice. In addition, the existing evidence is insufficient to provide clear answers to some important clinical issues in the diagnosis and treatment of RA, such as how to identify patients with poor response to csDMARDs, how to early identify D2T patients and provide effective treatment accordingly. Further studies are needed to further improve the treatment effect and prognosis of RA.
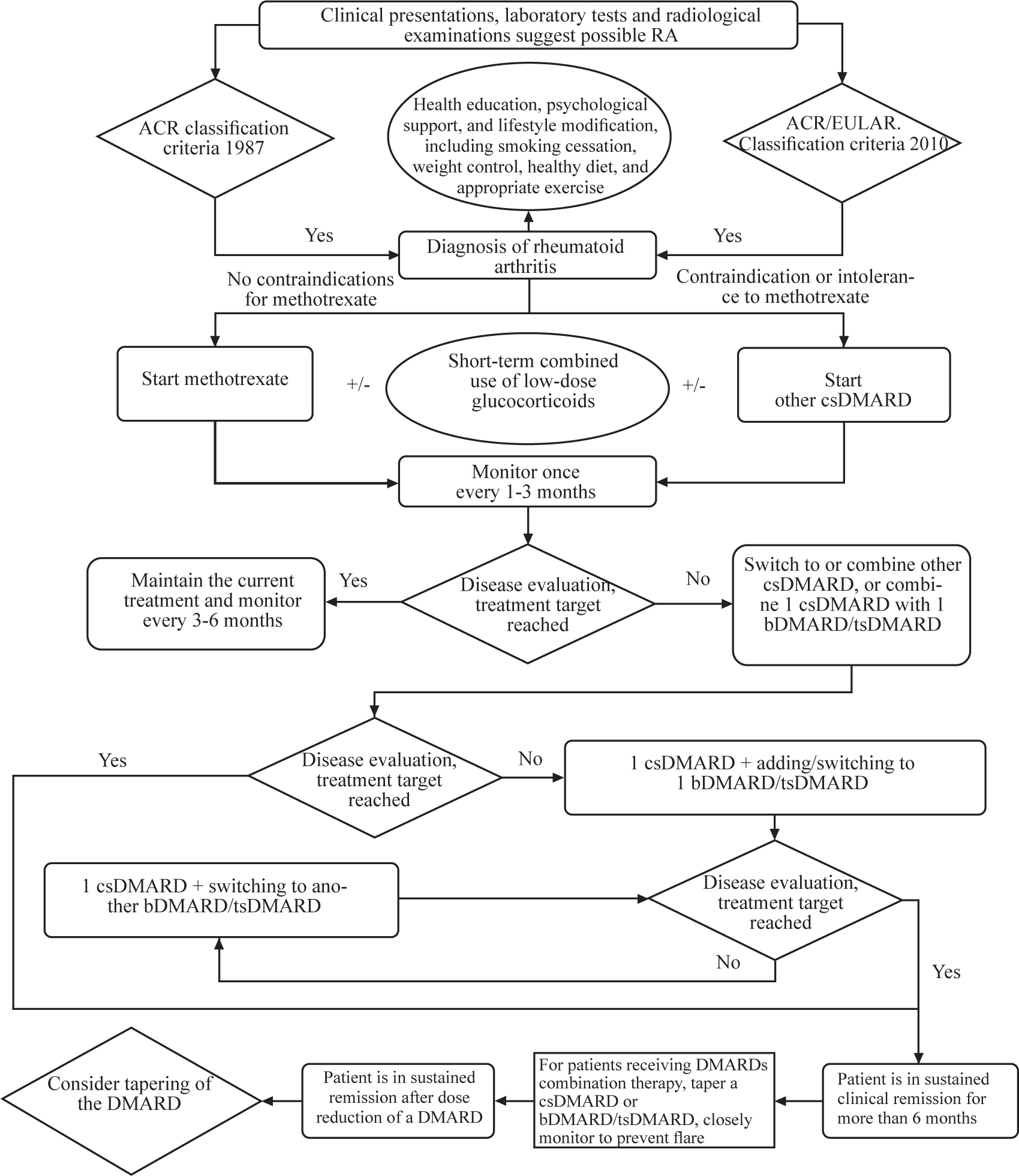
Recommended RA diagnosis and treatment process.
Methodology Panel
Yaolong Chen (Evidence-Based Social Science Research Centre, School of Public Health, Lanzhou University, Lanzhou University GRADE Center).
Drafting Group
Nan Jiang (Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Peking Union Medical College); Dagula (Department of Rheumatology, The Affiliated Hospital of Inner Mongolia Medical University); Shuang Liu (Department of Rheumatology and Immunology, First Affiliated Hospital of Kunming Medical University); Yaqian Liu (Department of Rheumatology and Immunology, the First Affiliated Hospital of Anhui Medical University); Jianda Ma (Department of Rheumatology and Immunology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University); Xiaofei Peng (Department of Rheumatology and Immunology.
The Second Xiangya Hospital of Central South University); Sun Yiduo (Department of Rheumatology and Immunology, The First Affiliated Hospital, Zhejiang University School of Medicine); Suli Wang (Department of Rheumatology and Immunology, Renji Hospital Affiliated to Shanghai Jiao Tong University School of Medicine).
Evidence Grading Panel
Xufei Luo (Evidence-Based Social Science Research Centre, School of Public Health, Lanzhou University, Lanzhou University GRADE Center); Ye Wang (Evidence-Based Social Science Research Centre, School of Public Health, Lanzhou University, Lanzhou University GRADE Center); Haodong Li (Evidence-Based Social Science Research Centre, School of Public Health, Lanzhou University, Lanzhou University GRADE Center); Renfeng Su (Evidence-Based Social Science Research Centre, School of Public Health, Lanzhou University, Lanzhou University GRADE Center).
Expert Panel
Guoqiang Chen (Department of Rheumatology and Immunology, The First People’s Hospital of Foshan); Yaolong Chen (Evidence-Based Social Science Research Centre, School of Public Health, Lanzhou University, Lanzhou University GRADE Center) ; Chen Zhen (Department of Rheumatology and Immunology, The Second Affiliated Hospital of Fujian Medical University); Lie Dai (Department of Rheumatology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University);; Feng Ding (Department of Rheumatology, Qilu Hospital of Shandong University); Xinwang Duan (Department of Rheumatology and Immunology, The Second Affiliated Hospital of Nanchang University); Yongfei Fang (Rheumatology and Immunology Department of Traditional Chinese Medicine, The Southwest Hospital of Army Medical University); Jieruo Gu (Department of Rheumatology and Immunology, the Third Affiliated Hospital, Sun Yat-Sen University); Dongyi He (Department of Rheumatology, Shanghai Guanghua Hospital of Integrated Traditional Chinese and Western Medicine); Lan He (Department of Rheumatology and Immunology, the First Affiliated Hospital of Xi’an Jiaotong University); Cibo Huang (Department of Rheumatology, South China Hospital, Health Science Center); Wenhui Huang (Department of Rheumatology and Immunology, Second Affiliated Hospital, Guangzhou Medical University); Lindi Jiang (Department of Rheumatology, Zhongshan Hospital, Fudan University); Zhenyu Jiang (Department of Rheumatology and Immunology, The First Hospital of Jilin University);); Caifeng Li (Department of Rheumatology, National Center for Children’s Health, Beijing Children’s Hospital, Capital Medical University); Fen Li (Department of Rheumatology and Immunology, The Second Xiangya Hospital of Central South University); Hongbin Li (Department of Rheumatology, The Affiliated Hospital of Inner Mongolia Medical University); Mengtao Li (Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College); Xiao Li (Department of Rheumatology, The Second Hospital of Shanxi Medical University); Xiaomei Li (Department of Rheumatology, The First Affiliated Hospital of University of Science and Technology of China); Xiaoxia Li (Department of Rheumatology and Allergy, Xuanwu Hospital, Capital Medical University); He Lin (Department of Rheumatology and Immunology, Fujian Provincial Hospital); Jin Lin (Department of Rheumatology, The First Affiliated Hospital, Zhejiang University School of Medicine); Dongzhou Liu (Department of Rheumatology and Immunology, Shenzhen People’s Hospital); Shengyun Liu (Department of Rheumatology and Immunology, The First Affiliated Hospital of Zhengzhou University); Yi Liu (Department of Rheumatology and Immunology, West China Hospital, Sichuan University); Hui Luo (Department of Rheumatology and Immunology, West China Hospital, Sichuan University); Liangjing Lu (Department of Rheumatology and Immunology, Renji Hospital Affiliated to Shanghai Jiao Tong University School of Medicine); Li Ma (Department of Rheumatology, China Japan Friendship Hospital);; Yifang Mei (Department of Rheumatology and Immunology, Shenzhen Third People’s Hospital); Haili Shen (Department of Rheumatology, Lanzhou University Second Hospital); Zongwen Shuai (Department of Rheumatology and Immunology, The First Affiliated Hospital of Anhui Medical University); Hui Song (Department of Rheumatology, Jishuitan Hospital, Beijing); Lingyun Sun (Department of Rheumatology, Nanjing Drum Tower Hospital of Nanjing University Medical School); Yin Su (Department of Rheumatology, Peking University People’s Hospital); Xinping Tian (Department of Rheumatology and Immunology, Peking Union Medical College Hospital); Caihong Wang (Department of Rheumatology and Immunology, The Second Hospital of Shanxi Medical University); Guochun Wang (Department of Rheumatology, China Japan Friendship Hospital); Jibo Wang (Department of Rheumatology & Clinical Immunology, Affiliated Hospital of Qingdao University);; Qian Wang (Department of Rheumatology and Immunology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences); Yongfu Wang (Department of Rheumatology, the First Affiliated Hospital of Baotou Medical College);; Youlian Wang (Department of Rheumatology and Immunology, Jiangxi Provincial People’s Hospital); Wei Wei (Department of Rheumatology and Immunology, Tianjin Medical University General Hospital); Huaxiang Wu (Department of Rheumatology and Immunology, the Second Affiliated Hospitalvof Zhejiang University, School of Medicine); Lijun Wu (Department of Rheumatology and Immunology, People’s Hospital of Xinjiang Uygur Autonomous Region);; Zhenbiao Wu (Department of Rheumatology and Immunology, Tangdu Hospital, the Second Affiliated Hospital of Air Force Medical University); Huji Xu (Department of Rheumatology, the Second Military Medical University Changzheng Hospital); Jian Xu (Department of Rheumatology and Immunology, First Affiliated Hospital of Kunming Medical University); Chengde Yang (Department of Rheumatology and Immunology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine); Min yang (Department of Rheumatology and Immunology, Nanfang Hospital, Southern Medical University); Pingting Yang (Department of Rheumatology and Immunology, The First Affiliated Hospital of China Medical University); Xiaofeng Zeng (Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College); Feng Zhan (Department of Rheumatology and Immunology, Hainan Provincial People’s Hospital); Fengxiao Zhang (Department of Rheumatology and Immunology, Hebei General Hospital); Miaojia Zhang (Department of Rheumatology, The First Affiliated Hospital of Nanjing Medical University); Wen Zhang (Department of Rheumatology and Immunology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences); Xiao Zhang (Department of Rheumatology and Immunology, Guangdong Provincial People’s Hospital); Xuewu Zhang (Department of Rheumatology and Immunology, Peking University People’s Hospital); Zhiyi Zhang (Department of Rheumatology and Immunology, The First Affiliated Hospital of Harbin Medical University); Zhuoli Zhang (Department of Rheumatology, the First Hospital of Peking University); Dongbao Zhao (Department of Rheumatology and Immunology, Changhai Hospital Affiliated to Naval Medical University); Yan Zhao (Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College); Yi Zheng (Department of Rheumatology and Immunology, Beijing Chaoyang Hospital Affiliated to Capital Medical University); Zhaohui Zheng (Department of Rheumatology and Immunology, First Affiliated Hospital of Air Force Medical University); Jing Zhu (Department of Rheumatology and Immunology, Sichuan Provincial People’s Hospital); Hejian Zou (Department of Rheumatology and Immunology, Huashan Hospital Affiliated to Fudan University).
Funding statement: Chinese National Key Technology R & D Program, Ministry of Science and Technology (2022YFC2504600), CAMS Innovation Fund for Medical Sciences (CIFMS) (2021-I2M-1-005, 2022-I2M-1-004, 2023-I2M-2-005), The Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2021-PT320-002, 2019-PT330-004), National High Level Hospital Clinical Research Funding (2022-PUMCH-B-013).
Acknowledgements
None.
-
Secondary Publication Declaration
This article was translated and published with permission from the Chinese language version first published by Chinese Journal of Internal Medicine in November, 2024.
-
Author contributions
Xinping Tia, Qian Wang and Nan Jiang-drraft preparation, Mengtao Li and Xiaofeng Zeng: conceptualization; Others: Reviewing and editing.
-
Informed consent
Not applicable.
-
Ethical approval
Not applicable.
-
Conflict of interest
Xiaofeng Zeng is the Editor-in-Chief of the journal; Xinping Tian is the Executive Editor-in-Chief; Mengtao Li and Yan Zhao are Associate Editors-in-Chief; Qian Wang, Yi Liu, and Huji Xu are an Editorial Board Member. The article was subjected to the standard procedures of the journal, with a review process independent of these editors and their research groups.
-
Data availability statement
No additional data is available.
References
[1] Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001.10.1038/nrdp.2018.1Suche in Google Scholar PubMed
[2] Zeng X, Zhu S, Tan A, et al. [A systematic evaluation of research on the disease burden and quality of life of rheumatoid arthritis in China.] Chin J Evid Based Med. 2013;13:300–307.Suche in Google Scholar
[3] Chen Yu, Mengtao Li, Xinwang Duan, et al. Chinese registry of rheumatoid arthritis (CREDIT): I. Introduction and prevalence of remission in Chinese patients with rheumatoid arthritis. Clin Exp Rheumatol. 2018;36:836–840.Suche in Google Scholar
[4] Jiang N, Li Q, Li H, et al. Chinese registry of rheumatoid arthritis (CREDIT) V: sex impacts rheumatoid arthritis in Chinese patients. Chin Med J (Engl). 2022;135:2210–2217.10.1097/CM9.0000000000002110Suche in Google Scholar PubMed PubMed Central
[5] Aletaha D, Smolen JS. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA. 2018;320:1360–1372.10.1001/jama.2018.13103Suche in Google Scholar PubMed
[6] Zhou Y, Wang X, An Y, et al. [A survey on disability and functional limitations in patients with multi center rheumatoid arthritis in China.] Chin J Rheumatol. 2013;17:526–532.Suche in Google Scholar
[7] Zhao S, Chen Y, Chen H. Sociodemographic factors associated with functional disability in outpatients with rheumatoid arthritis in Southwest China. Clin Rheumatol. 2015;34:845–851.10.1007/s10067-015-2896-zSuche in Google Scholar PubMed
[8] Figus FA, Piga M, Azzolin I, et al. Rheumatoid arthritis: Extra-articular manifestations and comorbidities. Autoimmun Rev. 2021;20:102776.10.1016/j.autrev.2021.102776Suche in Google Scholar PubMed
[9] Taylor PC, Atzeni F, Balsa A, et al. The Key Comorbidities in Patients with Rheumatoid Arthritis: A Narrative Review. J Clin Med. 2021;10:509.10.3390/jcm10030509Suche in Google Scholar PubMed PubMed Central
[10] Jin S, Li M, Fang Y, et al. Chinese Registry of rheumatoid arthritis (CREDIT): II. prevalence and risk factors of major comorbidities in Chinese patients with rheumatoid arthritis. Arthritis Res Ther. 2017;19:251.10.1186/s13075-017-1457-zSuche in Google Scholar PubMed PubMed Central
[11] GBD 2021 Rheumatoid Arthritis Collaborators. Global, regional, and national burden of rheumatoid arthritis, 1990–2020, and projections to 2050: a systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023;5:e594–e610.Suche in Google Scholar
[12] Li HB, Wu LJ, Jiang N, et al. Treatment satisfaction with rheumatoid arthritis in patients with different disease severity and financial burden: A subgroup analysis of a nationwide survey in China. Chin Med J (Engl). 2020;133:892–898.10.1097/CM9.0000000000000749Suche in Google Scholar PubMed PubMed Central
[13] Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2021;73:1108–1123.10.1002/art.41752Suche in Google Scholar PubMed
[14] Smolen JS, Landewé RBM, Bergstra SA, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. 2023;82:3–18.10.1136/ard-2022-223356corr1Suche in Google Scholar PubMed
[15] Lau CS, Chia F, Dans L, et al. 2018 update of the APLAR recommendations for treatment of rheumatoid arthritis. Int J Rheum Dis. 2019;22:357–375.10.1111/1756-185X.13513Suche in Google Scholar PubMed
[16] Rheuamtology Association of China Medical Association. Chiese guideline for the diagnosis and Treatment of Rheumatoid Arthritis: 2018. Chin J Intern Med. 2018;57:242-251 .Suche in Google Scholar
[17] Chen Y, Guyatt GH, Munn Z, et al. Clinical Practice Guidelines Registry: Toward Reducing Duplication, Improving Collaboration, and Increasing Transparency. Ann Intern Med. 2021;174:705–707.10.7326/M20-7884Suche in Google Scholar PubMed
[18] Vernooij RW, Sanabria AJ, Solà I, et al. Guidance for updating clinical practice guidelines: a systematic review of methodological handbooks. Implement Sci. 2014;9:3.10.1186/1748-5908-9-3Suche in Google Scholar PubMed PubMed Central
[19] World Health Organization. WHO handbook for guideline development. 2nd ed. Published December 2014. Available at: https://www.who.int/publications-detail-redirect/9789241548960. Accessed December 1, 2023.Suche in Google Scholar
[20] Chen Y, Yang K, Wang X, et al. [Guiding Principles for the Development/Revision of Clinical Diagnosis and Treatment Guidelines in China (2022 Edition)]. Chin Med J. 2022;102:697–703.Suche in Google Scholar
[21] Chen Y, Yang K, Marušic A, et al. A Reporting Tool for Practice Guidelines in Health Care: The RIGHT Statement. Ann Intern Med. 2017;166:128–132.10.7326/M16-1565Suche in Google Scholar PubMed
[22] Vernooij RW, Alonso-Coello P, Brouwers M, et al. Reporting Items for Updated Clinical Guidelines: Checklist for the Reporting of Updated Guidelines (CheckUp). PLoS Med. 2017;14:e1002207.10.1371/journal.pmed.1002207Suche in Google Scholar PubMed PubMed Central
[23] Shea BJ, Grimshaw JM, Wells GA, et al. Development of AM-STAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10.10.1186/1471-2288-7-10Suche in Google Scholar PubMed PubMed Central
[24] Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.10.1136/bmj.d5928Suche in Google Scholar PubMed PubMed Central
[25] Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536.10.7326/0003-4819-155-8-201110180-00009Suche in Google Scholar PubMed
[26] Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Published July 2014. Available at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed December 1, 2023.Suche in Google Scholar
[27] Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394.10.1016/j.jclinepi.2010.04.026Suche in Google Scholar PubMed
[28] Chen Y, Yao L, Norris S, et al. [The necessity and precautions of applying GRADE in system evaluation.] Chin J Evid Based Med. 2013;13:1401–1404.Suche in Google Scholar
[29] Yao L, Chen Y, Du L, Zeng X, et al. [Example analysis of the application of GRADE in the evaluation of diagnostic accuracy test systems.] Chin J Evid Based Med. 2014;14:1407–1412.Suche in Google Scholar
[30] Chen Y, Yao L, Du L, et al. [The principles, methods, challenges, and development trends of the application of GRADE in the evaluation of diagnostic accuracy test systems.] Chin J Evid Based Med. 2014;14:1402–1406.Suche in Google Scholar
[31] van der Heide A, Jacobs JW, Bijlsma JW, et al. The effectiveness of early treatment with “second-line” antirheumatic drugs. A randomized, controlled trial. Ann Intern Med. 1996;124:699–707.10.7326/0003-4819-124-8-199604150-00001Suche in Google Scholar PubMed
[32] Bukhari MA, Wiles NJ, Lunt M, et al. Influence of disease-modifying therapy on radiographic outcome in inflammatory polyarthritis at five years: results from a large observational inception study. Arthritis Rheum. 2003;48:46–53.10.1002/art.10727Suche in Google Scholar PubMed
[33] van Dongen H, van Aken J, Lard LR, et al. Efficacy of methotrexate treatment in patients with probable rheumatoid arthritis: a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2007;56:1424–1432.10.1002/art.22525Suche in Google Scholar PubMed
[34] Moon S, Lee CH, Kim YS, et al. Usefulness and Limitation of 2010 ACR/EULAR Classification Criteria in Korean Patients with Early RA. J Rheum Dis. 2012;19:326–333.10.4078/jrd.2012.19.6.326Suche in Google Scholar
[35] Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/ European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581.10.1002/art.27584Suche in Google Scholar PubMed
[36] Banal F, Dougados M, Combescure C, et al. Sensitivity and specificity of the American College of Rheumatology 1987 criteria for the diagnosis of rheumatoid arthritis according to disease duration: a systematic literature review and meta-analysis. Ann Rheum Dis. 2009;68:1184–1191.10.1136/ard.2008.093187Suche in Google Scholar PubMed
[37] Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324.10.1002/art.1780310302Suche in Google Scholar PubMed
[38] van der Linden MP, Knevel R, Huizinga TW, et al. Classification of rheumatoid arthritis: comparison of the 1987 American College of Rheumatology criteria and the 2010 American College of Rheumatology/European League Against Rheumatism criteria. Arthritis Rheum. 2011;63:37–42.10.1002/art.30100Suche in Google Scholar PubMed
[39] Berglin E, Dahlqvist SR. Comparison of the 1987 ACR and 2010 ACR/EULAR classification criteria for rheumatoid arthritis in clinical practice: a prospective cohort study. Scand J Rheumatol. 2013;42:362–368.10.3109/03009742.2013.776103Suche in Google Scholar PubMed
[40] Mjaavatten MD, Bykerk VP. Early rheumatoid arthritis: the performance of the 2010 ACR/EULAR criteria for diagnosing RA. Best Pract Res Clin Rheumatol. 2013;27:451–466.10.1016/j.berh.2013.09.001Suche in Google Scholar PubMed
[41] Kasturi S, Goldstein BL, Malspeis S, et al. Comparison of the 1987 American College of Rheumatology and the 2010 American College of Rheumatology/European League against Rheumatism criteria for classification of rheumatoid arthritis in the Nurses’ Health Study cohorts. Rheumatol Int. 2014;34:407–411.10.1007/s00296-013-2865-2Suche in Google Scholar PubMed PubMed Central
[42] Colebatch AN, Edwards CJ, Østergaard M, et al. EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. Ann Rheum Dis. 2013;72:804–814.10.1136/annrheumdis-2012-203158Suche in Google Scholar PubMed
[43] Silvagni E, Zandonella Callegher S, Mauric E, et al. Musculoskeletal ultrasound for treating rheumatoid arthritis to target-a systematic literature review. Rheumatology (Oxford). 2022;61:4590–4602.10.1093/rheumatology/keac261Suche in Google Scholar PubMed PubMed Central
[44] Sundin U, Sundlisater NP, Aga AB, et al. Value of MRI and ultrasound for prediction of therapeutic response and erosive progression in patients with early rheumatoid arthritis managed by an aggressive treat-to-target strategy. RMD Open. 2021;7:e001525.10.1136/rmdopen-2020-001525Suche in Google Scholar PubMed PubMed Central
[45] Barile A, Arrigoni F, Bruno F, et al. Computed Tomography and MR Imaging in Rheumatoid Arthritis. Radiol Clin North Am. 2017;55:997–1007.10.1016/j.rcl.2017.04.006Suche in Google Scholar PubMed
[46] Møller-Bisgaard S, Hørslev-Petersen K, Ejbjerg B, et al. Effect of Magnetic Resonance Imaging vs Conventional Treat-to-Target Strategies on Disease Activity Remission and Radiographic Progression in Rheumatoid Arthritis: The IMAGINE-RA Randomized Clinical Trial. JAMA. 2019;321:461–472.10.1001/jama.2018.21362Suche in Google Scholar PubMed PubMed Central
[47] Hotta M, Minamimoto R, Kaneko H, et al. Fluorodeoxyglucose PET/CT of Arthritis in Rheumatic Diseases: A Pictorial Review. Radiographics. 2020;40:223–240.10.1148/rg.2020190047Suche in Google Scholar PubMed
[48] Gravallese EM, Firestein GS. Rheumatoid Arthritis - Common Origins, Divergent Mechanisms. N Engl J Med. 2023;388:529–542.10.1056/NEJMra2103726Suche in Google Scholar PubMed
[49] Aletaha D, Maa JF, Chen S, et al. Effect of disease duration and prior disease-modifying antirheumatic drug use on treatment outcomes in patients with rheumatoid arthritis. Ann Rheum Dis. 2019;78:1609–1615.10.1136/annrheumdis-2018-214918Suche in Google Scholar PubMed PubMed Central
[50] Anderson J, Caplan L, Yazdany J, et al. Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis Care Res (Hoboken). 2012;64:640–647.10.1002/acr.21649Suche in Google Scholar PubMed PubMed Central
[51] Aletaha D, Smolen JS. Joint damage in rheumatoid arthritis progresses in remission according to the Disease Activity Score in 28 joints and is driven by residual swollen joints. Arthritis Rheum. 2011;63:3702–3711.10.1002/art.30634Suche in Google Scholar PubMed
[52] Felson DT, Smolen JS, Wells G, et al. American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum. 2011;63:573–586.10.1002/art.30552Suche in Google Scholar PubMed
[53] Zhu H, Li R, Da Z, et al. Remission assessment of rheumatoid arthritis in daily practice in China: a cross-sectional observational study. Clin Rheumatol. 2018;37:597–605.10.1007/s10067-017-3850-zSuche in Google Scholar PubMed
[54] Brites L, Rovisco J, Costa F, et al. High patient global assessment scores in patients with rheumatoid arthritis otherwise in remission do not reflect subclinical inflammation. Joint Bone Spine. 2021;88:105242.10.1016/j.jbspin.2021.105242Suche in Google Scholar PubMed
[55] Ferreira RJO, Carvalho PD, Ndosi M, et al. Impact of Patient’s Global Assessment on Achieving Remission in Patients With Rheumatoid Arthritis: A Multinational Study Using the METEOR Database. Arthritis Care Res (Hoboken). 2019;71:1317–1325.10.1002/acr.23866Suche in Google Scholar PubMed
[56] Studenic P, Aletaha D, de Wit M, et al. American College of Rheumatology/EULAR remission criteria for rheumatoid arthritis: 2022 revision. Ann Rheum Dis. 2023;82:74–80.10.1136/ard-2022-223413Suche in Google Scholar PubMed PubMed Central
[57] Janke K, Kiefer C, McGauran N, et al. A systematic comparison of different composite measures (DAS 28, CDAI, SDAI, and Boolean approach) for determining treatment effects on low disease activity and remission in rheumatoid arthritis. BMC Rheumatol. 2022;6:82.10.1186/s41927-022-00314-7Suche in Google Scholar PubMed PubMed Central
[58] Mian A, Ibrahim F, Scott DL. A systematic review of guidelines for managing rheumatoid arthritis. BMC Rheumatol. 2019;3:42.10.1186/s41927-019-0090-7Suche in Google Scholar PubMed PubMed Central
[59] Ramiro S, Landewé RB, van der Heijde D, et al. Is treat-to-target really working in rheumatoid arthritis? a longitudinal analysis of a cohort of patients treated in daily practice (RA BIODAM). Ann Rheum Dis. 2020;79:453–459.10.1136/annrheumdis-2019-216819corr1Suche in Google Scholar PubMed
[60] Grigor C, Capell H, Stirling A, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet. 2004;364:263–269.10.1016/S0140-6736(04)16676-2Suche in Google Scholar PubMed
[61] Muñoz-Femández S, Otón-Sánchez T, Carmona L, et al. Use of prognostic factors of rheumatoid arthritis in clinical practice and perception of their predictive capacity before and after exposure to evidence. Rheumatol Int. 2018;38:2289–2296.10.1007/s00296-018-4152-8Suche in Google Scholar PubMed
[62] Morel J, Combe B. How to predict prognosis in early rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2005;19:137–146.10.1016/j.berh.2004.08.008Suche in Google Scholar PubMed
[63] Archer R, Hock E, Hamilton J, et al. Assessing prognosis and prediction of treatment response in early rheumatoid arthritis: systematic reviews. Health Technol Assess. 2018;22:1–294.10.3310/hta22660Suche in Google Scholar PubMed PubMed Central
[64] Young A, Koduri G. Extra-articular manifestations and complications of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21:907–927.10.1016/j.berh.2007.05.007Suche in Google Scholar PubMed
[65] Fazeli MS, Khaychuk V, Wittstock K, et al. Rheumatoid arthritis-associated interstitial lung disease: epidemiology, risk/ prognostic factors, and treatment landscape. Clin Exp Rheumatol. 2021;39:1108–1118.10.55563/clinexprheumatol/h9tc57Suche in Google Scholar PubMed
[66] Qiu M, Jiang J, Nian X, et al. Factors associated with mortality in rheumatoid arthritis-associated interstitial lung disease: a systematic review and meta-analysis. Respir Res. 2021;22:264.10.1186/s12931-021-01856-zSuche in Google Scholar PubMed PubMed Central
[67] Rawla P. Cardiac and vascular complications in rheumatoid arthritis. Reumatologia. 2019;57:27–36.10.5114/reum.2019.83236Suche in Google Scholar PubMed PubMed Central
[68] Villa E, Sarquis T, de Grazia J, et al. Rheumatoid meningitis: A systematic review and meta-analysis. Eur J Neurol. 2021;28:3201–3210.10.1111/ene.14904Suche in Google Scholar PubMed
[69] Restivo V, Candiloro S, Daidone M, et al. Systematic review and meta-analysis of cardiovascular risk in rheumatological disease: Symptomatic and non-symptomatic events in rheumatoid arthritis and systemic lupus erythematosus. Autoimmun Rev. 2022;21:102925.10.1016/j.autrev.2021.102925Suche in Google Scholar PubMed
[70] Moshayedi S, Tasorian B, Almasi-Hashiani A. The prevalence of osteoporosis in rheumatoid arthritis patient: a systematic review and meta-analysis. Sci Rep. 2022;12:15844.10.1038/s41598-022-20016-xSuche in Google Scholar PubMed PubMed Central
[71] Lin JZ, Liang JJ, Ma JD, et al. Myopenia is associated with joint damage in rheumatoid arthritis: a cross-sectional study. J Cachexia Sarcopenia Muscle. 2019;10:355–367.10.1002/jcsm.12381Suche in Google Scholar PubMed PubMed Central
[72] Pan J, Zou YW, Zhu YY, et al. Muscle mass loss is associated with physical dysfunction in patients with early rheumatoid arthritis. Front Nutr. 2022;9:1007184.10.3389/fnut.2022.1007184Suche in Google Scholar PubMed PubMed Central
[73] Simon TA, Thompson A, Gandhi KK, et al. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther. 2015;17:212.10.1186/s13075-015-0728-9Suche in Google Scholar PubMed PubMed Central
[74] Baronnet L, Barnetche T, Kahn V, et al. Incidence of tuberculosis in patients with rheumatoid arthritis. A systematic literature review. Joint Bone Spine. 2011;78:279–284.10.1016/j.jbspin.2010.12.004Suche in Google Scholar PubMed
[75] Lin JZ, Liu Y, Ma JD, et al. Reduced skeletal muscle independently predicts 1-year aggravated joint destruction in patients with rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2020;12:1759720.10.1177/1759720X20946220Suche in Google Scholar PubMed PubMed Central
[76] Lin JZ, Ma JD, Yang LJ, et al. Myokine myostatin is a novel predictor of one-year radiographic progression in patients with rheumatoid arthritis: A prospective cohort study. Front Immunol. 2022;13:1005161.10.3389/fimmu.2022.1005161Suche in Google Scholar PubMed PubMed Central
[77] Rheumatology and Immunology Branch of the Chinese Medical Association. [Chinese expert consensus on the application of methotrexate in rheumatic diseases.] Chin J Int Med. 2018;57:719–722.Suche in Google Scholar
[78] Kameda H, Yamaoka K, Yamanishi Y, et al. Japan College of Rheumatology guidance for the use of methotrexate in patients with rheumatoid arthritis: Secondary publication. Mod Rheumatol. 2023;34:1–10.10.1093/mr/road098Suche in Google Scholar PubMed
[79] Shea B, Swinden MV, Tanjong Ghogomu E, et al. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. Cochrane Database Syst Rev. 2013;201:3.10.1111/jebm.12060Suche in Google Scholar PubMed
[80] Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res (Hoboken). 2021;73: 924–939.10.1002/acr.24596Suche in Google Scholar PubMed PubMed Central
[81] Alfaro-Lara R, Espinosa-Ortega HF, Arce-Salinas CA; PRE-CIS study group, et al. Systematic review and meta-analysis of the efficacy and safety of leflunomide and methotrexate in the treatment of rheumatoid arthritis. Reumatol Clin (Engl Ed). 2019;15:133–139.10.1016/j.reuma.2017.07.020Suche in Google Scholar PubMed
[82] Bae SC, Lee YH. Comparative efficacy and tolerability of monotherapy with leflunomide or tacrolimus for the treatment of rheumatoid arthritis: a Bayesian network meta-analysis of randomized controlled trials. Clin Rheumatol. 2018;37:323–330.10.1007/s10067-017-3857-5Suche in Google Scholar PubMed
[83] Suarez-Almazor ME, Belseck E, Shea B, et al. Sulfasalazine for rheumatoid arthritis. Cochrane Database Syst Rev. 2000;1998:CD000958.10.1002/14651858.CD000958Suche in Google Scholar PubMed PubMed Central
[84] Plosker GL, Croom KF. Sulfasalazine: a review of its use in the management of rheumatoid arthritis. Drugs. 2005;65:1825–1849.10.2165/00003495-200565130-00008Suche in Google Scholar PubMed
[85] Singh JA, Hossain A, Mudano AS, et al. Biologics or tofacitinib for people with rheumatoid arthritis naive to methotrexate: a systematic review and network meta-analysis. Cochrane Database Syst Rev. 2017 May 8;5:CD012657.10.1002/14651858.CD012657Suche in Google Scholar PubMed PubMed Central
[86] Donahue KE, Schulman ER, Gartlehner G, et al. Comparative Effectiveness of Combining MTX with Biologic Drug Therapy Versus Either MTX or Biologics Alone for Early Rheumatoid Arthritis in Adults: a Systematic Review and Network Meta-analysis. J Gen Intern Med. 2019;34:2232–2245.10.1007/s11606-019-05230-0Suche in Google Scholar PubMed PubMed Central
[87] Iwami RS, Moura MD, Sorrilha FB, et al. Effectiveness and safety of oral corticosteroids in the treatment of rheumatoid arthritis: a systematic review. Revista Brasileira de Farmácia Hospitalar e Serviços de Saúde. 2022;13:0749.10.30968/rbfhss.2022.131.0749Suche in Google Scholar
[88] Sanmartí R, Tornero J, Narváez J, et al. Efficacy and safety of glucocorticoids in rheumatoid arthritis: Systematic literature review. Reumatol Clin (Engl Ed). 2020;16:222–228.10.1016/j.reumae.2018.06.004Suche in Google Scholar
[89] Boers M, Hartman L, Opris-Belinski D, et al. Low dose, add-on prednisolone in patients with rheumatoid arthritis aged 65+: the pragmatic randomised, double-blind placebo-controlled GLORIA trial. Ann Rheum Dis. 2022;81:925–936.10.1136/annrheumdis-2022-eular.764Suche in Google Scholar
[90] Roubille C, Richer V, Starnino T, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal antiinflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74:480–489.10.1136/annrheumdis-2014-206624Suche in Google Scholar PubMed PubMed Central
[91] Dixon WG, Suissa S, Hudson M. The association between systemic glucocorticoid therapy and the risk of infection in patients with rheumatoid arthritis: systematic review and metaanalyses. Arthritis Res Ther. 2011;1:3.10.1186/ar3453Suche in Google Scholar PubMed PubMed Central
[92] Rheumatology and Immunology Branch of the Chinese Medical Association, the Rheumatology Branch of the Chinese Medical Association, the Osteoporosis and Bone Mineral Disease Branch of the Chinese Medical Association, and the National Clinical Medical Research Center for Dermatology and Immunological Diseases. [2020 Chinese Expert Consensus on the Prevention and Treatment of Glucocorticoid Induced Osteoporosis.] Chin J Int Med. 2021;60:9.Suche in Google Scholar
[93] Chen YR, Hsieh FI, Chang CC, et al. Effect on Risk of Stroke and Acute Myocardial Infarction of Nonselective Nonsteroidal Anti-Inflammatory Drugs in Patients With Rheumatoid Arthritis. Am J Cardiol. 2018;121:1271–1277.10.1016/j.amjcard.2018.01.044Suche in Google Scholar PubMed
[94] Hughes CD, Scott DL, Ibrahim F; TITRATE Programme Investigators. Intensive therapy and remissions in rheumatoid arthritis: a systematic review. BMC Musculoskelet Disord. 2018;19:389.10.1186/s12891-018-2302-5Suche in Google Scholar PubMed PubMed Central
[95] Albrecht K, Zink A. Poor prognostic factors guiding treatment decisions in rheumatoid arthritis patients: a review of data from randomized clinical trials and cohort studies. Arthritis Res Ther. 2017;19:68.10.1186/s13075-017-1266-4Suche in Google Scholar PubMed PubMed Central
[96] Curtis JR, Beukelman T, Onofrei A, et al. Elevated liver enzyme tests among patients with rheumatoid arthritis or psoriatic arthritis treated with methotrexate and/or leflunomide. Ann Rheum Dis. 2010;69:43–47.10.1136/ard.2008.101378Suche in Google Scholar PubMed PubMed Central
[97] Lee SW, Park HJ, Kim BK, et al. Leflunomide increases the risk of silent liver fibrosis in patients with rheumatoid arthritis receiving methotrexate. Arthritis Res Ther. 2012;1:4.10.1186/ar4075Suche in Google Scholar PubMed PubMed Central
[98] McEwen J, Purcell PM, Hill RL, et al. The incidence of pancytopenia in patients taking leflunomide alone or with methotrexate. Pharmacoepidemiol Drug Saf. 2007;16:65–73.10.1002/pds.1236Suche in Google Scholar PubMed
[99] Rempenault C, Combe B, Barnetche T, et al. Clinical and Structural Efficacy of Hydroxychloroquine in Rheumatoid Arthritis: A Systematic Review. Arthritis Care Res (Hoboken). 2020;72:36–40.10.1002/acr.23826Suche in Google Scholar PubMed
[100] Nazir AM, Koganti B, Gupta K, et al. Evaluating the Use of Hydroxychloroquine in Treating Patients With Rheumatoid Arthritis. Cureus. 2021;13:e19308.10.7759/cureus.19308Suche in Google Scholar PubMed PubMed Central
[101] Lv QW, Zhang W, Shi Q, et al. Comparison of Tripterygium wilfordii Hook F with methotrexate in the treatment of active rheumatoid arthritis (TRIFRA): a randomised, controlled clinical trial. Ann Rheum Dis. 2015;74:1078–1086.10.1136/annrheumdis-2013-204807Suche in Google Scholar PubMed
[102] Zhang X, Yang H, Zuo X, et al. Efficacy and safety of tripterygium wilfordii Hook F plus TNF inhibitor for active rheumatoid arthritis: A multicentre, randomized, double-blind, triple-dummy controlled trial. Clin Immunol. 2023;255:109749.10.1016/j.clim.2023.109749Suche in Google Scholar PubMed
[103] Shrestha S, Zhao J, Yang C, et al. Iguratimod combination therapy compared with methotrexate monotherapy for the treatment of rheumatoid arthritis: a systematic review and meta-analysis. Clin Rheumatol. 2021;40:4007–4017.10.1007/s10067-021-05746-zSuche in Google Scholar PubMed
[104] Ouyang D, Ma YZ, Zou J, et al. Effectiveness and Safety of Iguratimod Monotherapy or Combined With Methotrexate in Treating Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Front Pharmacol. 2022;13:911810.10.3389/fphar.2022.911810Suche in Google Scholar PubMed PubMed Central
[105] Zeng L, Yu G, Yang K, et al. The Effect and Safety of Iguratimod Combined With Methotrexate on Rheumatoid Arthritis: A Systematic Review and Meta-Analysis Based on a Randomized Controlled Trial. Front Pharmacol. 2022;12:780154.10.3389/fphar.2021.780154Suche in Google Scholar PubMed PubMed Central
[106] Luo J, Jin DE, Yang GY, et al. Total glucosides of paeony for rheumatoid arthritis: A systematic review of randomized controlled trials. Complement Ther Med. 2017;34:46–56.10.1016/j.ctim.2017.07.010Suche in Google Scholar PubMed
[107] Feng ZT, Xu J, He GC, et al. A systemic review and metaanalysis of the clinical efficacy and safety of total glucosides of peony combined with methotrexate in rheumatoid arthritis. Clin Rheumatol. 2018;37:35–42.10.1007/s10067-017-3770-ySuche in Google Scholar PubMed PubMed Central
[108] Weinblatt ME, Keystone EC, Furst DE, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48:35–45.10.1002/art.10697Suche in Google Scholar PubMed
[109] Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50:1400–1411.10.1002/art.20217Suche in Google Scholar PubMed
[110] Maini R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999;354:1932–1939.10.1016/S0140-6736(99)05246-0Suche in Google Scholar PubMed
[111] Lipsky PE, van der Heijde DM, St Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594–1602.10.1056/NEJM200011303432202Suche in Google Scholar PubMed
[112] Emery P, Fleischmann RM, Moreland LW, et al. Golimumab, a human anti-tumor necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum. 2009;60:2272–2283.10.1002/art.24638Suche in Google Scholar PubMed
[113] Keystone EC, Genovese MC, Klareskog L, et al. Golimumab, a human antibody to tumour necrosis factor {alpha} given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD Study. Ann Rheum Dis. 2009;68:789–796.10.1136/ard.2008.099010Suche in Google Scholar PubMed PubMed Central
[114] Smolen JS, Kay J, Doyle M, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor α inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther. 2015;17:14.10.1186/s13075-015-0516-6Suche in Google Scholar PubMed PubMed Central
[115] Keystone E, Heijde Dv, Mason D Jr, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 2008;58:3319–3329.10.1002/art.23964Suche in Google Scholar PubMed
[116] Fleischmann R, Vencovsky J, van Vollenhoven RF, et al. Efficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease-modifying antirheumatic therapy: the FAST4WARD study. Ann Rheum Dis. 2009;68:805–811.10.1136/ard.2008.099291Suche in Google Scholar PubMed PubMed Central
[117] Weinblatt ME, Kremer JM, Bankhurst AD, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340:253–259.10.1056/NEJM199901283400401Suche in Google Scholar PubMed
[118] Moreland LW, Baumgartner SW, Schiff MH, et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med. 1997;337:141–147.10.1056/NEJM199707173370301Suche in Google Scholar PubMed
[119] Moreland LW, Schiff MH, Baumgartner SW, et al. Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann Intern Med. 1999;130:478–486.10.7326/0003-4819-130-6-199903160-00004Suche in Google Scholar PubMed
[120] Combe B, Codreanu C, Fiocco U, et al. Etanercept and sulfasalazine, alone and combined, in patients with active rheumatoid arthritis despite receiving sulfasalazine: a double-blind comparison. Ann Rheum Dis. 2006;65:1357–1362.10.1136/ard.2005.049650Suche in Google Scholar PubMed PubMed Central
[121] Kalden JR, Nüsslein HG, Wollenhaupt J, et al. Combination treatment with infliximab and leflunomide in patients with active rheumatoid arthritis: safety and efficacy in an open-label clinical trial. Clin Exp Rheumatol. 2008;26:834–840.Suche in Google Scholar
[122] Combe B, Codreanu C, Fiocco U, et al. Efficacy, safety and patient-reported outcomes of combination etanercept and sulfasalazine versus etanercept alone in patients with rheumatoid arthritis: a double-blind randomised 2-year study. Ann Rheum Dis. 2009;68:1146–1152.10.1136/ard.2007.087106Suche in Google Scholar PubMed PubMed Central
[123] Strangfeld A, Hierse F, Kekow J, et al. Comparative effectiveness of tumour necrosis factor alpha inhibitors in combination with either methotrexate or leflunomide. Ann Rheum Dis. 2009;68:1856–1862.10.1136/ard.2008.098467Suche in Google Scholar PubMed
[124] Greenberg JD, Reed G, Kremer JM, et al. Association of methotrexate and tumour necrosis factor antagonists with risk of infectious outcomes including opportunistic infections in the CORRONA registry. Ann Rheum Dis. 2010;69:380–386.10.1136/ard.2008.089276Suche in Google Scholar PubMed PubMed Central
[125] Galloway JB, Hyrich KL, Mercer LK, et al. Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatology (Oxford). 2011;50:124–131.10.1093/rheumatology/keq242Suche in Google Scholar PubMed PubMed Central
[126] Holroyd CR, Seth R, Bukhari M, et al. The British Society for Rheumatology biologic DMARD safety guidelines in inflammatory arthritis-Executive summary. Rheumatology (Oxford). 2019;58:220–226.10.1093/rheumatology/key207Suche in Google Scholar PubMed
[127] Jones G, Sebba A, Gu J, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis. 2010;69:88–96.10.1136/ard.2008.105197Suche in Google Scholar PubMed PubMed Central
[128] Gabay C, Emery P, van Vollenhoven R, et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet. 2013;381:1541–1550.10.1016/S0140-6736(13)60250-0Suche in Google Scholar PubMed
[129] Buckley F, Finckh A, Huizinga TW, et al. Comparative Efficacy of Novel DMARDs as Monotherapy and in Combination with Methotrexate in Rheumatoid Arthritis Patients with Inadequate Response to Conventional DMARDs: A Network Meta-Analysis. J Manag Care Spec Pharm. 2015;21:409–423.10.18553/jmcp.2015.21.5.409Suche in Google Scholar PubMed PubMed Central
[130] Dougados M, Kissel K, Sheeran T, et al. Adding tocilizumab or switching to tocilizumab monotherapy in methotrexate inadequate responders: 24-week symptomatic and structural results of a 2-year randomised controlled strategy trial in rheumatoid arthritis (ACT-RAY). Ann Rheum Dis. 2013;72:43–50.10.1136/annrheumdis-2011-201282Suche in Google Scholar PubMed PubMed Central
[131] Kremer JM, Rigby W, Singer NG, et al. Sustained Response Following Discontinuation of Methotrexate in Patients With Rheumatoid Arthritis Treated With Subcutaneous Tocilizumab: Results From a Randomized, Controlled Trial. Arthritis Rheumatol. 2018;70:1200–1208.10.1002/art.40493Suche in Google Scholar PubMed
[132] Pombo-Suarez M, Gomez-Reino JJ. Abatacept for the treatment of rheumatoid arthritis. Expert Rev Clin Immunol. 2019;15:319–326.10.1080/1744666X.2019.1579642Suche in Google Scholar PubMed
[133] Weinblatt ME, Schiff M, Valente R, et al. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: findings of a phase IIIb, multinational, prospective, randomized study. Arthritis Rheum. 2013;65:28–38.10.1002/art.37711Suche in Google Scholar PubMed PubMed Central
[134] Mohamed Ahamada M, Wu X. Analysis of efficacy and safety of abatacept for rheumatoid arthritis: systematic review and meta-analysis. Clin Exp Rheumatol. 2023;41:1882–1900.10.55563/clinexprheumatol/2xjg0dSuche in Google Scholar PubMed
[135] Pugliesi A, de Oliveira AB, Oliveira AB, et al. Compared efficacy of rituximab, abatacept, and tocilizumab in patients with rheumatoid arthritis refractory to methotrexate or TNF inhibitors agents: a systematic review and network meta-analysis. Adv Rheumatol. 2023;63:30.10.1186/s42358-023-00298-zSuche in Google Scholar PubMed
[136] Fleischmann R, Mysler E, Hall S, et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet. 2017;390:457–468.10.1016/S0140-6736(17)31618-5Suche in Google Scholar PubMed
[137] van der Heijde D, Strand V, Tanaka Y, et al. Tofacitinib in Combination With Methotrexate in Patients With Rheumatoid Arthritis: Clinical Efficacy, Radiographic, and Safety Outcomes From a Twenty-Four-Month, Phase III Study. Arthritis Rheumatol. 2019;71:878–891.10.1002/art.40803Suche in Google Scholar PubMed PubMed Central
[138] Lee EB, Fleischmann R, Hall S, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014;370:2377–2386.10.1056/NEJMoa1310476Suche in Google Scholar PubMed
[139] Taylor PC, Keystone EC, van der Heijde D, et al. Baricitinib versus Placebo or Adalimumab in Rheumatoid Arthritis. N Engl J Med. 2017;376:652–662.10.1056/NEJMoa1608345Suche in Google Scholar PubMed
[140] van Vollenhoven R, Takeuchi T, Pangan AL, et al. Efficacy and Safety of Upadacitinib Monotherapy in Methotrexate-Naive Patients With Moderately-to-Severely Active Rheumatoid Arthritis (SELECT-EARLY): A Multicenter, Multi-Country, Randomized, Double-Blind, Active Comparator-Controlled Trial. Arthritis Rheumatol. 2020;72:1607–1620.10.1002/art.41384Suche in Google Scholar PubMed PubMed Central
[141] Smolen JS, Pangan AL, Emery P, et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): a randomised, placebo-controlled, double-blind phase 3 study. Lancet. 2019;393:2303–2311.10.1016/S0140-6736(19)30419-2Suche in Google Scholar PubMed
[142] Fleischmann R, Mysler E, Bessette L, et al. Long-term safety and efficacy of upadacitinib or adalimumab in patients with rheumatoid arthritis: results through 3 years from the SELECTCOMPARE study. RMD Open. 2022;8:e002012.10.1136/rmdopen-2021-002012Suche in Google Scholar PubMed PubMed Central
[143] Genovese MC, Fleischmann R, Combe B, et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet. 2018;391:2513–2524.10.1016/S0140-6736(18)31116-4Suche in Google Scholar PubMed
[144] Kvacskay P, Blank N, Lorenz HM, et al. Leflunomide in combination with JAK inhibitors in the treatment of rheumatoid arthritis: a case series. Rheumatology (Oxford). 2022;61:e280–e281.10.1093/rheumatology/keac240Suche in Google Scholar PubMed
[145] Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N Engl J Med. 2022;386:316–326.10.1056/NEJMoa2109927Suche in Google Scholar PubMed
[146] Hoisnard L, Pina Vegas L, Dray-Spira R, et al. Risk of major adverse cardiovascular and venous thromboembolism events in patients with rheumatoid arthritis exposed to JAK inhibitors versus adalimumab: a nationwide cohort study. Ann Rheum Dis. 2023;82:182–188.10.1136/ard-2022-222824Suche in Google Scholar PubMed
[147] Taylor PC, Takeuchi T, Burmester GR, et al. Safety of baricitinib for the treatment of rheumatoid arthritis over a median of 4.6 and up to 9.3 years of treatment: final results from long-term extension study and integrated database. Ann Rheum Dis. 2022;81:335–343.10.1136/annrheumdis-2021-221276Suche in Google Scholar PubMed PubMed Central
[148] Xie W, Huang Y, Xiao S, et al. Impact of Janus kinase inhibitors on risk of cardiovascular events in patients with rheumatoid arthritis: systematic review and meta-analysis of randomised controlled trials. Ann Rheum Dis. 2019;78:1048–1054.10.1136/annrheumdis-2018-214846Suche in Google Scholar PubMed
[149] European Medicines Agency. Direct healthcare professional communication (DHPC): Xeljanz (tofacitinib): increased risk of major adverse cardiovascular events and malignancies with use of tofacitinib relative to TNF-alpha inhibitors. Published June 2021. Available at: https://www.ema.europa.eu/en/documents/dhpc/direct-healthcare-professional-communication-dhpc-xeljanz-tofacitinib-increased-risk-major-adverse-cardiovascular-events-and-malignancies-use-tofacitinib-relative-tnf-alpha-inhibitors_en.pdf. Accessed December 1, 2023.Suche in Google Scholar
[150] Mariette X, Rouanet S, Sibilia J, Combe B, Le Loët X, Tebib J, et al. Evaluation of low-dose rituximab for the retreatment of patients with active rheumatoid arthritis: a non-inferiority randomised controlled trial. Ann Rheum Dis 2014;73:1508–1514.10.1136/annrheumdis-2013-203480Suche in Google Scholar PubMed
[151] Peterfy C, Emery P, Tak PP, Østergaard M, DiCarlo J, Otsa K, et al. MRI assessment of suppression of structural damage in patients with rheumatoid arthritis receiving rituximab: results from the randomised, placebo-controlled, double-blind RASCORE study. Ann Rheum Dis 2016;75:170–177.10.1136/annrheumdis-2014-206015Suche in Google Scholar PubMed PubMed Central
[152] Tak PP, Rigby W, Rubbert-Roth A, Peterfy C, van Vollenhoven RF, Stohl W, et al. Sustained inhibition of progressive joint damage with rituximab plus methotrexate in early active rheumatoid arthritis: 2-year results from the randomised controlled trial IMAGE. Ann Rheum Dis 2012;71:351–357.10.1136/annrheumdis-2011-200170Suche in Google Scholar PubMed PubMed Central
[153] Janke K, Biester K, Krause D, et al. Comparative effectiveness of biological medicines in rheumatoid arthritis: systematic review and network meta-analysis including aggregate results from reanalysed individual patient data. BMJ. 2020;370:m2288.10.1136/bmj.m2288Suche in Google Scholar PubMed PubMed Central
[154] Smolen JS, Kay J, Doyle MK, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet. 2009;374:210–221.10.1016/S0140-6736(09)60506-7Suche in Google Scholar PubMed
[155] Weinblatt ME, Fleischmann R, Huizinga TW, et al. Efficacy and safety of certolizumab pegol in a broad population of patients with active rheumatoid arthritis: results from the REALISTIC phase IIIb study. Rheumatology (Oxford). 2012;51:2204–2214.10.1093/rheumatology/kes150Suche in Google Scholar PubMed
[156] Gottenberg JE, Brocq O, Perdriger A, et al. Non-TNF-Targeted Biologic vs a Second Anti-TNF Drug to Treat Rheumatoid Arthritis in Patients With Insufficient Response to a First Anti-TNF Drug: A Randomized Clinical Trial. JAMA. 2016;316:1172–1180.10.1001/jama.2016.13512Suche in Google Scholar PubMed
[157] Gottenberg JE, Morel J, Perrodeau E, et al. Comparative effectiveness of rituximab, abatacept, and tocilizumab in adults with rheumatoid arthritis and inadequate response to TNF inhibitors: prospective cohort study. BMJ. 2019;364:l67.10.1136/bmj.l67Suche in Google Scholar PubMed PubMed Central
[158] Fleischmann RM, Genovese MC, Enejosa JV, et al. Safety and effectiveness of upadacitinib or adalimumab plus methotrexate in patients with rheumatoid arthritis over 48 weeks with switch to alternate therapy in patients with insufficient response. Ann Rheum Dis. 2019;78:1454–1462.10.1136/annrheumdis-2019-215764Suche in Google Scholar PubMed PubMed Central
[159] Singh JA, Cameron C, Noorbaloochi S, et al. Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis. Lancet. 2015;386:258–265.10.1016/S0140-6736(14)61704-9Suche in Google Scholar PubMed PubMed Central
[160] Uchida T, Iwamoto N, Fukui S, et al. Comparison of risks of cancer, infection, and MACEs associated with JAK inhibitor and TNF inhibitor treatment: a multicentre cohort study. Rheumatology (Oxford). 2023;62:3358–3365.10.1093/rheumatology/kead079Suche in Google Scholar PubMed
[161] Riek M, Scherer A, Möller B, et al. Serious infection risk of tofacitinib compared to biologics in patients with rheumatoid arthritis treated in routine clinical care. Sci Rep. 2023;13:17776.10.1038/s41598-023-44841-wSuche in Google Scholar PubMed PubMed Central
[162] Mikuls TR, Johnson SR, Fraenkel L, et al. American College of Rheumatology Guidance for the Management of Rheumatic Disease in Adult Patients During the COVID-19 Pandemic: Version 3. Arthritis Rheumatol. 2021;73:e1–e12.10.1002/art.41596Suche in Google Scholar PubMed
[163] Landewé RBM, Kroon FPB, Alunno A, et al. EULAR recommendations for the management and vaccination of people with rheumatic and musculoskeletal diseases in the context of SARS-CoV-2: the November 2021 update. Ann Rheum Dis. 2022;81:1628–1639.10.1136/annrheumdis-2021-222006Suche in Google Scholar PubMed
[164] Araujo FC, Goncalves J, Fonseca JE. Biosimilars in rheumatology. Pharmacol Res. 2019;149:104467.10.1016/j.phrs.2019.104467Suche in Google Scholar PubMed
[165] Chen Y, Zhou Q, Zeng B, et al. Meta analysis of clinical similarity assessment between biologically similar drugs and their reference drugs in the treatment of rheumatoid arthritis. Chin J New Drugs. 2021;30:723–731.Suche in Google Scholar
[166] Mikael Boesen, Lars Boesen, Karl Erik Jensen, et al. Clinical outcome and imaging changes after intraarticular (IA) application of etanercept or methylprednisolone in rheumatoid arthritis: magnetic resonance imaging and ultrasound-Doppler show no effect of IA injections in the wrist after 4 weeks. J Rheumatol. 2008;35:584–591.Suche in Google Scholar
[167] Fu Q, Feng P, Sun LY, et al. A double-blind, double-dummy, randomized controlled, multicenter trial of 99Tc-methylene diphosphonate in patients with moderate to severe rheumatoid arthritis. Chin Med J (Engl). 2021;134:1457–1464.10.1097/CM9.0000000000001527Suche in Google Scholar PubMed PubMed Central
[168] Mu R, Liang J, Sun L, et al. A randomized multicenter clinical trial of 99 Tc-methylene diphosphonate in treatment of rheumatoid arthritis. Int J Rheum Dis. 2018;21:161–169.10.1111/1756-185X.12934Suche in Google Scholar PubMed
[169] Roodenrijs NMT, Kedves M, Hamar A, et al. Diagnostic issues in difficult-to-treat rheumatoid arthritis: a systematic literature review informing the EULAR recommendations for the management of difficult-to-treat rheumatoid arthritis. RMD Open. 2021;7:e001511.10.1136/rmdopen-2020-001511Suche in Google Scholar PubMed PubMed Central
[170] Watanabe R, Hashimoto M, Murata K, et al. Prevalence and predictive factors of difficult-to-treat rheumatoid arthritis: the KURAMA cohort. Immunol Med. 2022;45:35–44.10.1080/25785826.2021.1928383Suche in Google Scholar PubMed
[171] Nagy G, Roodenrijs NMT, Welsing PM, et al. EULAR definition of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis. 2021;80:31–35.10.1136/annrheumdis-2020-217344Suche in Google Scholar PubMed PubMed Central
[172] de Hair MJH, Jacobs JWG, Schoneveld JLM, et al. Difficult-to-treat rheumatoid arthritis: an area of unmet clinical need. Rheumatology (Oxford). 2018;57:1135–1144.Suche in Google Scholar
[173] Nagy G, Roodenrijs NMT, Welsing PMJ, et al. EULAR points to consider for the management of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis. 2022;81:20–33.10.1136/annrheumdis-2021-220973Suche in Google Scholar PubMed PubMed Central
[174] Conran C, Kolfenbach J, Kuhn K, et al. A Review of Difficult-to-Treat Rheumatoid Arthritis: Definition, Clinical Presentation, and Management. Curr Rheumatol Rep. 2023;25:285–294.10.1007/s11926-023-01117-6Suche in Google Scholar PubMed
[175] Verhoef LM, Tweehuysen L, Hulscher ME, et al. bDMARD Dose Reduction in Rheumatoid Arthritis: A Narrative Review with Systematic Literature Search. Rheumatol Ther. 2017;4:1–24.10.1007/s40744-017-0055-5Suche in Google Scholar PubMed PubMed Central
[176] Wang X, Tang Z, Huang T, et al. Withdrawal of MTX in rheumatoid arthritis patients on bDMARD/tsDMARD plus methotrexate at target: a systematic review and meta-analysis. Rheumatology (Oxford). 2023;62:1410–1416.10.1093/rheumatology/keac515Suche in Google Scholar PubMed
[177] van Mulligen E, de Jong PHP, Kuijper TM, et al. Gradual tapering TNF inhibitors versus conventional synthetic DMARDs after achieving controlled disease in patients with rheumatoid arthritis: first-year results of the randomised controlled TARA study. Ann Rheum Dis. 2019;78:746–753.10.1136/annrheumdis-2018-214970Suche in Google Scholar PubMed
[178] van Mulligen E, Weel AE, Hazes JM, et al. Tapering towards DMARD-free remission in established rheumatoid arthritis: 2-year results of the TARA trial. Ann Rheum Dis. 2020;79:1174–1181.10.1136/annrheumdis-2020-217485Suche in Google Scholar PubMed PubMed Central
[179] van Mulligen E, Weel AE, Kuijper TM, et al. Two-year cost effectiveness between two gradual tapering strategies in rheumatoid arthritis: costutility analysis of the TARA trial. Ann Rheum Dis. 2020;79:1550–1556.10.1136/annrheumdis-2020-217528Suche in Google Scholar PubMed PubMed Central
[180] Emery P, Burmester GR, Bykerk VP, et al. Evaluating drug-free remission with abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann Rheum Dis. 2015;74:19–26.10.1136/annrheumdis-2014-206106Suche in Google Scholar PubMed PubMed Central
[181] Aguilar-Lozano L, Castillo-Ortiz JD, Vargas-Serafin C, et al. Sustained clinical remission and rate of relapse after tocilizumab withdrawal in patients with rheumatoid arthritis. J Rheumatol. 2013;40:1069–1073.10.3899/jrheum.121427Suche in Google Scholar PubMed
[182] Wu Z, Zhu Y, Wang Y, et al. The Effects of Patient Education on Psychological Status and Clinical Outcomes in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Front Psychiatry. 2022;13:848427.10.3389/fpsyt.2022.848427Suche in Google Scholar PubMed PubMed Central
[183] Fayet F, Pereira B, Fan A, et al. Therapeutic education improves rheumatoid arthritis patients’ knowledge about methotrexate: a single center retrospective study. Rheumatol Int. 2021;41:2025–2030.10.1007/s00296-021-04893-5Suche in Google Scholar PubMed
[184] Lopez-Olivo MA, Lin H, Rizvi T, et al. Randomized Controlled Trial of Patient Education Tools for Patients With Rheumatoid Arthritis. Arthritis Care Res (Hoboken). 2021;73:1470–1478.10.1002/acr.24362Suche in Google Scholar PubMed PubMed Central
[185] Taibanguay N, Chaiamnuay S, Asavatanabodee P, et al. Effect of patient education on medication adherence of patients with rheumatoid arthritis: a randomized controlled trial. Patient Prefer Adherence. 2019;13:119–129.10.2147/PPA.S192008Suche in Google Scholar PubMed PubMed Central
[186] McQuillan J, Andersen JA, Berdahl TA, et al. Associations of Rheumatoid Arthritis and Depressive Symptoms Over Time: Are There Differences by Education, Race/Ethnicity, and Gender? Arthritis Care Res (Hoboken). 2022;74:2050–2058.10.1002/acr.24730Suche in Google Scholar PubMed
[187] Brock J, Basu N, Schlachetzki JCM, et al. Immune mechanisms of depression in rheumatoid arthritis. Nat Rev Rheumatol.2023;19:790–804.10.1038/s41584-023-01037-wSuche in Google Scholar PubMed
[188] Ionescu CE, Popescu CC, Agache M, et al. Depression in Rheumatoid Arthritis: A Narrative Review-Diagnostic Challenges, Pathogenic Mechanisms and Effects. Medicina (Kaunas). 2022;58:1637.10.3390/medicina58111637Suche in Google Scholar PubMed PubMed Central
[189] Pei JH, Ma T, Nan RL, et al. Mindfulness-Based Cognitive Therapy for Treating Chronic Pain A Systematic Review and Meta-analysis. Psychol Health Med. 2021;26:333–346.10.1080/13548506.2020.1849746Suche in Google Scholar PubMed
[190] Nagy Z, Szigedi E, Takács S, et al. The Effectiveness of Psychological Interventions for Rheumatoid Arthritis (RA): A Systematic Review and Meta-Analysis. Life (Basel). 2023;13:849.10.3390/life13030849Suche in Google Scholar PubMed PubMed Central
[191] Lopez-Olivo MA, Sharma G, Singh G, et al. A systematic review with meta-analysis of the effects of smoking cessation strategies in patients with rheumatoid arthritis. PLoS One. 2022;17:e0279065.10.1371/journal.pone.0279065Suche in Google Scholar PubMed PubMed Central
[192] Zhao SS, Holmes MV, Zheng J, et al. The impact of education inequality on rheumatoid arthritis risk is mediated by smoking and body mass index: Mendelian randomization study. Rheumatology (Oxford). 2022;61:2167–2175.10.1093/rheumatology/keab654Suche in Google Scholar PubMed PubMed Central
[193] Nayebirad S, Javinani A, Javadi M, et al. The effect of smoking on response to methotrexate in rheumatoid arthritis patients: A systematic review and meta-analysis. Mod Rheumatol. 2023;34:68–78.10.1093/mr/road013Suche in Google Scholar PubMed
[194] Ohno T, Aune D, Heath AK. Adiposity and the risk of rheumatoid arthritis: a systematic review and meta-analysis of cohort studies. Sci Rep. 2020;10:16006.10.1038/s41598-020-71676-6Suche in Google Scholar PubMed PubMed Central
[195] Qin B, Yang M, Fu H, et al. Body mass index and the risk of rheumatoid arthritis: a systematic review and dose-response meta-analysis. Arthritis Res Ther. 2015;17:86.10.1186/s13075-015-0745-8Suche in Google Scholar PubMed PubMed Central
[196] Poudel D, George MD, Baker JF. The Impact of Obesity on Disease Activity and Treatment Response in Rheumatoid Arthritis. Curr Rheumatol Rep. 2020;22:56.10.1007/s11926-020-00933-4Suche in Google Scholar PubMed PubMed Central
[197] Flores-Alvarado DE, Esquivel-Valerio JA, Vega-Morales D, et al. Impact of obesity and overweight on C-reactive protein concentrations and disease activity in rheumatoid arthritis: A systematic review and meta-analysis. Int J Rheum Dis. 2023;26:2498–2508.10.1111/1756-185X.14948Suche in Google Scholar PubMed
[198] Kreps DJ, Halperin F, Desai SP, et al. Association of weight loss with improved disease activity in patients with rheumatoid arthritis: A retrospective analysis using electronic medical record data. Int J Clin Rheumtol. 2018;13:1–10.10.4172/1758-4272.1000154Suche in Google Scholar PubMed PubMed Central
[199] Ranganath VK, La Cava A, Vangala S, et al. Improved outcomes in rheumatoid arthritis with obesity after a weight loss intervention: randomized trial. Rheumatology (Oxford). 2023;62:565–574.10.1093/rheumatology/keac307Suche in Google Scholar PubMed
[200] Somers TJ, Blumenthal JA, Dorfman CS, et al. Effects of a Weight and Pain Management Program in Patients With Rheumatoid Arthritis With Obesity: A Randomized Controlled Pilot Investigation. J Clin Rheumatol. 2022;28:7–13.10.1097/RHU.0000000000001793Suche in Google Scholar PubMed
[201] Nelson J, Sjöblom H, Gjertsson I, et al. Do Interventions with Diet or Dietary Supplements Reduce the Disease Activity Score in Rheumatoid Arthritis? A Systematic Review of Randomized Controlled Trials. Nutrients. 2020;12:2991.10.3390/nu12102991Suche in Google Scholar PubMed PubMed Central
[202] Schönenberger KA, Schüpfer AC, Gloy VL, et al. Effect of Anti-Inflammatory Diets on Pain in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Nutrients. 2021;13:4221.10.3390/nu13124221Suche in Google Scholar PubMed PubMed Central
[203] Gwinnutt JM, Wieczorek M, Rodríguez-Carrio J, et al. Effects of diet on the outcomes of rheumatic and musculoskeletal diseases (RMDs): systematic review and metaanalyses informing the 2021 EULAR recommendations for lifestyle improvements in people with RMDs. RMD Open. 2022;8:e002167.10.1136/rmdopen-2021-002167Suche in Google Scholar PubMed PubMed Central
[204] Pan H, Li R, Li T, et al. Whether Probiotic Supplementation Benefits Rheumatoid Arthritis Patients: A Systematic Review and Meta-Analysis. Engineering. 2017;3:115–121.10.1016/J.ENG.2017.01.006Suche in Google Scholar
[205] Lanspa M, Kothe B, Pereira MR, et al. A Systematic Review of Nutritional Interventions on Key Cytokine Pathways in Rheumatoid Arthritis and Its Implications for Comorbid Depression: Is a More Comprehensive Approach Required? Cureus. 2022;14:e28031.10.7759/cureus.28031Suche in Google Scholar PubMed PubMed Central
[206] Peter WF, Swart NM, Meerhoff GA, et al. Clinical Practice Guideline for Physical Therapist Management of People With Rheumatoid Arthritis. Phys Ther. 2021 Aug 1;101:pzab127.10.1093/ptj/pzab127Suche in Google Scholar PubMed
[207] Wen Z, Chai Y. Effectiveness of resistance exercises in the treatment of rheumatoid arthritis: A meta-analysis. Medicine (Baltimore). 2021;100:e25019.10.1097/MD.0000000000025019Suche in Google Scholar PubMed PubMed Central
[208] Brady SM, Veldhuijzen van Zanten JJCS, Dinas PC, et al. Effects of lifestyle physical activity and sedentary behaviour interventions on disease activity and patient- and clinician- important health outcomes in rheumatoid arthritis: a systematic review with meta-analysis. BMC Rheumatol. 2023;7:27.10.1186/s41927-023-00352-9Suche in Google Scholar PubMed PubMed Central
[209] Sobue Y, Kojima T, Ito H, et al. Does exercise therapy improve patient-reported outcomes in rheumatoid arthritis? A systematic review and meta-analysis for the update of the 2020 JCR guidelines for the management of rheumatoid arthritis. Mod Rheumatol. 2022;32:96–104.10.1080/14397595.2021.1886653Suche in Google Scholar PubMed
[210] Ye X, Chen Z, Shen Z, et al. Yoga for Treating Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Front Med (Lausanne). 2020;7:586665.10.3389/fmed.2020.586665Suche in Google Scholar PubMed PubMed Central
[211] Wu H, Wang Q, Wen G, et al. The effects of Tai Chi on physical function and safety in patients with rheumatoid arthritis: A systematic review and meta-analysis. Front Physiol. 2023;14:1079841.10.3389/fphys.2023.1079841Suche in Google Scholar PubMed PubMed Central
[212] Küçükdeveci AA, Turan BK, Arienti C, et al. Overview of Cochrane Systematic Reviews of rehabilitation interventions for persons with rheumatoid arthritis: a mapping synthesis. Eur J Phys Rehabil Med. 2023;59:259–269.10.23736/S1973-9087.22.07833-9Suche in Google Scholar PubMed PubMed Central
© 2024 Xinping Tian, Qian Wang, Nan Jiang, Yan Zhao, Cibo Huang, Yi Liu, Huji Xu, Yaolong Chen, Lijun Wu, Jian Xu, Hongbing Li, Liangjing Lu, Jin Lin, Lie Dai, Fen Li, Zhenyu Jiang, Zhaohui Zheng, Zongwen Shuai, Shengqian Xu, Dongbao Zhao, Miaojia Zhang, Yunlin Sun, Shengyun Liu, Caifeng Li, Pingting Yang, Mengtao Li, Xiaofeng Zeng, published by De Gruyter on behalf of NCRC-DID
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Editorial
- Enhancing standardized diagnosis and treatment to improve the prognosis of patients with rheumatoid arthritis in China
- The potential for biosimilars to address unmet clinical need — Is the developed world missing out?
- Guideline
- Chinese guidelines for the diagnosis and treatment of rheumatoid arthritis: 2024 update
- Review
- Therapeutic potential of CD20/CD3 bispecific antibodies in the treatment of autoimmune diseases
- Original Article
- 14-3-3 Eta protein as a novel biomarker in early detection of uveitis in Egyptian juvenile idiopathic arthritis and rheumatoid arthritis patients: Diagnostic and prognostic value
- Immunomodulatory effects of novel nano micelle based curcumin in rheumatoid arthritis patients: A double blind randomized clinical trial
- Letter to the Editor
- McArdle’s disease presents with multiple large vessel lesions
- Images
- “Bouncing temporomandibular joint”: Multiple temporomandibular lesions in a patient with rheumatoid arthritis
Artikel in diesem Heft
- Editorial
- Enhancing standardized diagnosis and treatment to improve the prognosis of patients with rheumatoid arthritis in China
- The potential for biosimilars to address unmet clinical need — Is the developed world missing out?
- Guideline
- Chinese guidelines for the diagnosis and treatment of rheumatoid arthritis: 2024 update
- Review
- Therapeutic potential of CD20/CD3 bispecific antibodies in the treatment of autoimmune diseases
- Original Article
- 14-3-3 Eta protein as a novel biomarker in early detection of uveitis in Egyptian juvenile idiopathic arthritis and rheumatoid arthritis patients: Diagnostic and prognostic value
- Immunomodulatory effects of novel nano micelle based curcumin in rheumatoid arthritis patients: A double blind randomized clinical trial
- Letter to the Editor
- McArdle’s disease presents with multiple large vessel lesions
- Images
- “Bouncing temporomandibular joint”: Multiple temporomandibular lesions in a patient with rheumatoid arthritis

