Abstract
A simple fluorescent chemosensor, 5-(diethylamino)-2-((2-(pyrazin-2-yl) hydrazono)methyl)phenol, has been synthesized by Schiff-base condensation reaction. The chemosensor exhibited highly selective and sensitive “off-on” fluorescent responses toward Al3+ and Zn2+ but the signal of fluorescence emission varies. The detection limits were found to be 2.33 × 10−7 M for Al3+ and 1.68 × 10−7 M for Zn2+, respectively. The binding mechanisms between chemosensor and Al3+ or Zn2+ ions were supported by Job′s, 1H NMR, Fourier transform infrared spectra, and MS experiments. The sensing behavior was also studied with molecular logic functions of OR, AND, and NOT gates. In addition, the chemosensor was able to detect Al3+ and Zn2+ by producing distinct color changes observed by the naked eye on sensor-coated swabs. Moreover, the chemosensor was successfully applied to effectively detect Al3+ and Zn2+ in actual water and drug samples.
Graphical abstract

1 Introduction
Fluorescent chemosensors for selective detection of interesting analytes, such as ions and toxicants, have recently attracted great attention and are widely used in biological, industrial, and environmental analyses [1,2], because fluorescent chemosensors have some advantages of convenient operation, high selectivity, instantaneous response, and nondestructive detection [3,4,5,6]. As we all know, metal ions play very significant roles in biological and environmental systems. Among metals, as the third most widespread element, aluminum has been widely used in our daily life including food additives, aluminum-based medicines and storage/cooking utensils, and so on [7]. Therefore, these factors provide chances for increasing the concentration of Al3+ in the environment and biosome. As a non-essential element of the human body, nevertheless, excessive aluminum is harmful to the human nervous system and causes severe human health issues, such as Alzheimer’s disease, Parkinson’s disease, colic, rickets, and anemia [8,9]. In addition, the high content of Al3+ can impact the growth of plant roots and aquatic wildlife [10]. Consequently, the development of effective fluorescent chemosensors to detect Al3+ in biological and environmental systems is of important significance [11,12]. Likewise, Zn2+, being the second most abundant micronutrient transition metal ion plays multifunctional roles in fundamental biological processes including brain function and pathology, gene transcription and expression, cellular apoptosis, and neural signal transmission [13,14]. Moreover, Zn2+ is recognized to be an important structural cofactor and catalytic center of Zn2+-containing enzymes and DNA-binding proteins [15]. However, disruption of Zn2+ homeostasis may be associated with various pathological processes such as Alzheimer’s disease, diabetes, Parkinson’s disease, and hypoxia-ischemia [16]. And, Zn2+ is also a waste product derived from agriculture and food in the environment, and the excessive Zn2+ content may reduce the activity of microbes present in soil [17]. Therefore, designing selective and sensitive chemosensors for monitoring the presence of Zn2+ concentration level is becoming highly needed and is still full of challenges.
Owing to the significance of Al3+ and Zn2+, numerous fluorescent chemosensors for Al3+ or Zn2+ have been developed based on single-analyte responsive systems [18,19,20,21,22,23]. However, the development of a single fluorescent chemosensor for multi-analytes with distinct fluorescence responses is gradually becoming new research hot spot and is extremely challenging. In addition, single chemosensors for multi-analytes have some advantages, such as potential cost reduction and saving analytical time [24,25]. At present, although a few fluorescent chemosensors have been designed for simultaneous detection of Al3+ and Zn2+ based on different experimental conditions [26,27,28,29,30], the development of a single fluorescent chemosensor that can selectively detect and identify both Al3+ and Zn2+ is still a challenging work. In these considerations, as an easily composable group, Schiff base derivatives are well known to be good ligands and thereby can be developed a potential single chemosensor for the detection multi-analytes simultaneously.
With this in mind, we synthesized a simple pyrazinyl–salicylimine Schiff base fluorescent chemosensor. This chemosensor displayed favorable selectivity in the recognition and detection of Zn2+ and Al3+ under different emission wavelengths. The presence of other competitive metal ions and the effects of the system pH on detection were also studied. The chemosensor displayed a low detection limit for Zn2+ or Al3+ and showed good reversibility by the addition of Zn2+ or Al3+ and ethylenediamine tetraacetic acid (EDTA) that could be applied to build molecular logic circuits. Moreover, the chemosensor toward these two metal ions was also utilized for real water and drug samples quantitative analysis; also, cotton swabs containing the chemosensor were prepared for the rapid detection of Zn2+ and Al3+.
2 Experimental
2.1 Materials and instrumentations
All starting materials, metal ions, nitroaromatics, and reagents were obtained from the best-known commercial sources (Aladdin and Macklin) and were used without further purification. 1H NMR and 13C NMR spectra were recorded in d 6-DMSO with a Bruker 400 MHz spectrometer operating at 400 and 100 MHz for 1H and 13C NMR spectroscopy, respectively. Chemical shifts were reported in ppm downfield from the internal standard, tetramethylsilane. The ultraviolet (UV)-Vis absorption spectra were recorded on a Shimadzu UV-2550 spectrometer in 1 cm path length quartz cell. Fluorescence emission spectra were carried out on a Perkin Elmer fluorescence spectrophotometer equipped with quartz cuvettes of 1 cm path length. Fourier transform infrared spectra (FT-IR) were recorded on a Perkin Elmer FT-IR spectrophotometer using a KBr pellet and were reported in wavenumber (cm−1). Single crystal X-ray data were collected on Bruker Smart Apex II single-crystal X-ray diffractometer using graphite-monochromated Mo-Kα radiation (0.71073 Å) at 25°C. Images were generated using Mercury software.
2.2 Fluorescence experiments
First, the stock solutions of the chemosensor 1 (1 × 10−2 M) and the different metal ions (K+, Al3+, Na+, Ca2+, Co2+, Cu2+, Mn2+, Fe3+, Tb3+, Pb2+, Mg2+, Ni2+, Ag+, Zn2+, Cd2+ as perchlorates, 1 × 10−2 M) were prepared in ethanol and water (95:5, v/v). Then, the solution of 1 was diluted with ethanol-water (95:5, v/v) to 1 × 10−4 M. For the analytes selectivity experiments, 2.0 equivalents of the respective metal ions were added in 1.5 mL of 1 (1 × 10−4 M), and these mixed solutions were all diluted to 3 mL with ethanol-water (95:5, v/v) as the experimental subjects. For the competitive experiment of metal ions, the appropriate amounts of Al3+ or Zn2+ and a competitive metal ion were mixed with 1.5 mL of 1 solution, and then, these solutions were diluted with ethanol-water (95:5, v/v) to maintain the final concentration of 1 to be 5 × 10−5 M. In titration experiments, 15 mL solution of 1 (1 × 10−2 M) was taken in a quartz optical cell; then, the Al3+ or Zn2+ stock solutions were added gradually using a pipette, and the final concentration of 1 was 5 × 10−5 M. As for the reversible experiments, the chemosensor 1 and Al3+ or Zn2+ were first mixed, in which the EDTA was added after 5 min.
2.3 Calculations for detection limit
The detection limit (LOD) of the chemosensor 1 for Al3+ or Zn2+ was calculated based on 3σ/k, where σ is the standard deviation of the blank solutions, and k is the slope of the calibration curve.
2.4 Job’s plot experiments
Job’s continuation method was carried out to determine the sensing stoichiometry of 1 with Al3+ or Zn2+ using fluorescence emission spectroscopy. The concentrations of 1 and Al3+ or Zn2+ were varied but the sum of their concentrations was maintained at 5 × 10−5 M. Fluorescence intensity changes were plotted as a function of the mole fraction of Al3+ or Zn2+. The inflection points in the resulting Job’s plots corresponded to the mole fraction of Al3+ or Zn2+ in the complexes.
2.5 Synthesis of (E)-5-(diethylamino)-2-((2-(pyrazin-2-yl)hydrazono)methyl)phenol (1)
Into a 100 mL single-necked flask, 2-hydrazinopyrazine (0.20 g, 1.82 mM) and 4-(diethylamino)salicylaldehyde (0.35 g, 1.82 mM) were added and dissolved in 30 mL anhydrous ethanol. The mixture solution was stirred at 80oC for 6 h. The solid residue was collected by filtration and washed with cold ethanol, and then, the crude product was purified by recrystallization. After drying, chemosensor 1 was obtained as a yellow solid (0.45 g, 86%). FT-IR (KBr, cm−1) 3,439, 3,336, 3,179, 3,088, 3,049, 2,973, 1,632, 1,580, 1,523, 1,433, 1,352, 1,245, 1,126, 1,002, 919, 823, 782, 668. 1H NMR (DMSO-d 6, 400 MHz), δ (ppm): 10.91 (s, 1H), 10.47 (s, 1H), 8.30 (s, 1H), 8.18 (s, 1H), 8.09 (d, J = 4 Hz, 1H), 7.92 (d, J = 4 Hz, 1H), 7.31 (d, J = 8 Hz, 1H), 6.25 (d, J = 8 Hz, 1H), 6.13 (s, 1H), 3.35 (d, J = 8 Hz, 4H), 1.11 (d, J = 8 Hz, 6H). 13C NMR (DMSO-d 6, 100 MHz), δ (ppm): 157.90, 152.22, 149.27, 143.09, 142.03, 133.95, 129.95, 129.41, 107.40, 103.89, 97.42, 43.77, 12.57. Electrospray ionization-mass spectrometry (ESI-MS) (m/z): 286.26 [M + H]+.
3 Results and discussion
3.1 Synthesis and single crystal of 1
The chemosensor 1 was facilely synthesized by an one-pot aldehyde-hydrazine condensation reaction of 4-(diethylamino)salicylaldehyde and 2-hydrazinopyrazine in the presence of ethanol with excellent yield and high purity (Scheme 1). The molecular structure of 1 was confirmed using 1H NMR, 13C NMR, MS, and also single-crystal X-ray diffraction analyses.

Synthesis of the chemosensor 1.
A reasonable single crystal of chemosensor 1 for X-ray diffraction analysis was obtained from the slow evaporation of methanol–CH2Cl2 mixture (CCDC Number: 2121363). An oak ridge thermal ellipsoid plot (ORTEP) view (by Mercury software) and single-cell arrangement of 1 are given in Figure 1. The presence of the pyrazine and salicylaldehyde moieties could be easily observed and were linked by a hydrazone bond in the compound. It was also found that compound 1 was almost planar. An intramolecular hydrogen bond is present between N(3) and H(1) with a bond distance of 2.7 Å. The crystallographic data, refinement parameters (Table S1 in Supplementary material), and bond lengths and angles (Table S2) are presented in ESI.

(a) ORTEP view and the intramolecular hydrogen bond of the chemosensor 1; (b) unit cell of 1 in a single crystal. Hydrogen atoms were omitted for clarity.
3.2 Fluorescence response to metal ion
As an excellent chemosensor, the selective sensing behavior of 1 for various metal ions is a very important parameter, and the fluorescence experiment was investigated upon the addition of several metal ions such as Na+, K+, Ag+, Al3+, Ca2+, Pb2+, Co2+, Cd2+, Mg2+, Mn2+, Zn2+, Ni2+, Fe3+, Cu2+, and Tb3+ in ethanol–H2O (v:v = 95:5) at ambient conditions. As shown in Figure 2, upon excitation at 366 nm, 1 exhibited negligible fluorescence emission at 525 nm due to the photoinduced electron transfer (PET) process [31]. When 1 was treated with 2.0 equivalents of metal ions mentioned previously, Al3+ and Zn2+ induced apparent fluorescence enhancements. However, Al3+ and Zn2+ ions could be easily distinguished from the respective fluorescence emission wavelengths centered at 582 and 541 nm. The observed fluorescence enhancement should be attributed to the chelation-enhanced fluorescence (CHEF) effect by complexation of 1 with Al3+ and Zn2+ via the imine bond N, the phenolic hydroxyl O, and the pyrazine N [32,33], which inhibited of free rotation of 1 as well as eliminated the PET process. Such selective fluorescence changes of chemosensor 1 were not observed in the presence of other tested metal ions, which revealed a good selectivity of 1 toward Al3+ and Zn2+.

Fluorescence spectrum changes of 1 (50 μM) on addition of various metal ions (2.0 equiv.) in ethanol–water (95:5, v/v) (l ex = 366 nm).
The specificity fluorescence responses of 1 toward Al3+ and Zn2+ were examined by interference ion experiments, which were performed by taking the fluorescence of 1 with 2.0 equivalents of Al3+ or Zn2+ in the presence of 2.0 equivalents of other metal ions. As the fluorescence intensity of 1 increased at 582 nm and 541 nm in the presence of Al3+ and Zn2+, respectively, we monitored emission wavelength at 582 nm and 541 nm, respectively, to check the influence of other metal ions on the emission intensity. As shown in Figure 3a, except for the slight interference of Cu2+ and Fe3+, no significant fluorescence quenching at 582 nm was observed in 1–Al3+ solution upon the addition of other metal ions. This experiment showed that 1 could be used to detect Al3+ even in the presence of other relevant metal ions. Simultaneously, 1 was treated with Zn2+ in the presence of other metal ions, except for Cu2+, Fe3+, which quenched the fluorescence intensity at 541 nm; other metal ions did not significantly affect the intensity of the 1–Zn2+ solution (Figure 3b). It suggested that chemosensor 1 had a stronger binding affinity toward Cu2+ and Fe3+ than Zn2+. Although Cu2+ and Fe3+ caused certain interference, the chemosensor had potential application in the recognition of Zn2+ owing to its excellent selectivity [34]. From the competitive experiment, even coincidentally, the original fluorescence intensities were both enhanced to a certain extent by either Zn2+ added to 1–Al3+ solution or Al3+ added to 1–Zn2+ solution, indicating that there might be a certain synergistic effect between Al3+ and Zn2+ in fluorescence emission.

Competitive selectivity of 1 toward (a) Al3+ and (b) Zn2+ in the presence of other metals (2 equiv.).
In order to verify the sensitivity of the chemosensor 1 toward Al3+ and Zn2+, quantitative fluorescence titration experiments of Al3+ and Zn2+ to 1 had been carried out in ethanol–H2O (v:v = 95:5), respectively. The results are shown in Figure 4. For the titration of Al3+, the maximum emission wavelength red-shifted from 525 to 582 nm and a significant fluorescence emission enhancement at 582 nm was detected upon the progressive addition of Al3+. With an increase in Al3+ concentration up to 75 mM (1.5 equiv.), the fluorescence intensity showed almost no change. Plotting of the fluorescence emission intensity of 1–Al3+ at 582 nm versus the concentration of Al3+ (0–1.0 equiv.) showed a good linear relationship (R 2 = 0.99326), demonstrating that 1 could potentially be performed as a quantitative fluorescent chemosensor for detecting Al3+. The binding constant for the formation of the 1–Al3+ complex was calculated to be 2.03 × 104 M−1 using the Benesi–Hildebrand equation [35]. The detection limit was determined as low as 2.33 × 10−7 M for Al3+ on the basis of the IUPAC recommendation of the3σ/k method [36]. This detection limit was significantly below the enforceable drinking water standard for Al3+ (7.4 µM) proposed by the World Health Organization (WHO) [37]. Similarly, the binding ability of chemosensor 1 toward Zn2+ was also investigated by a fluorescence titration experiment in ethanol–H2O (v:v = 95:5), as shown in Figure 3b. Upon the addition of Zn2+ (0–2.0 equivalents) to the solution of 1, the fluorescence emission intensity at 542 nm increased gradually and then reached the maximum when the addition of Zn2+ was 90 μM (1.6 equiv.). And a good linear correlation between the emission intensity at 542 nm of 1–Zn2+ and the concentration ratio of [Zn2+]/[1] was observed in the range of 0–1.2 equivalents of Zn2+, which could be used for the determination of unknown Zn2+ in an aqueous solution containing ethanol. According to the titration profile, the corresponding detection limit was also calculated to be 1.68 × 10−7 M for Zn2+, which value was much lower than the WHO suggested tolerance level (76 µM) in drinking water [30]. And the binding constant between the chemosensor 1 and Zn2+ was calculated to be 1.15 × 104 M−1. From these results, therefore, chemosensor 1 had high sensitivity for Al3+ and Zn2+ and could be potentially used for the detection of Al3+ and Zn2+ in practical samples.

Fluorescence spectra changes of 1 (50 μM) upon incremental addition of (a) Al3+ (0–1.5 equiv.) and (c) Zn2+ (0–1.8 equiv.), the linear relationship the fluorescence intensity at (b) 582 nm versus the equivalents of Al3+ and (d) 543 nm versus the equivalents of Zn2+.
Generally, the solution pH value is also a primary factor affecting the response of the chemosensor. Thus, the effect of pH in the pH range 2.0–13.0 on the fluorescence emission of chemosensor 1 was evaluated in the absence and presence of Al3+ and Zn2+ separately (Figure S1 in Supplementary material). The fluorescence emission intensity of free chemosensor 1 showed little dependence on pH from 2.0 to 13.0. For Al3+ and Zn2+, emission intensity was monitored at 582 and 542 nm, respectively. In the presence of Al3+, significant fluorescence enhancements of 1 were observed upon the addition of Al3+ in the pH range from 4.0 to 10.0. By contrast, the fluorescence intensity decreased gradually in acidic (pH, < 4.0) and alkaline (pH, > 10.0) solutions. A similar fluorescence phenomenon was also found in the 1–Zn2+ system, and a drastic enhancement in emission intensity of 1 was caused in a range of pH 5.0–9.0 in the presence of Zn2+. The pH experiment suggested that the chemosensor 1 could form stable complexes with Al3+ or Zn2+ in weakly acidic, neutral, and weakly alkaline environments. We speculated that the strongly acidic conditions could induce dissociation of 1–Al3+ or 1–Zn2+ complexes because of the protonation of 1, and the strongly alkaline solutions could provide adequate hydroxyl ions to extract Al3+ or Zn2+ from complexes to form the corresponding hydroxide [38]. Taking the aforementioned results into consideration, these appropriate pH ranges of 1 toward Al3+ and Zn2+ made it suitable as a selective fluorescent chemosensor to recognize both Al3+ and Zn2+ in environmental systems.
3.3 Naked eye identification
Naked eye detection, as a simple method, was more practical for the rapid detection of target ions because it needed an uncomplicated program. Therefore, photographs of the single 1 and 1 with the above metal ions had been recorded under 365 nm UV light (Figure 5a). Except for Al3+ and Zn2+, single 1 and 1 with other metal ions did not produce any change in color under 365 nm irradiation. The chemosensor 1 exhibited yellow and greenish-yellow coloration in the presence of Al3+ and Zn2+, respectively, indicating that the chemosensor 1 could be used for a naked eye detection of Al3+ and Zn2+ over other metal ions by solution process. In addition, various concentrations of Al3+ or Zn2+ were added separately to verify the performance of 1 as an efficient chemosensor (Figure S2). The color of the solution was changed significantly in the presence of different Al3+ or Zn2+. With increasing amounts of Al3+ and Zn2+ to 0.1 and 0.02 equivalent, respectively, an obvious color change from pale yellow to yellow of 1 solution was observed under visible light and an enhancement of emission intensity was shown under UV 365 nm irradiation, showing that Al3+ and Zn2+ complexed with 1 to prevent the PET process and enhance the fluorescence intensity. These results indicated that chemosensor 1 could be conveniently used for the practical estimation of Al3+ and Zn2+ concentrations. Furthermore, for convenient use in an onsite analysis, swab-based chemosensor 1 for Al3+ and Zn2+ detection was also developed. As shown in Figure 5b, when the swap was dipped into the Al3+ and Zn2+ solution, respectively, a yellow and greenish-yellow emission was observed separately by the naked eye under a 365 nm UV lamp, indicating that Al3+ and Zn2+ complexed effectively with the chemosensor 1. Furthermore, when the swab was dipped in different ion solutions, the fluorescence of the swab dipped in Al3+ and Zn2+ strengthened and the fluorescence of the other swabs remained the same (Figure S3). The experiment of the swabs demonstrated that 1 was a portable chemosensor for the rapid detection of Al3+ and Zn2+. Undoubtedly, these data suggested that chemosensor 1 showed promising potential for the rapid and onsite identification of Al3+ and Zn2+.

(a) Photos of fluorescence changes of 1 on the addition of various metal ions (2.0 equiv.) in ethanol–water (95:5, v/v) under UV (365 nm) lamp. (b) The color changes of the 1-coated cotton swab in the absence and presence of Al3+ and Zn2+.
3.4 Reversibility for Al3+ and Zn2+
Reversibility, as an important factor to determine whether the chemosensor could be recycled, plays a significant role in practical applications. Therefore, the metal complexing agent EDTA was used as a chelating ligand and was added to the solutions of 1–Al3+ and 1–Zn2+, respectively (Figure 6a and b). In the case of 1–Al3+ solution, after the addition of 2.0 equivalents of EDTA, the yellow emission of the solution vanished accompanied by an obvious decrease in the fluorescence intensity at 582 nm, suggesting that EDTA extracted Al3+ from the 1–Al3+ complex to release the 1 free (Figure S4). Subsequently, when Al3+ was added again to the earlier mixture, the yellow emission as well as fluorescence intensity was both regenerated, and this reversible detection could be repeated at least four times by alternate addition of EDTA and Al3+. Coincidentally, in UV-Vis spectra, the free chemosensor 1 displayed a maximal absorption peak at 365 nm which was attributed to π–π* transition. Upon the addition of Al3+ to the solution of 1, the initial absorption band at 365 nm was decreased accompanied by a 15 nm blue-shift, and a simultaneous increase at 437 nm was observed. The new band at 437 nm could be attributed to metal to ligand charge transfer (MLCT). After then, the absorption spectrum of 1–Al3+ was similar to that of the free 1 upon the addition of EDTA (Figure S5). The same reversible cycle experiment was also implemented for the 1–Zn2+ system, and the greenish-yellow emission and fluorescence intensity at 542 nm could achieve “off-on-off” mode. In the absorption spectrum, the π–π* transition absorption wavelength of free 1 was decreased along with the shift of the 365 nm peak to 346 nm with the addition of Zn2+; in addition, a new MLCT absorption band at 429 nm emerged. Subsequently, the MLCT band disappeared, and the π–π* transition band was recovered upon the addition of EDTA. These results indicated that 1 was an effectively reversible chemosensor for Al3+ and Zn2+ detections.
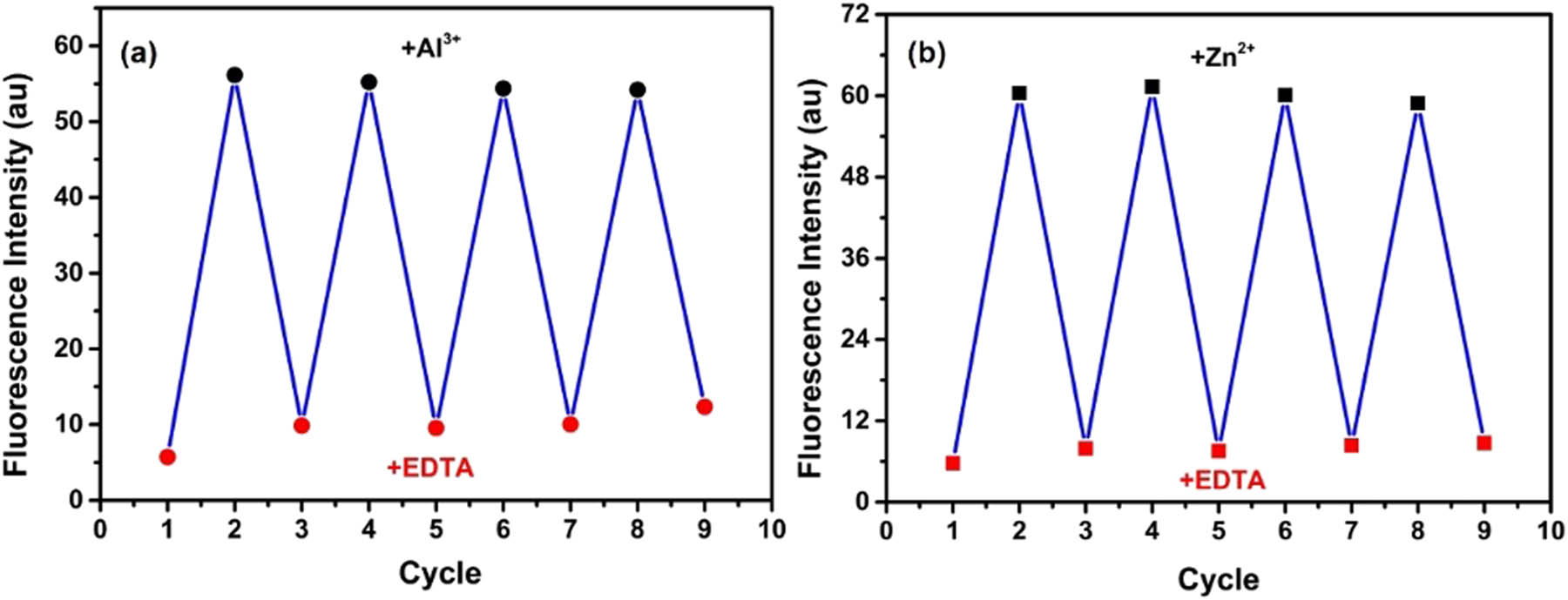
Fluorescence intensity of 1 at (a) 589 nm by alternate addition of Al3+ and EDTA and (b) 543 nm by alternate addition of Zn2+ and EDTA.
According to the aforementioned results, a molecular logic gate was further built, setting Al3+, Zn2+, and EDTA as multiple inputs. The group of Al3+ and Zn2+ was set as an OR logic gate, and EDTA merged the earlier logic gate to form an AND logic gate [39]. For input, the presence and absence of Al3+, Zn2+, and EDTA were assigned as 1 and 0, respectively. For output, turn-on fluorescence at 582 or 542 nm was recorded as 1, and quenched fluorescence was recorded as 0. As shown in Figure 7, in the absence of EDTA input, the fluorescence intensity of the chemosensor was enhanced (output = 1) either by Al3+ and Zn2+ input separately or by Al3+ and Zn2+ input together, and other input signals could cause the significant fluorescence quenching (output = 0).

(a) The possible logic gate circuit and (b) the truth table.
3.5 Sensing mechanism for Al3+ and Zn2+
To better understand the recognition mechanism of the chemosensor 1 to Al3+ and Zn2+, the binding stoichiometry of the complexation of 1 with Al3+ and Zn2+, respectively, was first verified using Job’s method. As shown in Figure 8, when the mole fraction of Al3+ or Zn2+ was reached around 0.33 and 0.50, respectively, the fluorescence intensity of the complex of 1–Al3+ at 582 nm and 1–Zn2+ at 542 nm reached the maximum, indicating the 2:1 stoichiometry for 1–Al3+ and 1:1 stoichiometry for 1–Zn2+ complex. Also, the linearity of the Bensi–Hildebrand (BH) graph showed the 1:1 bonding mechanism between 1 and Zn2+, whereas a poor linearity of BH graph was shown between 1 and Al3+ (Figure S6). In addition, the stoichiometric ratio has been confirmed using the free bindfit software, where a 2:1 and 1:1 stoichiometry of chemosensor 1 with Al3+ and Zn2+, respectively, gave highly satisfactory results (http://supramolecular.org). However, the fitting of the assumed conjugation of 1:2 and 1:1 resulted in lower values for binding constants of 1 with Al3+ as well as 1:2 and 2:1 resulted in negative values for binding constants of 1 with Zn2+ (Figure S7). In UV-Vis titration experiment, a strong absorbance maximum at 366 nm assigned to the π–π* transition of 1 was gradually weakened along with a simultaneous increase at 434 and 428 nm was observed upon progressive addition of Al3+ and Zn2+, respectively. And a clear isosbestic point at 398 nm for 1–Al3+ and 396 nm for 1–Zn2+ suggested the formation of a stable product (Figure S8). The results of UV–Vis absorption spectra indicated that the phenolic oxygen and imine nitrogen atoms in 1 might be involved in coordination with Al3+ and Zn2+ [40]. Furthermore, the signal peak of MALDI-TOF-MS at m/z = 595.3238 corresponding to [21 + Al-2H] (calcd. m/z 595.2838) and m/z = 627.7,871 corresponding to [1 + Zn + CH3OH + 2ClO4 + 2Na] (calcd. m/z 627.6,469) confirmed the 2:1 and 1:1 binding mode of 1 with Al3+ and Zn2+, respectively (Figure S9).

Job’s plot for determining the stoichiometry of 1 with (a) Al3+ and (b) Zn2+ with 366 nm excitation wavelength.
In addition, 1H-NMR titration experiment had been implemented for an in-depth understanding of the binding mode. In the case of Zn2+ (Figure 9), upon the addition of Zn2+, the phenolic-OH proton (Ha) peak at 10.47 ppm was weakened gradually and downfield shifted by 0.09 ppm. The signal at 10.91 ppm assigned to NH (Hb) exhibited a 0.10 ppm downfield shift. Meanwhile, the CH═N proton (Hc) at 8.30 ppm had been shifted downfield to 8.33 ppm. Also, the pyrazinyl proton signal Hg shifted to a lower field of about 0.04 ppm, and Hh and Hi had a little shift of about 0.02 ppm. Of all the benzene ring proton, Hd, He, and Hf shifted downfield about 0.08 ppm, 0.07 ppm, and 0.06 ppm, respectively, as well as these signals became blunt peaks because of the coordination reaction. Similarly, except for the proton signals of the benzene ring shifted upfield, other proton signals of 1 showed a trend of shifting downfield in the Al3+ titration experiment (Figure S10). In the FT-IR spectrum, the –OH stretching of 1 at 3,439 cm−1 disappeared upon the addition of Al3+ and Zn2+ with the appearance of a band at 3,370 cm−1. The peak at 1,634 cm−1 ascribed to C═N stretching moved toward a lower wavenumber around 1,628 and 1,624 cm−1 after complexation with Al3+ and Zn2+, respectively (Figure S11). The earlier results further demonstrated that phenol oxygen and imine nitrogen atoms of 1 are involved in the binding with Al3+ and Zn2+. Therefore, according to Job’s plot, MS, UV–Vis, 1H-NMR, and FT-IR results, the chemosensor 1 could provide three metal binding sites (phenol O, imine N, and pyrazine N) for Al3+ and Zn2+, and the reasonable coordination mode of 1 for Al3+, and Zn2+ is proposed in Scheme 2.

1H NMR spectra (400 MHz) measured during the titration of 1 with Zn2+ in d 6-DMSO.

Proposed reaction mechanism of 1 with Al3+ and Zn2+.
3.6 Practical applications
To explore the practical application as well as the sensitivity of chemosensor 1, experimental verification was implemented to detect Al3+ and Zn2+ in potable water and drug samples (Tables 1 and 2). All the results were measured three times in parallel. For the water samples, the accuracy was investigated via adding a known concentration of standard Al3+ or Zn2+ to the samples, and the results were analyzed using the linear relationship of the previously calculated fluorescence titrations. The results suggested that chemosensor 1 had excellent recoverability for the detection of Al3+ and Zn2+ in the tested water. Simultaneously, antacid and zinc granule supplementation drugs were selected as the test object for Al3+ and Zn2+, respectively. The content of Al3+ and Zn2+ in the drugs was calculated to be 0.954 and 42.81 mg·g−1, respectively, which was in good agreement with the actual content (42.81 mg·g−1 for Al3+ and 0.954 mg·g−1 for Zn2+). The results exhibited the effective content detection for target ions of the chemosensor 1. These results showed beyond doubt that chemosensor 1 could be used for the analysis and detection of Al3+ and Zn2+ in real water and drug samples.
Determination of Al3+ recovery in real samples
| Samples | Al3+ added (mM) | Al3+ found (n = 3, mM) | Recovery (n = 3, %) | RSD (%) | Relative error (%) |
|---|---|---|---|---|---|
| 20 | 19.14 | 95.70 | 0.79 | –0.86 | |
| Water | 30 | 28.66 | 95.53 | 1.52 | –1.34 |
| 40 | 39.41 | 98.53 | 1.07 | –0.59 | |
| Drug | — | 10.15 | — | 0.98 | — |
Determination of Zn2+ recovery in real samples
| Samples | Zn2+ added (mM) | Zn2+ found (n = 3, mM) | Recovery (n = 3, %) | RSD (%) | Relative error (%) |
|---|---|---|---|---|---|
| 30 | 30.26 | 100.89 | 1.32 | 0.26 | |
| Water | 45 | 45.58 | 101.28 | 0.87 | 0.58 |
| 55 | 53.78 | 97.78 | 1.65 | –1.22 | |
| Drug | — | 10.07 | — | 0.77 | — |
4 Conclusions
In summary, we have designed and developed a simple pyrazinyl-salicylimine Schiff base fluorescent chemosensor 1, which could achieve the identification and detection of Al3+ and Zn2+. Chemosensor 1 exhibited excellent sensitivity and selectivity toward Al3+ and Zn2+ in mixed-aqueous media (ethanol:H2O = 95:5) with distinct fluorescence “turn-on” signals. The fluorescence enhancement mechanisms of sensing Al3+ and Zn2+ were attributed to the CHEF effect and the stoichiometric ratios of 1 with Al3+ and Zn2+ appeared to be 2:1 and 1:1, respectively. Moreover, chemosensor 1 could reversibly identify Al3+ and Zn2+ upon the addition of EDTA, and thus, a reasonable logic circuit was constructed with fluorescence emission as an output signal. In addition, the easy-to-prepare 1-coated swabs could offer direct and rapid Al3+ and Zn2+ detection in real time by the naked eye. Importantly, chemosensor 1 was successfully applied to quantitative analysis of Al3+ and Zn2+ in actual samples. These findings provide a useful strategy for the design and construction of simple fluorescent chemosensors with multiple detection modes and potential utility in fluorescence analysis.
-
Funding information: This work was supported by the National Natural Science Foundation of China (No. 21703078), Science and Technology Research Project of the Department of Education of Jilin Province (No. JJKH20210454KJ).
-
Author contributions: Yucun Liu: writing – original draft, writing – review and editing, conceptualization, funding acquisition; Miao Wu: methodology, investigation; Jihan Zhao: methodology, formal analysis; Yuan Wang: validation, visualization; Yongling Zhang: funding acquisition, resources.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Kwon JY, Jang YJ, Lee YJ, Kim KM, Seo MS, Nam W, et al. A highly selective fluorescent chemosensor for Pb2+. J Am Chem Soc. 2005;127(28):10107–11. 10.1021/ja051075b.Search in Google Scholar
[2] Chen X, Pradhan T, Wang F, Kim JS, Yoon J. Fluorescent chemosensors based on spiroring-opening of xanthenes and related derivatives. Chem Rev. 2012;112(3):1910–56. 10.1021/cr200201z.Search in Google Scholar
[3] Yang C, Li Y, Wang J, He J, Hou H, Li K. Fast and highly selective detection of acetaldehyde in liquor and spirits by forming aggregation-induced emission luminogen. Sens Actuat B-Chem. 2019;285:617–24. 10.1016/j.snb.2019.01.104.Search in Google Scholar
[4] Sivaraman G, Iniya M, Anand T, Kotla NG, Sunnapu O, Singaravadivel S, et al. Chemically diverse small molecule fluorescent chemosensors for copper ion. Coord Chem Rev. 2018;357:50–104. 10.1016/j.ccr.2017.11.020.Search in Google Scholar
[5] Prodi L, Bargossi C, Montalti M, Zaccheroni N, Su N, Bradshaw JS, et al. An effective fluorescent chemosensor for mercury ions. J Am Chem Soc. 2000;122(28):6769–70. 10.1021/ja0006292.Search in Google Scholar
[6] Xu Z, Yoon J, Spring DR. Fluorescent chemosensors for Zn2+. Chem Soc Rev. 2010;39(6):1996–2006. 10.1039/B916287A.Search in Google Scholar
[7] Elkins KM, Nelson DJ. Spectroscopic approaches to the study of the interaction of aluminum with humic substances. Coord Chem Rev. 2002;228(2):205–25. 10.1016/S0010-8545(02)00040-1.Search in Google Scholar
[8] Perl DP, Gajdusek DC, Garruto RM, Yanagihara RT, Gibbs CJ. Intraneuronal aluminum accumulation in amyotrophic lateral sclerosis and parkinsonism-dementia of guam. Science. 1982;217(4564):1053–5. 10.1126/science.7112111.Search in Google Scholar
[9] Good PF, Olanow CW, Perl DP. Neuromelanin-containing neurons of the substantia nigra accumulate iron and aluminum in Parkinson’s disease: a LAMMA study. Brain Res. 1992;593(2):343–6. 10.1016/0006-8993(92)91334-B.Search in Google Scholar
[10] Alvim MN, Ramos FT, Oliveira DC, Isaias RMS, França MGC. Aluminium localization and toxicity symptoms related to root growth inhibition in rice (Oryza sativa L.) seedlings. J Biosci. 2012;37(1):1079–88. 10.1007/s12038-012-9275-6.Search in Google Scholar PubMed
[11] Gui S, Huang Y, Hu F, Jin Y, Zhang G, Yan L, et al. Fluorescence turn-on chemosensor for highly selective and sensitive detection and bioimaging of Al3+ in living cells based on ion-induced aggregation. Anal Chem. 2015;87(3):1470–4. 10.1021/ac504153c.Search in Google Scholar
[12] Samanta S, Goswami S, Hoque MN, Ramesh A, Das G. An aggregation-induced emission (AIE) active probe renders Al(III) sensing and tracking of subsequent interaction with DNA. Chem Commun. 2014;50(80):11833–6. 10.1039/C4CC05093B.Search in Google Scholar
[13] Berg JM, Shi Y. The galvanization of biology: A growing appreciation for the roles of zinc. Science. 1996;271(5252):1081–5. 10.1126/science.271.5252.1081.Search in Google Scholar
[14] Sun F, Zhang G, Zhang D, Xue L, Jiang H. Aqueous fluorescence turn-on sensor for Zn2+with a tetraphenylethylene compound. Org Lett. 2011;13(24):6378–81. 10.1021/ol2026735.Search in Google Scholar
[15] Finney LA, O’Halloran TV. Transition metal speciation in the cell: Insights from the chemistry of metal ion receptors. Science. 2003;300(5621):931–6. 10.1126/science.1085049.Search in Google Scholar
[16] Suh SW, Jensen KB, Jensen MS, Silva DS, Kesslak PJ, Danscher G, et al. Histochemically-reactive zinc in amyloid plaques, angiopathy, and degenerating neurons of Alzheimer’s diseased brains. Brain Res. 2000;852(2):274–8. 10.1016/S0006-8993(99)02096-X.Search in Google Scholar
[17] Na YJ, Hwang IH, Jo HY, Lee SA, Park GJ, Kim C. Fluorescent chemosensor based-on the combination of julolidine and furan for selective detection of zinc ion. Inorg Chem Commun. 2013;35:342–5. 10.1016/j.inoche.2013.07.011.Search in Google Scholar
[18] Tang L, Ding S, Zhong K, Hou S, Bian Y, Yan X. A new 2-(2′-hydroxyphenyl)quinazolin-4(3H)-one derived acylhydrazone for fluorescence recognition of Al3+. Spectrochim Acta A. 2017;174:70–4. 10.1016/j.saa.2016.11.026.Search in Google Scholar PubMed
[19] Li Y, Xu K, Si Y, Yang C, Peng Q, He J, et al. An aggregation-induced emission (AIE) fluorescent chemosensor for the detection of Al (III) in aqueous solution. Dye Pigm. 2019;171:107682. 10.1016/j.dyepig.2019.107682.Search in Google Scholar
[20] Qiu S, Cui S, Shi F, Pu S. Novel diarylethene-based fluorescent switching for the detection of Al3+ and construction of logic circuit. ACS omega. 2019;4(12):14841–8. 10.1021/acsomega.9b01432.Search in Google Scholar PubMed PubMed Central
[21] Xue L, Li G, Zhu D, Liu Q, Jiang H. Rational design of a ratiometric and targetable fluorescent probe for imaging lysosomal zinc ions. Inorg Chem. 2012;51(20):10842–9. 10.1021/ic301307v.Search in Google Scholar PubMed
[22] Mao Z, Hu L, Dong X, Zhong C, Liu B-F, Liu Z. Highly sensitive quinoline-based two-photon fluorescent probe for monitoring intracellular free zinc ions. Anal Chem. 2014;86(13):6548–54. 10.1021/ac501947v.Search in Google Scholar PubMed
[23] Ning P, Jiang J, Li L, Wang S, Yu H, Feng Y, et al. A mitochondria-targeted ratiometric two-photon fluorescent probe for biological zinc ions detection. Biosens Bioelectron. 2016;77:921–7. 10.1016/j.bios.2015.10.061.Search in Google Scholar PubMed PubMed Central
[24] Tang L, Li F, Liu M, Nandhakumar R. Single sensor for two metal ions: Colorimetric recognition of Cu2+and fluorescent recognition of Hg2+. Spectrochim Acta A. 2011;78(3):1168–72. 10.1016/j.saa.2010.12.072.Search in Google Scholar PubMed
[25] Long F, Qin JC, Li TR, Wang B, Yang ZY. A novel rhodamine chromone-based “Off–On” chemosensor for the differential detection of Al(III) and Zn(II) in aqueous solutions. Sens Actuat B-Chem. 2014;203:550–6. 10.1016/j.snb.2014.07.017.Search in Google Scholar
[26] Maity D, Govindaraju T. A differentially selective sensor with fluorescence turn-on response to Zn2+ and dual-mode ratiometric response to Al3+ in aqueous media. Chem Commun. 2012;48(7):1039–41. 10.1039/C1CC16064H.Search in Google Scholar PubMed
[27] Shellaiah M, Wu YH, Lin HC. Simple pyridyl-salicylimine-based fluorescence “turn-on” sensors for distinct detections of Zn2+, Al3+ and OH− ions in mixed aqueous media. Analyst. 2013;138(10):2931–42. 10.1039/C3AN36840H.Search in Google Scholar PubMed
[28] Goswami S, Paul S, Manna A. A differentially selective chemosensor for a ratiometric response to Zn2+ and Al3+ in aqueous media with applications for molecular switches. RSC Adv. 2013;3(47):25079–85. 10.1039/C3RA44539A.Search in Google Scholar
[29] Cao W, Zheng X-J, Sun J-P, Wong W-T, Fang D-C, Zhang J-X, et al. A highly selective chemosensor for Al (III) and Zn (II) and its coordination with metal ions. Inorg Chem. 2014;53(6):3012–21. 10.1021/ic402811x.Search in Google Scholar PubMed
[30] Tang Y, Sun J, Yin B. A dual-response fluorescent probe for Zn2+ and Al3+ detection in aqueous media: pH-dependent selectivity and practical application. Anal Chim Acta. 2016;942:104–11. 10.1016/j.aca.2016.08.048.Search in Google Scholar PubMed
[31] Liao ZC, Yang ZY, Li Y, Wang BD, Zhou QX. A simple structure fluorescent chemosensor for high selectivity and sensitivity of aluminum ions. Dye Pigment. 2013;97(1):124–8. 10.1016/j.dyepig.2012.12.017.Search in Google Scholar
[32] Goswami S, Das S, Aich K, Sarkar D, Mondal TK, Quah CK, et al. CHEF induced highly selective and sensitive turn-on fluorogenic and colorimetric sensor for Fe3+. Dalton Trans. 2013;42(42):15113–9. 10.1039/C3DT51974K.Search in Google Scholar PubMed
[33] Rahier R, Noiriel A, Abousalham A. Development of a direct and continuous phospholipase D assay based on the chelation-enhanced fluorescence property of 8-hydroxyquinoline. Anal Chem. 2016;88(1):666–74. 10.1021/acs.analchem.5b02332.Search in Google Scholar PubMed
[34] Qin J-C, Fan L, Wang B-D, Yang Z-Y, Li T-R. The design of a simple fluorescent chemosensor for Al3+/Zn2+via two different approaches. Anal Methods. 2015;7(2):716–22. 10.1039/C4AY02351J.Search in Google Scholar
[35] Benesi HA, Hildebrand J. A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J Am Chem Soc. 1949;71(8):2703–7. 10.1021/ja01176a030.Search in Google Scholar
[36] Liu H, Liu T, Li J, Zhang Y, Li J, Song J, et al. A simple Schiff base as dual-responsive fluorescent sensor for bioimaging recognition of Zn2+ and Al3+ in living cells. J Mater Chem B. 2018;6(34):5435–42. 10.1039/C8TB01743C.Search in Google Scholar PubMed
[37] Han T, Feng X, Tong B, Shi J, Chen L, Zhi J, et al. A novel “turn-on” fluorescent chemosensor for the selective detection of Al3+ based on aggregation-induced emission. Chem Commun. 2012;48(3):416–8. 10.1039/C1CC15681K.Search in Google Scholar PubMed
[38] Zhang J, Brutus TE, Cheng J, Meng X. Fluoride removal by Al, Ti, and Fe hydroxides and coexisting ion effect. J Env Sci. 2017;57:190–5. 10.1016/j.jes.2017.03.015.Search in Google Scholar PubMed
[39] Jain H, Deswal N, Joshi A, Ramachandran CN, Kumar R. Triazole-appended pyrano[2,3-c]pyrazolone based colorimetric chemosensors for recognition of Fe3+ ions and their molecular logic gate behavior. Anal Methods. 2019;11(25):3230–43. 10.1039/C9AY00515C.Search in Google Scholar
[40] Kong X-Y, Hou L-J, Shao X-Q, Shuang S-M, Wang Y, Dong C. A phenolphthalein-based fluorescent probe for the sequential sensing of Al3+ and F− ions in aqueous medium and live cells. Spectrochim Acta A. 2019;208:131–9. 10.1016/j.saa.2018.09.064.Search in Google Scholar PubMed
© 2022 Yucun Liu et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Abnormal retention of s-triazine herbicides on porous graphitic carbon
- One-factor-at-a-time method combined with ICP-MS for determining 11 elements in soy sauce and their migration from the containing glass bottles
- Analysis of initiator content of prepreg by near-infrared spectroscopy
- Simultaneous MEKC-DAD and smart spectrophotometric assays of thiocolchicoside and etoricoxib in challenging concentration ratio mixtures
- Alternative analytical methods for ibrutinib quantification in pharmaceutical formulation: A statistical comparison
- Chemometric determination of common cold infection drugs in human urine
- An effective, novel, and cheap carbon paste electrode for naproxen estimation
- Fabrication of ultra-sensitive carbon paste electrode with nanocomposite CdS modification for electroanalysis of rafoxanide in dosage form and biological fluids
- Purification and characterisation of phytochemicals extracted from Rhizophora mucronata: Their efficacy against Pseudomonas aeruginosa infection in Catla catla
- Review Articles
- Recent applications of quantitative analytical FTIR spectroscopy in pharmaceutical, biomedical, and clinical fields: A brief review
- Review of characteristics and analytical methods for determination of indomethacin
- A review of the application of comprehensive two-dimensional gas chromatography MS-based techniques for the analysis of persistent organic pollutants and ultra-trace level of organic pollutants in environmental samples
- Enrichment and analysis of glycated proteins
- Round robin tests of secondary raw materials: A systematic review of performance parameters
- Paper-based microfluidic devices: Fabrication, detection, and significant applications in various fields
- Applications of headspace solid-phase microextraction in human biological matrix analysis
- Chemometrics and infrared spectroscopy – A winning team for the analysis of illicit drug products
- Canagliflozin: A review with specific focus on analytical methods in biological matrices and pharmaceuticals
- RNA-based isothermal amplification technology and its clinical application in pathogen infection
- Detection of diarrheal shellfish toxins
- Special Issue: Nanomaterials with mimetic enzymatic properties for label-free sensors and biosensors (Guest Editors: Gang Wei and Zhiqiang Su)
- Preparation of cuprous oxide-supported silver-modified reduced graphene oxide nanocomposites for non-enzymatic electrochemical sensor
- Recent advances in matrix metalloproteinases-responsive nanoprobes for cancer diagnosis and therapy
- A simple salicylaldehyde-bearing pyrazine as a turn-on fluorescent chemosensor for Al3+ and Zn2+ recognition and its applications
- Preparation of silver nanosheet-assembled film as a surface-enhanced Raman scattering substrate
- Synthesis of group I–III–VI semiconductor quantum dots and its application in food safety testing
Articles in the same Issue
- Research Articles
- Abnormal retention of s-triazine herbicides on porous graphitic carbon
- One-factor-at-a-time method combined with ICP-MS for determining 11 elements in soy sauce and their migration from the containing glass bottles
- Analysis of initiator content of prepreg by near-infrared spectroscopy
- Simultaneous MEKC-DAD and smart spectrophotometric assays of thiocolchicoside and etoricoxib in challenging concentration ratio mixtures
- Alternative analytical methods for ibrutinib quantification in pharmaceutical formulation: A statistical comparison
- Chemometric determination of common cold infection drugs in human urine
- An effective, novel, and cheap carbon paste electrode for naproxen estimation
- Fabrication of ultra-sensitive carbon paste electrode with nanocomposite CdS modification for electroanalysis of rafoxanide in dosage form and biological fluids
- Purification and characterisation of phytochemicals extracted from Rhizophora mucronata: Their efficacy against Pseudomonas aeruginosa infection in Catla catla
- Review Articles
- Recent applications of quantitative analytical FTIR spectroscopy in pharmaceutical, biomedical, and clinical fields: A brief review
- Review of characteristics and analytical methods for determination of indomethacin
- A review of the application of comprehensive two-dimensional gas chromatography MS-based techniques for the analysis of persistent organic pollutants and ultra-trace level of organic pollutants in environmental samples
- Enrichment and analysis of glycated proteins
- Round robin tests of secondary raw materials: A systematic review of performance parameters
- Paper-based microfluidic devices: Fabrication, detection, and significant applications in various fields
- Applications of headspace solid-phase microextraction in human biological matrix analysis
- Chemometrics and infrared spectroscopy – A winning team for the analysis of illicit drug products
- Canagliflozin: A review with specific focus on analytical methods in biological matrices and pharmaceuticals
- RNA-based isothermal amplification technology and its clinical application in pathogen infection
- Detection of diarrheal shellfish toxins
- Special Issue: Nanomaterials with mimetic enzymatic properties for label-free sensors and biosensors (Guest Editors: Gang Wei and Zhiqiang Su)
- Preparation of cuprous oxide-supported silver-modified reduced graphene oxide nanocomposites for non-enzymatic electrochemical sensor
- Recent advances in matrix metalloproteinases-responsive nanoprobes for cancer diagnosis and therapy
- A simple salicylaldehyde-bearing pyrazine as a turn-on fluorescent chemosensor for Al3+ and Zn2+ recognition and its applications
- Preparation of silver nanosheet-assembled film as a surface-enhanced Raman scattering substrate
- Synthesis of group I–III–VI semiconductor quantum dots and its application in food safety testing


