Abstract
Gelatin, a natural functional material obtained from animal connective tissues, has been broadly applied in health-related products such as food, pharmacy, and cosmetic. But unclear labelling and false information of animal origin of gelatin in those products would violate religious rules and increase public health risks. Recently, animal origin identification of gelatin-based products has drawn more and more concerns. Among various identification methods, liquid chromatography-mass spectrometry (LC-MS) has specifically become a research hotspot for animal origin identification and quantitative analysis of gelatin-based products due to its superior reliability, selectivity, and sensitivity. The main desideratum of the current treatise is to review the recent progress on this subject with respect to: (1) the identification of animal in halal gelatin-based products, (2) the determination of the authenticity of gelatinous medicines from animal, especially from the highly homologous family species, and (3) the quantification of gelatin in gelatin-based products, using LC-MS method. We hope that this review could provide theoretical guidance and advanced strategies for developing animal origin identification technologies for gelatin-based products.
1 Introduction
Collagen, which is a long fibrous protein abundant in mammals, makes up 30% of the total protein mass in body [1]. So far, 28 types of collagen, called type I to type XXVIII based on the chronological order of their discovery, have been found and they mainly exist in animal connective tissues, such as skin, bone, blood vessel, tendon, cartilage, muscle, and ligament [2]. Usually, collagen from the animal raw material was hydrolyzed using dilute acid or alkali as the catalyst to the partial cleavage of cross-links of collagen and gelatin, as a common derivative of collagen, is formed. According to different catalysts, gelatin could be divided into type A gelatin (dilute acid as the catalyst) and type B gelatin (alkali as the catalyst) in the market [3]. The most common used gelatins in gelatin-based products are extracted from porcine, bovine, and fish [4]. Gelatin has been commonly used in the cosmetic, pharmaceutical, food, and photographic industries because of its unique structural stability and physio-chemical properties [5].
Nowadays, gelatin-based products require labelling and information about the animal origin which assist consumers to make choice about the products. Porcine gelatin (PG) is considered as non-halal materials because Muslims and Jews are not allowed to consume any products containing porcine ingredients [6]. On the other hand, there have been concerns that bovine gelatin (BG) may have the potential to carry certain diseases such as bovine spongiform encephalopathy [7]. Moreover, people may have allergy problems from exposure to certain animal-derived products. The use of inappropriate gelatin from animal origin can also induce the body's immune system responses, such as allergic reactions [8]. It has been reported that bovine gelatin in vaccine, erythropoietin products and suppositories could cause anaphylaxis [9]. Hence, there is an urgent requirement to develop a reliable method for identifying the animal origin of gelatin in the products.
The animal origin of the raw materials and the degree of processing have an effect on the physicochemical properties of the gelatin [10]. Due to the homology in the collagen structures of different animal species, the determination of gelatin animal origin is particularly challenging. Various analytical techniques are also developed for the determination of gelatin animal origin in gelatin-based products. The calcium phosphate precipitation was used as an identification strategy based on physicochemical properties to distinguish between bovine bone gelatin and porcine skin gelatin [11]. However, this technique has not been successful in detecting gelatin animal origin of mixtures containing gelatin and other substances.
Based on investigating the differences of functional groups in gelatins extracted from different animal origins using specific peaks, Fourier transform infrared (FTIR) spectroscopy could differentiate the animal origins of gelatins [12]. FTIR presents difficulties in identifying gelatin animal origin of mixtures containing gelatin and other substances. Polymerase chain reaction (PCR) serves as a powerful tool for tracing the origin of animals by exploring the specific markers of DNA [13]. However, degradation of DNA under hot water and acidic conditions reduces the accuracy of PCR identification of gelatin animal origin [14]. Proteins have a more stable primary structure than DNA during the preparation process. Enzyme-linked immunosorbent assay (ELISA) based on specific protein as marker has been used for speciation [15], but does not provide a species-specific identification for highly homologous gelatins [16]. At low concentrations of gelatin, liquid chromatography (LC) coupled with principle component analysis (PCA) provides sensitive analysis for the differentiation of gelatins from different animal origins [17]. Finally, liquid chromatography-mass spectrometry (LC-MS) is utilized to identify the animal origin of gelatin on the basis of marker peptides [18]. LC-MS method shows higher reliability than ELISA and PCR based techniques in tracking gelatin morphology [14].
Over the last few years, mass spectrometry methods, especially LC-MS method using marker peptides for determining the animal origin of gelatin, has been a hot topic. The LC-MS technology could be widely used in molecular weight detection of biomolecules [19], purification and analysis of bioactive peptides (antioxidant peptides, etc.) [20], and as a main proteomic analysis method [21]. Different LC-MS techniques have been adopted as a standard practice for protein analysis. The analysis of various gelatin-based products has been increasingly relied on proteomic approach using LC-MS method [22]. A schematic summary of available literature about the identification of gelatin animal origins through species-specific marker peptides is provided in Table 1. LC-MS method could simultaneously screen and use multiple marker peptides from the same species to differentiate between gelatins, thus increasing the selectivity of this method [23]. Amino acid sequence information for peptide fragments produced by trypsin digestion of gelatin is usually obtained by searching tandem mass spectrometry (MS/MS) databases. Based on the difference in collagen sequence, LC-MS method enables precise identification of gelatin animal origin. Moreover, in a recent research, quantitative analysis of gelatin could be performed by LC-MS method [24]. To a certain extent, the application of this method could ensure the safety, health, and reliability of gelatin-based products.
List of animal origins detectable with marker peptides in gelatin-based products using LC-MS method
| Animal origin | The range of animal origins | Marker peptide selected | Sequence difference of peptide from different animal origins | Marker peptide | Collagen type | Method in literature | Linearity | LOD | Percentage of detectability in mixtures |
|---|---|---|---|---|---|---|---|---|---|
| Porcine | Porcine | Marker selection | AGVMGPPGSR | AGVMGPpGSR | COL1A2 | UPLC-MS/MS (MRM mode) [33] | R2> 0.999 | 0.04% (wt) | 1% (pig in horse) |
| Horse | AGVMGPAGSR | ||||||||
| Cattle | AGVMGPAGSR | ||||||||
| Sheep | AGVMGPAGSR | ||||||||
| Tilapia | TGPVGMFGAR | ||||||||
| Deer | Donkey | Marker selection | AGETGASGPPGFAGEK | SGETGASGPpGFAGEK | COL1A2 | Nano-UPLC–MS/MS [40] | - | - | - |
| Cattle | SGETGASGPPGFVGEK | ||||||||
| Sheep | TGEPGAAGPPGFVGEK | ||||||||
| Porcine | TGETGASGPPGFAGEK | ||||||||
| Deer | SGETGASGPPGFAGEK | ||||||||
| Dog | TGETGASGPPGFTGEK | ||||||||
| Cat | TGETGASGPPGFAGEK | ||||||||
| Mouse | TGETGASGPPGFVGEK | ||||||||
| Rat | TGEIGASGPPGFAGEK | ||||||||
| Turtle | PGETGASGPPGFSGEK | ||||||||
| Donkey | Donkey Horse Hybrid Cattle Pig | SGQPGTVGPAGVR; GATGPAGVR | - | - | - | ||||
| Horse | Marker selection | - | SGQPGTVGPAGVR; GASGPAGVR | - | UPLC-ESI-MS/MS[26] | R2> 0.997 | 0.05% (wt) | 1% (horse in donkey) | |
| Hybrid | SGQPGTVGPAGVR ; GASGPAGVR; GATGPAGVR | R2> 0.997 | 0.10% (wt) | 20% (hybrid in donkey) | |||||
| Donkey | Donkey | Marker selection | - | GEAGPAGPAGPIGPVGAR | COL1A1 | HPLC-LTQ/Orbitrap MS/MS [44] | - | - | - |
| Porcine | Porcine | GETGPAGPAGPIGPVGAR | COL1A1 | ||||||
| Bovine | Bovine | GETGPAGPAGPIGPVGAR | COL1A1 | ||||||
| Donkey | Donkey Bovine | GEAGPAGPAGPIGPVGAR, GEAGPSGPGPTGAR | |||||||
| Bovine | Porcine Deer Tortoise | Marker selection | - | - | UPLC/Q-TOF-MS [39] | - | - | - | |
| Porcine | GEPGPTGVQGPPGPAGEEGK | ||||||||
| Bovine | Bovine | Marker selection | - | GPPGSAGSPGK PGEVGPPGPPGPAGEK TGPpGPSGISGPPGPPGPAGK GPPGSAGAPGK PGEVGPPGPPGPAGEK IGPpGPSGISGPPGPpGPAGK | COL1A1 | HPLC–MS/MS [23] | - | - | - |
| COL1A2 | |||||||||
| Porcine | Porcine | COL1A1 | |||||||
| COL1A2 | |||||||||
| Porcine | Porcine | Marker selection | - | GIpGEFGLpGPAGPR | COL1A2 | - | 0.05% (wt) | 10% (pig in bovine) | |
| Marker selection | - | GETGPAGPSGApGPAGSR | COL3A1 | UPLC-MS/MS [22] | - | - | - | ||
| Bovine | Bovine | Marker selection | GETGPAGPAGPIGPVGAR | COL1A1 | - | 0.05% (wt) | 10% (pig in bovine) | ||
| - | GEPGpTGIQGPpGPAGEEGK | ||||||||
| Bovine | Bovine | Sequence alignment | GETGPAGPpGApGAPGAPGPVGPAGK | COL1A1 | HPLC-LTQ-Orbitrap [24] | R2> 0.99 | 0.04 μg/mL | 1:10 (bovine in pig) | |
| Porcine | GETGPAGPAGPIGPVGAR | COL1A2 | |||||||
| GIpGPVGAAGATGAR | |||||||||
| GSTGEIGPAGPpGPPGLR | |||||||||
| GSTGEIGPAGPpGPpGLR | |||||||||
| EGPVGLpGIDGR | |||||||||
| GIpGEFGLpGPAGAR | |||||||||
| GPpGESGAAGPTGPIGSR | |||||||||
| GEpGVVGApGTAGPSGPSGLpGER | |||||||||
| TGppGPSGISGPPGPpGpAGK | |||||||||
| SGETGASGPpGFVGEK | |||||||||
| IGQPGAVGPAGIR | |||||||||
Animal origin identification of gelatin using LC-MS method in mixed matrix has become a challenging research hotspot in product detection. We mainly review the recent advances on this subject with respect to the animal origin identification and quantification of the gelatin-based products by LC-MS method. In this review, we will emphatically present the identification of animal components in halal gelatin-based products and the determination of the authenticity of gelatinous medicines from animal, using current detection techniques based on LC-MS method.
2 Sample preparation for LC-MS method
Since the gelatin with high molecular weight could not be detected by MS, it could be hydrolyzed by protease to detect the as-prepared hydrolysates. Trypsin, as a common protease used in LC-MS analysis, has been widely used in proteomic analysis due to its advantages of high specificity and the rich content of its restriction sites (lysine and arginine) in collagen [14,22,24,25]. In the enzymatic hydrolysis of gelatin procedure, gelatin solution and trypsin solution were mixed at the optimal operating pH of about 7.5–8.5 for trypsin. To obtain digestion solution, the mixture was incubated at the optimal operating temperature of trypsin (37°C) for about 10 h [26]. In addition, formic acid could be added in digestion solution to block digestion by creating an acidic environment for inactivating trypsin [14]. The insoluble components was removed from the digestion solution by centrifugation prior to analysis. Gelatin is digested to yield many kinds of peptides, including marker peptides for animal origin identification of gelatin.
3 The animal origin identification and quantification of the gelatin-based products by LC-MS method
3.1 Animal origin identification of gelatin-based products
Sequence analysis of collagens obtained from various animals indicates that some partial sequences are relatively specific, which could be used as marker peptides for gelatin differentiation [18]. Therefore, after gelatin was hydrolyzed by specific enzyme, some specific marker peptides have been obtained for identifying the animal origin of gelatin by LC-MS method. Scheme 1 shows the strategy of animal origin identification of gelatin-based products by LC-MS method.

Strategy of animal origin identification of gelatin-based products by LC-MS methods.
The gelatin to be detected can usually be digested by proteases such as trypsin [27]. The animal origins of gelatins are then identified through analysis of specific marker peptides in the enzymatic solution using LC-MS method. Table 1 lists some animal origins of gelatins detected by LC-MS method in literature. The identification of marker peptides is generally carried out by sequence alignment and selection of marker ions obtained from MS spectrum. Sequence alignment can compare the protein sequences from different animal origins in the database for direct peptide sequence analysis [23]. The peptide sequence obtained by this method is relatively comprehensive, but the results of this method are greatly affected by hydroxylation modification, such as hydroxyproline (Hyp) and hydroxylysine which show as proline and lysine in the protein sequence. In addition, the inability to determine the signal strength of peptides also limits the use of sequence alignment methods. The information of ions obtained from MS is rich and comprehensive. Therefore, it is necessary to conduct ion screening to obtain marker ions. Compared to sequence alignment, this method for obtaining marker peptide sequence is not disturbed by protein modification. However, inevitable and unconquerable errors from data processing tools, unstable signal and low repeatability will also affect the selection of marker peptides and analysis of peptide sequences [28]. The validation of candidate marker peptides selected is performed via basic local alignment search tool (BLAST) search, peptide synthesis and sample analysis for peptide.
Remarkably, the same mass difference among leucine (Leu), isoleucine (Ile), and Hyp are present in different peptides, which cannot be distinguished by low resolution mass spectrometry (Triple Quadrupole or Triple Quadrupole Ion Trap). High resolution mass spectrometry with the high mass accuracy can distinguish between Hyp and Leu (or Ile), but it is hard to distinguish between Leu and Ile. Therefore, attention should be paid to the presence of Leu and Ile in the screening and determination of marker peptides. MS/MS analysis was well used to solve the problem of determining the modified site of each marker peptide. Collision induced dissociation (CID) is a common parent ion fragmentation mechanism in MS/MS analysis. Different locations of the fractures produce different ion pairs (a–x, b–y, and c–z ions pairs). Figure 1 shows the fracture locations of the different ion pairs (a–x, b–y, and c–z ions pairs) produced by collision induced dissociation (CID). Compared to other ion pairs, b–y ion pairs are more easily detected due to their easier electrical charge. Therefore, accurate identification of b–y ion pairs obtained by MS/MS is the key to identify the sequence of the marker peptides [23,29].
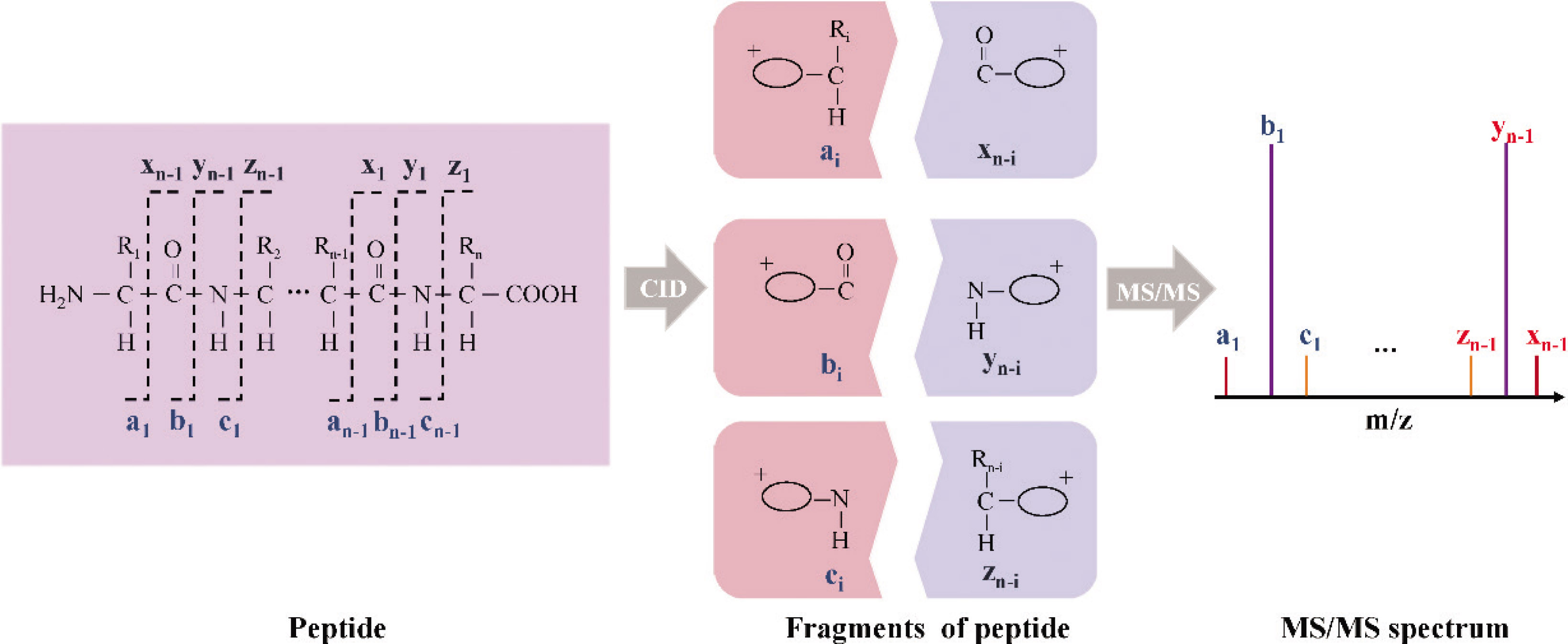
The fracture locations of the different ion pairs, a–x, b–y and c–z ions pairs produced by collision induced dissociation (CID).
3.1.1 Identification of the animal origin of gelatin in halal products
A common non halal substances found in gelatin-based products is PG. In terms of law and religious beliefs, the presence of PG is prohibited in all halal products. Accurately distinguishing PG from other gelatins that meet halal requirements is the key to ensuring the authenticity of halal gelatin-based products. BG was frequently used as a halal gelatin. Muslims also accepted other alternative animal origin gelatins (chicken gelatin and fish gelatin) as halal gelatin-based products besides BG [30]. It is necessary to ensure that PG is not exist in these other alternative animal origin gelatins with the purpose of respecting people religious beliefs and dietary habits.
During the identification of gelatin animal origin, the same mass amino acid such as Hyp, Leu and Ile may affect the judgment results of amino acid sequence, leading to misidentification of the animal origin [31]. For example, the marker peptide with the sequence G308AAGLP313GVAGAPGLPGPR325 is found in bovine COL1A2, and the marker peptide sequence G310AACLL315GVAGAPGLPGPR327 is also found in porcine COL1A2. Once Pro313 is hydroxylated, it might be confused with amino acids of the same mass, such as Leu and Ile, when deducing peptide sequence. The high resolution mass spectrometry improved mass accuracy of peptide analysis to the extent that Hyp, Leu, and Ile could be distinguished, which was not possible with conventional low resolution mass spectrometry. Therefore, high resolution mass spectrometry could be the preferred choice to analyze the primary structure of marker peptide, even with lower abundant. For example, nano-ultra-performance liquid chromatography and electrospray ionization quadrupole time-of-flight mass spectrometry (nano-UPLC-ESI-q-TOF-MS) was successfully used to identify PG from different animal origins of dairy products (cheese, yoghurt, and ice cream) by detecting marker peptides of PG [32]. The percent of peptides identified from the gelatin extracted from dairy products was lower than that from the gelatin samples, but unique marker peptides could still be analyzed to identify PG. The utilization of high resolution mass spectrometry achieved the detection of a unique porcine peptide (the sequence GFpGSpGNVGPAGK) with two hydroxylations (p: hydroxyproline). It might be possible to detect low contamination PG in BG, because they identified PG in the test sample prepared by mixing BG and PG in the weight ratio of 9:1. In order to improve the efficiency of identification, a rapid and simple UPLC-MS/MS multiple reaction monitoring (MRM) method with highly sensitivity has managed to identify PG in mixture of gelatins from different animal origins (cattle, horse, fish, and sheep) [33]. Important marker peptides with the sequence 414AGVMGPpGSR423 of PG that were utilized to identify PG in the mixture could be easily detected at an amount over 1% of the total weight.
The high resolution mass spectrometry used in previous studies is not as widely available in control and regulatory laboratories as low resolution mass spectrometry due to some reasons, such as higher purchase and maintenance costs. Compared to using LC-MS (high resolution mass spectrometry) alone, LC-MS (low resolution mass spectrometry) combined with chemometrics classification methods provide a simple way to reduce the workload on screening marker peptides from LC-MS data and the cost of tracing the animal origin of gelatin.
Principal component analysis (PCA) and partial least square discriminant analysis (PLS-DA) are the two most representative modeling methods in chemometrics. LC-MS techniques combined with PCA and PLS-DA were used to distinguish PG from separate origins, namely BG and fish gelatin (FG) [34]. Gelatin samples were analyzed by HPLC-ESI-QTRAP to generate a series of LC-MS data sets for providing PCA and PLS-DA objects. PCA as a descriptive method intuitively described the differences of PG, BG, and FG through the analysis of the first three principal components (species type, the process of gelatin preparation and geographical conditions) in gelatin sample. PCA was adjusted to get knowledge for setting the PLS-DA parameters. PLS-DA was applied to establish a suitable identification model using the control samples (gelatin samples derived from different animal origins) to determine the origin of the tested gelatin. The proposed methods are successful in the identification of PG in commercial pure gelatin and also food and drug products. After cross-evaluation of LC-MS gelatin animal origin analysis results and real-time PCR, the consistent results of both methods indicated that this established LC-MS method could be used for halal certification analysis of PG-based products. However, it is necessary to further analyze to obtain the sequence of marker peptide.
The development of these LC-MS analysis method can provide support for the halal gelatin-based product industries, such as halal foods (gummy, chewable, marshmallow) and pharmaceuticals (capsule shell). In this way, the authenticity of gelatin species will be determined and the increasing demand for animal origin constituent consistent with the label in halal gelatin-based products will be met.
3.1.2 Identification of the animal origin of gelatin in gelatin-based products for health
Detecting species origin is also important from health and commercial perspectives. Gelatin might be considered a potential risk for certain infectious diseases, such as bovine spongiform encephalopathy, avian leukosis virus, and Newcastle disease virus, etc., associated with animal origin. The establishment of LC-MS method has helped to eliminate the concerns of consumers about the existence of BG. For example, electrospray ionisation-liquid chromatography mass spectrometry (ESI-LC-MS/MS) was used to determine the animal origins of the commercial solid gelatins [14]. In commercial sample testing, undeclared bovine gelatin-based additives were discovered in commercial injection matrices labelled as containing chicken only.
In terms of health, certain diseases, such as bovine spongiform encephalopathy, carry a much higher risk of transmission in bovine bone gelatin than gelatin extracted from skins [35]. Thus, the detection of tissue origin is as important as the detection of the animal origin. The majority of the animal tissue origins of collagen and gelatin on the market are skin and bone. Skin gelatin and bone gelatin from the same animal origin have the higher degree of similarity. However, small but significant differences exist in the composition of amino acid. On the basis of the slight differences between skin gelatin and bone gelatin, Ghorbani et al. [36] by establishing the PCA and PLS-DA optimal model using HPLC-MS/MS data sets, conducted a study to distinguish the gelatins extracted from bovine bone and bovine skin. The successful differentiation of skin gelatin and bone gelatin has promoted the further development of the LC-MS method in animal tissue traceability. A database search of the selected peptide was performed to determine type of collagen which the peptide belonged to, so as to make a preliminary judgment on the origin of tissues. Three similar marker peptides extracted from bovine skin and bone, namely GETGPAGPSGApGPAGSR (COL3A1), GETGPAGPAGPIGPVGAR (COL1A1), and GEpGPAGAVGPAGAVGPR (COL1A2) were acquired by UPLC-MS/MS method [37]. These marker peptides have similar composition and arrangement of the amino acid. It was reported that the most prominent difference found in both bovine skin gelatin and bovine bone gelatin samples is that the marker peptide from bovine skin gelatin, GETGPAGPSGApGPAGSR (COL3A), almost undetectable in bovine bone gelatin. The reasons for this difference is due to the fact that the main protein in bone is type I collagen, while that in skin is type I and Type III collagen [38].
In addition, medicinal gelatins such as donkey-hide gelatin are the important animal-derived gelatinous medicines, which are very popular in Asian countries, especially in China. Generally, animal origin can associate with the efficacy of medicinal gelatins. Gelatin derived from unknown animal origin may pose certain risks to people. Hence, identification of the animal origin of the gelatin ensures the efficacy and authenticity of gelatin-based products. Some smart strategies were satisfactorily applied for identifying marker peptide in highly processed gelatinous medicines. A sample profiling method using ultra performance liquid chromatography/time-of-flight mass spectrometry (UPLC/Q-TOF-MS) coupled with PCA has been successfully utilized to distinguished donkey-hide gelatin, bovine-hide gelatin, pig-hide gelatin, deer-horn gelatin and tortoise shell glue [39]. At low concentration levels, tryptic peptides of gelatins were analyzed to measured important markers of differences. Some unique novel peptides were detected in these five gelatins such as GEAGPAGPAGPIGPVGAR with m/z 765.8556 for donkey-hide gelatin, unknown sequence with m/z 758.3530 for glue of tortoise shell and unknown sequence with m/z 732.8282 for deer-horn gelatin. These peptide markers could be successfully used for identification and differentiation of gelatins in mixtures. To further ensure the uniqueness of marker peptide, Liu et al. [40] proposed a simple strategy for identifying marker peptides of deer-hide gelatin using untargeted mass spectrometry (MS) and targeted MS. The resulting Nano-LC–MS/MS data were processed by mathematical set theory to ascertain peptides in the intersection sets of target species and other species, and the uniqueness of the peptides selected is verified using BLAST. The two peptides, including SGETGASGPpGFAGEK (at m/z 732.84) and GNAGPVGTAGApGPQGPVGPTGK (at m/z 1147.56) were recognized as the markers in deer-hide gelatin. As for the targeted MS, the two peptides were validated by the LC–MS/MS MRM analysis mentioned above to verify their species specificity.
Homologous degree of repetitive amino acids sequence among different species reveals their evolutionary relationship in a certain degree. The degree of homology is very high for gelatins from the homologous family species, which greatly increases the difficulty for their differentiation. In the case of high homology, the use of marker peptides from a single animal species to trace the origin of gelatin could make a definite impact on the accuracy of identification results. The combination of gelatin marker peptides from multiple animals can increase the accuracy of gelatin differentiation in animals of the same family. Currently, animal origin identification of gelatin obtained from the highly homologous species such as donkey, horse, and their hybrids (hinny and mule) continues to be a major challenge. Figure 2 shows the different strategies for the identification of donkey-derived gelatin from different animal origins with different homology degree. Marker peptides from a single animal species have been used to identify gelatin of different animal origins with low homology. A peptides combination method with highly sensitive and rapid detection was proposed for the animal origin identification of products containing donkey-hide gelatin [26]. Three peptides were selected from equine family species gelatin as a combination marker by high performance liquid chromatography coupled with linear ion traporbitrap mass spectrometry (HPLC-LTQ-Orbitrap). One of the marker peptides (SGQPGTVGPAGVR) could distinguish Equidae gelatin from other animals (BG and PG). Two other marker peptides (GASGPAGVR and GATGPAGVR) could also be utilized to differentiate donkey gelatin from horse gelatin and the hybrids gelatin. The LC-MS method can accurately accomplish the recognition of donkey, horse, and their hybrids in gelatin-based products according to the combination of the three peptides. The marker peptides combination method is helpful for identifying the animal origin of gelatin-based products from highly homologous family species.
![Figure 2 The strategies for identification of donkey-derived gelatin from animal origins with different homology degree. (a) Identification of donkey-derived gelatin from PG and BG with lower homology degree using signal marker peptide (Copyright 2018, Elsevier, License Number 4936280137359) [44]. (b) Identification of donkey-derived gelatin from horse-hide gelatin and hinny-hide gelatin with higher homology degree using marker peptide combination (Copyright 2020, Elsevier, License Number 4936370542390) [26].](/document/doi/10.1515/revac-2020-0121/asset/graphic/j_revac-2020-0121_fig_002.jpg)
The strategies for identification of donkey-derived gelatin from animal origins with different homology degree. (a) Identification of donkey-derived gelatin from PG and BG with lower homology degree using signal marker peptide (Copyright 2018, Elsevier, License Number 4936280137359) [44]. (b) Identification of donkey-derived gelatin from horse-hide gelatin and hinny-hide gelatin with higher homology degree using marker peptide combination (Copyright 2020, Elsevier, License Number 4936370542390) [26].
Depending on these studies mentioned above, LC-MS method has been verified to be particularly helpful in quality control of gelatin-based products.
3.2 LC-MS quantitative analysis method for gelatin from different animal origin
Gelatin-based products such as gelatin films, capsule shells, thickeners for frozen, premium fudge, ice cream, yogurt, and clarifier for fruit wine are widely used. Product frauds are a critical issue in the field of product safety and quality. Quantification of the gelatin from an animal in gelatin-based products is required for health safety concerns. Gelatin-based products from the single animal need to be tested and labelled to ensure animal constituent unicity and prevent illegal addition of other animal origins gelatins. It is necessary to measure whether the actual gelatin content of the gelatin-based products conforms to the content shown on the ingredient list. Considering the cost and practical application, triple quadrupole MS, which belong to low resolution mass spectrometry, is the preferred method for quantifying animal composition in gelatin. Some LC-MS methods have been validated on commercial products, using specially defined calibration lines to quantify the gelatin from different animal species [22,24]. Various indexes including linear correlation coefficient (R2), the limit of detection (LOD), the limit of quantification (LOQ) and reproducibility are used to evaluate these quantitative methods [41].
Some marker peptides with relatively poor and unstable response value are inappropriate for the identification and quantification of gelatin. In addition to experimental considerations such as sensitivity and peak shape, Kleinnijenhuis et al. [22] proposed a range of theoretical peptide selection criteria to screen out efficient quantitative marker peptides for gelatin: (1) peptide length between 6 and 20–25 amino acids (suitable length for MS detection), (2) no methionine, cysteine, asparagine, glutamine, (3) no post-translational modification (PTM) site except Hyp, and (4) uniqueness for target animal species. In particular, Hyp, which is very abundant in gelatin, could lead to an increase in gel strength and tensile strength [42]. Therefore, the identification of the proline hydroxylation-modified marker peptide is an important step before quantification of gelatin. Furthermore, a LC-MS method has been developed for determining the presence of porcine gelatin in bovine gelatin and vice versa by screening the marker peptides under the influence of different factors such as production process, animal origin, animal tissue, factory origin, and region [22].
Stable isotope labeled internal standard peptides was applied in the UPLC-MS/MS method, which was demonstrated to own acceptable and reliable superiority on the single sample level [22]. This validated method was adequately suited to detect PG traces in BG and vice versa, given the large variation in commercial gelatin due to different ingredients and processing methods. The lower limit of quantification for the detection of PG in the BG by this validated method was 0.05% and vice versa.
However, isotopic labeling methods suffered from disadvantages that limit their application in gelatin traceability research. For example, label-based methods, such as isotope labeling, may experience the risk of tag shedding over time [43]. The label-free method for quantitative BG and its film developed using HPLC-LTQ-Orbitrap with selected ion monitoring (SIM) mode has been reported to show excellent performance [24]. To ensure the effectiveness of the scheme, stable marker peptides that were detectable under the concentration of 1.00 μg/mL were used to quantify BG. The calibration equation with a high R2 value established by the relationship between the marker peptide and the gelatin content could be used to measure the unknown concentration of gelatin-based products in food system. What is most remarkable is that the effects of food matrices must be assessed at length before quantifying the target gelatin in gelatin-based products using this method. In other modes of MS, selected reaction monitoring (SRM) experiments using LC coupled with triple quadrupole tandem mass spectrometry have been extensively used in targeted quantitative proteomics. The relationship between SRM peak area and the content of marker peptide added was utilized to draw calibration curves with good linearity and all R2 greater than 0.997 [26]. Among these gelatin mixtures with the quantitative method, the good truth value of donkey-hide gelatin was found to be in the range of 90–110% after repetition of analysis. In gelatin-based products, horse-hide gelatin and hybrid-hide hide gelatin could be detected at 0.05% and 0.10%, respectively. This SRM method has high sensitivity to the quantification of Equidae origin gelatins (donkey, horse, and their hybrid). Moreover, multiple transitions can be monitored simultaneously in MRM experiments carried out on a triple quadrupole tandem mass spectrometry. Quantifying a peptide in complex gelatin samples generally requires multiple transitions, but the most abundant single transition may be sufficient to monitor peptides of specific proteins with high abundance in order to simplify the method. An accurate and sensitive quantitative analysis method for PG was established by UPLC-MS/MS in MRM mode involved in screening and synthesizing one unique PG marker peptide [33]. A standard curve with good linearity (R2 > 0.999) was plotted to explore the relationship between the injected solutions of peptide with various concentrations and the MRM peak area. Based on the capability of this quantitation method, PG could be detected when the content was above 0.04%. It indicated that the LC-MS quantitative methods possess good application and potential for the quantitative detection of gelatin-based products.
4 Conclusion
Gelatin, derived from animal species such as cattle, pig, fish, horse, and donkey, is widely used in many pharmaceutical, food, and cosmetic products. Porcine gelatin is banned to be consumed by Muslims and Jews, and bovine gelatin might be involved in certain diseases from cattle. In addition, product frauds such as unclear labeling and information distortion are a critical issue in the field of product safety and quality. It is crucially important to develop reliable methods for identifying gelatin animal origins and content in gelatin-based products.
The purpose of this mini review is to summarize a strategy for the identification of gelatin animal origin and the quantification of animal composition in gelatin using LC-MS methods. The efficiency of the identification of gelatin animal origin and the quantification of animal composition in gelatin depends on the analysis speed of MS. During the identification of gelatin animal origin, high resolution mass spectrometry with higher sensitivity is the first choice to detect the primary structure of peptide with high signal intensity. Considering the cost and practical application, triple quadrupole MS, which belong to low resolution mass spectrometry, is the preferred method for quantifying animal composition in gelatin. Compared with the LC-MS method used for the identification of gelatin animal origin, the LC-MS method used for quantification could shorten the analysis time and improve the detection efficiency by optimizing the elution gradient to the optimal state.
We have tried to outline the up-to-date outcomes of the identification and detection of gelatin-based products using LC-MS method. Most of the studies have revealed that LC-MS occupies a reliable position in proteomics. LC-MS method plays an important role in the animal origin identification and quantitative analysis of gelatin due to its superior selectivity and sensitivity. The LC-MS method developed for the identification of the composition of PG in halal gelatin-based products and the differentiation of the gelatin animal origins of gelatinous medicines are successful examples of such approaches. At the tissue level, the tissue origin of the gelatin from the same animal can be identified by LC-MS method. Additionally, the isotopic labeling methods and label-free methods developed by LC-MS method realized the quantification of gelatin by establishing mathematical models in different modes of MS. The purity of gelatin animal constituent in the gelatin-based products can be detected to ensure animal constituent unicity and prevent illegal addition of other animal origin gelatins. Therefore, LC-MS is one of the important tools to ensure the safety and authenticity of gelatin-based products. With the development of science and technology, LC-MS technology is also improving, and it still has great application potential in the animal origin identification of gelatin-based products.
Research funding: Authors state no funding involved.
Author contribution: Guiya Deng: Writing – original draft, Writing – review and editing, Investigation, Methodology; Shangwei Guo: Writing – review and editing, Investigation, Methodology; Fakhar Zaman: Writing – review and editing, Investigation; Tianyu Li: Writing – review and editing, Supervision; Yaqin Huang: Writing – review and editing, Supervision, Validation.
Conflict of interest: Authors state no conflict of interest.
Data availability statement: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
[1] Luca S, Nunzia G, Lucia NM, Lorena C, Paola L, Marta M, et al. Marine collagen and its derivatives: Versatile and sustainable bio-resources for healthcare. Mater Sci Eng C. 2020:110963.10.1016/j.msec.2020.110963Suche in Google Scholar PubMed
[2] Hong H, Fan H, Chalamaiah M, Wu J. Preparation of low-molecular-weight, collagen hydrolysates (peptides): Current progress, challenges, and future perspectives. Food Chem. 2019;301:125222.10.1016/j.foodchem.2019.125222Suche in Google Scholar PubMed
[3] Michelini L, Probo L, Farè S, Negrini NC. Characterization of gelatin hydrogels derived from different animal sources. Mater Lett. 2020;272(1):127865.10.1016/j.matlet.2020.127865Suche in Google Scholar
[4] Karim AA, Bhat R. Fish gelatin: properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocolloid. 2009;23(3):563–76.10.1016/j.foodhyd.2008.07.002Suche in Google Scholar
[5] Gómez-Guillén M, Giménez B, López-Caballero Ma, Montero M. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocolloid. 2011;25(8):1813–27.10.1016/j.foodhyd.2011.02.007Suche in Google Scholar
[6] Lubis HN, Mohd-Naim NF, Alizul NN, Ahmed MU. From market to food plate: Current trusted technology and innovations in halal food analysis. Trends Food Sci Tech. 2016;58:55–68.10.1016/j.tifs.2016.10.024Suche in Google Scholar
[7] Morris C. A review of genetic resistance to disease in Bos taurus cattle. Vet J. 2007;174(3):481–91.10.1016/j.tvjl.2006.09.006Suche in Google Scholar PubMed
[8] Kuno-Sakai H, Kimura M. Removal of gelatin from live vaccines and DTaP - an ultimate solution for vaccine-related gelatin allergy. Biologicals. 2003;31(4):245–9.10.1016/S1045-1056(03)00063-0Suche in Google Scholar PubMed
[9] Hori H, Hattori S, Inouye S, Kimura A, Irie S, Miyazawa H, et al. Analysis of the major epitope of the alpha2 chain of bovine type i collagen in children with bovine gelatin allergy. J Allergy Clin Immun. 2002 110(4):652–7.10.1067/mai.2002.127862Suche in Google Scholar PubMed
[10] Lin L, Regenstein JM, Lv S, Lu J, Jiang S. An overview of gelatin derived from aquatic animals: Properties and modification. Trends Food Sci Tech. 2017;68:102–12.10.1016/j.tifs.2017.08.012Suche in Google Scholar
[11] Hidaka S, Liu S. Effects of gelatins on calcium phosphate precipitation: a possible application for distinguishing bovine bone gelatin from porcine skin gelatin. J Food Compos Anal. 2003;16(4):477–83.10.1016/S0889-1575(02)00174-6Suche in Google Scholar
[12] Cebi N, Durak MZ, Toker OS, Sagdic O, Arici M. An evaluation of Fourier transforms infrared spectroscopy method for the classification and discrimination of bovine, porcine and fish gelatins. Food Chem. 2016;190:1109–15.10.1016/j.foodchem.2015.06.065Suche in Google Scholar PubMed
[13] Shabani H, Mehdizadeh M, Mousavi SM, Dezfouli EA, Solgi T, Khodaverdi M, et al. Halal authenticity of gelatin using species-specific PCR. Food Chem. 2015;184:203–6.10.1016/j.foodchem.2015.02.140Suche in Google Scholar PubMed
[14] Grundy H, Reece P, Buckley M, Solazzo C, Dowle A, Ashford D, et al. A mass spectrometry method for the determination of the species of origin of gelatine in foods and pharmaceutical products. Food Chem. 2016;190:276–84.10.1016/j.foodchem.2015.05.054Suche in Google Scholar PubMed
[15] Venien A, Levieux D. Differentiation of bovine from porcine gelatines using polyclonal anti-peptide antibodies in indirect and competitive indirect ELISA. J Pharmaceut Biomed Anal. 2005;39(3–4):418–24.10.1016/j.jpba.2005.04.013Suche in Google Scholar PubMed
[16] Nhari RMHR, Ismail A, Che Man YB. Analytical methods for gelatin differentiation from bovine and porcine origins and food products. J Food Sci. 2012;77(1):R42–R6.10.1111/j.1750-3841.2011.02514.xSuche in Google Scholar PubMed
[17] Nemati M, Oveisi M, Abdollahi H, Sabzevari O. Differentiation of bovine and porcine gelatins using principal component analysis. J Pharmaceut Biomed Anal. 2004;34(3):485–92.10.1016/S0731-7085(03)00574-0Suche in Google Scholar
[18] Zhang G-F, Liu T, Wang Q, Lei J-D, Ma G-H, Su Z-G. Identification of marker peptides in digested gelatins by high performance liquid chromatography/mass spectrometry. Chinese J Anal Chem. 2008;36(11):1499–504.10.1016/S1872-2040(09)60003-7Suche in Google Scholar
[19] Wu W, Li B, Hou H, Zhang H, Zhao X. Identification of iron-chelating peptides from Pacific cod skin gelatin and the possible binding mode. J Funct Food. 2017;35:418–27.10.1016/j.jff.2017.06.013Suche in Google Scholar
[20] Zhang Y, Duan X, Zhuang Y. Purification and characterization of novel antioxidant peptides from enzymatic hydrolysates of tilapia (Oreochromis niloticus) skin gelatin. Peptides. 2012;38(1):13–21.10.1016/j.peptides.2012.08.014Suche in Google Scholar PubMed
[21] Kasprzyk J, Stępień E, Piekoszewski W. Application of nano-LC-MALDI-TOF/TOF-MS for proteomic analysis of microvesicles. Clin Biochem. 2017;50(4–5):241–3.10.1016/j.clinbiochem.2016.11.013Suche in Google Scholar PubMed
[22] Kleinnijenhuis AJ, Van Holthoon FL, Herregods G. Validation and theoretical justification of an LC-MS method for the animal species specific detection of gelatin. Food Chem. 2018;243:461–7.10.1016/j.foodchem.2017.09.104Suche in Google Scholar PubMed
[23] Zhang G, Liu T, Wang Q, Chen L, Lei J, Luo J, et al. Mass spectrometric detection of marker peptides in tryptic digests of gelatin: A new method to differentiate between bovine and porcine gelatin. Food Hydrocolloid. 2009;23(7):2001–7.10.1016/j.foodhyd.2009.03.010Suche in Google Scholar
[24] Sha X-M, Wang G-Y, Li X, Zhang L-Z, Tu Z-C. Identification and quantification of gelatin by a high-resolution mass spectrometry-based label-free method. Food Hydrocolloid. 2020;101:105476.10.1016/j.foodhyd.2019.105476Suche in Google Scholar
[25] Burkhart JM, Schumbrutzki C, Wortelkamp S, Sickmann A, Zahedi RP. Systematic and quantitative comparison of digest efficiency and specificity reveals the impact of trypsin quality on ms-based proteomics. J Proteomics. 2012;75(4):1454–62.10.1016/j.jprot.2011.11.016Suche in Google Scholar PubMed
[26] Guo S, Deng G, Duan X, Zhou X, Huang Y. Marker peptide combination for source identification of gelatins obtained from Equidae hides by LC–MS/MS detection. Polym Test. 2020:106576.10.1016/j.polymertesting.2020.106576Suche in Google Scholar
[27] Cheison SC, Schmitt M, Leeb E, Letzel T, Kulozik U. Influence of temperature and degree of hydrolysis on the peptide composition of trypsin hydrolysates of β-lactoglobulin: Analysis by LC–ESI-TOF/MS. Food Chem. 2010;121(2):457–67.10.1016/j.foodchem.2009.12.065Suche in Google Scholar
[28] Tian H, Li B, Shui G. Untargeted LC-MS data preprocessing in metabolomics. J Anal Test. 2017;1:187–92.10.1007/s41664-017-0030-8Suche in Google Scholar
[29] Ocaña MF, Neubert H, Przyborowska A, Parker R, Bramley P, Halket J, et al. BSE Control: Detection of gelatine-derived peptides in animal feed by mass spectrometry. Analyst. 2004;129(2):111–5.10.1039/B312593ASuche in Google Scholar PubMed
[30] Zhang T, Sun R, Ding M, Li L, Tao N, Wang X, et al. Commercial cold-water fish skin gelatin and bovine bone gelatin: Structural, functional, and emulsion stability differences. LWT. 2020:109207.10.1016/j.lwt.2020.109207Suche in Google Scholar
[31] Rohman A, Windarsih A, Erwanto Y, Zakaria Z. Review on analytical methods for analysis of porcine gelatine in food and pharmaceutical products for halal authentication. Trends Food Sci Tech. 2020;101:122–32.10.1016/j.tifs.2020.05.008Suche in Google Scholar
[32] Yilmaz MT, Kesmen Z, Baykal B, Sagdic O, Kulen O, Kacar O, et al. A novel method to differentiate bovine and porcine gelatins in food products: NanoUPLC-ESI-Q-TOF-MSE based data independent acquisition technique to detect marker peptides in gelatin. Food Chem. 2013;141(3):2450–8.10.1016/j.foodchem.2013.05.096Suche in Google Scholar PubMed
[33] Guo S, Xu X, Zhou X, Huang Y. A rapid and simple UPLC-MS/MS method using collagen marker peptides for identification of porcine gelatin. RSC Adv. 2018;8(7):3768–73.10.1039/C7RA12539ASuche in Google Scholar
[34] Jannat B, Ghorbani K, Shafieyan H, Kouchaki S, Behfar A, Sadeghi N, et al. Gelatin speciation using real-time PCR and analysis of mass spectrometry-based proteomics datasets. Food Control. 2018;87:79–87.10.1016/j.foodcont.2017.12.006Suche in Google Scholar
[35] Wenz B, Oesch B, Horst MJB. Analysis of the risk of transmitting bovine spongiform encephalopathy through bone grafts derived from bovine bone. Biomaterials. 2001;22(12):1599–606.10.1016/S0142-9612(00)00312-4Suche in Google Scholar PubMed
[36] Jannat B, Ghorbani K, Kouchaki S, Sadeghi N, Eslamifarsani E, Rabbani F, et al. Distinguishing tissue origin of bovine gelatin in processed products using LC/MS technique in combination with chemometrics tools. Food Chem. 2020;319:126302.10.1016/j.foodchem.2020.126302Suche in Google Scholar PubMed
[37] Kleinnijenhuis AJ, van Holthoon FL, Herregods G. Validation and theoretical justification of an LC-MS method for the animal species specific detection of gelatin. Food Chem. 2018;243:461–7.10.1016/j.foodchem.2017.09.104Suche in Google Scholar PubMed
[38] Gelse K, Poschl E, Aigner T. Collagens - structure, function, and biosynthesis. Adv Drug Deliver Rev. 2003;55(12):1531–46.10.1016/j.addr.2003.08.002Suche in Google Scholar PubMed
[39] Cheng X-L, Wei F, Xiao X-Y, Zhao Y-Y, Shi Y, Liu W, et al. Identification of five gelatins by ultra performance liquid chromatography/time-of-flight mass spectrometry (UPLC/Q-TOF-MS) using principal component analysis. J Pharmaceut Biomed Anal. 2012;62:191–5.10.1016/j.jpba.2011.12.024Suche in Google Scholar PubMed
[40] Liu R, Huang Y, Xu H, Zheng Y, Liu Y, Han S, et al. A strategy for identifying species-specific peptide biomarkers in deer-hide gelatin using untargeted and targeted mass spectrometry approaches. Anal Chim Acta. 2019;1092:32–41.10.1016/j.aca.2019.09.064Suche in Google Scholar PubMed
[41] Li L, Wang H, Shuang Y, Li L. The preparation of a new 3, 5-dichlorophenylcarbamated cellulose-bonded stationary phase and its application for the enantioseparation and determination of chiral fungicides by LC-MS/MS. Talanta. 2019;202:494–506.10.1016/j.talanta.2019.05.011Suche in Google Scholar PubMed
[42] Aykın-Dinçer E, Koç A, Erbaş M. Extraction and physicochemical characterization of broiler (Gallus gallus domesticus) skin gelatin compared to commercial bovine gelatin. Poultry Sci. 2017;96(11):4124–31.10.3382/ps/pex237Suche in Google Scholar PubMed
[43] Xin Y, Wan B. A label-free quantification method for measuring graphene oxide in biological samples. Anal Chim Acta. 2019;1079:103–10.10.1016/j.aca.2019.06.036Suche in Google Scholar PubMed
[44] Sha X-M, Zhang L-J, Tu Z-C, Zhang L-Z, Hu Z-Z, Li Z, et al. The identification of three mammalian gelatins by liquid chromatography-high resolution mass spectrometry. LWT. 2018;89:74–86.10.1016/j.lwt.2017.10.001Suche in Google Scholar
© 2020 Guiya Deng et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Review Articles
- Liquid-phase microextraction of polycyclic aromatic hydrocarbons: A review
- Colorimetric hand-held sensors and biosensors with a small digital camera as signal recorder, a review
- Mini-Review of Analytical Methods used in Quantification of Ellagic Acid
- Highly Sensitive and Robust Capillary Electrophoresis-Electrospray Ionization-Mass Spectrometry: Interfaces, Preconcentration Techniques and Applications
- Spectroscopic Determination of Two Beta-Blockers – Atenolol and Propanolol by Oxidative Derivatization Using Potassium Permanganate in Alkaline Medium
- Review on analytical methods for quantification of ADHD drugs in human biological samples
- Benzene, toluene, ethylbenzene, and xylene: Current analytical techniques and approaches for biological monitoring
- Advances in neurochemical measurements: A review of biomarkers and devices for the development of closed-loop deep brain stimulation systems
- Review on stationary phases and coating methods of MEMs gas chromatography columns
- Research Article
- Xanthene based resonance Rayleigh scattering and spectrofluorimetric probes for the determination of cyclobenzaprine: Application to content uniformity test
- Special Issue: SPECIAL ISSUE ON 25TH INTERNATIONAL SYMPOSIUM ON SEPARATION SCIENCES
- Chromatographic analysis of bio-oil formed in fast pyrolysis of lignocellulosic biomass
- Urinary carboxylic acids (UCAs) in subjects with autism spectrum disorder and their association with bacterial overgrowth
- Separation procedures in the identification of the hydrogenation products of biomass-derived hydroxymethylfurfural
- The effect of selenium, zinc and copper on the excretion of urinary modified nucleobases in rats treated with prostate cancer cells
- Analytical approaches and preparation of biological, food and environmental samples for analyses of zearalenone and its metabolites
- Nanosized zinc, epigenetic changes and its relationship with DMBA induced breast cancer in rats
- Special Issue: Bioanalytical Methods and Their Applications
- Single-molecule force spectroscopy: A facile technique for studying the interactions between biomolecules and materials interfaces
- Biological nanoscale fluorescent probes: From structure and performance to bioimaging
- Detection of metal ions in biological systems: A review
- Recent advances in animal origin identification of gelatin-based products using liquid chromatography-mass spectrometry methods: A mini review
Artikel in diesem Heft
- Review Articles
- Liquid-phase microextraction of polycyclic aromatic hydrocarbons: A review
- Colorimetric hand-held sensors and biosensors with a small digital camera as signal recorder, a review
- Mini-Review of Analytical Methods used in Quantification of Ellagic Acid
- Highly Sensitive and Robust Capillary Electrophoresis-Electrospray Ionization-Mass Spectrometry: Interfaces, Preconcentration Techniques and Applications
- Spectroscopic Determination of Two Beta-Blockers – Atenolol and Propanolol by Oxidative Derivatization Using Potassium Permanganate in Alkaline Medium
- Review on analytical methods for quantification of ADHD drugs in human biological samples
- Benzene, toluene, ethylbenzene, and xylene: Current analytical techniques and approaches for biological monitoring
- Advances in neurochemical measurements: A review of biomarkers and devices for the development of closed-loop deep brain stimulation systems
- Review on stationary phases and coating methods of MEMs gas chromatography columns
- Research Article
- Xanthene based resonance Rayleigh scattering and spectrofluorimetric probes for the determination of cyclobenzaprine: Application to content uniformity test
- Special Issue: SPECIAL ISSUE ON 25TH INTERNATIONAL SYMPOSIUM ON SEPARATION SCIENCES
- Chromatographic analysis of bio-oil formed in fast pyrolysis of lignocellulosic biomass
- Urinary carboxylic acids (UCAs) in subjects with autism spectrum disorder and their association with bacterial overgrowth
- Separation procedures in the identification of the hydrogenation products of biomass-derived hydroxymethylfurfural
- The effect of selenium, zinc and copper on the excretion of urinary modified nucleobases in rats treated with prostate cancer cells
- Analytical approaches and preparation of biological, food and environmental samples for analyses of zearalenone and its metabolites
- Nanosized zinc, epigenetic changes and its relationship with DMBA induced breast cancer in rats
- Special Issue: Bioanalytical Methods and Their Applications
- Single-molecule force spectroscopy: A facile technique for studying the interactions between biomolecules and materials interfaces
- Biological nanoscale fluorescent probes: From structure and performance to bioimaging
- Detection of metal ions in biological systems: A review
- Recent advances in animal origin identification of gelatin-based products using liquid chromatography-mass spectrometry methods: A mini review

