Nanosized zinc, epigenetic changes and its relationship with DMBA induced breast cancer in rats
-
Barbara Bobrowska-Korczak
, Kamila Domanska
Abstract
The aim of the research was to compare the impact of nano- and micro-sized-zinc on the kinetics of changes in the level of 3-methyladenine, 7-methylguanine, 7-methylguanosine, O-methylguanosine, 1-methyladenosine, N6-methyl-2’-deoxyguanosine in urine of rats with breast cancer. Female Sprague-Dawley rats divided into 3 groups were used in the study. Animals were fed only a control diet or diets supplemented with the nano and micro-sized zinc particles. To induce the mammary cancer (adenocarcinoma), rats were treated with 7,12-dimethylbenz[a]anthracene (DMBA). Modified nucleosides were determined by a validated high performance liquid chromatography coupled to mass spectrometry method. In the first stage of investigations a synergistic activity of nanosized Zn with DMBA on the growth of the neoplastic process was found. During that time a statistically significant increase in the levels of all six examined markers in the rats’ urine was observed. However, as the experiment continued, the supplementation with nanosized zinc caused inhibition of tumour growth, being followed by regression and remission of tumours, as well as, a statistically significant systematic reduction of the levels of methyl derivatives in the urine. Biopsy images indicated grade 1 tumours with multiple inflammatory infiltrates in the group treated with zinc nanoparticles, whereas, in the other groups, moderately-differentiated grade 2 adenocarcinoma was identified. It was found that the biological activity of zinc depends on the size of applied particles, as the treatment with zinc microparticles has not had much effect on cancer progression.
1 Introduction
Recent studies have shown a potential role of using nano-particles of zinc in biomedicine and therapeutics. Several studies suggested an increase in in vitro cytotoxicity with nanosized zinc as compared with larger bulk-sized (micro and larger) particles for certain types of cancer including breast [1,2], colon [3], lung [4], ovarian [5], bone [6], leukemia [7], cervical [8], and liver [9]. Numerous publications showed that the activity of zinc nanoparticles is mainly due to the formation of reactive oxygen species (ROS) [1]. ROS production is proposed as a key cytotoxic mechanism of zinc nanoparticles leading to cell death via an apoptotic mechanism. The loss of the mitochondrial membrane potential could open outer membrane pores which would result in the release of some related apoptotic proteins including cytochrome c into the cytosol and activate the caspase. Moghaddam et al. [1] demonstrated that the possibility of using zinc nanoparticles in treatment of MCF-7 breast cancer and excessive ROS generation caused upre-gulation of the pro-apoptotic p53, p21, Bax, and JNK genes, whereas anti-apoptotic genes Bcl-2, AKT1, and ERK1/2 were downregulated in a dose-dependent manner. Zn2+ nanoparticles exposure was shown to induce the production of a variety of proinflammatory cytokines, including TNF-α, IFN-γ, IL-12, IL1β [10, 11, 12]. Nanoparticle-induced cytokines could also facilitate effective anti-cancer actions by eliciting a cytokine profile crucial for directing the development of Th1-mediated immunity [13,14]. The Th1 lymphocyte subset plays an essential role in enhancing the natural cytotoxic potential of natural killer cells and T cytotoxic cells against cancer cells. Excessive ROS generation which, if effectively targeted at the cancer cells, will lead to their selective destruction. This mechanism was confirmed in several experiments using laboratory animals [15, 16, 17]. Nanoparticles can lead to an increase in ROS production and oxidative DNA damage, which may affect the ability of methyltransferases activity leading to DNA hypomethylation and altered expression of methylated-regulated genes. However, the effect of nanoparticles on epigenetics/methylation has not been fully investigated yet. The changes in global or gene-specific methylation patterns can affect the overall processes related to the course of cancer transformations proceeding by way of both genetic and epigenetic changes. Recent studies have highlighted the application of the combination of the autophagy inhibitor 3-methyladenine and radioche-motherapy in the diagnosis and treatment of cancer. This combination is known to effectively reduce the proliferation and induce apoptosis of cancer cells [18, 19, 20]. More information is needed, especially on physicochemical properties of nanoparticles, their behaviour in environments of cancer and their interaction with the biological system.
The aim of the present research was to assess the impact of nano- and micro-sized-zinc on the kinetics of changes (9-11-17-20 week of a rat’s life) in the level of 3-methyladenine, 7-methylguanine, 7-methylguanosine, O-methylguanosine, N6-methyl-2’-deoxyguanosine 1-methy-ladenosine in urine of rats with mammary cancer (adenocarcinoma) induced with 7,12-dimethylbenz[a] anthracene. The studies of the impact of dietary components on the growth and development of the neoplastic process and on selected biomarkers can be very important both in cancer prevention and in pharmacological treatment of cancer. According to the opinion of Food and Drug Administration zinc is generally recognized as biosafe (GRAS) [21].
2 Materials and methods
2.1 Synthesis of zinc nanoparticles
1.36 g of anhydrous zinc chloride and 50 mL of 1,3,5-tri-methylbenzene (dried over activated 4Å molecular sieves) were placed in a 100 mL round-bottomed flask. 15 mL of solvent was removed using Dean-Stark technique with stirring. Next, 330 mg of PVP and 15 mL of 1,3,5-trimethyl-benzene were added and another 15 mL of solvent was removed using Dean-Stark technique. After that, the reaction mixture was cooled to 0°C and 760 mg of LiAlH4 was added. Dean-Stark apparatus was replaced with a reflux condenser, and the reaction mixture was refluxed for 24 h with vigorous stirring. In the further step, the reaction mixture was allowed to cool to room temperature, and cold methanol was added until LiAlH4 decomposition. Resulted zinc nanoparticles were transferred to appropriate container, centrifuged (3500 rpm, 6-min) and washed with a cold methanol 3 times, then dried overnight [22].
2.2 Synthesis of zinc microparticles
Zinc microparticles were synthesized in the similar way as nanoparticles except the step, when the reaction mixture was heated at 100°C instead of refluxed. The synthesized zinc micro- and nanoparticles were stabilized with polyvinylpyrrolidone K15 (PVP, Mw 10 000, TCI EUROPE N.V., Zwijndrecht, Belgium) to prevent oxidation and aggregation.
2.3 Measurement
The average particle size and zeta potential of the particles were determined by a dynamic light scattering (DLS) technique using a Zetasizer Nano ZS instrument (Malvern Instruments, Westborough, MA, USA) equipped with a red laser at a wavelength of 633 nm and scattering angle of 173° at 25°C (Table 1, Figure 1).
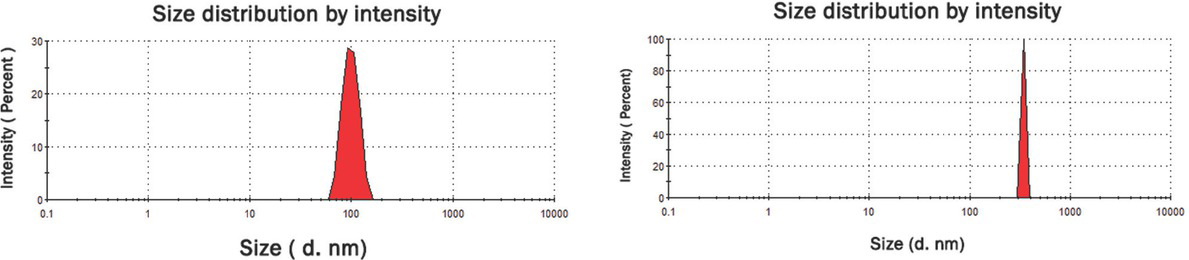
DLS size distribution graph for synthesized zinc nano- (left) and microparticles (right).
2.4 Laboratory animals
Female Sprague-Dawley rats (n = 24) were obtained from the Animal Laboratory, Department of General and Experimental Pathology, Medical University of Warsaw. The study was approved by the Ethics Committee of Medical University of Warsaw (code 645/2018). All rats were provided Labofeed H standard diet (standard diet: Labofeed H, Żurawia 19, 89-240 Kcynia, Poland) and water ad libitum and housed in an environmentally-controlled room maintained at 22°C with a 12-h light-dark cycle.
2.5 Experimental procedure
The experiment was conducted over 100 days. After the adaptation period (10 days) the animals were randomly divided into three main experimental groups: (i) rats, which were fed only the standard diet – without supplementation, receiving 0.4 mL of water, orally, via gavage (ii) rats with the micro-sized zinc particles (342 nm) supplementation at a dose of 4.6 mg/mL, administered in 0.4 mL of water, orally, via gavage, (iii) rats with the nano-sized zinc particles (99 nm) supplementation, administered orally, via gavage, at the same dose of 4.6 mg/mL in 0.4 mL of water. The rats were fed extra supplements from 40 days until 20 weeks of age (Figure 2). Doses of supplemented trace elements were determined on the basis of values used in the Labofeed H diet (extrapolated on the rats’ body weight). It was exceeded the standard diet zinc content two times, i.e. 76.9 mg/kg diet. Identifying the results of zinc activity in selected dose may allow its application in human cancer prevention or for improvement of pharmacological treatment.
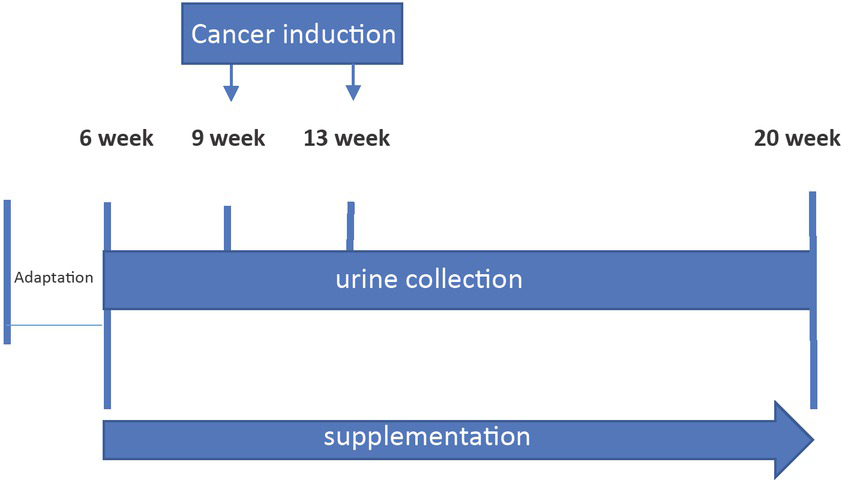
Scheme of the experimental procedure.
To induce the mammary cancer (adenocarcinoma), rats were treated twice via gavage with DMBA dissolved in a rapeseed oil (7,12-dimethyl-1,2-benz[a]anth-racene; Sigma-Aldrich, St. Louis, MO, USA). The first treatment was performed at 60 days of age (80 mg/kg of body weight), followed by another dose of 40 mg/kg of body weight at 90 days of age. The animals were examined by palpation during the study to characterize the time course of tumour development. In order to obtain urine samples, each animal was individually placed in a metabolic cage for 24 h. Urine samples were collected once a week during the 9-11-17-20th weeks of rodent’s age and stored at a temperature of −70°C until the test time. At 140 days of age, animals were sacrificed by intraperitoneal injection of morbital followed by spinal cord disruption. Amount of administered morbital used for euthanasia was 0.3 mL/kg, active substances: sodium pentobarbital + pentobarbital. 1 mL of the product contains 133.3 mg of sodium pentobarbital and 26.7 mg of pentobarbital, i.e. per kg of body weight it will be administered 39.99 mg (0.3 mL) of sodium pentobarbital and 8.01 mg (0.3 mL) of pentobarbital. During the autopsy, tumours were identified, differentiated with other anatomical structures and harvested. The tumours were then weighed, packed and sent to the laboratory for histopathological examination.
2.5 Histopathological analysis of tumours
Tumours collected from rats after decapitation were subjected to histopathological examination. Tissues were recorded in a buffered formalin solution. Then they were dehydrated, sealed in paraffin and cut into 4 μm thick scraps. The hematoxylin and eosin staining of tissue and cell sections was applied and sections were evaluated using a BX43 Olympus research microscope.
2.6 LC-MS/MS analysis
Reference standards i.e. 1-methyladenine, 3-methyladenine, 7-methylguanine, 1-methyladenosine, 7-methylguanosine, N6-methyl-2’-deoxyguanosine as well as internal standard (tubercidin) were purchased from Sigma Aldrich (St Louis, Mo, USA). Modified nucleosides and nucleobases were determined by validated high performance liquid chromatography coupled to mass spectrometry (LC-MS/MS) method using multiple reaction monitoring (MRM) mode on Agilent 1260 Infinity (Agilent Technologies, Santa Clara, CA, US) coupled to QTRAP 4000 (AB Sciex, Framingham, MA, US). MRM transitions, declustering potential (DP), and collision energy (CE) for O-methylguanosine, 1-methyladenosine, 7-methylguanosine, 7-methylguanine, 3-methyladenine and N6-methyl-2-deoxyadenosine were: (m/z) 298 > 152 (DP = 51 V, CE = 17 V), 282 > 55 (DP = 66 V, CE = 87 V), 298 > 166 (DP = 71 V, CE = 19 V), 166 > 79 (DP = 96 V, CE = 43 V), 150 > 123 (DP = 86 V, CE = 31 V) and 266 > 150 (DP = 61 V, CE = 23 V), respectively. Chromatographic separation was achieved using SeQuant® ZIC®-HILIC (50 × 2.1 mm, 5 μm, Merck) column. The column was maintained at 25°C at the flow rate of 0.5 mL min−1. The mobile phases consisted of 20 mM ammonium acetate as the eluent A and acetonitrile with 0.2% formic acid as the eluent B. The gradient (%B) was as follows: 0 min 95%; 1 min 95%; 7 min 50%, 8 min 50%. The injection volume was 5 μL. Urine samples (0.1 mL) prior to injection to LC were mixed with tubercidin (0.1 mL, 1 μg/mL) and acetonitrile (0.6 mL), vortexed in high speed (3 min) and centrifuged (5 min at 10,000 g). The method was validated according to the guidelines of the European Medicines Agency (EMA) [23].
2.7 Statistics
The Statistica 12.0 software (StatSoft, USA) was used for statistical analysis. The normal distribution of the data was tested using the Shapiro-Wilk method. For the normal data, the Student’s test and ANOVA, followed by Tukey’s test were used for analysis. The non-normal data was analyzed with Mann-Whitney U nonparametric test. In order to verify the results of tumor incidence, the relative risk (RR) calculation was used. The results were considered statistically significant when p < 0.05.
3 Results
As a result of the performed investigations it was found that the supplementation of rats with nanosized zinc inhibits the formation of tumours. In the case of rats that were not supplemented and obtained a standard diet the incidence of cancer was 100%, the weight of tumours was in the range 0.1–7.8 g and the number of tumours per rat ranged from 2 to 9 (4.63 ± 2.39) (Table 2). Similar results were obtained in the case of rats supplemented with microsized zinc (incidence 100%, tumour weight 0.06–7.41 g, number of tumours per rat 1-6 (3.75 ± 1.39)). Whereas in the case of rats supplemented with nano-sized zinc in week 16 of the rats’ lifetime, i.e. 7 weeks after DMBA administration the first tumours were observed in all rats from that group, which could suggest that there is a synergistic effect of nanosized zinc and DMBA as concerns the development of the neoplastic process (Table 3).
Tumour induction in 7,12-dimethylbenz[a]anthracene treated groups in relation to supplementation
| Supplementation | The week when first tumour occurred | Number of tumours per animal (week 20) mean ± SD | Tumour incidence (%) (week 20) | Tumour weight (g) (week 20) |
|---|---|---|---|---|
| standard | 16 (2/8) | 4.63 ± 2.39a (2-9) | 100% | 0.10–7.80 |
| micro-zinc | 16 (6/8) | 3.75 ± 1.39b (1-6) | 100% | 0.06–7.41 |
| nano-zinc | 16 (8/8) | 1.75 ± 1.04ab (0-3) | 88% | 0.01–1.79 |
Values sharing a letter indicate statistically significant differences between groups (p < 0.01).
Tumour incidence (week 9-11-16-17-20) (%) (number animals that developed tumors)
| Supplementation | Tumour incidence (%) | ||||
|---|---|---|---|---|---|
| week 9 | week 11 | week 16 | week 17 | week 20 | |
| standard | 0/8 | 0/8 | 2/8 (25%) | 5/8 (63%) | 8/8 (100%) |
| micro-zinc | 0/8 | 0/8 | 6/8 (75%) | 8/8 (100%) | 8/8 (100%) |
| nano-zinc | 0/8 | 0/8 | 8/8 (100%) | 8/8 (100%) | 7/8 (88%) |
However, as the experiment continued, the supplementation with nanosized zinc caused inhibition of tumour growth, being followed by regression and remission of tumours (incidence 88%, number of tumours per rat 0-3 (1.75 ± 1.04) while the weight of tumours at the end of the experiment was 0.01–1.79 g) (Table 2). Moderately differentiated tumour cells, with areas of increased proliferation, characteristic of grade 2 adenocarcinoma, were found both in the control and in the group supplemented with zinc microparticles (Figures 3a,b). In contrast, in the group supplemented with zinc nanoparticles, the grade 1 tumours were found and multiple inflammatory infiltrates were observed, with abundant lymphocytes around tumour sites (Figure 3c).
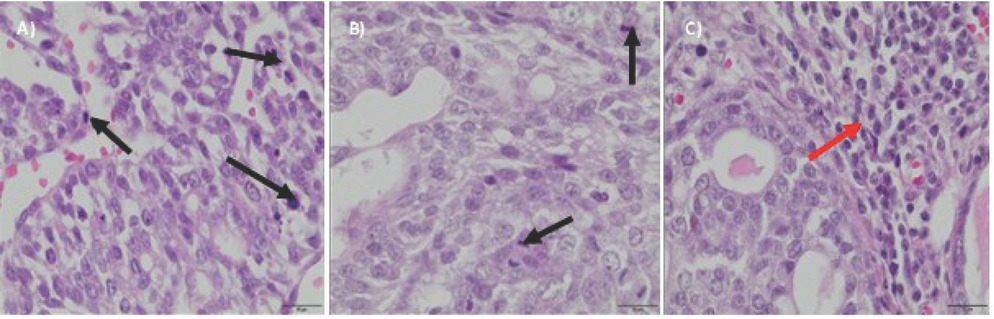
Biopsy images of tumour samples with 40x magnification; hematoxylin and eosin staining was applied. DMBA-induced tumour in the control group – II grade adenocarcinoma (a), in the group with the zinc microparticles supplementation – II grade adenocarcinoma (b), in the group supplemented with zinc nanoparticles – I grade adenocarcinoma (c). Black arrows indicate areas of the increased proliferation. Red arrow indicate inflammatory infiltrates.
It was shown that the kinetics of changes in the levels of selected methyl derivatives in urine depends on the size of Zn particles (nano- or micro-sized) with which the rats were supplemented (Figure 4). Urine taken for the investigations was from week 9-11-17-20 of the rats’ lifetime, that is from the period just before DMBA administration (three weeks after the supplementation began), two weeks after the rats were implanted with DMBA, the period when the first tumours appeared, and also at the end of the experiment (when the tumours were fully developed). It was shown that the supplementation of rats with nanosized zinc resulted in a significant increase of the concentration of 3-methyladenine, 7-methylguanine, 7-methylguanosine, O-methylguano-sine, N6-methyl-2’-deoxyguanosine, 1-methyladenosine in urine from week 9 and 11, as concerns both the rats which obtained the standard diet and those whose diet was supplemented with microsized zinc. The supplementation with nanosized zinc resulted in regression and remission of tumours, as well as, a statistically significant reduction of the levels of methyl derivatives in urine from week 20 of the rats’ lifetime. In the case of rats that obtained only the standard diet as well as those supplemented with microsized zinc an increase of the levels of the examined methyl derivatives was found together with the development of the neoplastic process (week 9 → 20).
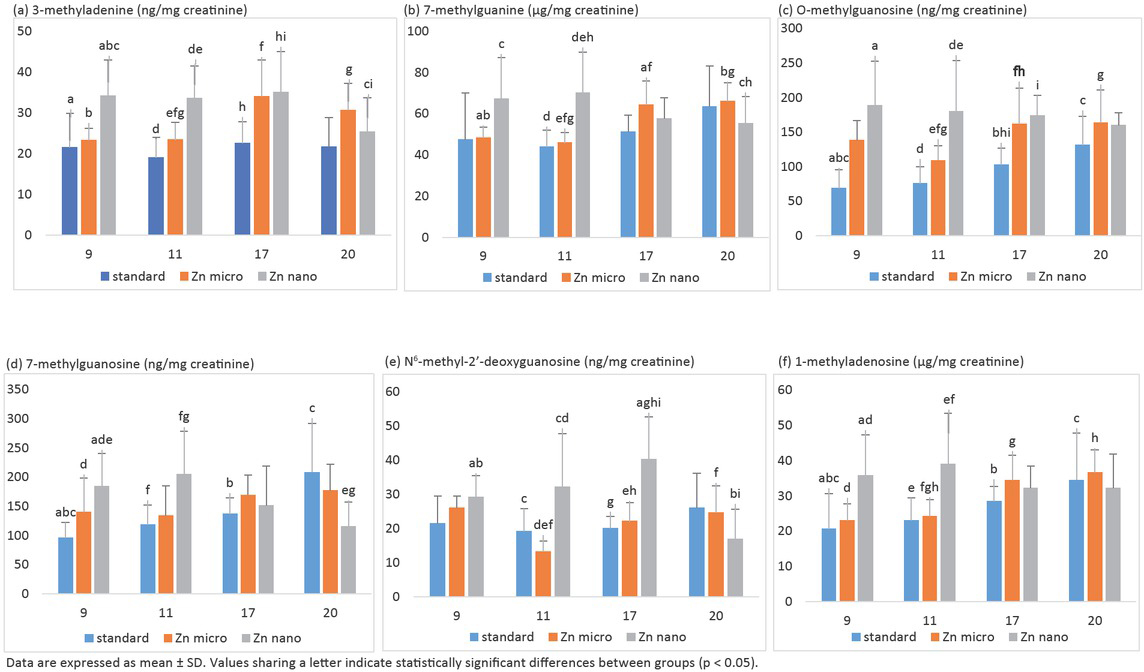
The effect of nano- and micro-sized-zinc on the kinetics of changes (9-11-17-20 week of rat’s life) in the level of: (a) 3-methyladenine, (b) 7-methylguanine, (c) O-methylguanosine, (d) 7-methylguanosine (ng/mg creatinine), (e) N6-methyl-2’-deoxyguanosine, and (f) 1-methyladenosine in urine of rats treated with DMBA.
4 Discussion
For many years modified nucleosides have been suggested as tumour markers [24, 25, 26, 27, 28, 29]. It was found that their levels are frequently elevated in patients with oncogenic disease. However, so far few studies have been published concerning the kinetics of changes in the profile of the above-mentioned biomarkers at the early step of tumour growth as well as the assessment of the impact of nanosized components on this process. To the best of our knowledge, these are the first investigations into the effect of nanosized zinc on the concentration of 3-methyladenine, 7-methylguanine, 7-methylguanosine, O-methylguanosine, N6-methyl-2’-deoxyguanosine and 1-methyladenosine in urine of rats that were implanted with 7,12-dimethylbenz[a]anthracene to induce mammary cancer. Moreover, it is interesting to note that 3-methyladenine, is a potent autophagy inhibitor. Autophagy, which is a catabolic process of self-digestion, plays a key role in various physiopathological processes, such as cell death and survival, development and tumorigenesis. Connections with cellular life-and-death decisions and with cancer are now emerging [30, 31, 32].
Zinc is an essential micronutrient for many living organisms [33]. As the main component of various enzyme systems, zinc takes part in metabolic processes and plays an essential role in proteins and nucleic acid synthesis, hematopoiesis and neurogenesis. A wide range of physiological defects, including skin disorders, growth retardation and impaired neurological, reproductive and immune systems are associated with zinc deficiency [34]. In the last few years considerable progress has been observed in investigations into better understanding of the dependence of the diet and expression of certain genes and their influence on the initiation and progression of multiple diseases. The reversibility of epigenetic transformations raises hope that some dietary components can be used both in prevention and treatment of certain diseases, especially cancer. It was shown that zinc plays the role of a cofactor for several enzymes of the methionine cycle transsulfuration pathway, which is a key pathway creating the donors of methyl groups such as S-adenosyl-L-methionine (SAM) or betaine. Betaine-homocysteine methyltransferase and methionine synthase are the other enzymes whose activity depends on the presence of zinc [35]. Serine hydroxymethyltransferase which is the main enzyme metabolizing folic acid and transferring methyl groups from serine to the methionine cycle is regulated by zinc-dependent transcription factors, including transcription factor 1. The above data provides strong foundations to state that zinc is an important dietary component which participates in maintaining the correct state of methylation in the cells. Zinc deficiency can result in the deficiency of methyl groups, similarly as in the case of a low supply of other donors of methyl groups such as folic acid. Many patients with cancer, especially of the lungs, breast, head and neck, have a decreased level of zinc in the blood [36,37]. Studies have shown that the combined zinc and melatonin therapy can contribute to the prevention of tumor growth by improving the disruption in element metabolism, and an increase in the immunity parameters and reducing tissue damage that causes the cancer [38,39].
The studies on zinc in nanosized form have become particularly important these days. In this form zinc is currently used in the production of foods (as a source of Zn nutrient), plastics, paper, ceramics, glass, cement, rubber, textiles and as ingredients of personal care products including cosmetics and toothpaste [40,41]. The nano form changes zinc bioavailability. Zinc bioavailability is defined in three basic steps: absorption, penetration into systemic circulation and use in the cells. Reduction of materials of the nanoscale can sometimes lead to the development of new structural, phytochemical, electronic, and magnetic properties that are not present in larger bulk-sized (micro and larger) particles comprised in the same material system. In comparison with their bulky counterparts, nanoparticles have novel/ different physical properties such as an increased surface area to volume ratio, reactive sites, charge, shape, mobility and thermal properties [40]. The nano form of zinc can unambiguously change the properties of zinc which affect our bodies.
In the performed studies it was shown that the supplementation of rats with nanosized zinc affects the development of the cancer process and the kinetics of changes as concerns the level of selected biomarkers in the rats’ urine. In the first stage of investigations a synergistic activity of nanosized Zn with DMBA on the growth of the neoplastic process was found. In week 16, malignant tumours were found by palpation in all the nano-sized Zn supplemented rats (in the case of rats obtaining a standard diet and those supplemented with micro-sized zinc the per cent amount was 25% and 75%, respectively). In the case of short-term exposure nanosized zinc was found to stimulate the growth of cancer. What is interesting to note, during that time a statistically significant increase of the levels of all six examined markers: 3-methyladenine, 7-methylguanine, 7-methylguanosine, O-methylguanosine, N6-methyl-2’-deoxyguanosine and 1-methyladenosine in the rats’ urine was observed. Elevated levels of the discussed compounds were found in urine from patients suffering from breast [24], lung [25], ovarian [26], bladder [27], colon [28], and leukemia [29] cancer. The changes in association/dissociation of specific proteins with methylated bases can disturb normal biological functions. Recent studies indicated that auto-phagy defection increases cancer cell growth [30]. Interestingly, 3-methyladenine decreased the Zn-induced autophagy [42,43]. However, starting from week 17–18 the supplementation with nanosized zinc resulted in regression and remission of tumours as well as a statistically significant systematic reduction of the levels of methyl derivatives in urine. No such dependence was found either in the case of unsupplemented rats obtaining only the standard diet or those supplemented with microsized zinc. In both cases an increase of the level of the examined methylderivatives together with the growth of cancer process was found (week 9 → 20). The inhibiting effect of nanosized zinc was confirmed by the results of cancer incidence, tumour weight and the number of tumours per rat. As a result of the performed investigations it can be concluded that the supplementation of rats with nano-sized zinc particles affects the growth of cancer process also through the methylation and epigenetic mechanisms. Zn inhibits cell proliferation and induces apoptosis and autophagy. High concentrations of Zn induces DNA fragmentation and nuclear morphological changes [42,43]. Rapidly growing evidence has linked exposure to nanoparticles with epigenetic variations, including changes in DNA methylation, histone modifications, and mRNA [44]. Some of such epigenetic changes were associated with variation in gene expression. Patil et al. [44] showed a direct correlation between the concentration of ZnO nanoparticles, global DNA methylation and expression levels of DNA methyltransferase 1, 3A, and 3 B genes upon exposure. Gong et al. [45] examined the epigenetic response to nm-SiO2 particles in human HaCaT cells. It was found that nanosized-SiO2 treatment induced global hypoacetylation implying a global epigenetic response. The levels of DNMT1, DNMT3a and methyl-CpG binding protein 2 (MBD2) were also decreased in a dose dependent manner at mRNA and protein level. The Ag-nanoparticles treatment caused a significant reduction of the global methylation level for histone 3 (H3) in erythroid MEL cells in sublethal concentrations [46]. The analyses demonstrated that methylation of H3 at lysine (Lys) 4 (H3K4) and Lys 79 (H3K79) on the β-globin locus was greatly reduced. The reduction in methylation could be attributed to decreased histone methyltransferase DOT-1L and MLL levels as well as the direct binding between nanosized Ag to H3/H4. By contrast, Ag ion-treated cells showed no alterations at the histone methylation level. Alterations in the methylation status of global DNA and transposable elements in mouse lung were observed after exposure to CuO [47]. In the literature there is an increasing number of articles confirming the effect of nanocompounds on the genome. Our investigations into nanosized zinc have confirmed that this is the right direction of research which could be important while searching for compounds with anticancer properties. The reversibility of epigenetic changes raises great hopes that some substances found in food can be used both in prevention and treatment of diseases, particularly cancer.
It can be concluded that the development of cancer, as well as, the kinetics of changes in the level of selected methylderivatives in urine depends on the size of particles (micro or nano) with which the rats were supplemented.
References
[1] Moghaddam AB, Moniri M, Azizi S, Rahim RA, Ariff AB, Navaderi M, et al. Eco-friendly formulated zinc oxide nanoparticles: induction of cell cycle arrest and apoptosis in the MCF-7 cancer cell line. Genes. 2017;8:281–96.10.3390/genes8100281Suche in Google Scholar PubMed PubMed Central
[2] De Angelis I, Barone F, Zijno A, Bizzarri L, Russo MT, Pozzi R, et al. Comparative study of ZnO and TiO2 nanoparticles: physicochemical characterisation and toxicological effects on human colon carcinoma cells. Nanotoxicology. 2013;7:1361–72.10.3109/17435390.2012.741724Suche in Google Scholar PubMed
[3] Condello M, De Berardis B, Ammendolia MG, Barone F, Condello G, Degan P, et al. ZnO nanoparticle tracking from uptake to gentotoxic damage in human colon carcinoma cells. Toxicol Vitr. 2016;35:169–79.10.1016/j.tiv.2016.06.005Suche in Google Scholar PubMed
[4] Bai KJ, Chuang KJ, Ma CM, Chang TY, Chuang HC. Human lung adenocarcinoma cells with an EGFR mutation are sensitive to non-autophagic cell death induced by zinc oxide and aluminium-doped zinc oxide nanoparticles. J Toxicol Sci. 2017;42:437–44.10.2131/jts.42.437Suche in Google Scholar PubMed
[5] Bai DP, Zhang XF, Zhang GL, Huang YF, Gurunathan S. Zinc oxide nanoparticles induce apoptosis and autophagy in human ovarian cancer cells. Int J Nanomedicine. 2017;12:6521–35.10.2147/IJN.S140071Suche in Google Scholar PubMed PubMed Central
[6] Nair S, Sasidharan A, Divya RV, Menon D, Nair S, Manzoor K, et al. Role of size scale of ZnO nanoparticles and microparticles on toxicity toward bacteria and osteoblast cancer cells. J Mater Sci Mater Med. 2009;20:235–41.10.1007/s10856-008-3548-5Suche in Google Scholar PubMed
[7] Guo D, Wu C, Jiang H, Li Q, Wang X, Chen B. Synergistic cytotoxic effect of different sized ZnO nanoparticles and daunorubicin against leukemia cancer cells under UV irradiation, J Photochem Photobiol B. 2008;93:119–26.10.1016/j.jphotobiol.2008.07.009Suche in Google Scholar PubMed
[8] Pandurangan M, Enkhtaivan G, Kim DH. Anticancer studies of synthesized ZnO nanoparticles against human cervical carcinoma cells. J Photochem Photobiol B. 2016;158:206–11.10.1016/j.jphotobiol.2016.03.002Suche in Google Scholar PubMed
[9] Sharma V, Anderson D, Dhawan A. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2). Apoptosis. 2012;17:852–70.10.1007/s10495-012-0705-6Suche in Google Scholar PubMed
[10] Poon WL, Alenius H, Ndika J, Fortino V, Kolhinen V, Meščeriakovas A, et al. Nano-sized zinc oxide and silver, but not titanium di-oxide, induce innate and adaptive immunity and antiviral response in differentiated THP-1 cells. Nanotoxicology. 2017;11:936–51.10.1080/17435390.2017.1382600Suche in Google Scholar PubMed
[11] Sayes CM, Reed KL, Warheit DB. Assessing toxicity of fine and nanoparticles: comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicol Sci. 2007;97:163–80.10.1093/toxsci/kfm018Suche in Google Scholar PubMed
[12] Hanley C, Thurber A, Hanna C, Punnoose A, Zhang J, Wingett DG. The influences of cell type and ZnO nanoparticle size and immune cell cytotoxicity and cytokine induction. Nanoscale Res Lett. 2009;4:1409–20.10.1007/s11671-009-9413-8Suche in Google Scholar PubMed PubMed Central
[13] Lappin MB, Campbell JD. The Th1-Th2 classification of cellular immune responses: concepts, current thinking and applications in hematological malignancy. Blood Rev. 2000;14:228–39.10.1054/blre.2000.0136Suche in Google Scholar PubMed
[14] Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–85.10.1038/nri2526Suche in Google Scholar PubMed PubMed Central
[15] Hejazy M, Koohi MK. Effect of subacute exposure of nano zinc particles on oxidative stress parameters in rats. Iran J Vet Med. 2017;11:155–63.Suche in Google Scholar
[16] Ansar S, Abudawood M, Alaraj ASA, Hamed SS. Hesperidin alleviates zinc oxide nanoparticle induced hepatotoxicity and oxidative stress. BMC Pharmacol Toxicol. 2018;19:1–6.10.1186/s40360-018-0256-8Suche in Google Scholar PubMed PubMed Central
[17] Kuang H, Yang P, Yang L, Aguilar ZP, Xu H. Size dependent effect of ZnO nanoparticles on endoplasmic reticulum stress signaling pathway in murine liver. J Hazard Mater. 2016;317:119–26.10.1016/j.jhazmat.2016.05.063Suche in Google Scholar PubMed
[18] Liu B, Zhang B, Zhang W, Zhang Y, Feng W, Li Y, Cao X. Effect of 3-methyladenine on apigenin-induced autophagy and apoptosis in the breast cancer T47D cell line. Chinese J Clin Oncol. 2011;38:1318–21.Suche in Google Scholar
[19] Zhang X, Zhao W. Effect of autophagy inhibitor 3-methylad-enine combined with TP chemotherapy on nasopharyngeal carcinoma and EGFR and VEGF levels in tissues. Int J Clin Exp Med. 2019;12(5):6403–10.Suche in Google Scholar
[20] Zhang R, Wang R, Chen Q, Chang H. Inhibition of autophagy using 3-methyladenine increases cisplatin-induced apoptosis by increasing endoplasmic reticulum stress in U251 human glioma cells. Mol Med Rep. 2015;12:1727–32.10.3892/mmr.2015.3588Suche in Google Scholar PubMed PubMed Central
[21] Rasmussen JW, Martinez E, Louka P, Wingett DG. Zinc Oxide Nanoparticles for Selective Destruction of Tumor Cells and Potential for Drug Delivery Applications. Expert Opin Drug Deliv. 2010;7:1063–77.10.1517/17425247.2010.502560Suche in Google Scholar PubMed PubMed Central
[22] Ghanta SR, Rao MH, Muralidharan K. Single-pot synthesis of zinc nanoparticles, borane (BH3and closo-dodecaborate (B12H122− using LiBH4 under mild conditions. Dalton Trans. 2013;42:8420–5.10.1039/c3dt00092cSuche in Google Scholar PubMed
[23] Raćkowska E, Bobrowska-Korczak B, Giebułtowicz J. Development and validation of a rapid LC-MS/MS method for determination of methylated nucleosides and nucleobases in urine. J Chromatogr B. 2019;1128:121775.10.1016/j.jchromb.2019.121775Suche in Google Scholar PubMed
[24] Zheng YF, Kong HW, Xiong JH, Lv S, Xu GW. Clinical significance and prognostic value of urinary nucleosides in breast cancer patients. Clin Biochem. 2005;38:24–30.10.1016/j.clinbiochem.2004.09.021Suche in Google Scholar
[25] Seidel M, Seidel P, Manuwald O, Herbarth O. Modified nucleosides as biomarkers for early cancer diagnose in exposed populations. Environ Toxicol. 2015;30:956–67.10.1002/tox.21970Suche in Google Scholar
[26] Oerlemans F, Lange F. Major and modified nucleosides as markers in ovarian cancer: a pilot study. Gynecol Obstet. 1986;22:212–17.10.1159/000298916Suche in Google Scholar
[27] Saad AA, O’Connor PJ, Mostafa MH, Metwalli NE, Cooper DP, Margison GP, et al. Bladder tumor contains higher N7-methylguanine levels in DNA than adjacent normal bladder epithelium. Cancer Epidemiol Biomark Prev. 2006;15:740–3.10.1158/1055-9965.EPI-05-0813Suche in Google Scholar
[28] Zheng YF, Yang J, Zhao XJ, Feng B, Kong HW, Chen YJ, et al. Urinary nucleosides as biological markers for patients with colorectal cancer. World J Gastroenterol. 2005;11:3871–6.10.3748/wjg.v11.i25.3871Suche in Google Scholar
[29] Itoh K, Konno T, Sasaki T, Ishiwata S, Ishida N, Misugaki M. Relationship of urinary pseudouridine and methyladenosine to activity of leukemia and lymphoma. Clin Chim Acta. 1992;206:181–9.10.1016/0009-8981(92)90087-7Suche in Google Scholar
[30] Denton D, Nicolson S, Kumar S. Cell death by autophagy: Facts and apparent artefacts. Cell Death Differ. 2012;19:87–95.10.1038/cdd.2011.146Suche in Google Scholar PubMed PubMed Central
[31] Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–75.10.1038/nature06639Suche in Google Scholar PubMed PubMed Central
[32] Karantza-Wadsworth V, Patel S, Kravchuk O, Chen G, Mathew R, Jin S, et al. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev. 2007;21:1621–35.10.1101/gad.1565707Suche in Google Scholar PubMed PubMed Central
[33] Prasad AS. Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp Gerontol. 2008;43:370–7.10.1016/j.exger.2007.10.013Suche in Google Scholar PubMed
[34] Chasapis CT, Loutsidou AC, Spiliopoulou CA, Stefanidou ME. Zinc and human health: an update. Arch Toxicol. 2012;86:521–34.10.1007/s00204-011-0775-1Suche in Google Scholar PubMed
[35] Dhawan DK, Chadha VD. Zinc: a promising agent in dietary chemoprevention of cancer. Indian J Med Res. 2010;132:676–82.Suche in Google Scholar
[36] Prasad AS. Zinc in human health: effect of zinc on immune cells. Mol Med Camb Mass. 2008;14:353–7.10.2119/2008-00033.PrasadSuche in Google Scholar PubMed PubMed Central
[37] Alam S, Kelleher SL. Cellular mechanisms of zinc dysregulation: a perspective on zinc homeostasis as an etiological factor in the development and progression of breast cancer. Nutrients. 2012;4:875–903.10.3390/nu4080875Suche in Google Scholar PubMed PubMed Central
[38] Baltaci SB, Mogulkoc R, Baltaci AK, Emsen A, Artac H. The effect of zinc and melatonin supplementation on immunity parameters in breast cancer induced by DMBA in rats. Arch Physiol Biochem. 2018;124:247–52.10.1080/13813455.2017.1392580Suche in Google Scholar PubMed
[39] Gulbahce-Mutlu E, Baltaci SB, Menevsa E, Mogulkoc R, Baltaci AK. The Effect of Zinc and Melatonin Administration on Lipid Peroxidation, IL-6 Levels, and Element Metabolism in DMBA-Induced Breast Cancer in Rats. Biol Trace Elem Res 2020; DOI: 10.1007/s12011-020-02238-0.10.1007/s12011-020-02238-0Suche in Google Scholar PubMed
[40] Ma H, Williams PL, Diamond SA. Ecotoxicity of manufactured ZnO nanoparticles – a review. Environ Pollut. 2013;172: 76–85.10.1016/j.envpol.2012.08.011Suche in Google Scholar PubMed
[41] Nohynek GJ, Dufour EK, Roberts MS. Nanotechnology, cosmetics and the skin: is there a health risk? Skin Pharmacol Physiol. 2008;21:136–49.10.1159/000131078Suche in Google Scholar PubMed
[42] John E, Laskow TC, Buchser WJ, Pitt BR, Basse PH, Butterfield LH, et al. Zinc in innate and adaptive tumor immunity. J Transl Med 2010;8:1–16.10.1186/1479-5876-8-118Suche in Google Scholar PubMed PubMed Central
[43] Zhou X, Li Y, Li CM. Autophagy plays a positive role in zinc-induced apoptosis in intestinal porcine epithelial cells. Toxicol in Vitro. 2017;44:392–402.10.1016/j.tiv.2017.08.006Suche in Google Scholar PubMed
[44] Patil NA, Gade WN, Deobagkar DD. Epigenetic modulation upon exposure of lung fibroblast to TiO2 and ZnO nanoparticles: alternations in DNA methylation. Int J Nanomedicine. 2016;11:4509–19.10.2147/IJN.S110390Suche in Google Scholar PubMed PubMed Central
[45] Gong C, Tao G, Yang L, Liu J, Liu Q, Zhuang Z. SiO2 nanoparticles induce global genomic hypomethylation in HaCaT cells. Biochem Biophys Res Commun. 2010;397:397–400.10.1016/j.bbrc.2010.05.076Suche in Google Scholar PubMed
[46] Qian Y, Zhang J, Hu Q, Xu M, Chen Y, Hu G, et al. Silver nanoparticle-induced hemoglobin decrease involves alteration of histone 3 methylation status. Biomaterials. 2015;70:12–22.10.1016/j.biomaterials.2015.08.015Suche in Google Scholar PubMed
[47] Lu X, Miousse IR, Pirela SV, Moore JK, Melnyk S, Koturbash I, et al. In vivo epigenetic effects induced by engineered nanomaterials: A case study of copper oxide and laser printer-emitted engineered nanoparticles. Nanotoxicology. 2016;10(5):629–39.10.3109/17435390.2015.1108473Suche in Google Scholar PubMed PubMed Central
© 2020 Barbara Bobrowska-Korczak et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Review Articles
- Liquid-phase microextraction of polycyclic aromatic hydrocarbons: A review
- Colorimetric hand-held sensors and biosensors with a small digital camera as signal recorder, a review
- Mini-Review of Analytical Methods used in Quantification of Ellagic Acid
- Highly Sensitive and Robust Capillary Electrophoresis-Electrospray Ionization-Mass Spectrometry: Interfaces, Preconcentration Techniques and Applications
- Spectroscopic Determination of Two Beta-Blockers – Atenolol and Propanolol by Oxidative Derivatization Using Potassium Permanganate in Alkaline Medium
- Review on analytical methods for quantification of ADHD drugs in human biological samples
- Benzene, toluene, ethylbenzene, and xylene: Current analytical techniques and approaches for biological monitoring
- Advances in neurochemical measurements: A review of biomarkers and devices for the development of closed-loop deep brain stimulation systems
- Review on stationary phases and coating methods of MEMs gas chromatography columns
- Research Article
- Xanthene based resonance Rayleigh scattering and spectrofluorimetric probes for the determination of cyclobenzaprine: Application to content uniformity test
- Special Issue: SPECIAL ISSUE ON 25TH INTERNATIONAL SYMPOSIUM ON SEPARATION SCIENCES
- Chromatographic analysis of bio-oil formed in fast pyrolysis of lignocellulosic biomass
- Urinary carboxylic acids (UCAs) in subjects with autism spectrum disorder and their association with bacterial overgrowth
- Separation procedures in the identification of the hydrogenation products of biomass-derived hydroxymethylfurfural
- The effect of selenium, zinc and copper on the excretion of urinary modified nucleobases in rats treated with prostate cancer cells
- Analytical approaches and preparation of biological, food and environmental samples for analyses of zearalenone and its metabolites
- Nanosized zinc, epigenetic changes and its relationship with DMBA induced breast cancer in rats
- Special Issue: Bioanalytical Methods and Their Applications
- Single-molecule force spectroscopy: A facile technique for studying the interactions between biomolecules and materials interfaces
- Biological nanoscale fluorescent probes: From structure and performance to bioimaging
- Detection of metal ions in biological systems: A review
- Recent advances in animal origin identification of gelatin-based products using liquid chromatography-mass spectrometry methods: A mini review
Artikel in diesem Heft
- Review Articles
- Liquid-phase microextraction of polycyclic aromatic hydrocarbons: A review
- Colorimetric hand-held sensors and biosensors with a small digital camera as signal recorder, a review
- Mini-Review of Analytical Methods used in Quantification of Ellagic Acid
- Highly Sensitive and Robust Capillary Electrophoresis-Electrospray Ionization-Mass Spectrometry: Interfaces, Preconcentration Techniques and Applications
- Spectroscopic Determination of Two Beta-Blockers – Atenolol and Propanolol by Oxidative Derivatization Using Potassium Permanganate in Alkaline Medium
- Review on analytical methods for quantification of ADHD drugs in human biological samples
- Benzene, toluene, ethylbenzene, and xylene: Current analytical techniques and approaches for biological monitoring
- Advances in neurochemical measurements: A review of biomarkers and devices for the development of closed-loop deep brain stimulation systems
- Review on stationary phases and coating methods of MEMs gas chromatography columns
- Research Article
- Xanthene based resonance Rayleigh scattering and spectrofluorimetric probes for the determination of cyclobenzaprine: Application to content uniformity test
- Special Issue: SPECIAL ISSUE ON 25TH INTERNATIONAL SYMPOSIUM ON SEPARATION SCIENCES
- Chromatographic analysis of bio-oil formed in fast pyrolysis of lignocellulosic biomass
- Urinary carboxylic acids (UCAs) in subjects with autism spectrum disorder and their association with bacterial overgrowth
- Separation procedures in the identification of the hydrogenation products of biomass-derived hydroxymethylfurfural
- The effect of selenium, zinc and copper on the excretion of urinary modified nucleobases in rats treated with prostate cancer cells
- Analytical approaches and preparation of biological, food and environmental samples for analyses of zearalenone and its metabolites
- Nanosized zinc, epigenetic changes and its relationship with DMBA induced breast cancer in rats
- Special Issue: Bioanalytical Methods and Their Applications
- Single-molecule force spectroscopy: A facile technique for studying the interactions between biomolecules and materials interfaces
- Biological nanoscale fluorescent probes: From structure and performance to bioimaging
- Detection of metal ions in biological systems: A review
- Recent advances in animal origin identification of gelatin-based products using liquid chromatography-mass spectrometry methods: A mini review

