Abstract
Gas chromatography (GC) is an important and widely used technique for separation and analysis in the field of analytical chemistry. Micro gas chromatography has been developed in response to the requirement for on-line analysis and on-site analysis. At the core of micro gas chromatography, microelectromechanical systems (MEMs) have the advantages of small size and low power consumption. This article introduces the stationary phases of micro columns in recent years, including polymer, carbon materials, silica, gold nanoparticles, inorganic adsorbents and ionic liquids. Preparation techniques ranging from classical coating to unusual sputtering of stationary phases are reviewed. The advantages and disadvantages of different preparation methods are analyzed. The paper introduces the separation characteristics and application progress of MEMs columns and discusses possible developments.
1 Introduction
The separation and analysis of mixtures is key in the field of analytical chemistry. Chromatography is a separation and analysis method, which has a very wide range of applications in analytical chemistry, organic chemistry, biochemistry and other fields [1]. Chromatography techniques can be divided into gas chromatography (GC) and liquid chromatography (LC) according to different mobile phases. Gas chromatography (GC) is a common technique that is widely used for separating and measuring semi-volatile and volatile compounds. Commercially available GC analyzers use conventionally manufactured components whose size, weight and power consumption often limit portability and reduce suitability for on-site use.
In a gas chromatography separation system, the column is the core part. Traditional chromatographic systems mainly use either a capillary column or a packed column to allow separation. The inner wall of the separation column is coated or filled with a stationary phase. As the components of the mixture pass through the chromatographic column within the mobile phase (carrier gas), the differing retentions of the components allows the separation of the mixture. Columns used in gas chromatography are predominately either capillary columns or packed columns.
GC micro columns made by microelectromechanical systems (MEMs) technology have appeared since 1979 [2]. Subsequently, most reports published in the area of microchip GC are developments dedicated to column technology. The micro-column has the advantages of small size and small heat capacity, so related research has become one of the focuses in the field of gas chromatography [3,4]. Commercial micro gas chromatographs have begun to use MEMs columns, including the FROG-4000 portable GC from Defiant Technologies and the Zebra GC system from Virginia Tech [5]. However, micro-chromatographic columns are still very different from traditional capillary columns. Due to the limitation of column length and column defects, the separation performance is relatively low, which temporarily limits its application. With the development of MEMs technology, different types of micro-columns have appeared (see Figure 1) [6], include capillary micro-columns, multicapillary micro-columns (MCC), and packed and semi-packed micro-columns. Column performance is affected by many factors, such as the material of the column, the structure of the column channel, stationary phase, and the heating distribution of the column.

Cross-sectional view of different conventional GC column and MEMs column.
The choice and coating of stationary phases remain a challenge in the fabrication of microelectromechanical systems (MEMs) columns. This article introduces the stationary phases of MEMs columns and their preparation methods. It introduces polymer-based stationary phases, carbon materials based stationary phases, silicon-based materials, inorganic adsorbents and ionic liquids used in MEMS columns. The article also describes the preparation methods of these stationary phases, including static coating, dynamic coating, chemical vapor deposition (CVD), physical vapor deposition (PVD), electroplating, spin coating, layer-to-layer deposition (LbL), surface segregation, sol-gel method, particle packing and the monolithic column method. The separation characteristics and application of these MEMs columns are reported in this paper.
2 Polymer-based stationary phase
In traditional gas chromatography columns, various polymers such as organopolysiloxane are used in capillary or packed columns as stationary phase materials. Polymer-based stationary phases have advantages derived from both organic and inorganic properties such as chemical inertness, thermal stability, and a wide operating temperature range. Polysiloxane polymers include (in order of increasing polarity) non-polar polydimethylsiloxane, phenylmethylpolysiloxane, and trifluoromethylmethylpolysiloxane. Differing polarities can be used to tailor the separation of specific analytes.
Polysiloxane polymers have been widely used in microelectromechanical systems (MEMs) column. The main coating methods are static coating and a dynamic coating. The stationary phase is usually applied by either static or dynamic coating methods as wall coated stationary phases. The static coating is the method most frequently used for columns. In the static method, the column is filled with the stationary phase dissolved in a volatile solvent. Then one end of the column is sealed, a vacuum is applied, and the solvent is further evaporated, leaving a coating of the stationary phase on the column wall. The thickness of the stationary phase is calculated from the weight and density of the filling solution. Reidy et al. have described that the performance of the static coating of MEMs column is significantly better than the methods of dynamic coating.
Sun et al. [7] used a static method to coat the stationary phase of polydimethylsiloxane (PDMS) in MEMs column for the separation of benzene and toluene. Ali et al. [8] prepared a semi-packed MEMs column with a rectangular configuration, which was prepared by the static method, and then passed through azo-tert-butane vapor to cross-link to form a stationary phase. Han et al. [9] prepared a microcolumn for the detection of respiratory gas in patients with non-alcoholic fatty liver disease. PDMS was fabricated as a stationary phase by the static method. The static coating method is the most widely used method and allows film thickness to be precisely controlled. The disadvantage of this method is the formation of gas bubbles by the rough surfaces and varied geometries in the microchannel. When the column is placed under vacuum, the evaporation of air bubbles in the solvent can cause the stationary phase to be destroyed. Air bubbles may be generated in the microcavity at the interface of the microcolumn or the surface of the channel. When the microcolumn has a rough surface or a different geometry, the air bubbles will expand under vacuum, causing the coating solution to fall out of the microcolumn. A solution to overcome this involves pressurizing the coating solution in the channel before vacuum evaporation [10]. Another method to reduce air bubbles is removal in an ultrasonic bath during static coating [11].
Radadia et al. [12] prepared a microelectromechanical systems (MEMs) column using OV-5 as a stationary phase using a dynamic method. A stationary phase solvent was injected onto the GC column and flowed through under pressure. The thickness of the stationary phase can be controlled by altering the flow rate and the concentration of the stationary phase solvent. Although the dynamic coating method and equipment have the advantage of being simple, dynamic coated columns are generally not as effective as static coated columns and it is difficult to obtain a perfect coating film due to cavitation. Lambertus et al. reported that cavitation was observed to occur primarily at the interface where the capillary leads were connected to the MEMs column.
Rankin and Suslick [13] first reported a microcolumn consisting of a single microtextured thermoset polymer composite which acts as both the structural material and the stationary phase. The stationary phase was prepared by the surface segregation method, where diethoxydimethylsilane was prepared in a flexible epoxy resin. This cheap disposable GC MEMs column can separate volatile organic compounds. The surface segregation method has the advantage of simple operation steps, but the consistency of the stationary phase needs more investigation.
Breshike et al. [14] researched a simple and new method for preparing microelectromechanical systems (MEMs) columns. A 0.3 m-long micro-column channel was prepared by pouring epoxy resin into the mold. The spin coating method was used to prepare a stationary phase; uniformly OV-1 spin coating a flat surface with the stationary phase and creating a column by pressing a lid, with micro-fabricated ridges, down onto the coated substrate as shown in Figure 2. This molding method can produce multiple molds which can dramatically scale up production. The spin coating method is simple and fast, but defects are easily caused by impurities, bubbles, and operating conditions.

Schematic diagram of MEMs column prepared by an epoxy resin mold.
Nakai et al. [15,16] used a physical vapor deposition method to prepare a MEMs column with a deposition of an aminofunctionalized parylene as the stationary phase. In physical vapor deposition, the source materials are transformed into a vapor or plasma using a physical process, such as heating, sublimation, or physical bombardment before wafer bonding. This fabrication method has the advantages of a uniform and conformal coating. Physical vapor deposition has suitable for a wide range of stationary phase materials such as polymers and metals; however, this method is complicated and uses complex equipment.
New technologies related to micro GC (μGC) are constantly being developed, and during these studies, polysiloxane polymers have also been used as stationary phases. Qin and Gianchandani [17] studied a μGC system that comprised a Knudsen pump with bidirectional capability. This μGC did not utilize traditional air pumps, valves, pre-concentration, and column separation methods; instead, valveless μGC architecture was used which was comprised of a temperature-regulated Knudsen pump, preconcentrator, and a separation column. Hsieh and Kim [18] prepared a circulatory separation μGC. The circulatory column system consisted of two 25-cm micro columns and six mini valves to produce circulatory chromatography separation. The circulatory separation of target gas molecules extended up to 10 turns through a set of two 25-cm micro columns, which is equivalent to a column of 5 m in length. This research team developed a closed-loop [19]. which demonstrated 16 times elongation of a virtual column length up to 800 cm by utilizing two 25 cm microcolumns which can reduce column length and head pressure. Cesar et al. [20] studied the application of correlation technology in μGC, which involves the injection of a pseudorandom stream in a chromatography column and recovery of the chromatography by cross correlation with the output signal. The results showed that correlation chromatography has the capability to improve signal-to-noise ratio in uGC. Wang et al. [21] used programmed axial thermal gradient gas chromatography (TGGC) technology for field analysis. TGGC improves column separation performance; compared with traditional programmed gas chromatography, the separation time of TGGC is shortened by 20%. There were three steps involved during separation. First, the column was at room temperature during injections. Then, the inlet end of the column was rapidly heated to 180°C while the outlet end was cooled by air flow; analytes were separated and focused in this step. After the inlet end reached 180°C, the cooling air was turned off and the outlet end was raised by heat conduction to 140°C. Analytes were separated during this step. Gaddes et al. [22] reported research to improve the technique by using a vespel/graphite ferrule based compression sealing technique. This sealing technique enabled separation at temperatures up to 350°C on the uGC column.
Much research on the influence of μGC column structure configuration has also used polymer-based stationary phases. Radadia et al. [23] compared the separation performance of serpentine, circular-spiral and square-spiral MEMs columns by dynamic methods. The results showed that the serpentine columns allowed higher separation plate numbers. Sun and Cui [24] developed a microelectromechanical systems (MEMs) column containing embedded micro-posts with a higher sample capacity. The group of Sun et al. [25] used the dynamic method to apply the mixed solution of SE-54 and PEG-20M as a stationary phase for the detection of benzene compounds. Li et al. [26] prepared a semi-packed MEMs column. The column had a higher number of plates than a 30-meter HP-5 capillary column. Tian et al. [27] prepared 2 and 4 m MEMs columns with embedded ellipitic cylindrical posts, giving a resolution between methane and ethane of 1.02. et al. [28] developed a wavy configuration MEMs column consisting of upper arcs and lower arcs which were alternatively connected in series. The stationary phase was prepared by the static method using ozone as a cross-linking agent. Tian et al. [29] prepared MEMs column containing embedded elliptic cylindrical posts, which had a higher stationary phase content and lower pressure drop. The column efficiency was improved by 129% for toluene.
MEMS columns have also been used in the study of uGC detectors. Reddy et al. [30] developed a μGC system that comprised a MEMs column, and nondestructive Fabry-Perot (FP) vapor sensors on a silicon chip. The performance of the OV-215 stationary phase micro column was better than the OV-1 micro column. Narayanana et al. [31] fabricated a μGC with micro helium discharge ionization detectors (μHDID). The absolute limit of detection was 60 pg for octane in air at 3.3 mW. Chang et al. [32] reported the integration of a microelectromechanical systems (MEMs) column with an ion trap mass spectrometer. The system demonstrated the separation of VOCs and achieved a plate height of 0.78cm.
Other types of polymers have also been used in the research of MEMs columns. Serrano et al. [33] prepared microcolumns of OV-1 and OV-215 stationary phases. After pretreatment in air at 200°C, the retention time was stable. Other stationary phases used in MEMs columns include polyethylene glycol [34,35], trifluoropropyl-substituted polydimethylsiloxane [36], etc. The structures of some polymeric stationary phase materials are shown in Figure 3. Polymer microspheres can also be used as stationary phases. Kaanta et al. [37] prepared a monolithic GC component with micro column and a thermal conductivity detector. The stationary phase of the column was HayeSep A microspheres. Sun et al. [38] fabricated a micro packed gas chromatograph column integrated with a micro heater by using laser etching technology (LET) for analyzing environmental gases. LET is a powerful tool to etch deep well-shaped channels. Porapak Q was packed into the column as the stationary phase. The separation efficiency was about 5800 plates/m.
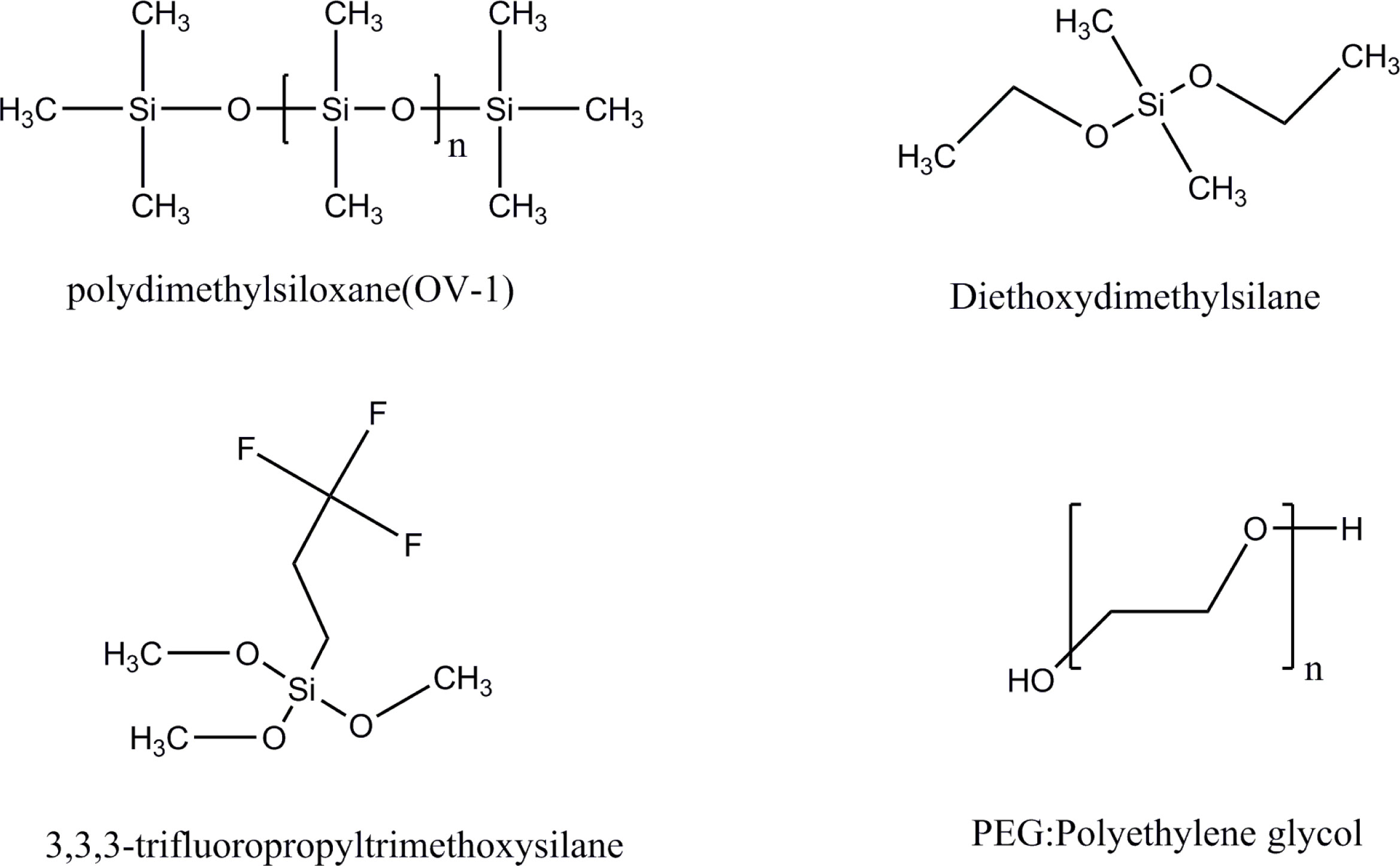
structures of some polymeric stationary phase materials.
3 Carbon materials based stationary phase
Carbon sorbents are common types of stationary phases used in gas chromatography. Carbon adsorbents such as graphitized carbon and carbon molecular sieves are used both as chromatographic column packings and liquid stationary phase supports.. In recent years, new nano carbon materials such as carbon nanotubes and graphene have been used in MEMs columns. Mogensen and Kutter [39] reviewed the carbon nanotube stationary phases for μGC. Carbon nanotubes are used in GC due to their unique geometry, chemical stability, and high surface-to-volume ratio. In addition, graphene oxide and graphene have a large surface area and are easy to modify.
Particulate carbon adsorbents are often used as stationary phases in MEMs columns. Zampoli et al. [40] used two types of graphitized carbon and polyethylene glycol to pack into microelectromechanical systems (MEMs) columns. The column was used in combination with metal oxide gas sensors for detection. Subsequently, Carbograph 2 and polyethylene glycol were also used to monitor aromatic VOCs by μGC. Sklorz et al. [41] packed MEMs columns with carbon molecular sieves and Carboxen 1000 and coupled them with SnO2 detectors for the detection of ethylene. Zampolli et al. [42] prepared a flexible integration portable μGC system to be used in field sensing. The MEMs columns were packed with carbograph and carbosieve as stationary phases, for the detection of natural gas and benzene series. The particle packing method is simple and gives a large column capacity, although column efficiency can be limited.
Li et al. [43] prepared a graphene functionalized PDMS open tubular and semi-packed column, which adsorbed graphene on the surface of the PDMS coating and functionalized. The characteristics of the column were a rough and porous surface and improved separation performance. Li et al. [44] also applied a dynamic method to twice coat microchannels to become a stationary phase. First, PDMS was coated, and then the MWCNTs suspension was passed into the microchannels, followed by heating and curing to become a functionalized column. Compared with pure PDMS coated columns the functionalized column improved separation performance. Yuan et al. [45] reported that ZnO particles were prepared by a sol-gel method in MCC, and then the graphene solution was coated by the static method as the stationary phase.
Reid et al. [46] used chemical vapor deposition (CVD) to deposit single-walled carbon nanotubes (SWNTs) with a high specific surface area in MEMs columns for ultra-fast (26°/s) GC analysis. Nakai et al. [16] deposited SWNTs as a stationary phase by catalytic vapor deposition on the surface of metal particles, reporting that the preparation of thin carbon nanotube films was a way to improve the resolution. Houdebourg et al. [47] reported a method of physical vapor deposition (PVD) using graphite as a stationary phase by physical sputtering. The characteristics and preparation methods of various chromatographic columns are shown in Table 1.
Summary of MEMs column characteristics and stationary phases
| Stationary phases | Column features | Length×width×depth (cm, um, um) | Fabrication | Application | Nmax | Year |
|---|---|---|---|---|---|---|
| OV-1 | Serpentine | 6.0×100×100 | static | Benzene, toluene | 4850 | 2009 |
| OV-1 | Semi-packed | 1×150×180 | static | Mixture(8) | 10000 | 2009 |
| PDMS | Serpentine | (1,3)×30×250 | static | Array pillar | NR | 2019 |
| SE-54 | Serpentine | 5.9×158×80 | static | C10–C40 | NR | 2017 |
| PDMS | Array pillar | 0.7×619×75 | static | Alkanes (6) | 90000 | 2017 |
| OV-5 | Serpentine | 0.34×100×100 | dynamic | DMMP(10) | 2564 | 2009 |
| DEDMS | Serpentine | 1×250×500 | S-S | Trichloroethylene(12) | 1800 | 2015 |
| OV-1 | Semicircle | 0.3×500×300 | spin-coat | C12–C16 | NR | 2020 |
| Parylene | Semi-packed | 1×180×230 | PVD | Alcohols(5) | 166 | 2009 |
| OV-1 | Capillary | 0.25×230×230 | static | Benzene(3) | NR | 2014 |
| OV-1 | Spiral | 0.25×150×370 | static | C10–C16 | 12720 | 2014 |
| OV-1 | Spiral | 0.25×150×370 | static | C5–C6 | 68696 | 2016 |
| OV-1 | Rectangular | 5×120×300 | static | VOCs | NR | 2015 |
| SE-54 | Serpentine | 1.4×(60-150) ×60 | static | Toluene(19) | 6300 | 2014 |
| PDMS | Spiral | 2×100×100 | dynamic | PAHs | 28000 | 2014 |
| OV-5 | Serpentine | 3×100×100 | dynamic | C5–C13 | 18700 | 2010 |
| SE-54;etc | Serpentine | 200×300×350 | dynamic | Aromatic (5) | 9500 | 2013 |
| SE-54;PEG-20M | Semi-packed | 2×300×350 | dynamic | Styrene(3) | NR | 2013 |
| SE-54 | Semi-packed | 1×160×250 | static | Alkanes | 55366 | 2014 |
| OV-1;OV-215 | Rectangular | 0.3×120×400 | static | Toluene, C10–C12 | NR | 2017 |
| SE-54 | Wave post | 2×40×300 | static | CWAs simulant | 1354 | 2017 |
| PDMS,PMPS | Semi-packed | 2×250×300 | static | Ethylbenzene(6) | NR | 2018 |
| OV-1 | Semi-packed | (2-4)×250×300 | static | C1–C4 | NR | 2013 |
| PDMS | Capillary | 1×80×240 | static | Octane | NR | 2015 |
| OV-1 | Rectangular | 6×30×250 | static | Mixture(16) | NR | 2020 |
| PDMS,OV-215 | Rectangular | 0.5–3×150×240 | static | Alcohols ketones | 14400 | 2009 |
| Carbowax | Serpentine | 320×250×250 | static | Toluene | 9750 | 2011 |
| Styrene | Packed | 0.52×440×500 | packing | C1–C3 | NR | 2009 |
| OV-1;OV-215 | Rectangular | 0.25×400×200 | static | DMMP, Alkanes | NR | 2011 |
| HayeSep A | Serpentine | 0.5×400×200 | packing | Methan(4) | NR | 2010 |
| Porapak Q | Rectangular | 1.2×600×1200 | packing | CO, Alkanes (8) | 5800 | 2016 |
| Carbograph2; | Spiral | 0.5×NR×NR | packing | VOCs | NR | 2009 |
| CMS | Spiral | 75×1000×800 | packing | Ethylene | 962 | 2013 |
| Carbosieve | Rectangular | 0.5–6 length | packing | VOCs | NR | 2020 |
| RGO,SWNT | Semi-packed | NR×(30–300)×300 | dynamic | Alkanes (13) | NR | 2016 |
| SWNT/PDMS | Capillary | 1.5–3×NR×300 | dynamic | Alkanes (11) | NR | 2016 |
| RGO | MCC | 0.5×30×300 | sol-gel | C5–C12 | 11363 | 2017 |
| SWNT | Serpentine | 0.3×50×50 | CVD | C6–C11 | NR | 2009 |
| SWNT | Serpentine | 1×160×250 | CVD | Alkanes | 1613 | 2009 |
| Graphite, silicon | Capillary | 2.2×100×100 | PVD | C1–C9 | 2500 | 2013 |
| Silicon | Serpentine | 2.2×75×100 | PVD | Alkanes (4) | 5000 | 2011 |
| Graphite, silicon | Serpentine | 220×75×100 | PVD | Alkanes (4) | NR | 2013 |
| SNP | MCC | 0.25×30×240etc | LbL | VOCs(10) | 4750 | 2013 |
| SNP | W-M | 1×8 (20–120) ×250 | LbL | C8–C10 | 5500 | 2013 |
| Porous silicon | Serpentine | 1.33×80×80 | CVD | Benzene | 2626 | 2013 |
| Porous silicon | Packed | 2×350×320 | dynamic | Styrene(6) | 10,000 | 2014 |
| MS | Semi-packed | 2×250×300 | sol-gel | C1–C4 | 11420 | 2018 |
| MS | Semi-packed | 2×250×220 | sol-gel | (C5–C10) | NR | 2017 |
| MS | Semi-packed | 2×250×220 | sol-gel | C1–C10 | NR | 2018 |
| MS-PDMS | Semi-packed | 2×250×300 | sol-gel | Benzene | 9290 | 2018 |
| MS | Pillars packed | NR×250×300 | sol-gel | Alkanes | 14458 | 2019 |
| Silicon nanowire | Rectangular | 2×100×300 | etching | C6–C10 | 23647 | 2019 |
| Au- Thiol | MCC | 25×25×250 | E-P | Alkanes, ethanol | 20000 | 2010 |
| Au- Thiol | MCC | 0.25×30×x250 | E-P | Alkanes(9) | 7300 | 2011 |
| Au- Thiol | MCC | 0.25×30×250 | E-P | Butanol(5) | 7200 | 2012 |
| Au- Thiol | MCC | 0.25×30×250 | PVD/LbL | Alkanes(9) | 5400 | 2013 |
| Au- Thiol | Semi-packed | 1×190×220 | PVD/LbL | C10–C15 | NR | 2015 |
| Al | Semi-packed | 1.1×200×100 | PVD | Alkanes | 4500 | 2014 |
| Al-Chlorosilane | Rectangular | NR×20–200×50 | LbL | NR | NR | 2017 |
| Al | Semi-packed | 1×190×180 | ALD | Mixture(8) | 4200 | 2015 |
| Al-Chlorosilane | Semi-packed | NR×190×240 | ALD | VOCs(8) | 4200 | 2017 |
| Al-Chlorosilane | Semi-packed | 3×150×250 | ALD | Mixture(20) | 8000 | 2017 |
| Al-Chlorosilane | Semi-packed | 1×190×240 | ALD | VOCs(12) | NR | 2015 |
| RTIL | Serpentine | 1×190×240 | dynamic | Mixture(15) | 2300 | 2017 |
| Al-RTIL | Serpentine | 1×190×240 | ALD | Toxic (21) | 8000 | 2018 |
| [BMIM]PF6 | Semi-packed | 1×190×240 | static | Alkanes(9) | 2568 | 2019 |
| Et-β–CD | Spiral | 2×80×80 | static | Enantiomer | 7200 | 2016 |
Maximum theoretical plate: Nmax; Not reported: NR; physical vapor deposition: PVD; chemical phase deposition: CVD; layer-to-layer deposition: LBL; atomic layer deposition: ALD; Carbon nanotubes: CNT; Surfacesegregation: S-S; Silicon nanoparticles: SNP; Width modulation: W-M; Room temperature ionic liquids: RTIL; diethoxydimethylsilane: DEDMS; polyethylene glycol: PEG; reduced graphene oxide: RGO; mesoporous silica: MS; multicapillary column: MCC; electroplating: E-P; Polycyclic aromatic hydrocarbons: PAHs; Carbon molecular sieve: CMS; chemcial warfare agent: CWAs; 1-butyl-3-methylimidazolehexafluorophosphate:[BMIM]PF6
4 Silica based stationary phase
Silica can be modified to produce different adsorption properties and can be used in a MEMs column as a stationary phase or a stationary phase support layer. Vial et al. [48] prepared a stationary phase by silica sputtering using a physical vapor deposition (PVD) method. Sputtering also enables vapor deposition of a thin film on a substrate. The mechanism involves the ionization of a gas, generated ions are then guided towards the sputtering target where collision results in ejection of the target atoms that are deposited on the substrate surface. Houdebourg et al. [47] used a method of physically sputtering silicon and graphite on the surface of a single-layer silicon wafer. The silicon wafer bonded with a pyrex glass to form a chromatographic column. The separation time of C1 to C9 was 15 s.
Wang et al. [49] prepared silicon nano-particles (SNP) using a layer-by-layer self-assembly technique method. This technique involved alternating immersion of a substrate into two oppositely charged solutions with rinsing in between. Thin films of the oppositely charged materials are electrostatically bound together as multilayers. The process is terminated after the desired thickness is achieved (as shown in Figure 4).
Shakeel et al. [50] developed a width-modulated micro column for chromatographic separation. The LbL method was used to prepare a stationary phase using SNPs and PAH. The layer-by-layer deposition method can produce uniform coatings and film thickness can be precisely controlled, however a limited range of stationary phase materials can be used.

Schematic process flow of SNPs coating using layer-by-layer self-assembly technique.
Ricoul et al. [51] used a chemical vapor deposition (CVD) method to deposit porous silicone films in micropillars. The chemical vapor deposition method involves the passing of precursor gas into a heated chamber containing the object to be coated. Reactions occur on/near the hot surface, resulting in the deposition of a coating on the surface. Sun et al. [52] used an electrochemical anodization method to prepare porous silicon as a stationary phase support layer. Subsequently, the SE-54 and PEG-20M layers were coated with a dynamic method to become a stationary phase. The study compared the column efficiency of the micro-pillars with and without a silicon support layer. The results showed that the column samples with a porous silicon support layer had a larger capacity and higher column efficiency. The chemical vapor deposition method can produce pure, uniform coatings of metals or polymers even on rough surfaces but has the disadvantage of a low deposition rate.
Tian et al. [53] developed μGC with an integrated MEMs column and microthermal conductivity cell detector. A sol-gel method was used to prepare mesoporous silica (MS) nanoparticles as a stationary phase. This technique involved hydrolysis and condensation of precursor material to form a glassy material. During this process, a “sol” or colloidal suspension of particles was converted into a “gel” by polymerization. Hou et al. [54,55] studied semi-packed GC columns coated with two kinds of MS as a stationary phase. MSs with pore sizes of 2 and 5 nm were prepared using two different soft-templates and deposited on the inner surface of the GC columns by dip-coating. The results showed that the analytes have longer retention times in the larger pore size columns. Lou et al. [56] used MS as the stationary phase carrier, and then PDMS as the stationary phase by static coating. The results showed that the column with the carrier had a higher resolution. Yang et al. [57] fabricated a semi-packed micro column with embedded micro-elliptical pillars. A mesoporous silica (MS) film with a 2nm pore size was used as a stationary phase. The resolution of pentane and solvent was 3.6. The sol-gel formation method gives a uniform stationary phase with high strength. One disadvantage of this method is that it is difficult to obtain satisfactory control over the thickness.
Feng et al. [58] reported a technique where silicon nanowires were grown in-situ in the micro channels as the support layer of the stationary phase. OV-101 was coated as a stationary phase, as shown in Figure 5. The separation efficiency of the μGC column was as high as 23647 plates/m, which could be attributed to the large surface area of the silicon nanowires.

The fabrication process of the μGC column which use silicon nanowires as the stationary phase support and is coated with OV-101.
5 Inorganic adsorbents based stationary phase
Inorganic adsorbent stationary phases in GC are usually silica gel and alumina. The use of inorganic adsorbents in micro chromatographic columns began with Reston and Kolesar used copper phthalocyanine for the detection of ammonia and nitrogen dioxide [59]. In recent years, inorganic coatings such as gold nanoparticles and alumina have also been used in MEMs columns as stationary phases.
Jahromi and Agah [60] reported the use of electroplating to prepare monolayer-protected gold as a stationary phase. This method involved the deposition of a metal coating onto a substrate by giving it a negative charge and introducing it into an electrolyte solution of the coating metal. The gold layer can be functionalized by self-assembly of octadecyl mercaptan. Shakeel and Agah [61] used a similar method to fabricate MCCs with gold-supported stationary phases. The column temperature programming was only 4 min from 36.5°C to 302°C. This group [62] fabricated reconfigurable chromatographic separation columns, where the gold surface can be functionalized with different groups such as octadecyl mercaptan and 6-mercapto-1-hexanol, becoming both a polar and non-polar stationary phase. The column can be reconfigurable into a different column by replacing the thiol functional group. The electroplating method can produce uniform coatings, but only a limited range of stationary phase materials can be used.
The group [63] compared the preparation of gold-supported stationary phases with two different deposition methods, including physical vapor deposition (PVD) and layer-to-layer deposition (LbL). The results showed that the column using the PVD method had a slightly higher column efficiency. In a MEMs column [64], the gold layer can be functionalized by different thiol groups such as octyl mercaptan and 6-mercapto-1-hexanol, as shown in Figure 6. Haudebourg et al. [65] also reported a method of PVD using sputtered alumina as a column stationary phase for the separation of alkane mixtures.

Schematic diagram of MEMs column and Alkane thiol functionalized thin gold films.
Hoogerwerf and Durante [66] reported the design of the integrated microcolumn at the front end of the mass spectrometer. The article analyzed the possible nonuniform layer thickness of the stationary phase caused by the liquid coating in a rectangular column. The process involved alumina and dodecylsilane deposition in the column to form polar and non-polar stationary phases by molecular vapor deposition methods.
Shakeel et al. [67] utilized atomic layer deposited alumina as a gas solid stationary phase medium for microelectromechanical system (MEMs) columns. After atomic layer deposition (ALD) of aluminum oxide, a chloroalkylsilane was utilized to functionalize the oxide surface to improve peak symmetry and retention times as shown in Figure 7. Akbar et al. [68,69] reported the preparation of microcolumns by a similar method. Shakeel et al. [70] optimized the conditions of the method, including precursor exposure time and deposition temperature. High performance MEMs columns termed high-density semi-packed columns were fabricated using this optimized method.

Schematic diagram of MEMs column prepared by chemical vapor deposition.
6 Ion liquids based stationary phase
Room temperature ionic liquid RTILs are a special type of solvent. They usually consist of organic cations (such as alkylimidazolium quaternary phosphorus salts) and inorganic anions containing N and P. Due to the low volatility, high thermal stability, excellent selectivity, and good wetting properties, they are suitable for use as a chromatographic stationary phase.
Regmi et al. [71] used two kinds of ILs (trihexyltetradecylphosphonium bis(trifluoromethylsulfonyl)imide ([P66614][NTf2]) and 1-butylpyridinum bis(trifluoromethylsulfonyl)imide ([BPyr][NTf2])) as stationary phases to prepare MEMs columns. Comparing the dynamic/static coating methods showed the dynamic coated columns to be more effective. Regmi et al. [72] demonstrated the influence of an intermediate layer of aluminum oxide on the separation performance of a room temperature ionic liquid (RTIL)-coated gas chromatography column. A thin layer of alumina was then deposited on the surface of the channels by atomic layer deposition. Following the alumina deposition, the channels were coated with an RTIL. The plate of alumina pretreated columns showed a 2.1-fold increase compared to the column with no alumina layer. The results showed that the alumina coating layer promotes the formation of a more uniform RTIL film. Chan and Agah [73] fabricated density-modulated semi-packed columns. The column was modulated by the number and density of support pillars from the inlet to the outlet. The stationary phase of the column was 1-butyl-3-methylimidazole hexafluorophosphate and the performance of the column was characterized by the determination of C9–C15.
7 Miscellaneous
With the development of microelectromechanical systems (MEMs) columns, cyclodextrin and other stationary phases that can be used for chiral enantiomeric separation have also appeared. Cagliero et al. [74] prepared MEMs columns from 1.68 to 2 m length by the static method. The columns included non-polar hexamethylsilane columns, polar FFAP columns, and β-cyclodextrin chiral columns. The plates number of the chiral column was 5,600.
8 Conclusion
This review covered the recent development of stationary phases and fabrication method for MEMs columns. The review focused on differences between micro columns and traditional GC columns. Six kinds of stationary phases used for microelectromechanical systems (MEMs) columns in recent years were introduced. The future development of microcolumn stationary phases will further improve the performance of MEMs columns. New coating material and other strategies are expected to improve column capacity and separation performance. This article also reported on the separation characteristics and application of micro chromatographic columns. With the continuous development of stationary phase materials and column preparation technology, high-performance MEMs columns will appear in micro chromatography instruments, online monitoring instruments, and field low-power monitoring equipment. MEMs column will play an important role in the future of analytical systems.
Abbreviations
- GC
gas chromatography
- MEMs
microelectromechanical systems
- MCC
multicapillary columns
- MS
mass spectrometer
- CVD
chemical vapor deposition
- PVD
physical vapor deposition
- CWAs
chemical warfare agents
- VOCs
volatile organic compounds
- DMMP
dimethyl methylphosphonate
References
[1] Braga M. A Handbook of Chromatography. Germany: Verlag Omniscriptam; 2017.Search in Google Scholar
[2] Terry SC, Jerman JH, Angell JB. Gas-chromatographic air analyzer fabricated on a silicon-wafer. IEEE Trans Electron Dev. 1979;26(12):1880–6.10.1109/T-ED.1979.19791Search in Google Scholar
[3] Haghighi F, Talebpour Z, Sanati-Nezhad A. Through the years with on-a-chip gas chromatography: a review. Lab Chip. 2015;15:2559.10.1039/C5LC00283DSearch in Google Scholar PubMed
[4] Hussain CM. Nanomaterials in Chromatography Current Trends in Chromatographic. Research Technology and Techniques Book. Elsevier; 2018.Search in Google Scholar
[5] Garg A, Zebra GC. A Fully Integrated Micro Gas Chromatography System [thesis]. Blacksburg: Virginia Polytechnic Institute; 2014.10.1109/ICSENS.2014.6985088Search in Google Scholar
[6] Regmi BP, Agah M. Micro Gas Chromatography: An Overview of Critical Components and Their Integration. Anal Chem. 2018;90:13133–50.10.1021/acs.analchem.8b01461Search in Google Scholar PubMed
[7] Sun JH, Cui DF, Li YT. A high resolution MEMS based gas chromatography column for the analysis of benzene and toluene gaseous mixtures. Sensor Actuat B-Chem. 2009;141(2):431–5.10.1016/j.snb.2009.06.047Search in Google Scholar
[8] Ali S, Ashraf-Khorassani M, Taylor LT, Agah M. MEMS-based semi-packed gas chromatography columns. Sensor Actuat B-Chem. 2009;141(1):309–15.10.1016/j.snb.2009.06.022Search in Google Scholar
[9] Han BQ, Wu GS, Huang H, Liu TH. A semi-packed micro GC column for separation of the NAFLD exhaled breath VOCs. Surf Coat Tech. 2019;363:322–9.10.1016/j.surfcoat.2019.02.049Search in Google Scholar
[10] Ghosh A, Johnson JE, Nuss JG, Stark BA. Extending the upper temperature range of gas chromatography with all-silicon microchip columns using a heater/clamp assembly. J Chromatogr A. 2017;1517:134–41.10.1016/j.chroma.2017.08.036Search in Google Scholar PubMed
[11] Jespers S, Schlautmann S, Gardeniers H, Malsche WD. Chip-Based Multicapillary Column with Maximal Interconnectivity to Combine Maximum Efficiency and Maximum Loadability. Anal Chem. 2017;89:11605–13.10.1021/acs.analchem.7b03036Search in Google Scholar PubMed
[12] Radadia AD, Morga RD, Masel RI, Shannon MA. Partially buried microcolumns for micro gas analyzers. Anal Chem. 2009;81(9):3471–7.10.1021/ac8027382Search in Google Scholar PubMed
[13] Rankin JM, Suslick KS. The development of a disposable gas chromatography microcolumn. Chem Commun. 2015;51(43):8920–3.10.1039/C4CC09915JSearch in Google Scholar
[14] Breshike CJ, Furstenberg R, Dominguez D, Kusterbeck A, Kozak D, Stievater T, et al. Gas chromatography using a spin-coated stationary phase and a molded elastomer micro-channel. J Chromatogr A. 2020;1610:460555.10.1016/j.chroma.2019.460555Search in Google Scholar PubMed
[15] Nakai T, Okawa J, Takada S, Shuzo M, Shiomi J, Yamada I. Carbon nanotube stationary phase in a microfabricated column for high-performance gas chromatography. AIP Conf Proc. 2009;1137(1):249–52.10.1063/1.3156518Search in Google Scholar
[16] Nakai T, Nishiyama S, Shuzo M, Delaunay J, Yamada I. Micro-fabricated semi-packed column for gas chromatography by using functionalized parylene as a stationary phase. J Micromech Microeng. 2009;19(6):065032.10.1088/0960-1317/19/6/065032Search in Google Scholar
[17] Qin YT, Gianchandani YB. A micro gas chromatograph with integrated bi-directional pump for quantitative analyses. IEEE 27 Int Conf Micro Electro Mech Systems (MEMS). 2014:26–30.10.1109/MEMSYS.2014.6765634Search in Google Scholar
[18] Hsieh HC, Kim H. Miniature circulatory column system for gas chromatography IEEE 27 Int Conf Micro Electro Mech Systems (MEMS). 2014:26–3010.1109/MEMSYS.2014.6765814Search in Google Scholar
[19] Hsieh HC, Kim H. A miniature closed-loop gas chromatography system. Lab Chip. 2016;16:1002–12.10.1039/C5LC01553GSearch in Google Scholar
[20] Cesar W, Flourens F, Kaiser C, Sutour C. Enhanced Microgas Chromatography Using Correlation Techniques for Continuous Indoor Pollutant Detection. Anal Chem. 2015;87:5620–5.10.1021/acs.analchem.5b00687Search in Google Scholar PubMed
[21] Wang A, Hynynen S, Hawkins AR, Tolley SE, Tolley HD, Lee MI. Axial thermal gradients in microchip gas chromatography. J Chromatogr A. 2014;1374:216–23.10.1016/j.chroma.2014.11.035Search in Google Scholar PubMed
[22] Gaddes D, Westland J, Dorman FL, Tadigadapa S. Improved micromachined column design and flfluidic interconnects for programmed high-temperature gas chromatography separations. J Chromatogr A. 2014;1349:96–104.10.1016/j.chroma.2014.04.087Search in Google Scholar PubMed
[23] Radadia AD, Salehi-Khojin A, Masel RI, Shannon MA. The effect of microcolumn geometry on the performance of micro-gas chromatography columns for chip scale gas analyzers. Sensor Actuat B-Chem. 2010;150:456–64.10.1016/j.snb.2010.07.002Search in Google Scholar
[24] Sun JH, Cui DF. Fabrication and characterization of microelectromechanical systems-based gas chromatography column with embedded micro-posts for separation of environmental carcinogens. J Chromatogr A. 2013;1291(24):122–8.10.1016/j.chroma.2013.03.022Search in Google Scholar
[25] Sun JH, Guan FY, Cui DF. An improved photoionization detector with a micro gas chromatography column for portable rapid gas chromatography system. Sensor Actuat B-Chem. 2013;188:513–8.10.1016/j.snb.2013.07.066Search in Google Scholar
[26] Li Y, Du XS, Wang Y, Jiang YD. Improvement of column efficiency in MEMS-Based gas chromatography column. RSC Adv. 2014;4:3742–7.10.1039/C3RA44255ASearch in Google Scholar
[27] Tian B, Feng F, Hou L, Wu YH. Research on micro-fabricated gas chromatographic columns with embedded ellipitic cylindrical posts; IEEE 30 Int Conf Micro Electro Mech Systems (MEMS). 2017:16708024.10.1109/MEMSYS.2017.7863669Search in Google Scholar
[28] Yuan H, Du XS, Tai HL, Yang X, Xu M. MEMS-based column coated with reduced graphene oxide as stationary phase for gas chromatography. RSC Adv. 2017;7:32749–56.10.1039/C7RA03271DSearch in Google Scholar
[29] Tian BW, Feng F, Zhao B, Luo B, Yang XL, Zhau HM, et al. Study of Monolithic Integrated Micro Gas Chromatography Chip. Chin J Anal Chem. 2018;46(9):1363–71.10.1016/S1872-2040(18)61110-7Search in Google Scholar
[30] Reddy K, Liu J, Fan XD, Khaing Oo MK, Fan X. Integrated Separation Columns and Fabry-Perot Sensors for Microgas Chromatography Systems. J Microelectromech S. 2013;22(5):1174–9.10.1109/JMEMS.2013.2262582Search in Google Scholar
[31] Narayanana S, Rice G, Agaha M. Characterization of a micro-helium discharge detector for gas chromatography. Sensor Actuat B-Chem. 2015;206:190–7.10.1016/j.snb.2014.09.014Search in Google Scholar
[32] Chang T-H, Struk D, Navaei M, Doroshenko VM, Laiko V, Moskovtes E, et al. Separation of volatile organic compounds using a MEMS separation column integrated with ion trap mass spectrometer. Sensor Actuat B-Chem. 2020;307:127588.10.1016/j.snb.2019.127588Search in Google Scholar
[33] Serrano G, Reidy SM, Zellers ET. Assessing the reliability of wall-coated microfabricated gas chromatographic separation columns. Sensor Actuat B-Chem. 2009;141(1):217–26.10.1016/j.snb.2009.05.003Search in Google Scholar
[34] Lee CY, Liu CC, Chen SC, Chiang C.M. High-performance MEMS-based gas chromatography column with integrated micro heater. Microsyst Technol. 2011;17(4):523–31.10.1007/s00542-010-1165-ySearch in Google Scholar
[35] Yamamoto Y, Akao S, Sakuma M, Kobari K, Noguchi K. Development of packed column for surface acoustic wave gas chromatograph using anodically bonded silicon-glass structure with a compression jacket. Jpn J Appl Phys. 2009;48(7).10.1143/JJAP.48.07GG12Search in Google Scholar
[36] Liu J, Gupta NK, Wise KD, Gianchandani YB, Fan XD. Demonstration of motionless Knudsen pump based micro-gas chromatography featuring micro-fabricated columns and on-column detectors. Lab Chip. 2011;11(20):3487–92.10.1039/c1lc20511kSearch in Google Scholar PubMed
[37] Kaanta B, Chen H, Zhang X. Monolithic micro gas chromatographic separation column and detector. IEEE Int Conf Micro Electro Mech Systems (MEMS). 2010:907–10.10.1109/MEMSYS.2010.5442354Search in Google Scholar
[38] Sun JH, Guan FY, Zhu XF, Ning ZW. Micro-fabricated packed gas chromatography column based on laser etching technology. J Chromatogr A. 2016;1429:311–6.10.1016/j.chroma.2015.12.001Search in Google Scholar PubMed
[39] Mogensen KB, Kutter JP. Carbon nanotube based stationary phases for microchip chromatography. Lab Chip. 2012;12(11):1951–8.10.1039/c2lc40102aSearch in Google Scholar PubMed
[40] Zampolli S, Elmi I, Mancarella F, Severi M. Real-time monitoring of sub-ppb concentrations of aromatic volatiles with a MEMS-enabled miniaturized gas-chromatograph. Sensor Actuat B-Chem. 2009;141(1):322–8.10.1016/j.snb.2009.06.021Search in Google Scholar
[41] Sklorz A, Janßen S, Lang W. Application of a miniaturised packed gas chromatography column and a SnO2 gas detector for analysis of low molecular weight hydrocarbons with focus on ethylene detection. Sensor Actuat B-Chem, 2013;180:43–49.10.1016/j.snb.2011.12.110Search in Google Scholar
[42] Zampolli S, Elmi I, Cardinali GC, Masini L, Bonafe F, Zardi F, Compact-GC platform: A flexible system integration strategy for a completely microsystems-based gas-chromatograph. Sensor Actuat B-Chem. 2020;305:127444.10.1016/j.snb.2019.127444Search in Google Scholar
[43] Li Y, Zhang R, Wang T, Wang Y, Zhao W, Wang X, et al. A micro gas chromatography with separation capability enhanced by polydimethylsiloxane stationary phase functionalized by carbon nanotubes and graphene. Talanta. 2016;154:99–108.10.1016/j.talanta.2016.03.037Search in Google Scholar PubMed
[44] Li YB, Zhang RZ, Wang T, Wang YH, Xu TB, Li LF, et al. Determination of n-alkanes contamination in soil samples by micro gas chromatography functionalized by multi-walled carbon nanotubes. Chemosphere. 2016;158:154–62.10.1016/j.chemosphere.2016.05.068Search in Google Scholar PubMed
[45] Yuan H, Du XS, Tai HL, Li Y, Zhao XL, Guo PF. The effect of the channel curve on the performance of micromachined gas chromatography column. Sensor Actuat B-Chem. 2017;239:304–10.10.1016/j.snb.2016.08.003Search in Google Scholar
[46] Reid R, Stadermann M, Bakajin O, Synovec RE. High-speed, temperature programmable gas chromatography utilizing a microlfabricated chip with an improved carbon nanotube stationary phase. Talanta. 2009;77(4):1420–5.10.1016/j.talanta.2008.09.023Search in Google Scholar PubMed
[47] Haudebourg R, Vial J, Thiebaut D, Danaie K, Breviere J, Sassiat P, et al. Temperature-programmed sputtered micromachined gas chromatography columns: an approach to fast separations in oilfield applications. Anal Chem. 2013;85(1):114–20.10.1021/ac3022136Search in Google Scholar PubMed
[48] Vial J, Thiébaut D, Marty F, Guibal P, Haudebourg R, Nachef K, et al. Silica sputtering as a novel collective stationary phase deposition for microelectromechanical system gas chromatography column: feasibility and first separations. J Chromatogr A. 2011;1218(21):3262–6.10.1016/j.chroma.2010.12.035Search in Google Scholar PubMed
[49] Wang D, Shakeel H, Lovette J, Rice GW, Heflflin JR, Agah M. Highly Stable Surface Functionalization of Microg as Chromatography Columns Using Layer-by-Layer Self-Assembly of Silica Nanoparticles. Anal Chem. 2013;85:8135–41.10.1021/ac401080uSearch in Google Scholar PubMed
[50] Shakeel H, Wang D, Heflin R, Agah M. Width-modulated microgas chromatography separation columns with silica nanoparticles stationary phase. IEEE Sens J. 2013:1–4.10.1109/ICSENS.2013.6688124Search in Google Scholar
[51] Ricoul F, Lefebvre D, Bellemin-Comte A, David N, Bourlon B, Jousseaume V, et al. Novel stationary phase for silicon gas chromatography microcolumns. IEEE Sens Proc. 2014:206–8.10.1109/ICSENS.2014.6984969Search in Google Scholar
[52] Sun J, Cui DF, Guan FY, Chen X, Zhang LL. High resolution microfabricated gas chromatography column with porous silicon acting as support. Sensor Actuat B-Chem. 2014;201:19–24.10.1016/j.snb.2014.04.076Search in Google Scholar
[53] Tian B, Zhao B, Feng F, Luo F. A micro gas chromatographic column with embedded elliptic cylindrical posts. J Chromatogr A. 1565;2018(31):130–7.10.1016/j.chroma.2018.06.036Search in Google Scholar
[54] Hou L, Feng F, You W.B, Xu PC, Effect of pore size of stationary phase on the separation performance of chip-based gas chromatography columns. IEEE 19 Int Conf Solid-State Sensors Actuators Microsystems. 2017:17078990.10.1109/TRANSDUCERS.2017.7994347Search in Google Scholar
[55] Hou L, Feng F, You WB, Xu PC. Pore size effect of mesoporous silica stationary phase on the separation performance of microfabricated gas chromatography columns. J Chromatogr A. 1552;2018(1):73–8.10.1016/j.chroma.2018.04.006Search in Google Scholar
[56] Luo F, Zhao B, Feng F, Hou L, You WB, Improved separation of micro gas chromatographic column using mesoporous silica as a stationary phase support; Talanta. 188, 1,2018, 546–51.10.1016/j.talanta.2018.06.020Search in Google Scholar
[57] Yang XL, Zhao B, Feng F, Zhou HM. High Performance Micro Gas Chromatography Column Using Mesoporous Silica as Stationary Phase. Chin J Anal Chem. 2019;47(6):832–7.10.1016/S1872-2040(19)61164-3Search in Google Scholar
[58] Feng F, Zhao B, Luo F. The Exploration of Silicon Nanowires as a Stationary Phase Support for Micro Gas Chromatographic Columns. IEEE 32 Int Conf Micro Electro Mech Systems (MEMS). 2019:19128150.10.1109/MEMSYS.2019.8870687Search in Google Scholar
[59] Reston RR, Kolesar ES. Silicon-micromachined gas-chromatography system used to separate and detect ammonia and nitrogen-dioxide. 1. Design, fabrication, and integration of the gas-chromatography system. J Microelectromech Syst. 1994;3(4):134–46.10.1109/84.338634Search in Google Scholar
[60] Jahromi MA, Agah M. Microfabricated gas chromatography columns with monolayer-protected gold stationary phases. J Microelectromech Syst. 2010;19(2):294–304.10.1109/JMEMS.2009.2038936Search in Google Scholar
[61] Shakeel H, Agah M. High-Performance Multicapillary Gas Separation Columns with MPG Stationary Phases. 2011 IEEE. Sens J. 1909–12.10.1109/ICSENS.2011.6127406Search in Google Scholar
[62] Shakeel H, Rice G, Agah. M. First reconfigurable MEMS separation columns for micro gas chromatography. IEEE 25 Int Conf Micro Electro Mech Systems (MEMS). 2012:823–26.10.1109/MEMSYS.2012.6170312Search in Google Scholar
[63] Shakeel H, Agah M. Self-patterned gold-electroplated multicapillary gas separation columns with MPG stationary phases. J Microelectromech Syst. 2013;22(1):62–70.10.1109/JMEMS.2012.2213068Search in Google Scholar
[64] Shakeel H, Wang D, Heflin JR, Agah M. Improved self-assembled thiol stationary phases in microfluidic gas separation columns. Sensor Actuat B-Chem. 2015;216:349–57.10.1016/j.snb.2015.03.107Search in Google Scholar
[65] Haudebourg R, Matouk Z, Zoghlami EI, Azzouz K, Thiebaut D, Vial J. Sputtered alumina as a novel stationary phase for micro machined gas chromatography columns. Anal Bioanal Chem. 2014;406(4):1245–7.10.1007/s00216-013-7289-zSearch in Google Scholar PubMed
[66] Hoogerwerf A, Durante GS. A Mems-based Gas Chromatograph Front-end for a Miniature Spectrometer. Symposium on Design, Test, Integration and Packaging of MEMS/MOEMS (DTIP). 2017.10.1109/DTIP.2017.7984483Search in Google Scholar
[67] Shakeel H, Rice GW, Agah M. Semipacked columns with atomic layer-deposited alumina as a stationary phase. Sensor Actuat B-Chem. 2014;203:641–6.10.1016/j.snb.2014.06.017Search in Google Scholar
[68] Akbar M, Restaino M, Agah M. Chip-scale gas chromatography: from injection through detection. Microsyst Nanoeng. 2015;1:1–7.10.1038/micronano.2015.39Search in Google Scholar
[69] Akbar M, Shakeel H, Agah M. GC-on-chip: integrated column and photoionization detector. Lab Chip. 2015;15(7):1748–58.10.1039/C4LC01461HSearch in Google Scholar PubMed
[70] Shakeel H, Agah M. High density semipacked separation columns with optimized atomic layer deposited phases. Sensor Actuat B-Chem. 2017;242: 215–23.10.1016/j.snb.2016.11.046Search in Google Scholar
[71] Regmi BP, Chan R, Agah M. Ionic liquid functionalization of semi-packed columns for high-performance gas chromatographic separations. J Chromatogr A. 2017;1510(11):66–72.10.1016/j.chroma.2017.06.050Search in Google Scholar PubMed
[72] Regmi BP, Chan R, Atta A, Agah M. Ionic liquid-coated aluminapretreated micro gas chromatography columns for high-efficient separations. J Chromatogr A. 2018:1566,124–34.10.1016/j.chroma.2018.06.058Search in Google Scholar PubMed
[73] Chan R, Agah M. Semi-Packed Gas Chromatography Columns With Density Modulated Pillars. J Microelectromech Syst. 2019;28(1):114–24.10.1109/JMEMS.2018.2881532Search in Google Scholar
[74] Cagliero C, Galli S, Galli M, Elmi I. Conventional and enantioselective gas chromatography with microfabricated planar columns for analysis of real-world samples of plant volatile fraction. J Chromatogr A. 2016;1429:329–39.10.1016/j.chroma.2015.12.037Search in Google Scholar PubMed
© 2020 Liu Yang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Review Articles
- Liquid-phase microextraction of polycyclic aromatic hydrocarbons: A review
- Colorimetric hand-held sensors and biosensors with a small digital camera as signal recorder, a review
- Mini-Review of Analytical Methods used in Quantification of Ellagic Acid
- Highly Sensitive and Robust Capillary Electrophoresis-Electrospray Ionization-Mass Spectrometry: Interfaces, Preconcentration Techniques and Applications
- Spectroscopic Determination of Two Beta-Blockers – Atenolol and Propanolol by Oxidative Derivatization Using Potassium Permanganate in Alkaline Medium
- Review on analytical methods for quantification of ADHD drugs in human biological samples
- Benzene, toluene, ethylbenzene, and xylene: Current analytical techniques and approaches for biological monitoring
- Advances in neurochemical measurements: A review of biomarkers and devices for the development of closed-loop deep brain stimulation systems
- Review on stationary phases and coating methods of MEMs gas chromatography columns
- Research Article
- Xanthene based resonance Rayleigh scattering and spectrofluorimetric probes for the determination of cyclobenzaprine: Application to content uniformity test
- Special Issue: SPECIAL ISSUE ON 25TH INTERNATIONAL SYMPOSIUM ON SEPARATION SCIENCES
- Chromatographic analysis of bio-oil formed in fast pyrolysis of lignocellulosic biomass
- Urinary carboxylic acids (UCAs) in subjects with autism spectrum disorder and their association with bacterial overgrowth
- Separation procedures in the identification of the hydrogenation products of biomass-derived hydroxymethylfurfural
- The effect of selenium, zinc and copper on the excretion of urinary modified nucleobases in rats treated with prostate cancer cells
- Analytical approaches and preparation of biological, food and environmental samples for analyses of zearalenone and its metabolites
- Nanosized zinc, epigenetic changes and its relationship with DMBA induced breast cancer in rats
- Special Issue: Bioanalytical Methods and Their Applications
- Single-molecule force spectroscopy: A facile technique for studying the interactions between biomolecules and materials interfaces
- Biological nanoscale fluorescent probes: From structure and performance to bioimaging
- Detection of metal ions in biological systems: A review
- Recent advances in animal origin identification of gelatin-based products using liquid chromatography-mass spectrometry methods: A mini review
Articles in the same Issue
- Review Articles
- Liquid-phase microextraction of polycyclic aromatic hydrocarbons: A review
- Colorimetric hand-held sensors and biosensors with a small digital camera as signal recorder, a review
- Mini-Review of Analytical Methods used in Quantification of Ellagic Acid
- Highly Sensitive and Robust Capillary Electrophoresis-Electrospray Ionization-Mass Spectrometry: Interfaces, Preconcentration Techniques and Applications
- Spectroscopic Determination of Two Beta-Blockers – Atenolol and Propanolol by Oxidative Derivatization Using Potassium Permanganate in Alkaline Medium
- Review on analytical methods for quantification of ADHD drugs in human biological samples
- Benzene, toluene, ethylbenzene, and xylene: Current analytical techniques and approaches for biological monitoring
- Advances in neurochemical measurements: A review of biomarkers and devices for the development of closed-loop deep brain stimulation systems
- Review on stationary phases and coating methods of MEMs gas chromatography columns
- Research Article
- Xanthene based resonance Rayleigh scattering and spectrofluorimetric probes for the determination of cyclobenzaprine: Application to content uniformity test
- Special Issue: SPECIAL ISSUE ON 25TH INTERNATIONAL SYMPOSIUM ON SEPARATION SCIENCES
- Chromatographic analysis of bio-oil formed in fast pyrolysis of lignocellulosic biomass
- Urinary carboxylic acids (UCAs) in subjects with autism spectrum disorder and their association with bacterial overgrowth
- Separation procedures in the identification of the hydrogenation products of biomass-derived hydroxymethylfurfural
- The effect of selenium, zinc and copper on the excretion of urinary modified nucleobases in rats treated with prostate cancer cells
- Analytical approaches and preparation of biological, food and environmental samples for analyses of zearalenone and its metabolites
- Nanosized zinc, epigenetic changes and its relationship with DMBA induced breast cancer in rats
- Special Issue: Bioanalytical Methods and Their Applications
- Single-molecule force spectroscopy: A facile technique for studying the interactions between biomolecules and materials interfaces
- Biological nanoscale fluorescent probes: From structure and performance to bioimaging
- Detection of metal ions in biological systems: A review
- Recent advances in animal origin identification of gelatin-based products using liquid chromatography-mass spectrometry methods: A mini review

