Selective actinide(III) separation using 2,6-bis[1-(propan-1-ol)-1,2,3-triazol-4-yl]pyridine (PyTri-Diol) in the innovative-SANEX process: laboratory scale counter current centrifugal contactor demonstration
-
Andreas Wilden
, Dimitri Schneider
, Zaina Paparigas
, Maximilian Henkes
, Fabian Kreft
, Andreas Geist
, Eros Mossini
, Elena Macerata
, Mario Mariani
, Maria Chiara Gullo
, Alessandro Casnati
and Giuseppe Modolo
Abstract
An innovative-SANEX process for the selective separation of the trivalent actinides americium and curium from a simulated PUREX raffinate solution was successfully demonstrated on the laboratory scale using a 16-stage 1 cm annular centrifugal contactor setup. The solvent was composed of 0.2 mol L−1 N,N,N′,N′-tetra-n-octyl-diglycolamide (TODGA) and 5% v/v 1-octanol in a kerosene diluent. Zr(IV) and Pd(II) co-extraction was prevented using trans-1,2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid (CDTA) as a masking agent in the feed. The actinide(III) selective back-extraction was achieved using 2,6-bis[1-(propan-1-ol)-1,2,3-triazol-4-yl]pyridine (PyTri-Diol) in 0.45 mol L−1 HNO3 as a CHON alternative to the sulfur-containing stripping agent used in a previous version of the innovative-SANEX process. The new process described in this paper showed excellent performance for the recovery of An(III). An An(III) product with a quasi-quantitative recovery of americium and curium (≥99.9%) and very good separation from fission and activation products was obtained (decontamination factors ≥4000). Only a slight contamination with Zr and Ru was observed. This test demonstrates the successful use of molecules containing only carbon, hydrogen, oxygen, and nitrogen atoms (so-called CHON molecules) for the selective separation of An(III) from a simulated PUREX raffinate solution. By avoiding sulfur- or phosphorous-containing molecules, the generation of secondary radioactive waste during process operation can be reduced drastically.
1 Introduction
Nuclear electricity production is currently applied in 32 countries worldwide with 439 operating nuclear power reactors and 51 reactors under construction, as of beginning 2022 [1]. Globally, used fuel in storage accumulates at an annual rate of ca. 7000 metric tons of heavy metal (t HM) and the stored inventory is approaching 300,000 t HM [2]. Additionally, ca. 3000 t HM used fuel can be treated annually in reprocessing facilities [3]. Most countries use a once-through fuel cycle, while only a few countries apply reprocessing on an industrial scale (e.g. France, Russia) for the recovery of uranium and plutonium [4]. For the future application of nuclear electricity production and improvements of sustainability, further advancements of the nuclear fuel cycle and innovative reactor concepts are considered [5], [6], [7], [8]. In Europe, several research projects were funded by the European Commission, addressing innovative hydrometallurgical processes for the separation of actinides from used nuclear fuel [9], [10], [11], [12], [13], [14], [15], [16], [17]. One of the objectives was the development of a process for the separation of the trivalent minor actinides (An(III)) americium and curium from a PUREX (Plutonium Uranium Reduction Extraction) process raffinate [18].
The extractant N,N,N′,N′-tetraoctyl diglycolamide (TODGA, Figure 1) is the most prominent extractant of the class of diglycolamide extractants. It has a high affinity for the extraction of trivalent metal ions, especially An(III) and trivalent lanthanides (Ln(III)) [19], [20], [21], [22]. Several separation processes have been studied using TODGA or a related diglycolamide extractant [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34]. Commonly, An(III) and Ln(III) are effectively extracted, but the undesired co-extraction of Sr(II), Pd(II), Zr(IV) and Ru (numerous nitrosyl complexes) [35] is also observed [36]. While the co-extraction of Pd(II) and Zr(IV) can be overcome using trans-1,2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid (CDTA, Figure 1) as a masking agent [37], and Sr(II) can be scrubbed using less concentrated nitric acid, Ru co-extraction is still problematic and has to be addressed during solvent regeneration. Nevertheless, the co-extracted Ru is not recovered in the An(III) product [24, 26, 33, 34]. TODGA proved to be hydrolytically stable and the radiolytic stability was also found to be sufficient for long-term operation of an An(III) selective separation process [23, 38], [39], [40], [41], [42], [43], [44], [45]. As TODGA alone extracts both An(III) and Ln(III) and a separation of the two groups is not possible, selective stripping of An(III) from the loaded solvent and separation from Ln(III) was accomplished using different approaches. On the one hand, An(III) selective stripping was achieved using hydrophilic polyaminocarboxylic acids (e.g., diethylenetriamine-N,N,N′,N’’,N’’-pentaacetic acid (DTPA)). However, the pH of the An(III) stripping section needed to be controlled precisely in a very narrow range [25, 30, 33, 34]. The use of hydrophilic complexants containing only N-donor atoms, on the other hand, yielded much higher selectivity for An(III) and enabled stripping at higher HNO3 concentrations compared to the polyaminocarboxylic acids [46]. The use of 2,6-bis(5,6-di(sulfophenyl)-1,2,4-triazin-3-yl)pyridine (SO3-Ph-BTP, Figure 1) was successfully demonstrated on the laboratory scale in the innovative-SANEX process using centrifugal contactors [31]. It showed excellent performance for the recovery of An(III). The use of SO3-Ph-BTP was an improvement over the formerly used buffered polyaminocarboxylic acid solutions, as precise pH control and salting out agents were no longer needed. SO3-Ph-BTP was also used in the TRU-SANEX process, a combination of the EURO-GANEX [47, 48] and innovative-SANEX processes for heterogenous recycling of the actinides from Np to Cm [49]. However, the SO3-Ph-BTP molecule contains sulfur and is thus incompatible with the CHON principle, which describes that all chemical reagents used in a process should be totally destructible into gases, therefore only containing atoms of carbon (C), hydrogen (H), oxygen (O), and nitrogen (N) [50].
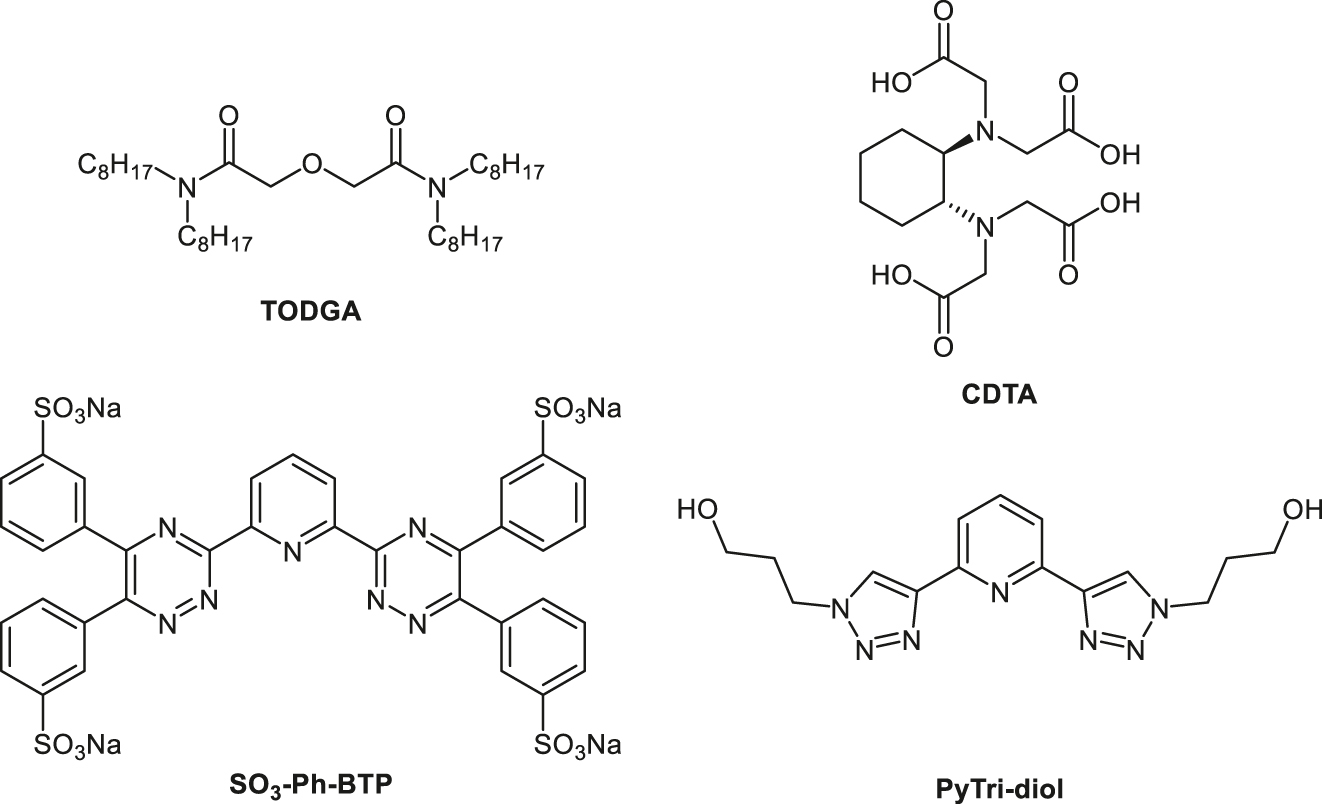
Chemical structures of TODGA, CDTA, SO3-Ph-BTP, and PyTri-Diol.
To overcome that issue, the applicability of 2,6-bis[1-(propan-1-ol)-1,2,3-triazol-4-yl]pyridine (PyTri-Diol, Figure 1) as a highly selective water-soluble stripping agent for trivalent actinides in the innovative-SANEX process was studied. It was shown that PyTri-Diol provides good separation of An(III) from Ln(III) under process-relevant conditions and good radiolytic and hydrolytic stability [51], [52], [53], [54], [55]. The stripping kinetics were shown to be sufficient to be used in a centrifugal contactor setup [56]. A flow sheet has been calculated using batch extraction data and kinetics data from the single centrifugal contactor tests with the SX Process code [56], [57], [58].
The present paper describes the results of a laboratory scale counter current demonstration of the innovative-SANEX process using PyTri-Diol as the An(III)-selective stripping agent.
2 Materials and methods
2.1 Chemicals and reagents
TODGA was purchased from Technocomm Ltd. (Wellbrae, Falkland, Scotland). PyTri-Diol was synthesized at Università di Parma, Parma, Italy, according to the procedure described elsewhere [51]. TPH (hydrogenated tetrapropene, a kerosene-like diluent commonly used in the French reprocessing facility) was obtained from CEA, Marcoule, France. CDTA (purity ≥ 99.0%) was purchased from Sigma-Aldrich, Germany. Nitric acid solutions (Merck AG) were prepared by dilution from a 65% nitric acid solution EMSURE® for analysis using ultra-pure water (18.2 MΩ cm). All commercially available chemicals were purchased in high purity and used without further purification. The radiotracers 241Am, 244Cm, and 152Eu were purchased from Isotopendienst M. Blaseg GmbH, Waldburg, Germany, Oak Ridge National Laboratory, Oak Ridge, USA, and Eckert & Ziegler Nuclitec GmbH, Braunschweig, Germany.
A synthetic PUREX raffinate was used as the feed. The composition is shown in Table 1. It was prepared by a specific protocol for the dissolution of the reagents, which is mainly based on the use of metal nitrate salts [59]. It corresponds to a PUREX raffinate with a volume of 5000 L t−1 (t = metric ton) UO2 fuel with an initial 235U enrichment of 3.5% and thermal burn-up of 33,000 MW d tHM −1 after 3 years of cooling [60], [61], [62]. The relatively high concentrations of Na and Fe in the raffinate are due to purification steps for the U/Pu product produced by the PUREX process and solvent clean-up steps. The initial HNO3 concentration was 4.4 mol L−1 HNO3. 0.05 mol L−1 CDTA was added as a masking agent for Zr and Pd to the innovative SANEX feed [37].
Composition of the synthetic PUREX raffinate solution used as the feed.
| Component | Concentration [mg L−1 or as shown] | Component | Concentration [mg L−1] | Component | Concentration [mg L−1] |
|---|---|---|---|---|---|
| Na | 2000 | Al | 2 | Cr | 106 |
| Fe | 1900 | Ni | 84 | Cu | 21 |
| Se | 6 | Rb | 73 | Sr | 184 |
| Y | 99 | Zr | 720 | Mo | 684 |
| Ru | 385 | Rh | 80 | Pd | 221 |
| Ag | 9 | Cd | 21 | Sn | 0.2 |
| Sb | 3 | Te | 109 | Cs | 532 |
| Ba | 298 | La | 261 | Ce | 594 |
| Pr | 240 | Nd | 757 | Sm | 156 |
| Eu | 35 | Gd | 27 | ||
| HNO3 | 4.4 mol L−1 | ||||
| CDTA | 0.05 mol L−1 |
2.2 Centrifugal contactor setup
The demonstration of the process was carried out using 1 cm annular miniature centrifugal contactors produced by the Institute of Nuclear Energy Technology, Tsinghua University, Beijing, China, with the rotors made of titanium and the stator housings made of stainless steel [63, 64]. The process was run in counter current mode with a rotator speed of 4500 rpm. The speed was checked regularly during the experiment with a stroboscope tachometer. The contactor battery setup consists of four batteries with four stages each, resulting in a total available number of 16 stages. As the extraction/scrubbing sections of the innovative-SANEX process have been demonstrated in the same setup before [31], and no changed were made to the solvent composition, only the An-stripping and Ln re-extraction sections were tested in the centrifugal contactor setup. Calibrated syringe pumps (Kent Scientific Corp., Torrington, CT, USA) were used to deliver the organic and aqueous flows. The flow rates were optimized in single-stage centrifugal contactor experiments and 30 mL h−1 were found to be optimal for each the An stripping, loaded solvent, and fresh solvent flows [56]. The flow rates are also given in the flow sheet shown in Figure 2.

Flow sheet of the innovative SANEX demonstration process with PyTri-Diol.
2.3 Procedures and analytics
Batch solvent extraction experiments for the extraction and three consecutive scrubbing steps were carried out at room temperature (ca. 22 °C) using equal volumes of 300 mL of each phase in glass bottles with Teflon screw caps. The bottles were shaken by hand for 30 min at room temperature for the extraction step, and 15 min at room temperature for each of the three scrubbing steps. After shaking, the bottles were left for phase separation. After phase separation was complete, the phases were sampled for analysis and the organic phase was transferred to another bottle for the next step according to the flow sheet given in Figure 2. 152Eu, 241Am, and 244Cm tracer were added in the last scrubbing step (Scrub 3) to reduce radiation dose to personnel, as the metal distribution ratios at 0.5 mol L−1 HNO3 are very high. Adding the tracers in the first extraction step would only have caused unnecessary dose with only tiny changing the composition of the loaded solvent. The counter current demonstration was run in the 16-stage centrifugal contactor setup. During process operation, aliquots of the spent solvent and An product were sampled periodically to assess the evolution of the steady state. At the end of the experiment, aliquots of aqueous and organic phases were collected from each centrifugal contactor. All these samples were analyzed by gamma and alpha spectrometry, ICP-MS, and acid-base titration. Gamma measurements of 241Am (60 keV) and 152Eu (122 keV) were carried out using an Eurisys EGC 35-195-R germanium coaxial N-type detector and spectra were evaluated using the GammaVision Software. Samples were measured directly without further treatment. Alpha measurements were carried out for 241Am (5486 keV) and 244Cm (5805 keV) using an Ortec Octête-pc eight chamber alpha measurement system equipped with PIPS detectors. Sample preparation for alpha measurement was done by homogenizing a 10 μL aliquot in 100 μL of a mixture of Zapon varnish and acetone (1:100 v/v). This mixture was distributed over a stainless-steel plate obtained from Berthold, Bad Wildbad, Germany. The sample was dried under a heating lamp and annealed into the stainless-steel plate using a gas-flame burner. For stable elements, inductively coupled plasma mass spectrometry (ICP-MS) was conducted using a Perkin Elmer NexION 2000C. The ICP-MS measurements were calibrated against certified metal solution standards. Aqueous samples were measured after dilution in 1% v/v nitric acid solution without further treatment. Organic samples were measured directly in a matrix containing the surfactant Triton-X-100 in 1% v/v HNO3. The ICP-MS measurements of several elements (Eu, Am, Cm) were compared with measurements from other analytical methods and found to be in very good agreement. Acid concentration was measured by titration against 0.1 mol L−1 or 0.10 mol L−1 standardized NaOH solutions using a 798 MPT Titrino, purchased from Metrohm GmbH & Co. KG (Filderstadt, Germany).
Distribution ratios D were calculated as the ratio of activity or concentration of a metal ion in the organic phase versus the activity or concentration of the metal ion in the aqueous phase. Distribution ratios between 0.01 and 100 exhibit an uncertainty of ±5%, while lower/higher values exhibit larger uncertainties.
To evaluate the results of the full counter current centrifugal contactor demonstration test, process decontamination factors, DFfeed/An product, were calculated according to Eq. (1), where Q is the volumetric flow rate and C is the metal ion (M) concentration. The An/M decontamination factors were calculated according to Eq. (2). For the evaluation of the demonstration test discussed in this paper, the loaded solvent composition and flow rates were considered as feed, according to Eqs. (1) and (2).
3 Results and discussion
The innovative-SANEX with PyTri-Diol process is based on the innovative-SANEX process using SO3-Ph-BTP, which was demonstrated previously [31]. It uses an identical solvent composition (0.2 mol L−1 TODGA and 5% v/v 1-octanol in TPH). As only 16 contactors are available in the laboratory setup, only the An stripping and Ln re-extraction sections were actually run in the centrifugal contactor battery, as PyTri-Diol is only used in those stages. The loaded solvent used as the organic feed solution for this demonstration was produced by batch contacts, resembling the extraction and scrubbing steps of the innovative-SANEX process, as these steps were already demonstrated [31] and are not within the scope of this work. Therefore, the solvent was contacted with the synthetic PUREX raffinate solution (composition given in Table 1), containing 0.05 mol L−1 CDTA to prevent Zr and Pd co-extraction [31, 37]. Consecutively, the loaded solvent was washed three times with 0.5 mol L−1 HNO3. The TODGA solvent is known to co-extract a certain amount of nitric acid [65, 66], which was considered in the flow sheet development [56]. As the TODGA solvent extracts An(III) and Ln(III) even from 0.5 mol L−1 HNO3 with high distribution ratios, the radioactive tracer elements 152Eu, 241Am, and 244Cm were added in the third scrubbing step only, to reduce radiation dose to personnel. The loaded organic phase from the third scrubbing step was used as the organic feed for the centrifugal contactor test. The composition of the loaded solvent after the third scrubbing step is given in Table 2. The loaded solvent, which was prepared by batch contact here, compares well with the loaded solvent produced in the counter current centrifugal contactor test of the prior innovative-SANEX test [31]. Most of the feed elements were not extracted and thus not detected in the loaded solvent. In comparison to the prior test, Pd, Mo, and Sr were also not detected, while the Zr concentration was slightly, and the Ru concentration significantly higher. These effects were probably caused by a longer contact time and more efficient mixing in the batch contacts compared to the centrifugal contactor tests. The higher Zr concentrations can be explained by the very slow Zr extraction kinetics with TODGA in the presence of CDTA, which was observed before and seems to be due to very slow decomplexation kinetics of Zr-CDTA complexes in the aqueous phase [37]. The concentration of some light lanthanides was lower in the loaded solvent (esp. La and Ce), as the light lanthanides are least extracted with TODGA [20].
Composition of the loaded solvent used as the organic feed for the centrifugal contactor test.
| Component | Concentration [mg L−1 or as shown] | Component | Concentration [mg L−1] | Component | Concentration [mg L−1] |
|---|---|---|---|---|---|
| Na | ≤10a | Al | ≤0.1a | Cr | ≤0.1a |
| Fe | ≤1a | Ni | ≤0.1a | Cu | ≤0.1a |
| Se | ≤0.1a | Rb | ≤0.1a | Sr | ≤0.1a |
| Y | 82 | Zr | 18 | Mo | ≤0.1a |
| Ru | 97 | Rh | ≤0.1a | Pd | ≤0.1a |
| Ag | ≤0.1a | Cd | ≤0.1a | Sn | ≤0.1a |
| Sb | ≤0.1a | Te | ≤0.1a | Cs | ≤0.1a |
| Ba | ≤0.1a | La | 90 | Ce | 314 |
| Pr | 159 | Nd | 612 | Sm | 145 |
| Eu | 33 | Gd | 23 | ||
| 152Eu | 10.4 MBq L−1 | ||||
| 241Am | 5.7 MBq L−1 | ||||
| 244Cm | 4.4 MBq L−1 | ||||
| HNO3 | 0.02 mol L−1 |
-
adetection limit.
The An stripping and Ln re-extraction sections were run in the 1 cm annular miniature centrifugal contactor setup, installed in the laboratories of Forschungszentrum Jülich, Germany. The tested flow sheet is shown in Figure 2. The Ln stripping was not tested here, as the previous innovative-SANEX demonstration showed that Ln(III) back extraction can easily be achieved in four stages using 0.5 mol L−1 citric acid at pH 3 [31].
The outlets of the centrifugal contactor battery (An product and spent solvent) were monitored during process operation by sampling and quick gamma measurements of 241Am and 152Eu. These preliminary gamma spectrometry results were used to assess the approach to steady state during the test. They showed very quick development of a constant 152Eu concentration in the spent solvent, already after 60 min run time. The 241Am concentration in the An product increased more slowly and reached a plateau after 240 min. The centrifugal contactor test was continued further to enable built-up of a steady state also for the elements, which could not be monitored during the test by gamma spectrometry and stopped after 360 min. Full analysis by gamma and alpha spectrometry and ICP-MS showed that the steady-state had been reached for all elements already after 240 min. No hydrodynamic problems (phase entrainment, third phase formation, or precipitation) were observed during the process demonstration. After stopping the test (stopping pumps and contactors), the contents of the mixing chambers were quickly transferred to test tubes and centrifuged to achieve quantitative phase separation. Both phases were sampled and analyzed by gamma and alpha spectrometry and ICP-MS, and the HNO3 concentration was measured. The results of several elements analyzed by different analytical techniques were compared to each other and found to be in very good agreement (e.g., Am profiles determined by gamma, alpha, and ICP-MS measurements were in very good agreement).
The Am, Cm, and Eu stage profiles of the innovative-SANEX with PyTri-Diol demonstration test are shown in Figure 3. Am and Cm were both stripped very well from the loaded solvent with PyTri-Diol and showed distribution ratios of ca. 0.23 and 0.28, respectively, in all stages. Both elements were efficiently routed to the An product and separated from the lanthanides. The slightly better stripping of Am(III) in comparison with Cm(III) was observed previously [56] and is reflected in the stage profiles of stages 11–16. The An(III) distribution ratios observed here were slightly lower compared to the single centrifugal contactor test [56]. This was probably due to a slightly lower HNO3 concentration in the loaded solvent compared to the loaded solvent used in the single centrifugal contactor test. Here, the solvent was scrubbed three times with 0.5 mol L−1 HNO3, while it was only scrubbed once during the single centrifugal contactor test. The average aqueous nitric acid concentration in all stages was 0.46 mol L−1 HNO3 and varied only slightly.

Am, Cm, and Eu stage profiles of the innovative-SANEX with PyTri-Diol demonstration test (241Am and 152Eu data from gamma spectrometry and 244Cm data from alpha spectrometry measurements).
Europium was not stripped and stayed in the organic phase with distribution ratios of ca. 50. Therefore, it was routed to the spent solvent outlet. The other Ln(III) were also routed to the spent solvent, as shown in Figure 4 for the least extracted elements La, Ce, and Pr (average distribution ratios of 2.4, 3.3, and 4.6, respectively). The An(III)/Ln(III) separation with PyTri-Diol worked very well and the number of stages for An stripping and Ln re-extraction were sufficient. In comparison with the prior innovative-SANEX test using SO3-Ph-BTP, four more stages were used (8 + 8 stages here, instead of 6 + 6 stages in the prior test for An stripping and Ln re-extraction), as PyTri-Diol shows a lower An(III)/Ln(III) selectivity compared to SO3-Ph-BTP [46, 51, 56].

La, Ce, and Pr stage profiles of the innovative-SANEX with PyTri-Diol demonstration test (data from ICP-MS measurements).
The An(III) product was very clean with only very low contaminations as shown in Table 3. The only measurable contaminants in the product were Zr and Ru. The Zr and Ru stage profiles are shown in Figure 5. Both elements showed high distribution ratios in the An stripping section and low distribution ratios in the Ln re-extraction section and the stage profiles were flat in the corresponding sections. Comparable stage profiles were observed for Ru in the prior innovative-SANEX process (the Zr concentration was too low) [31]. For both elements, this behavior could be explained by the existence of different Zr and Ru species, which have different solubility in the aqueous and organic phases, respectively. The existence of different zirconium and ruthenium species and their different extraction behavior in solvent extraction systems has been studied previously (mainly under PUREX process conditions), ascribed to different hydroxide, nitrate, nitrite and other nitrous oxide (mixed) complexes [35, 67], [68], [69], [70], [71]. Further process development has to be conducted to address these issues, although the higher Zr concentration in the current process demonstration was due to the batch preparation of the loaded solvent and is expected to be less impacting in a counter current process including also the extraction and scrubbing stages. The loaded solvent in the prior innovative-SANEX process contained much lower Zr concentrations [31].
Mass balances, recoveries, process, and An/M decontamination factors (DF) obtained during the innovative-SANEX with PyTri-Diol test (only the elements detectable in the loaded solvent are shown, cf. Table 2).
| Component | An product [%] | Spent solvent [%] | DFfeed/An product | DFAm/M | DFCm/M |
|---|---|---|---|---|---|
| Y | ≤0.1 | ≥99.9 | ≥5000 | ≥5000 | ≥5000 |
| Zr | 10 | 90 | 10 | 11 | 10 |
| Ru | 5 | 95 | 20 | 22 | 20 |
| La | ≤0.1 | ≥99.9 | ≥4000 | ≥4000 | ≥4000 |
| Ce | ≤0.1 | ≥99.9 | ≥5000 | ≥5000 | ≥5000 |
| Pr | ≤0.1 | ≥99.9 | ≥5000 | ≥5000 | ≥5000 |
| Nd | ≤0.1 | ≥99.9 | ≥5000 | ≥5000 | ≥5000 |
| Sm | ≤0.1 | ≥99.9 | ≥5000 | ≥5000 | ≥5000 |
| Eu | ≤0.1 | ≥99.9 | ≥5000 | ≥5000 | ≥5000 |
| Gd | ≤0.1 | ≥99.9 | ≥5000 | ≥5000 | ≥5000 |
| 241Am | ≥99.9 | ≤0.1 | 1.0 | 1.0 | 0.9 |
| 244Cm | ≥99.9 | ≤0.1 | 1.0 | 1.1 | 1.0 |

Zr and Ru stage profiles of the innovative-SANEX with PyTri-Diol demonstration test (data from ICP-MS measurements).
The separation from Ln(III) worked very well and the corresponding An(III)/Ln(III) decontamination factors were very high (≥4,000, Table 3). The lanthanide distribution ratios in the An stripping and Ln re-extraction sections were well above one (DLa ≥2.0) and followed the known extraction pattern (i.e. increasing distribution ratios with decreasing ionic radius) [20, 56]. The Ln(III) distribution ratios observed in the present demonstration test and stage profiles in the An stripping and Ln re-extraction sections are comparable to the ones of the prior innovative-SANEX test [31]. The performance of the present demonstration test exceeded the expectations from flow sheet calculations [56]. The use of six stages each for An stripping and Ln re-extraction (as in the prior innovative-SANEX test) would probably result in a loss of 0.5% Am and 0.9% Cm to the spent solvent, but a contamination of the An(III) product with light lanthanides would still be negligible (ca. 0.1% La(III), all other Ln(III) even lower). PyTri-Diol therefore is a very good alternative stripping agent for the recovery of An(III) from PUREX raffinate, which also fulfills the CHON criterion.
4 Conclusions
The innovative-SANEX with PyTri-Diol process demonstration described in this paper showed excellent performance for the recovery of An(III) from a simulated feed solution using the same TODGA-based solvent as in the prior innovative-SANEX process. With the new process, the separation of An(III) in a single cycle becomes possible using chemicals made of C, H, O, and N atoms, using only four stages more than in the prior process. This is a great improvement over the prior innovative-SANEX process, which used a sulfur-containing stripping agent. Therefore, the generation of secondary radioactive waste during process operation can be reduced drastically. The successful demonstration of the use of PyTri-Diol also promises its usability in other processes, e.g., EURO-GANEX [48], TRU-SANEX [49] or TALSPEAK [72].
Funding source: GEN IV Integrated Oxide Fuels Recycling Strategies 10.13039/501100000780
Award Identifier / Grant number: 730227
Funding source: Partitioning and Transmuter Research Initiative in a Collaborative Innovation Action 10.13039/501100000780
Award Identifier / Grant number: 945077
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Funding for this research was provided by the European Commission through the GEN IV Integrated Oxide Fuels Recycling Strategies (GENIORS) project, grant agreement number 730227 and the Partitioning and Transmuter Research Initiative in a Collaborative Innovation Action (PATRICIA) project, grant agreement number 945077.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. International Atomic Energy Agency (IAEA) PRIS – power reactor information system. https://pris.iaea.org/pris/home.aspx (accessed Jan 05, 2022).Search in Google Scholar
2. International Atomic Energy Agency (IAEA) Nuclear Technology Review 2021, Vienna, Austria, 2021, September 2021.Search in Google Scholar
3. OECD-NEA Nuclear Energy Data – Données sur l’énergie nucléaire 2020; OECD/NEA Publishing: Paris, France, 2021. Vol. NEA No. 7556.Search in Google Scholar
4. International Atomic Energy Agency (IAEA) Status and Trends in Spent Fuel and Radioactive Waste Management, Vienna, Austria, 2022.Search in Google Scholar
5. OECD-NEA Transition Towards a Sustainable Nuclear Fuel Cycle; OECD-NEA: Paris, France, 2013.Search in Google Scholar
6. OECD-NEA State-of-the-Art Report on the Progress of Nuclear Fuel Cycle Chemistry; OECD Nuclear Energy Agency: Paris, France, 2018. Vol. NEA No. 7267.Search in Google Scholar
7. OECD-NEA Strategies and Considerations for the Back End of the Fuel Cycle; OECD Nuclear Energy Agency (NEA): Boulogne-Billancourt, France, 2021. Vol. NEA No. 7469.Search in Google Scholar
8. International Atomic Energy Agency (IAEA) Nuclear Energy for a Net Zero World, Vienna, Austria, 2021, September 2021.Search in Google Scholar
9. Madic, C., Hudson, M. J., Liljenzin, J.-O., Glatz, J.-P., Nannicini, R., Facchini, A., Kolarik, Z., Odoj, R. Recent achievements in the development of partitioning processes of minor actinides from nuclear wastes obtained in the frame of the NEWPART European programme (1996–1999). Prog. Nucl. Energy 2002, 40, 523–526; https://doi.org/10.1016/s0149-1970(02)00046-x.Search in Google Scholar
10. Madic, C., Testard, F., Hudson, M. J., Liljenzin, J. O., Christiansen, B., Ferrando, M., Facchini, A., Geist, A., Modolo, G., Gonzales-Esperanto, A., Mendoza, J. D. PARTNEW – New Solvent Extraction Processes for Minor Actinides – Final Report. CEA-report 6066, 2004.Search in Google Scholar
11. Madic, C., Ouvrier, N. EUROPART: EUROpean research program for the PARTitioning of minor actinides from high active wastes arising from the reprocessing of spent nuclear fuels. Radiochim. Acta 2008, 96, 183–185; https://doi.org/10.1524/ract.2008.1477.Search in Google Scholar
12. Bourg, S., Hill, C., Caravaca, C., Rhodes, C., Ekberg, C., Taylor, R., Geist, A., Modolo, G., Cassayre, L., Malmbeck, R., Harrison, M., de Angelis, G., Espartero, A., Bouvet, S., Ouvrier, N. ACSEPT – partitioning technologies and actinide science: towards pilot facilities in Europe. Nucl. Eng. Des. 2011, 241, 3427–3435; https://doi.org/10.1016/j.nucengdes.2011.03.011.Search in Google Scholar
13. Bourg, S., Geist, A., Narbutt, J. SACSESS – the EURATOM FP7 project on actinide separation from spent nuclear fuels. Nukleonika 2015, 60, 809–814; https://doi.org/10.1515/nuka-2015-0152.Search in Google Scholar
14. Geist, A., Taylor, R., Ekberg, C., Guilbaud, P., Modolo, G., Bourg, S. The SACSESS hydrometallurgy domain: an overview. Procedia Chem. 2016, 21, 218–222; https://doi.org/10.1016/j.proche.2016.10.031.Search in Google Scholar
15. Geist, A., Adnet, J.-M., Bourg, S., Ekberg, C., Galán, H., Guilbaud, P., Miguirditchian, M., Modolo, G., Rhodes, C., Taylor, R. An overview of solvent extraction processes developed in Europe for advanced nuclear fuel recycling, Part 1 – heterogeneous recycling. Separ. Sci. Technol. 2021, 56, 1866–1881; https://doi.org/10.1080/01496395.2020.1795680.Search in Google Scholar
16. Authen, T. L., Adnet, J.-M., Bourg, S., Carrott, M., Ekberg, C., Galán, H., Geist, A., Guilbaud, P., Miguirditchian, M., Modolo, G., Rhodes, C., Wilden, A., Taylor, R. An overview of solvent extraction processes developed in Europe for advanced nuclear fuel recycling, Part 2 – homogeneous recycling. Separ. Sci. Technol. 2021. accepted; https://doi.org/10.1080/01496395.2021.2001531.Search in Google Scholar
17. Bourg, S. GENIORS project homepage, 2022. http://www.geniors.eu/ (accessed Jan 05, 2022).Search in Google Scholar
18. Modolo, G., Geist, A., Miguirditchian, M. Minor actinide separations in the reprocessing of spent nuclear fuels: recent advances in Europe. In Reprocessing and Recycling of Spent Nuclear Fuel. Chap. 10; Taylor, R., Ed.; Woodhead Publishing: Oxford, 2015, pp. 245–287; https://doi.org/10.1016/b978-1-78242-212-9.00010-1.Search in Google Scholar
19. Stephan, H., Gloe, K., Beger, J., Muhl, P. Liquid-liquid-extraction of metal-ions with amido podands. Solvent Extr. Ion Exch. 1991, 9, 459–469; https://doi.org/10.1080/07366299108918064.Search in Google Scholar
20. Sasaki, Y., Sugo, Y., Suzuki, S., Tachimori, S. The novel extractants, diglycolamides, for the extraction of lanthanides and actinides in HNO3-n-dodecane system. Solvent Extr. Ion Exch. 2001, 19, 91–103; https://doi.org/10.1081/sei-100001376.Search in Google Scholar
21. Ansari, S. A., Pathak, P., Mohapatra, P. K., Manchanda, V. K. Chemistry of diglycolamides: promising extractants for actinide partitioning. Chem. Rev. 2012, 112, 1751–1772; https://doi.org/10.1021/cr200002f.Search in Google Scholar PubMed
22. Whittaker, D., Geist, A., Modolo, G., Taylor, R., Sarsfield, M., Wilden, A. Applications of diglycolamide based solvent extraction processes in spent nuclear fuel reprocessing, Part 1: TODGA. Solvent Extr. Ion Exch. 2018, 36, 223–256; https://doi.org/10.1080/07366299.2018.1464269.Search in Google Scholar
23. Modolo, G., Asp, H., Schreinemachers, C., Vijgen, H. Development of a TODGA based process for partitioning of actinides from a PUREX raffinate Part I: batch extraction optimization studies and stability tests. Solvent Extr. Ion Exch. 2007, 25, 703–721; https://doi.org/10.1080/07366290701634578.Search in Google Scholar
24. Modolo, G., Asp, H., Vijgen, H., Malmbeck, R., Magnusson, D., Sorel, C. Demonstration of a TODGA-based continuous counter-current extraction process for the partitioning of actinides from a simulated PUREX raffinate, Part II: centrifugal contactor runs. Solvent Extr. Ion Exch. 2008, 26, 62–76; https://doi.org/10.1080/07366290701784175.Search in Google Scholar
25. Hérès, X., Sorel, C., Miguirditchian, M., Camès, B., Hill, C., Bisel, I., Espinoux, D., Eysseric, C., Baron, P., Lorrain, B. Results of recent counter-current tests on An(III)/Ln(III) separation using TODGA extractant. In Proceedings of GLOBAL, Paris, France, 2009, pp. 1127–1132, 6–11 September, paper 9384.Search in Google Scholar
26. Magnusson, D., Christiansen, B., Glatz, J.-P., Malmbeck, R., Modolo, G., Serrano-Purroy, D., Sorel, C. Demonstration of a TODGA based extraction process for the partitioning of minor actinides from a PUREX raffinate Part III: centrifugal contactor run using genuine fuel solution. Solvent Extr. Ion Exch. 2009, 27, 26–35; https://doi.org/10.1080/07366290802544726.Search in Google Scholar
27. Ansari, S. A., Pathak, P., Mohapatra, P. K., Manchanda, V. K. Aqueous partitioning of minor actinides by different processes. Separ. Purif. Rev. 2011, 40, 43–76; https://doi.org/10.1080/15422119.2010.545466.Search in Google Scholar
28. Magnusson, D., Geist, A., Wilden, A., Modolo, G. Direct selective extraction of actinides (III) from PUREX raffinate using a mixture of CyMe4-BTBP and TODGA as 1-cycle SANEX solvent Part II: flow-sheet design for a counter-current centrifugal contactor demonstration process. Solvent Extr. Ion Exch. 2013, 31, 1–11; https://doi.org/10.1080/07366299.2012.700596.Search in Google Scholar
29. Wilden, A., Modolo, G., Schreinemachers, C., Sadowski, F., Lange, S., Sypula, M., Magnusson, D., Geist, A., Lewis, F. W., Harwood, L. M., Hudson, M. J. Direct selective extraction of actinides (III) from PUREX raffinate using a mixture of CyMe4BTBP and TODGA as 1-cycle SANEX solvent Part III: demonstration of a laboratory-scale counter-current centrifugal contactor process. Solvent Extr. Ion Exch. 2013, 31, 519–537; https://doi.org/10.1080/07366299.2013.775890.Search in Google Scholar
30. Gelis, A. V., Lumetta, G. J. Actinide lanthanide separation process-ALSEP. Ind. Eng. Chem. Res. 2014, 53, 1624–1631; https://doi.org/10.1021/ie403569e.Search in Google Scholar
31. Wilden, A., Modolo, G., Kaufholz, P., Sadowski, F., Lange, S., Sypula, M., Magnusson, D., Müllich, U., Geist, A., Bosbach, D. Laboratory-scale counter-current centrifugal contactor demonstration of an innovative-SANEX process using a water soluble BTP. Solvent Extr. Ion Exch. 2015, 33, 91–108; https://doi.org/10.1080/07366299.2014.952532.Search in Google Scholar
32. Marie, C., Kaufholz, P., Vanel, V., Duchesne, M.-T., Russello, E., Faroldi, F., Baldini, L., Casnati, A., Wilden, A., Modolo, G., Miguirditchian, M. Development of a selective americium separation process using H4TPAEN as water-soluble stripping agent. Solvent Extr. Ion Exch. 2019, 37, 313–327; https://doi.org/10.1080/07366299.2019.1643569.Search in Google Scholar
33. Gelis, A. V., Kozak, P., Breshears, A. T., Brown, M. A., Launiere, C., Campbell, E. L., Hall, G. B., Levitskaia, T. G., Holfeltz, V. E., Lumetta, G. J. Closing the nuclear fuel cycle with a simplified minor actinide lanthanide separation process (ALSEP) and additive manufacturing. Sci. Rep. 2019, 9, 12842; https://doi.org/10.1038/s41598-019-48619-x.Search in Google Scholar PubMed PubMed Central
34. Wilden, A., Kreft, F., Schneider, D., Paparigas, Z., Modolo, G., Lumetta, G. J., Gelis, A. V., Law, J. D., Geist, A. Countercurrent actinide lanthanide separation process (ALSEP) demonstration test with a simulated PUREX raffinate in centrifugal contactors on the laboratory scale. Appl. Sci. 2020, 10, 7217; https://doi.org/10.3390/app10207217.Search in Google Scholar
35. Dirks, T., Dumas, T., Solari, P. L., Charbonnel, M.-C. Ruthenium nitrosyl structure in solvent extraction systems: a comparison of tributyl phosphate, tetrabutyl urea, N-methyl, N-octyl ethylhexanamide, and N,N,N’,N’-tetraoctyl diglycolamide. Ind. Eng. Chem. Res. 2019, 58, 14938–14946; https://doi.org/10.1021/acs.iecr.9b02555.Search in Google Scholar
36. Stephan, H., Gloe, K., Beger, J., Muhl, P. Liquid-liquid-extraction of strontium with amido podands. Solvent Extr. Ion Exch. 1991, 9, 435–458; https://doi.org/10.1080/07366299108918063.Search in Google Scholar
37. Sypula, M., Wilden, A., Schreinemachers, C., Malmbeck, R., Geist, A., Taylor, R., Modolo, G. Use of polyaminocarboxylic acids as hydrophilic masking agents for fission products in actinide partitioning processes. Solvent Extr. Ion Exch. 2012, 30, 748–764; https://doi.org/10.1080/07366299.2012.700591.Search in Google Scholar
38. Sugo, Y., Taguchi, M., Sasaki, Y., Hirota, K., Kimura, T. Radiolysis study of actinide complexing agent by irradiation with helium ion beam. Radiat. Phys. Chem. 2009, 78, 1140–1144; https://doi.org/10.1016/j.radphyschem.2009.06.031.Search in Google Scholar
39. Gujar, R. B., Ansari, S. A., Bhattacharyya, A., Kanekar, A. S., Pathak, P. N., Mohapatra, P. K., Manchanda, V. K. Radiolytic stability of N,N,N′,N′-tetraoctyl diglycolamide (TODGA) in the presence of phase modifiers dissolved in n-dodecane. Solvent Extr. Ion Exch. 2012, 30, 278–290; https://doi.org/10.1080/07366299.2011.609389.Search in Google Scholar
40. Galán, H., Zarzana, C. A., Wilden, A., Núñez, A., Schmidt, H., Egberink, R. J. M., Leoncini, A., Cobos, J., Verboom, W., Modolo, G., Groenewold, G. S., Mincher, B. J. Gamma-radiolytic stability of new methylated TODGA derivatives for minor actinide recycling. Dalton Trans. 2015, 44, 18049–18056.10.1039/C5DT02484FSearch in Google Scholar
41. Zarzana, C. A., Groenewold, G. S., Mincher, B. J., Mezyk, S. P., Wilden, A., Schmidt, H., Modolo, G., Wishart, J. F., Cook, A. R. A comparison of the γ-radiolysis of TODGA and T(EH)DGA using UHPLC-ESI-MS analysis. Solvent Extr. Ion Exch. 2015, 33, 431–447; https://doi.org/10.1080/07366299.2015.1012885.Search in Google Scholar
42. Peterman, D., Geist, A., Mincher, B., Modolo, G., Galán, M. H., Olson, L., McDowell, R. Performance of an i-SANEX system based on a water-soluble BTP under continuous irradiation in a γ-radiolysis test loop. Ind. Eng. Chem. Res. 2016, 55, 10427–10435; https://doi.org/10.1021/acs.iecr.6b02862.Search in Google Scholar
43. Hubscher-Bruder, V., Mogilireddy, V., Michel, S., Leoncini, A., Huskens, J., Verboom, W., Galán, H., Núñez, A., Cobos Sabate, J., Modolo, G., Wilden, A., Schmidt, H., Charbonnel, M.-C., Guilbaud, P., Boubals, N. Behaviour of the extractant Me-TODGA upon gamma irradiation: quantification of the degradation compounds and individual influences on complexation and extraction. New J. Chem. 2017, 41, 13700–13711; https://doi.org/10.1039/c7nj02136d.Search in Google Scholar
44. Horne, G. P., Zarzana, C., Rae, C., Cook, A. R., Mezyk, S. P., Zalupski, P., Wilden, A., Mincher, B. J. Does addition of 1-octanol as a phase modifier provide radical scavenging radioprotection for N,N,N’,N’-tetraoctyldiglycolamide (TODGA)? Phys. Chem. Chem. Phys. 2020, 22, 24978–24985; https://doi.org/10.1039/d0cp04310a.Search in Google Scholar PubMed
45. Malmbeck, R., Banik, N. L. Radiolytic behaviour of a TODGA based solvent under alpha irradiation. J. Radioanal. Nucl. Chem. 2020, 326, 1609–1615; https://doi.org/10.1007/s10967-020-07444-7.Search in Google Scholar
46. Geist, A., Müllich, U., Magnusson, D., Kaden, P., Modolo, G., Wilden, A., Zevaco, T. Actinide(III)/lanthanide(III) separation via selective aqueous complexation of actinides(III) using a hydrophilic 2,6-Bis(1,2,4-Triazin-3-yl)-Pyridine in nitric acid. Solvent Extr. Ion Exch. 2012, 30, 433–444; https://doi.org/10.1080/07366299.2012.671111.Search in Google Scholar
47. Carrott, M., Bell, K., Brown, J., Geist, A., Gregson, C., Hères, X., Maher, C., Malmbeck, R., Mason, C., Modolo, G., Müllich, U., Sarsfield, M., Wilden, A., Taylor, R. Development of a new flowsheet for Co-separating the transuranic actinides: the “EURO-GANEX” process. Solvent Extr. Ion Exch. 2014, 32, 447–467; https://doi.org/10.1080/07366299.2014.896580.Search in Google Scholar
48. Malmbeck, R., Magnusson, D., Bourg, S., Carrott, M., Geist, A., Hérès, X., Miguirditchian, M., Modolo, G., Müllich, U., Sorel, C., Taylor, R., Wilden, A. Homogenous recycling of transuranium elements from irradiated fast reactor fuel by the EURO-GANEX solvent extraction process. Radiochim. Acta 2019, 107, 917–929; https://doi.org/10.1515/ract-2018-3089.Search in Google Scholar
49. Carrott, M., Maher, C., Mason, C., Sarsfield, M., Taylor, R. “TRU-SANEX”: a variation on the EURO-GANEX and i-SANEX processes for heterogeneous recycling of actinides Np-Cm. Separ. Sci. Technol. 2016, 51, 2198–2213; https://doi.org/10.1080/01496395.2016.1202979.Search in Google Scholar
50. Madic, C., Hudson, M. J. High-Level Liquid Waste Partitioning by Means of Completely Incinerable Extractants; EUR 18038 EN, European Commission: Luxembourg, 1998.Search in Google Scholar
51. Macerata, E., Mossini, E., Scaravaggi, S., Mariani, M., Mele, A., Panzeri, W., Boubals, N., Berthon, L., Charbonnel, M.-C., Sansone, F., Arduini, A., Casnati, A. Hydrophilic clicked 2,6-Bis-triazolyl-pyridines endowed with high actinide selectivity and radiochemical stability: toward a closed nuclear fuel cycle. J. Am. Chem. Soc. 2016, 138, 7232–7235; https://doi.org/10.1021/jacs.6b03106.Search in Google Scholar PubMed
52. Wagner, C., Mossini, E., Macerata, E., Mariani, M., Arduini, A., Casnati, A., Geist, A., Panak, P. J. Time-resolved laser fluorescence spectroscopy study of the coordination chemistry of a hydrophilic CHON 1,2,3-Triazol-4-yl pyridine ligand with Cm(III) and Eu(III). Inorg. Chem. 2017, 56, 2135–2144; https://doi.org/10.1021/acs.inorgchem.6b02788.Search in Google Scholar PubMed
53. Mossini, E., Macerata, E., Brambilla, L., Panzeri, W., Mele, A., Castiglioni, C., Mariani, M. Radiolytic degradation of hydrophilic PyTri ligands for minor actinide recycling. J. Radioanal. Nucl. Chem. 2019, 322, 1663–1673; https://doi.org/10.1007/s10967-019-06772-7.Search in Google Scholar
54. Weßling, P., Trumm, M., Macerata, E., Ossola, A., Mossini, E., Gullo, M. C., Arduini, A., Casnati, A., Mariani, M., Adam, C., Geist, A., Panak, P. J. Activation of the aromatic core of 3,3 ’-(Pyridine-2,6-diylbis(1H-1,2,3-triazole-4,1-diyl))bis(propan-1-ol) – effects on extraction performance, stability constants, and basicity. Inorg. Chem. 2019, 58, 14642–14651; https://doi.org/10.1021/acs.inorgchem.9b02325.Search in Google Scholar PubMed PubMed Central
55. Mossini, E., Macerata, E., Boubals, N., Berthon, C., Charbonnel, M.-C., Mariani, M. Effects of gamma irradiation on the extraction properties of innovative stripping solvents for i-SANEX/GANEX processes. Ind. Eng. Chem. Res. 2021, 60, 11768–11777; https://doi.org/10.1021/acs.iecr.1c01236.Search in Google Scholar
56. Mossini, E., Macerata, E., Wilden, A., Kaufholz, P., Modolo, G., Iotti, N., Casnati, A., Geist, A., Mariani, M. Optimization and single-stage centrifugal contactor experiments with the novel hydrophilic complexant PyTri-diol for the i-SANEX process. Solvent Extr. Ion Exch. 2018, 36, 373–386; https://doi.org/10.1080/07366299.2018.1507134.Search in Google Scholar
57. Magnusson, D., Geist, A., Malmbeck, R. SX process-A code for solvent extraction processes in centrifugal contactors simulation. Chem. Eng. Sci. 2013, 99, 292–297; https://doi.org/10.1016/j.ces.2013.06.008.Search in Google Scholar
58. Magnusson, D., Geist, A., Malmbeck, R., Müllich, U. Study of the mass transfer behavior in a centrifugal contactor and verification of the solvent extraction model for the SX process program. Solvent Extr. Ion Exch. 2013, 31, 578–589; https://doi.org/10.1080/07366299.2013.806748.Search in Google Scholar
59. Heits, B. Zusammensetzung und Herstellung von HAW/HAWC-Simulaten gemäß den Planungsgrundlagen der DWK und den Erfahrungen der WAK; Deutsche Gesellschaft für Wiederaufarbeitung von Kernbrennstoffen mbH: Karlsruhe, 1982.Search in Google Scholar
60. Cecille, L., Landat, D., Mannone, F. Separation of actinides from solutions of high activity nuclear wastes (HAW raffinates). 1. Description of separation by TBP process. Radiochem. Radioanal. Lett. 1977, 31, 19–28.Search in Google Scholar
61. Cecille, L., Lestang, M., Mannone, F. Separation of actinides from solutions of high activity nuclear wastes (HAW raffinates). 2. Description of separation by HDEHP process. Radiochem. Radioanal. Lett. 1977, 31, 29–37.Search in Google Scholar
62. Kolarik, Z. Separation of actinindes and long-lived fission products from high-level radioactive wastes (a review). In KfK 4945; Kernforschungszentrum Karlsruhe GmbH: Karlsruhe, 1991.Search in Google Scholar
63. Leonard, R. A. Design principles and applications of centrifugal contactors for solvent extraction. In: Moyer, B. A., Ed.; Ion Exchange and Solvent Extraction, A Series of Advances; CRC Taylor and Francis: Boca Raton, FL, USA, Vol. 19, 2009, pp. 563–616. Chap. 10; https://doi.org/10.1201/9781420059700-c10.Search in Google Scholar
64. Duan, W., Zhao, M., Wang, C., Cao, S. Recent advances in the development and application of annular centrifugal contactors in the nuclear industry. Solvent Extr. Ion Exch. 2014, 32, 1–26; https://doi.org/10.1080/07366299.2013.833741.Search in Google Scholar
65. Geist, A. Extraction of nitric acid into alcohol: kerosene mixtures. Solvent Extr. Ion Exch. 2010, 28, 596–607; https://doi.org/10.1080/07366299.2010.499286.Search in Google Scholar
66. Woodhead, D., McLachlan, F., Taylor, R., Müllich, U., Geist, A., Wilden, A., Modolo, G. Nitric acid extraction into a TODGA solvent modified with octanol. Solvent Extr. Ion Exch. 2019, 37, 173–190; https://doi.org/10.1080/07366299.2019.1625201.Search in Google Scholar
67. Siczek, A. A., Steindler, M. J. Chemistry of ruthenium and zirconium in the PUREX solvent-extraction process. Atom. Energy Rev. 1978, 16, 575–618.Search in Google Scholar
68. Lefebvre, C., Dumas, T., Charbonnel, M. C., Solari, P. L. Speciation of ruthenium in organic TBP/TPH organic phases: a study about acidity of nitric solutions. Procedia Chem. 2016, 21, 54–60; https://doi.org/10.1016/j.proche.2016.10.008.Search in Google Scholar
69. Lefebvre, C., Dumas, T., Tamain, C., Ducres, T., Solari, P. L., Charbonnel, M.-C. Addressing ruthenium speciation in tri-n-butyl-phosphate solvent extraction process by fourier transform infrared, extended X-ray absorption fine structure, and single crystal X-ray diffraction. Ind. Eng. Chem. Res. 2017, 56, 11292–11301; https://doi.org/10.1021/acs.iecr.7b02973.Search in Google Scholar
70. Moeyaert, P., Miguirditchian, M., Masson, M., Dinh, B., Hérès, X., De Sio, S., Sorel, C. Experimental and modelling study of ruthenium extraction with tri-n-butylphosphate in the purex process. Chem. Eng. Sci. 2017, 158, 580–586; https://doi.org/10.1016/j.ces.2016.10.035.Search in Google Scholar
71. Verma, P. K., Mohapatra, P. K. Ruthenium speciation in radioactive wastes and state-of-the-art strategies for its recovery: a review. Separ. Purif. Technol. 2021, 275, 119–148; https://doi.org/10.1016/j.seppur.2021.119148.Search in Google Scholar
72. Wu, F. F., Lv, H. B., He, X. H., Cheng, Z. P., Jia, H. W., Xie, S. B., Liu, Y. K., Ye, G. A., He, H. Selective Am(III) stripping with water-soluble PyTri-Diol in nitric acid from HDEHP organic phase. J. Radioanal. Nucl. Chem. 2020, 323, 283–289; https://doi.org/10.1007/s10967-019-06961-4.Search in Google Scholar
© 2022 Andreas Wilden et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Editorial: Diamond Jubilee Issue
- Sixty years of Radiochimica Acta: a brief overview with emphasis on the last 10 years
- A. Chemistry of Radioelements
- Five decades of GSI superheavy element discoveries and chemical investigation
- Chemistry of the elements at the end of the actinide series using their low-energy ion-beams
- Sonochemistry of actinides: from ions to nanoparticles and beyond
- Theoretical insights into the reduction mechanism of neptunyl nitrate by hydrazine derivatives
- The speciation of protactinium since its discovery: a nightmare or a path of resilience
- On the volatility of protactinium in chlorinating and brominating gas media
- The aqueous chemistry of radium
- B. Energy Related Radiochemistry
- Selective actinide(III) separation using 2,6-bis[1-(propan-1-ol)-1,2,3-triazol-4-yl]pyridine (PyTri-Diol) in the innovative-SANEX process: laboratory scale counter current centrifugal contactor demonstration
- Fate of Neptunium in nuclear fuel cycle streams: state-of-the art on separation strategies
- Uranium adsorption – a review of progress from qualitative understanding to advanced model development
- Targeted synthesis of carbon-supported titanate nanofibers as host structure for nuclear waste immobilization
- Progress of energy-related radiochemistry and radionuclide production in the Republic of Korea
- C. Nuclear Data
- How accurate are half-life data of long-lived radionuclides?
- Status of the decay data for medical radionuclides: existing and potential diagnostic γ emitters, diagnostic β+ emitters and therapeutic radioisotopes
- An overview of nuclear data standardisation work for accelerator-based production of medical radionuclides in Pakistan
- An overview of activation cross-section measurements of some neutron and charged-particle induced reactions in Bangladesh
- Nuclear reaction data for medical and industrial applications: recent contributions by Egyptian cyclotron group
- Nuclear data for light charged particle induced production of emerging medical radionuclides
- D. Radionuclides and Radiopharmaceuticals
- The role of chemistry in accelerator-based production and separation of radionuclides as basis for radiolabelled compounds for medical applications
- Production of neutron deficient rare earth radionuclides by heavy ion activation
- Evaluation of 186WS2 target material for production of high specific activity 186Re via proton irradiation: separation, radiolabeling and recovery/recycling
- Special radionuclide production activities – recent developments at QST and throughout Japan
- China’s radiopharmaceuticals on expressway: 2014–2021
- E. Environmental Radioactivity
- A summary of environmental radioactivity research studies by members of the Japan Society of Nuclear and Radiochemical Sciences
Articles in the same Issue
- Frontmatter
- Editorial: Diamond Jubilee Issue
- Sixty years of Radiochimica Acta: a brief overview with emphasis on the last 10 years
- A. Chemistry of Radioelements
- Five decades of GSI superheavy element discoveries and chemical investigation
- Chemistry of the elements at the end of the actinide series using their low-energy ion-beams
- Sonochemistry of actinides: from ions to nanoparticles and beyond
- Theoretical insights into the reduction mechanism of neptunyl nitrate by hydrazine derivatives
- The speciation of protactinium since its discovery: a nightmare or a path of resilience
- On the volatility of protactinium in chlorinating and brominating gas media
- The aqueous chemistry of radium
- B. Energy Related Radiochemistry
- Selective actinide(III) separation using 2,6-bis[1-(propan-1-ol)-1,2,3-triazol-4-yl]pyridine (PyTri-Diol) in the innovative-SANEX process: laboratory scale counter current centrifugal contactor demonstration
- Fate of Neptunium in nuclear fuel cycle streams: state-of-the art on separation strategies
- Uranium adsorption – a review of progress from qualitative understanding to advanced model development
- Targeted synthesis of carbon-supported titanate nanofibers as host structure for nuclear waste immobilization
- Progress of energy-related radiochemistry and radionuclide production in the Republic of Korea
- C. Nuclear Data
- How accurate are half-life data of long-lived radionuclides?
- Status of the decay data for medical radionuclides: existing and potential diagnostic γ emitters, diagnostic β+ emitters and therapeutic radioisotopes
- An overview of nuclear data standardisation work for accelerator-based production of medical radionuclides in Pakistan
- An overview of activation cross-section measurements of some neutron and charged-particle induced reactions in Bangladesh
- Nuclear reaction data for medical and industrial applications: recent contributions by Egyptian cyclotron group
- Nuclear data for light charged particle induced production of emerging medical radionuclides
- D. Radionuclides and Radiopharmaceuticals
- The role of chemistry in accelerator-based production and separation of radionuclides as basis for radiolabelled compounds for medical applications
- Production of neutron deficient rare earth radionuclides by heavy ion activation
- Evaluation of 186WS2 target material for production of high specific activity 186Re via proton irradiation: separation, radiolabeling and recovery/recycling
- Special radionuclide production activities – recent developments at QST and throughout Japan
- China’s radiopharmaceuticals on expressway: 2014–2021
- E. Environmental Radioactivity
- A summary of environmental radioactivity research studies by members of the Japan Society of Nuclear and Radiochemical Sciences


