Recent progress on the morphology and thermal cycle of phase change materials (PCMs)/conductive filler composites: a mini review
-
Andiswa Kaleni
, Teboho Clement Mokhena
Abstract
The current society is experiencing serious problems in terms of energy and environmental pollution. Environmentally friendly and renewable sources such as phase change materials have been employed as a reliable and effective source of energy storage. Phase change materials (PCMs) are known to absorb or release sizeable amount of energy during melting and solidification process. PCMs have been employed in both thermal protection and energy storage systems, more in active as well as passive cooling applications. However, PCMs are associated with low thermal conductivity, which hinders the process of heat rate exchange during the melting/solidification cycles. To improve the thermal conductivity of PCMs, various conductive fillers have been incorporated to such systems. Thermal cycle test is done to verify the stability of PCMs in thermal energy storage systems. This review emphasizes on the morphology, preparation methods and thermal cycle of various types of PCMs in the presence of conductive filler. Furthermore, the bibliometric analysis based on key research areas, top ten leading countries, and citations by country on phase change materials for energy storage research are reported in this review paper. The bibliometric analysis shows the dominance of the People’s Republic of China on PCMs for energy storage research.
Nomenclature
- AgNPs
-
silver nanoparticles
- Aℓ2O3
-
alumina nanoparticles
- BPC
-
biological porous carbon
- CBCF
-
carbon bonded carbon fiber
- CNF
-
carbon nanofibers
- CNTs
-
carbon nanotubes
- CVD
-
chemical vapour deposition
- DSC
-
differential scanning calorimetry
- EG
-
expanded graphite
- EGP
-
expanded graphite plate
- ENG-TSA
-
expanded natural graphite treated with sulfuric acid
- EVM
-
expanded vermiculite
- F-CB
-
functionalized carbon black
- Fe3O4
-
nanomagnetite
- F-MWCNTs
-
functionalize multiwalled carbon nanotubes
- FTIR
-
Fourier transform infra-red
- GNPs
-
graphene nanoplatelets
- GNS
-
graphene nanosheets
- GO
-
graphite oxide
- L-MWCNTs
-
long multiwalled carbon nanotubes
- OD
-
1-octadecanol
- O-GNs
-
oriented graphite nanosheets
- PCMs
-
phase change materials
- PEG
-
polyethylene glycol
- RD
-
raw diatomite
- R-GNs
-
randomly distributed graphite nanosheets
- SA
-
sebacic acid
- SCFs
-
short carbon fiber
- SEM
-
scanning electron microscopy
- SiC NWs
-
silicon carbide nanowires
- S-MWCNT
-
short multi-walled CNTs
- SSL
-
sodium stearoyl lactylate
- TGA
-
thermogravimetric analysis
- UFG
-
ultrathin-graphite foam
- xGnP
-
exfoliated graphite nanoplatelets
- XRD
-
X-ray diffraction
1 Introduction
The utilization of renewable and sustainable energy resources to reduce the greenhouse gases emission and dependence on the fossil fuels has been amongst the major research subjects in the past decades [1], [2], [3]. One of the best solutions in reducing the greenhouse emission and utilizing ecofriendly energy resources is to implement PCMs. Figure 1 illustrates the rapid increase in studies on phase change materials since the year 2015 due to the energy crisis caused by the increasing demands of energy, resulting from the rising population growth, wars, and market manipulations. PCMs are recognized by absorbing or releasing energy when they undergo a change in physical state, which includes solid–liquid, solid–solid, and gas–liquid. Figure 2 shows the top 10 countries that are actively involved in utilization of PCMs for energy storage. The analysis reveals that the dominant country is the People’s Republic of China (36.8%). In second position is the United States of America (10.7%) which is closely followed by India (10%). Figure 3 illustrates citation network by countries. The bibliometric network further proves the dominance of Chinese on PCMs for energy storage. The size of the frame is positively correlated with the number of citations. The thickness of the lines and proximity of the frames represent the strength of the citation link between countries. The People’s Republic of China is the most cited country regarding this research topic (42358 citations) with total link strength of 75717.

Annual number of scientific publications from 2015 to 2021, using the search terms “phase change materials for energy storage” and “thermal conductivity of phase materials”. Data analysis was completed using the Web of Science core collection databases on 06 April 2021.
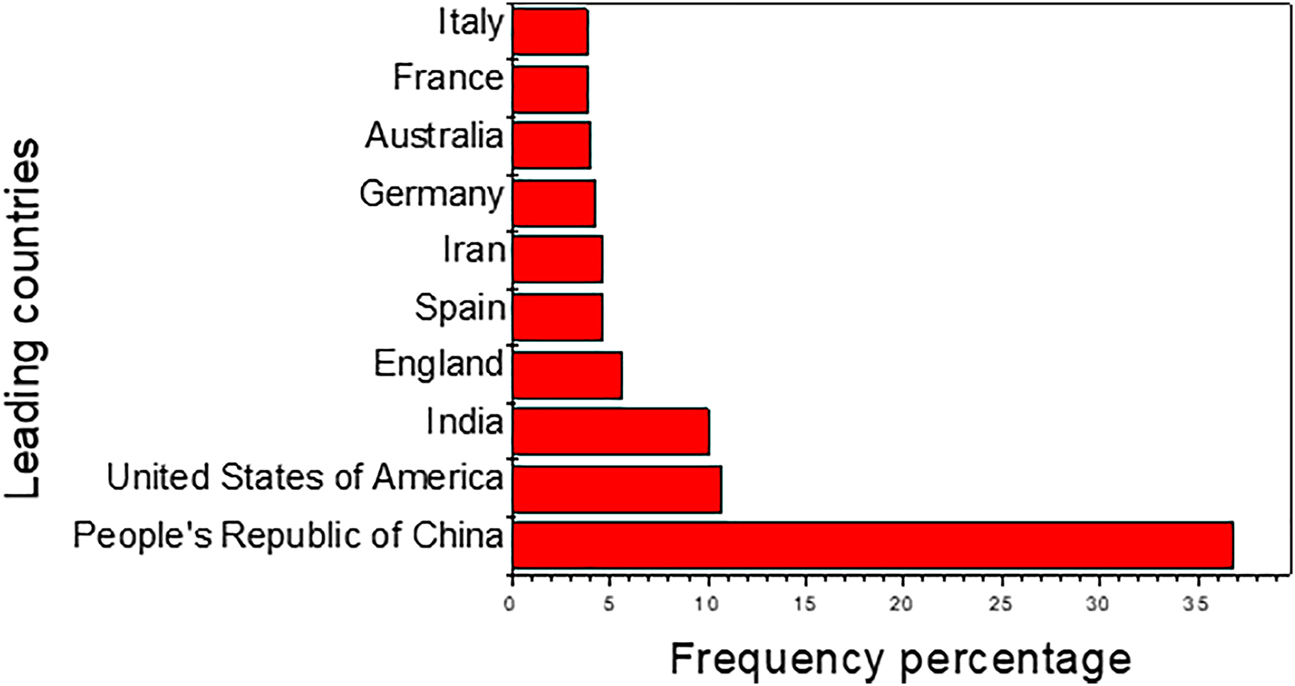
Top ten leading countries active on research based on PCMs as sustainable energy storage materials (2015–2021).

PCMs as sustainable energy storage materials bibliometric network visualization of citations by country using VOS viewer version 1.6.15 software. Data was extracted from the WoS core collection database.
Phase change materials are categorized as inorganic, organic, or eutectic mixture of inorganic–organic (Figure 4) [4], [5], [6], [7], [8]. Organic materials can be subdivided into non-paraffins and paraffins [4], [5], [6, 9, 10]. Most of the organic phase change materials undergo self-nucleation, meaning crystallization with negligible or no super cooling. Paraffins include paraffin wax, which is composed of straight chain n alkanes. Paraffin wax may be regarded as an effective heat storage material because it is reliable, safe, predictable, cheap, noncorrosive, and shows little volume change during melting [6, 11]. Non-paraffins (viz: formic acid, caprylic acid, glycerin, phenol, etc.) have a variety of properties, when compared with paraffins, which have indistinguishable properties. Some properties of non-paraffins, include: (i) inflammability, (ii) excellent heat of fusion, (iii) a low flash point, (iv) a low thermal conductivity, (v) varying degree of toxicity, and (vi) unstable at elevated temperatures. Inorganic phase change materials are divided into metallics and salt hydrates [12], [13], [14]. The inorganic PCMs do not super cool in an appropriate manner and there is no degradation during cycling. Salt hydrates are consisted of inorganic salts and water that produce a more crystalline solid. Salt hydrates have been used because of: (i) high thermal conductivity (ii) small volume changes during melting and (iii) high latent of fusion. Another type of inorganic PCMs is metallics, which has not been widely investigated due to their weight problems and corrosiveness. However, one major advantage of the metallic PCMs in comparison to other PCMs is their excellent thermal conductivity with acceptable heat of fusion and volume changes during melting. Besides other PCMs classification mentioned above, there is another type, in the form of eutectic PCM, which has a minimum melting temperature between mixtures of components where melting and freezing occur congruently, producing a mixture of crystals during crystallization. One important feature of eutectics PCMs is that they melt and freeze without segregation.
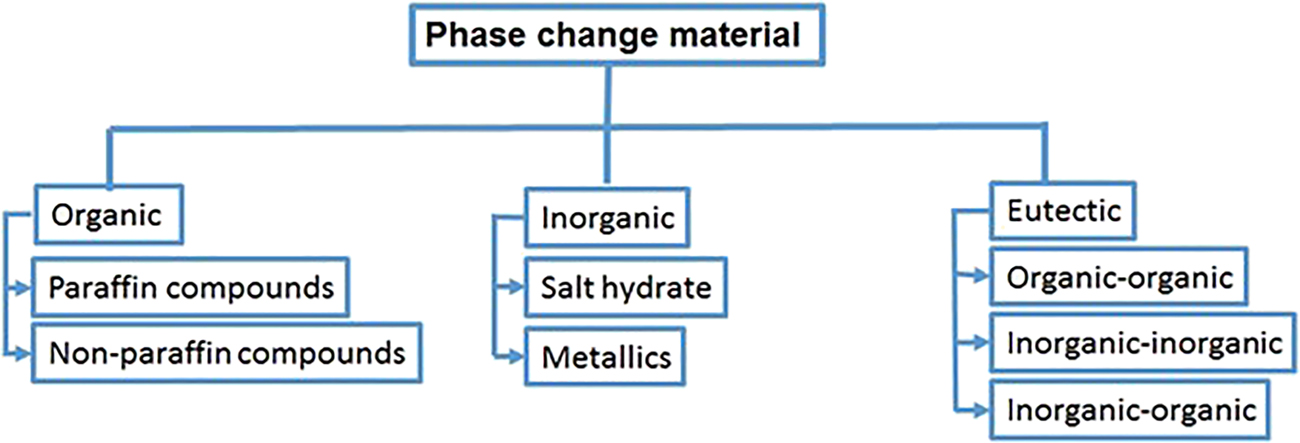
Classification of phase change materials.
Most PCMs (except for the metallics) have low thermal conductivity, irrespective of their classification [15], [16], [17], [18], [19], [20], [21]. This property hinders their widespread applications because of little heat transfer during storing or releasing of thermal energy. Numerous studies were conducted (Figure 1) to overcome this issue by incorporating highly conductive fillers into PCMs. Conductive fillers are added to PCMs for two reasons, viz. (i) to facilitate thermal conductivity within PCM system and (ii) to adsorb and prevent PCMs leakage during transition. Out of the top 10 countries conducting research incorporating conductive filler to promote thermal conductivity within PCM systems, the People’s Republic of China (39.2%), United States of America (15%), and India (9.2%) are amongst the leading countries, with the People’s Republic of China as leaders (see Figure 5). The bibliometric analysis further reveals that the People’s Republic of China (58472 citations) is the most cited country regarding the inclusion of conductive filler in order to improve overall thermal conductivity of PCMs (see Figure 6). The United States of America (28 620 citations) is ranked second and it is strongly linked with third placed India regarding citations.

Top ten leading countries on thermal conductivity of phase change materials research (2015–2021).

Thermal conductivity of phase change materials bibliometric network visualization of the citations by country using VOS viewer version 1.6.15 software. Data was extracted from the WoS core collection database.
Reviews and research papers on thermal conductivity and thermal storage properties of PCMs are well documented, while reports on their thermal stability as well as their reliability are fairly scarce [4], [5], [6], [7], [8], [9], [10]. The success for widespread usage of phase change materials is limited by poor long-term thermal stability. The long-term performance of the storage system can be established by monitoring the differences in the thermo-physical properties of the PCMs after being subjected to a numerous number of thermal cycles. The current review paper reports on the morphology, preparation methods and thermal stability of the various types of PCMs in the presence of a conductive filler.
2 Preparation and morphology of PCMs/conductive filler systems
The development of conductive PCM composites was done not only to enhance the thermal conductivity of the ensuing system (viz. resulting in a rapid energy storage and release), even so for prevention of paraffin leakage in the molten state [22], [23], [24]. The morphological characterization is an important tool because it can provide more information on the interaction and compatibility between pure PCMs and the conductive nanofillers [25]. A number of factors, such as: different preparation methods, content of the filler, type of the PCM, type of the conductive filler, orientation, and the functionalization of the conductive nanoparticles, will naturally, affect the morphology of conductive PCM composites [25], [26], [27], [28], [29]. Table 1 summarizes the preparation methods and morphology of different PCMs/conductive filler systems. In most PCMs/conductive filler systems, the relevant methods for preparation of such composites is the immersion of the conductive filler (viz. expanded graphite, ultrathin graphite foams etc.) in a hot liquid wax and/or cold compression method, as summarized in Table 1. In the compression method, solid PCMs and fillers are mixed and then compressed with or without heat [26]. The method is preferred for the preparation of conductive PCMs composites because the fillers normally occupy the voids between the PCM particles during melt compression and this process facilitating the formation of a percolating network within the system. Irrespective of the classification of PCMs (Figure 4) and the preparation method (Table 1), in most cases, if expanded graphite (EG) was used as a conductive filler and/or matrix for PCMs, the PCMs were found to occupy the pores within the honeycomb network of EG. This indicates that EG has a strong affinity for the PCMs [27]. It is believed that the EG consists of two types of pores i.e., the macro-pores and meso-pores. The macro pores are mainly distributed in the gap between two different worm-like skeletons and the meso-pores are found through the separation of graphite layers. This means that the PCMs are, basically, absorbed in either the meso-pores or macro-pores structures of EG. Huang et al. [28] investigated wood alloy/expanded graphite composite for electronic thermal management. The authors reported absorption of wood alloys into the micro-pores due to capillary forces, while less absorption occurred into the meso-pores structures at 70 wt% of PCM in the composite. The reason for less absorption into the mesoporous is attributed to the closing of the mesopores after being through the physical mixing process. The same method of adding the conductive filler (viz. metal foam, carbon nanotubes, and carbon nanofiber etc.), other than expanded graphite into the melted PCMs was investigated elsewhere in the literature and the resultant morphology differed depending on the type of conductive filler used [29]. The ultrathin-graphite foam (UFG)-erythritol composite was investigated in a study by Ji et al. [29]. This was done by submerging the foam in hot liquid wax. In this study, the conductive filler in the form of ultrathin-graphite foam (UFG) was synthesized on nickel foam as a template through chemical vapor deposition (CVD) method. The template was then removed using wet chemical etching process, thereby, leaving behind ultrathin-graphite struts that were connected in continuous foam. The grown UFG are hydrophobic and as a result, they can easily be filled into the hydrophobic paraffin wax. Scanning electron microscopy (SEM) and micro-CT scan sheets of the UFG-erythritol composites, revealed that the hollow interior of the UFG struts were occupied with PCM.
Summary for the preparation and morphology of different PCM composite system.
| PCM/conductive filler composites | Preparation method | Comments on morphology | References |
|---|---|---|---|
| 1-Octadecanol (OD)/MWCNT |
|
The OD-grafted MWCNTs composites were more stable due to stronger colloidal attractions between OD-G-MWCNT and OD. The nanofillers were well-distributed throughout the surface of the OD |
[22] |
| Fatty acids/CNTs | Fatty acids were grafted onto CNTs and mixed with fatty acids | The nanofillers were well-distributed within the composite structure | [23] |
| LiNO3-KCℓ-NaNO3/expanded graphite | Capillary adsorption method | The particles were disseminated inside the honeycomb web structure of EG | [35] |
| Ultrathin-graphite foams (UFG)/erythritol | Immersion of foam into liquid PCM | UFG struts filled with PCM | [29] |
| Graphite foam/commercial paraffin | Heat of 335 K was applied on graphite foam and paraffin wax filled beaker for about 5 h in a vacuum oven. The heat applied was above the melting of paraffin wax (321.83 K) | PCM was infused into the openings of the graphite foam | [36] |
| Wood’s alloy/expanded graphite | Three step method:
|
Surface of the graphite particles were covered with wood’s alloy | [28] |
| Paraffin wax (PW)/carbon nanotubes sponges | Paraffin wax penetrated the inner pores of the nanotubes sponge through solvent-assisted infiltration | The paraffin wax filled the internanotube spacing and nanotube covered by paraffin wax membranes | [37] |
| Erythritol/carbon fiber | The melt-dispersion (MD) method together with a novel hot-press (HP) method was used | Carbon fiber uniformly dispersed when the composites were prepared using melt-dispersion. In the case of hot-pressing method, the carbon fiber seemed to be found near the phase change material particles of the PCC | [26] |
| n-Octadecane/mesoporous silica (MPSiO2) | Fabricated by separating MPSiO2 in phase change material | Permeability and spherical particles were high | [38] |
| (KNO3/NaNO3)/expanded natural graphite with added sulfuric acid | Cold-compression method | SEM images showed that the composite have a coated structure with “salts surrounded”. Furthermore, the preferential orientation of the ENG-TSA particles in the composite sample is induced by the pressing tension | [39] |
| Paraffin/copper foam and paraffin/nickel foam | The vacuum impregnation method was employed | Irrespective of the type of metal foam used, paraffin was totally compatible with the metal foam | [40] |
| Paraffin(n-nonacosane)/exfoliated graphite nanoplatelets (xGnP) | Mixed paraffin with xGnP or graphene was used to prepare composites in hot toluene followed by solvent extraction and vacuum drying | The studied nano-graphite particles had a marked difference in the absorption ability of PCM. Most of the paraffin was not well absorbed by graphene as conductive filler; however, paraffin was efficiently absorbed between the graphite flake spaces | [41] |
| Paraffin with randomly distributed graphite nanosheets (R-GNs) and oriented graphite nanosheets (O-GNs) | The melted paraffin was added with both types of graphite and then stirred for 30 min 75 °C | Uniform dispersal of GNs in paraffin was found, even at higher loading of GNs | [42] |
| Stearyl alcohol/palm triple pressed acid/(GNPs) | Stearyl alcohol/palm triple pressed was melted in hot plate at 80 °C; the blend was stirred and melted for 5 min and GNPs were added into the melted mixture. It was followed by evaporation and drying | GNPs appeared to be covered by the PCM | [43] |
| Commercial grade paraffin (RT27)/graphene nanoplatelets (xGnP) (dispersed and non-dispersed) | Vacuum impregnation method | xGnP particles were found to be partially submerged into the paraffin of composite PCM | [44] |
| Sebacic acid (SA)/carbon nanotube sponge | The SA was kept at a temperature of 150 °C in a thermostat. The CNT sponge was then immersed into molten SA to be encapsulated. The composites were refloated followed by filtration and dried to fabricate SA/CNT sponge composite. | SA was sucked into the pores of the CNT sponge. Furthermore, the XRD pattern of the composite exhibited all the peaks belonging to both SA and the CNT sponge. It is observed that no new peaks are produced, even though sebacic acid relatively produces higher peaks | [45] |
| Carbon bonded carbon fiber (CBCF) monoliths/paraffin wax | Composites of CBCF-PCM with different carbon fibers volume fractions were obtained by saturating melted PW into the carbon bonded carbon fiber pores under a vacuum | SEM images showed penetration of PW into the interconnected 3D permeable network without any spaces | [46] |
| Manganese dioxide nanowires and nanotubes based PCM nanocomposites (MnO2 NWs/PCM and MnO2 NTs/PCM); PCMs used are myristic acid (MA), palmitic acid and stearic acid | Melt-impregnation method | The SEM and TEM images showed that the MnO2 nanowires surfaces were covered by PCMs (viz: Palmitic acid and stearic acid) | [47] |
| Pentaerythritol/alumina | Alumina nanoparticles were mixed with pentaerythritol with the aid of the low energy ball mill set at 200 rpm for about 90 min | SEM images of pentaerythritol showed loose microstructures and individual lamellae. The addition of alumina provided a condensed lamellar microstructure to pentaerythritol | [48] |
| MgCl2-KCℓ/expanded graphite/graphite paper | A mixing-compression heating process was employed to develop a composite | MgCℓ2-KCℓ/EG/(GP) composite showed compact microstructures in comparison with MgCℓ2-KCℓ/EG counterpart | [49] |
| Erythritol/short carbon fiber (SCFs) | Method: Conductive filler added to melted PCM: Erythritol was melted at 125 °C and then poured into a beaker which was stirred for 20 min using a magnetic stirring system. Carbon nanofibers were added into the melted to the PCM, melted composite was poured into a cylindrical mould |
SEM images showed no agglomerations or entanglements of SCFs. Furthermore, a homogenous distribution of the SCFs was observed due to stirring process during the preparation of the composites | [50] |
Furthermore, the effects of different carbon nanofillers i.e., short multiwalled carbon nanotubes, long multi-walled carbon nanotubes, carbon nanofibers and graphene nanoplatelets were explored for enhancing thermal conductivity of paraffin-based nanocomposite PCMs [30]. PCM/carbon nanofillers were prepared via a two-step process. Firstly, the nanofillers were incorporated into the molten paraffin wax and secondly, the suspension was poured into a rectangular mold and then allowed to solidify into composite samples. SEM images of raw carbon nanotubes (CNTs) and carbon nanofibers (CNF) (Figure 7(a)–(c)) were found to be agglomerated, whereas CNPs were confirmed [30] to be planar and stacked together (Figure 7(d)). However, in the PCM/carbon nanofillers composites, carbon nanotubes and carbon nanofibers were well distributed within paraffin wax, and the large clusters were broken down into smaller particles during preparation (Figure 8(a)–(c)). The stacked GNPs were also separated into thin graphene sheets that were well distributed in the PCM system (Figure 8(d)).
![Figure 7:
SEM images of the raw (a) S-MWCNTs, (b) L-MWCNTs, (c) CNFs, and (d) GNPs with different sizes and shapes. (d) Insert showing an individual GNP sheet and its thickness [30]. Copyright Elsevier.](/document/doi/10.1515/polyeng-2022-0020/asset/graphic/j_polyeng-2022-0020_fig_007.jpg)
SEM images of the raw (a) S-MWCNTs, (b) L-MWCNTs, (c) CNFs, and (d) GNPs with different sizes and shapes. (d) Insert showing an individual GNP sheet and its thickness [30]. Copyright Elsevier.
![Figure 8:
TEM images showing the sizes and distributions of: (a) S-MWCNTs, (b) L-MWCNTs, (c) CNFs and (d) GNPs filled in the paraffin wax [30]. Copyright Elsevier.](/document/doi/10.1515/polyeng-2022-0020/asset/graphic/j_polyeng-2022-0020_fig_008.jpg)
TEM images showing the sizes and distributions of: (a) S-MWCNTs, (b) L-MWCNTs, (c) CNFs and (d) GNPs filled in the paraffin wax [30]. Copyright Elsevier.
The influence of the filler content was reported by Ren, et al. [31]. The system was composed of Ca(NO3)2-NaNO3/expanded graphite with EG loadings being varied throughout the preparation process. The prepared composite proved that the content of EG had an influence on the resultant morphology of the system [31]. Macro-cracks were observed in the 5 wt% of EG composite, whereas the leakage of PCM in a liquid form was observed for 6 wt% EG composite. Finally, the surface of 7 wt% of EG was smooth and homogenous without cracks and liquid leakage. It is well known that the morphology of any composites system affects the final properties of the system. It was reported in the literature that conductive particles experience increases in surface interaction and are more likely to undergo a particle adhesion through immediate contact, via three forces i.e. electrostatic, van der Waals, and magnetic forces due to high surface area and energy [32]. Chemical functionalization was also used to enhance the compatibility between conductive filler and phase change material. Chemical functionalization became a popular method because it is more effective in improving the compatibility when compared to the physical absorption of surfactants [33]. Functionalized carbon black (F-CB)/octadecane composites were investigated for room temperature electrical and thermal regulation [33]. The F-CB particles were combined with various amounts of octadecane and rigorously stirred at 70 °C for 2 h in order to produce the F-CB/octadecane composites. Carbon black nanoparticles were functionalized through hydroxylation and esterification and it was found that the modified carbon black particles dispersed uniformly in octadecane. Similarly, Sun and co-workers [34] fabricated carbon nanotubes (CNT)/hexadecane composites for room temperature electrical and thermal switching. In order to functionalize multiwalled carbon nanotubes (F-MWCNTs), oxidation process was employed in order to introduce carboxylic acid groups on the surface of the CNTs and then functionalized MWCNTs (F-MWCNTs) were obtained from thermal mixing of oxidized MWCNT and octadecylamine (ODA). An obvious multi-layered structure with smooth surface in the nonfunctionalized MWCNT, however for the functionalized MWCNT, an amorphous substance that was attached onto the surface of MWCNT was seen, which was attributed to the grafting of long-chain amine groups. Nonfunctionalized MWCNT and functionalized MWCNT were dispersed in hexadecane and the samples were allowed to stand for a month to analyse the stability of the two types of carbon nanotubes in hexadecane. After a month, the resultant micrographs showed sediment in the nonfunctionalized MWCNT/hexadecane composite, whereas for the functionalized MWCNT/hexadecane composite, there was no sediment, which proved that F-MWCNT had a better stability in hexadecane.
The specific surface area and orientation of the conductive filler can also affect the morphology of the conductive PCMs composite. The specific surface area of conductive filler plays a significant role in shape stabilization. In addition, a conductive filler with a high specific surface area, is expected to be well dispersed in the paraffin as a result, this makes it convenient for the particles to form a mechanically interconnected structure, even at lesser contents and provide better mechanical reinforcement. The overall idea is to obtain better reproducibility and uniformity of the conductive PCMs composite systems. Shi et al. [41] investigated the shape stabilized PCMs using two nanographite fillers (viz. exfoliated graphite nanoplatelets and graphene) with different specific surface area. The specific surface area of graphene and exfoliated graphite nanoplatelets were reported to be 876.2 and 13.8 m2 g−1, respectively. Because of the higher specific surface area of graphene nanoplatelets, SEM images, at 2 wt% loading of the nanofiller, showed no white areas which proved that paraffin was well immersed into the spaces between the graphene nanoplatelets. The result is an indication that graphene, as a conductive filler can assist in terms of reducing the content of additives required for shape stabilization. However, there were no SEM images for an exfoliated paraffin composite system; however, it can be deduced from the composite’s low dropping points (67.0 °C), when compared to the paraffin/graphene composite (185.2 °C) that it must have experienced some degree of liquid leakage, during its solid change. This symbolizes excessive paraffin not being absorbed by the exfoliated graphite. Depending on the type of application required, the ideal orientation of the conductive nanoparticles is very important. The assumption of random distribution and orientation of the conductive filler was demonstrated by Chen et al. [42]. In this study, the authors fabricated two types of paraffin composites, with both the randomly distributed as well as oriented nanosheets termed R-GPNs and O-GPNs, respectively. Irrespective of graphite nanosheets (GPNs) orientation, SEM images of the composites showed that GPNs well dispersed in paraffin, even at higher GPNs content. In the case of R-GPNs/paraffin composites, wrinkles were observed at higher GPNs content, i.e. 5 wt%, whereas, for the O-GPNs/paraffin composite, the GPNs were oriented relatively parallel, due to the shearing effect during the ball rolling process. Based on the morphology above, it can be expected that the wrinkled dispersion of the GPNs will have better thermal conductivity in comparison to the parallel orientation of the nanographite; due to the better GPNs contact, resulting in better pathways for heat transfer.
3 Stability test of PCMs-conductive filler composites
One of the most important requirements of PCM/conductive filler composites is their ability to withstand multiple melting and solidification cycles. A satisfactory thermal reliability is essential for the application of PCM-based composite, since it ensures a more stable phase change in temperature and the latent heat of PCM composites after a numerous number of cycles. The idea is such that there must be insignificant or no change in the thermal property for a long-term usage. Ji et al. [29] studied thermal cycle stability of the composite by analysing the electrical resistance after numerous melting and solidification cycles. In their study, two types of PCM composites (viz: UGF-wax and UGF-erythritol) were investigated. During melting process, the liquid PCM remained within the pores of the ultrathin-graphite foams (UGF), due to surface tension. This simply implies that there was no significant mass loss recorded during electrical measurement, even after 100 melting-solidification cycles. The use of other techniques, such as: XRD, FTIR, TGA, and DSC are usually employed to confirm whether there are any changes in crystallinity, chemical, and thermal properties of the PCM composites, after the stability test. In most cases, the crystallinity, thermal stability, and chemical structure of the resulting composite materials do not change after the stability test. However, no changes were recorded regarding latent heat, as well as melting and freezing temperatures, depending on the system, as summarized in Table 2. The changes in these properties are within experimental error, which makes it difficult to conclude whether the PCM composite system is losing its crystallinity, thermal and chemical integrities during the stability test. On the other hand, in most of these studies, the test methods differ, with some been in-house constructed testers, which make it difficult to compare the results obtained with other studies. The standardization of the stability test of PCM composites is needed to evaluate thoroughly and compare the results regarding the ability of the conductive filler to absorb the PCM material without observable leakage. For instance, the use of DSC to study the stability of the composites is controversial regarding the fact that the PCM composite is closed in a pan and even if there is leakage from the composite material, the PCM will still be in the pan. Thus, these types of methods must be carefully evaluated to establish their reliability towards stability testing of PCM composites. Moreover, the morphological characterization of the PCM composites after thermal cycle must be done and compared with unanalysed samples in order to thoroughly understand whether there is any leakage after testing. FTIR serves as important tool to evaluate any chemical changes, especially for PCM materials that change their chemical structures during charging and discharging, whereas for other PCMs, e.g., paraffin wax, since leakage is the only important aspect, researchers’ can overlook such techniques. Nonetheless, a simple method for the evaluation of leakage of the PCM composites was conducted by heating the composite material to a temperature above the melting temperature of the PCM, and then hold it for 20 min. It was found that the presence of halloysite is important to prevent seepage, as shown in Figure 9, while additional graphite and CNTs improves the thermal conductivity of the resulting PCM composite product [51].
Stability test for PCM-conductive filler composites.
| PCM/conductive filler composites | Test method | Comments on thermal cycling | References |
|---|---|---|---|
|
Cyclic test was conducted by repetitively heating 30 mg of specimen to 800 °C and cooled to 500 °C at a rate of 50 K min−1. This was done using an Al2O3 container in an air flow of 200 ml min−1 | After 10 cycles, both phases change temperature and latent heat of the phase change materials were almost consistent with first cycle | [52] |
| Expanded natural graphite treated with sulfuric acid (ENG-TSA) (10 and 15%)/mixture of KNO3/NaNO3 1:1 | Cyclic test using DSC | Experiment findings after 10 cycles showed that the melting temperatures of shifted to higher temperatures in the presence of ENG-TSA as well as the freezing temperatures because of the force of capillary in the ENG-TSA matrix | [39] |
| Erythritol/vermiculite/graphite | A self-constructed cyclic heating-cooling device was used to measure the thermal cycling performance of the materials. The limits (upper and lower) were set at 200° and 20 °C, respectively. Within a total cycle of 300 cycles, the heating frequency was set at 20 °C/min, although the cooling frequency was about 30 °C/min | FT-IR test result confirmed that the composition of the sample remained unchanged. On the other hand, the enthalpy of the sample decreased with 3.41% while thermal conductivity declined by only 4.58%. Each phase of the sample was distributed evenly with no cracks and significant phase separation after 300 thermal cycles. However, the microstructure of the sample showed that there were pores which may be attributed to multiple cycles | [53] |
| MgCℓ2-graphite foam | The thermal cycling experimental procedure was as follows:
|
Severe corrosion after the cycling tests was observed | [54] |
| Carbon nanotube sponges (CNTS)/paraffin wax | Cyclic test using DSC | A composite containing 90.5 wt % tested for 100 cycles under the same settings demonstrated small shifts of temperatures in relation to wax melting and freezing have a very small shift for all the cycles. Similar observations were reported for composites with paraffin wax loadings of 76 and 91 wt % after being tested for 11 cycles | [37] |
| Paraffin and graphite oxide (GO) composite | The self-constructed cyclic heating-cooling device were used to measure the performance of the thermal cycle | The latent heat values measured using DSC for composite PCM changed by −3.10%, −4.03% and −1.73% for melting process. In contrast, for freezing process the values were −3.42%, −4.81%, and 2.74% after 500, 1500, and 2500 cycles which are acceptable values for LHTES applications | [55] |
| Carbon-coated aluminum nanoparticles/paraffin wax | Thermal cycling performance of the materials was measured using a self-constructed cyclic heating-cooling device | The difference in change during the melting and freezing process was less than 5% after 200, 600, and 1000 cycles when compared to that of 0 cycles | [56] |
| Crystalline TiO2 shell and n-eicosane core attached with graphene nanosheets | DSC with 200-cycle heating-cooling scans. The specimen were examined by FTIR spectroscopy and TGA after DSC measurement. |
|
[57] |
| Erythritol (C4H8O4)/carbon fiber/indium | Thermal cycling tests were conducted in furnace by heating the samples to 125 °C and keeping them there for 15 min then cooled to room temperature for up to 10 cycles | The stability of the PCC during thermal cycling was boosted with increasing indium content, whereas the CF filler network was stabilized through welding by the indium | [58] |
| Polyethylene glycol (PEG)/porous carbon synthesized from radish and potato under different temperatures (i.e., 400, 800, 1100, 1300, and 1600 °C) with the optimal temperature being 1300 °C for 2 h | Not stated | FT-IR spectra, XRD, DSC curves, and TGA curves of prepared PCMs for pre and post thermal cycling were similar which justifies that chemical and structural stability was conserved after 200 cycles | [59] |
| Paraffin/nanomagnetite (Fe3O4) | Stability was carried out by subjecting all samples to successive melting and freezing cycles using a programmable thermostatic water bath with temperature ranging from 25 to 70 °C for 100, 200, 300, 400, and 500 cycles | DSC thermograms after 0, 100, 200, 300, 400, and 500 cycles overlapped each other such that they had the same melting range, melting, and freezing peaks | [60] |
| Palmitic acid (PA)/graphene nanoplatelets (GNPs) | The test method used was the in-house thermal cycling system design which runs up to 2500 cycles | Only small changes in melting and freezing temperatures of composites were detected after 2500 cycles indicating good thermal reliability. Moreover, there was no new secondary peaks were observed on DSC curves which justifies good chemical stability | [61] |
| Bi nanoparticles embedded in an Ag matrix | The composite samples were subjected to 100 melt-freeze cycles | There were no prominent changes in melting temperature or enthalpy throughout the cycles | [62] |
| Octadecane/carbon-decorated diatomite | The composite samples were subjected to 400 melt-freeze cycles | FT-IR spectra and XRD analysis of the composites before and after thermal cycle showed similar shape and frequency bands. Furthermore, the overlapped curves indicated that the crystal structure was not destroyed. The repeated melting and freezing cycle did not degrade the chemicals | [63] |
| Pentaerythritol [2,2-bis(hydroxymethyl)-1,3-propanediol]/alumina nanoparticles (Aℓ2O3) | Using thermal cycling unit, the samples were subjected to heating from 30 °C and the heating rate continued at 10 °C/min up to 210 °C. The samples were kept at 210 °C for between 5 and 10 minand then cooled to 30 °C at 10 °C/min. The samples were ten held at 30 °C for a further 10 min. The thermal cycle was repeated 100 times |
|
[48] |
| Polyethylene glycol (PEG600)/raw diatomite (RD)/carbon nanotubes (CNTs) | Thermal cycler (BIOER TC-25/H model, China) and the analysis before and after thermal cycling for 500 times were carried out by DSC, and FTIR |
|
[64] |
| Polyethylene glycol (PEG)/silicon carbide nanowires (SiC NWs) network/expanded vermiculite (EVM) | The thermal reliability of the samples was determined through 200 phase change cycles | FTIR absorption is at its highest point in the spectrum after 200 cycles did not change indicating good chemical compatibility, whereas no significant difference was observed in both endothermic and exothermic DSC curves revealing that the samples have excellent thermal reliability and thermal stability | [64] |
| Paraffin/copper nanoparticles | The thermal reliability of the samples was determined through 100 phase change cycles | The latent heat and phase-change temperatures change slightly after 100 thermal cycles with a maximum change of −1.6% for melting temperature. Furthermore, −1.9% change is observed for freezing temperature | [65] |
| paraffin/porous TiO2 foams | The thermal cycling tests of the composite PCMs were carried out by placing each sample on a plate covered with a filter paper and then placed in a thermostatic chamber and maintained at 80 °C. The composite PCMs were exposed alternately to room temperature and to 80 °C (thermostatic chamber). During this process, the filter paper was changed after every 10 cycles, with each cycle including a melting and solidification step. The PCMs were tested for 200 cycles. |
Comparing the melting and freezing temperatures before cycling and after cycling, there was a slight change of less than 2 °C in the Tm and Tf values, while enthalpy values decreased by 20 J/g after 200 melting and freezing cycles | [66] |
| Paraffin/aluminum honeycomb | The self-constructed cyclic heating–cooling device was used to measure the performance of the thermal cycle. | The thermal conductivity of the prepared shape-stabilized phase change material was considered stable near the average value, and the thermal physical properties of the shape-stabilized phase change material kept stable after multiple thermal cycles of melting and solidification | [67] |
![Figure 9:
Photographs of (a) pure wax, (b) wax/halloysite (50/50%), (c) wax/graphite (90/10%), (d) wax/halloysite/graphite (45/45/10%) and (e) wax/halloysite/graphite/carbon nanotubes (45/45/5/5%) held at 90 °C for 20 min [51]. IOP Publishing (open access).](/document/doi/10.1515/polyeng-2022-0020/asset/graphic/j_polyeng-2022-0020_fig_009.jpg)
Photographs of (a) pure wax, (b) wax/halloysite (50/50%), (c) wax/graphite (90/10%), (d) wax/halloysite/graphite (45/45/10%) and (e) wax/halloysite/graphite/carbon nanotubes (45/45/5/5%) held at 90 °C for 20 min [51]. IOP Publishing (open access).
4 Effect of conductive filler on melting enthalpy
Often, the thermal performance of PCMs is investigated by the melting temperature (Tm), heat of fusion and thermal conductivity. PCMs systems are fabricated in such a way that there should be a specific melting temperature and high heat of fusion in to be in tandem with the energy density requirements for a large adoption of PCM-based thermos-physical storage technologies. Several studies on the incorporation of conductive fillers into a phase change material have shown to change the melting enthalpy (of PCM) because the dispersed conductive fillers may interrupt the local bonding environment of the PCM molecules, as summarized in Table 3. It is noteworthy to note that two observations, viz.: (i) some studies reported a decrease in the melting enthalpy values after filler loading were made in relation to the melting enthalpy [59, 68] whereas (ii) other studies reported a negligible change in melting enthalpy values. Sani and Etesani [69] investigated the effect of TiO2 nanoparticles on the thermal properties of paraffin. In their study, surfactant, i.e., sodium stearoyl lactylate (SSL) was employed to facilitate the dispersion of the nanoparticles in the chosen paraffin. It was found that the PCM composite with or without surfactant showed similar phase change behavior. However, maximum latent heat was obtained for samples prepared with surfactant, especially at high mass ratio of nanoparticles, when compared to PCM composites without surfactant (Figure 10(a)). This was attributed to the ability of the surfactant to facilitate the dispersion of the nanoparticles in the paraffin. In the case of the melting temperature, it was found that the increase in nanoparticles from 0.5 wt% to 1 wt% in both samples, with or without surfactant, decreased the melting temperature, however, a further increase led to an increase in melting temperatures (Figure 10(b)). This was ascribed to the aggregation of the particles at higher loadings and reduction of the interaction between the paraffin molecules and the nanoparticles. The type and/or strength of interaction between the conductive fillers and PCM material, has the most significant influence on the thermal phase change behavior of the resulting PCM composite material, regardless of conductive-filler type employed. If there is no chemical interaction between the filler and PCM, that means it is only physical interaction, hence the melting temperature and/or freezing temperatures of the PCM composites are often similar, as depicted in Figure 11(a) and (b) [70]. It was reported that there was a weak interaction between the filler and PCM materials.
Selective studies on heat of fusion and melting temperature of PCM in PCM/conductive filler composites.
| PCM/conductive filler composites | Heat of fusion of PCM | Heat of fusion of composites | Melting temperature (°C) | Comments based on the results | References |
|---|---|---|---|---|---|
| 1-Octadecanol (stearyl alcohol) (Tm = ∼66 °C)/graphene nanosheets (GNS) | ∼250 J/g | 1 wt% GNS- ∼230 J/g 2 wt% GNS- ∼228 J/g 3 wt% GNS- ∼201 J/g |
|
A decrease in melting enthalpy was expected due to the replacement of the PCM volume by the graphene sheets that do not undergo the phase change process, whereas no significant change was observed in melting temperature when filler’ content was increased | [68] |
| Eicosane (Tm = ∼36.4 °C)/silver nanoparticles (AgNPs) | 247.5 J/g |
|
|
The decrease in the latent heat of fusion was attributed to the colligative properties of the composites and probably the unbound ligands of Oleoyl Sarcosine (OS) (stabilizing agent for Ag particles) which are well-spread in eicosane affecting eicosane crystal structure and grain size. It was explained that if the solvent-ligand attraction force overcomes solvent-solvent interaction, then it is expected that the required de-crystallization energy will be very low | [71] |
| Crystalline TiO2 shell and n-eicosane core attached with graphene nanosheets (n-eicosane (Tm = 40.95 °C) @ TiO2@graphene microcapsules) | 246.5 J/g | ∼160 | ∼41 | A decrease in melting enthalpy was expected due to the replacement of the PCM volume by the graphene sheets that do not undergo the phase change process, whereas no significant change was observed in melting temperature when filler’ content was increased | [57] |
| PEG (Tm = 58.8 °C)/honeycomb-like structured biological porous carbon (BPC) | 205.7 J/g | ∼159 | ∼57 |
|
[59] |
| Paraffin (42.4–49.8 °C)/nanomagnetite (Fe3O4) | 134.9 J/g |
|
|
Latent heat values were increased with increase in nanoparticles content, while the melting temperature ranges determined from onset and end temperatures remained almost similar. The increment in Latent heat values was ascribed to large surface to volume ratio of nanomaterials and the intermolecular attractions based on approximation from Lennard–Jones potential between nanoparticles and paraffin | [60] |
| n-eicosane (Tm = ∼35.7 °C)/graphene nanoplatelets (GNPs) | ∼260 J/g |
|
∼36 |
|
[72] |
| Pentaerythritol (Tm = 187.82 °C)/alumina nanoparticles (Aℓ2O3) | 263.90 J/g |
|
∼188 |
|
[48] |
| Polyethylene glycol (PEG) (Tm = 62.93 °C)/silicon carbide nanowires (SiC NWs) network/expanded vermiculite (EVM) | 200.41 J/g |
|
|
The DSC curves of composites were significantly moved towards the lower-temperature direction with the increase in the mass fraction of SiC NWs even though PEG weight fractions were increased in these samples, hence a decline in latent heat values. This was ascribed to the strong restrictive effect of the nanoscale pore structures of EVM and the surface interactions of EVM and SiC NWs. It was further explained that inhibiting the thickening of thin lamellar PEG crystallites into stable crystal sliding motion of PEG molecular chains could be attributed to the restriction due to the confinement of active points or surface of EVM | [64] |
![Figure 10:
(a) Latent heat and (b) melting temperature changes for TiO2/paraffin composites, composed different TiO2 nanoparticles loadings with (and without) SSL [69]. Copyright Elsevier.](/document/doi/10.1515/polyeng-2022-0020/asset/graphic/j_polyeng-2022-0020_fig_010.jpg)
(a) Latent heat and (b) melting temperature changes for TiO2/paraffin composites, composed different TiO2 nanoparticles loadings with (and without) SSL [69]. Copyright Elsevier.
![Figure 11:
Melting and crystallization temperatures for SSPCM-filled with different concentrations of (a) expanded graphite plate (EGP) or (b) MWCNT [70]. MDPI (open access).](/document/doi/10.1515/polyeng-2022-0020/asset/graphic/j_polyeng-2022-0020_fig_011.jpg)
Melting and crystallization temperatures for SSPCM-filled with different concentrations of (a) expanded graphite plate (EGP) or (b) MWCNT [70]. MDPI (open access).
5 Future trends based on phase change materials
Majority of phase change materials have been hosted by porous carbon-based materials, which seemed to have performed well as host materials. However, there is need for researchers explore the possibility of hybridization of the fillers that can absorb PCMs more than single conductive fillers to avoid their leaching during change of state. These fillers can be chosen based on the structural properties as well as their thermal conductivity. Such modification can improve overall properties of the ensuing composite material. Furthermore, in order for PCMs/conductive filler(s) to meet certain requirements in some applications, the PCMs composite must have great thermal reliability, shape, and structural stability. To achieve these properties, it is very important for scientist/researchers to try and modify the conductive fillers in future in order to enhance the properties, as a result expanding the applications of the PCMs. It is well-known that the storage of PCMs composites depends on the composition of the PCM in the composite; as such there is a need to enhance the storage system to about 70% of the PCMs without compromising the strength of the composite. Furthermore, it has been recognized that PCMs tends to reduce the thermal stability and mechanical properties, as a result researchers might have to explore other possibilities of adding fillers such as natural fibers into the PCMs system, to enhance both thermal stability and mechanical properties.
6 Conclusions
The review paper discussed an in-depth overview of the morphology and thermal cycle of the various PCMs/conductive filler(s) composites. It has been highlighted from different studies that factors such as preparation methods, content of the filler, type of the PCM, type of the conductive filler, orientation, and the functionalization of the conductive nanoparticles affect the morphology of the resultant composite, as a result influences on the overall properties of the composites. Of all conductive fillers, carbon-based filler, such as graphite, has good properties, not only to improve the overall conductivity of the resulting composite material, but also the ability to absorb more PCM material, especially paraffin wax into its porous structure and prevent leakage during the charging and releasing processes. It was further realized in this review paper that, as much as PCMs are employed for energy storage, there is a need for ensuring that the PCM systems can withstand long term usage, which makes the studies on the thermal cycle of such systems very important. In most cases, the crystallinity, thermal stability, and chemical structure of the composites do not change after the stability test. However, insignificant changes were reported regarding latent heat, as well as melting and freezing temperatures, depending on the system. In future, standardization on the stability test should be considered for comparison of the results, not only in the same study, but also in the overall studies that are based on PCM-composite materials.
Funding source: National Research Foundation, South Africa
Award Identifier / Grant number: (Grant UID: 129347) and (Grant UID: 127278)
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: The authors would like to acknowledge the following institutions for funding: National Research Foundation (NRF), South Africa and Department of Science and Innovation- National Research Foundation (DSI-NRF), South Africa.
-
Conflict of interest statement: The authors declare that they have no conflicts of interest regarding this article.
References
1. Liu, Y., Xia, Y., Kan, A., Huang, C., Cui, W., Wei, S., Ji, R., Xu, F., Zhang, H., Sun, L. Fabrication and characterization of novel meso-porous carbon/n-octadecane as form-stable phase change materials for enhancement of phase-change behavior. J. Mater. Sci. Technol. 2019, 35, 939–945. https://doi.org/10.1016/j.jmst.2018.11.001.Search in Google Scholar
2. Mochane, M. J., Mokhena, T. C., Motaung, T. E., Linganiso, L. Z. Shape-stabilized phase change materials of polyolefin/wax blends and their composites. J. Therm. Anal. Calorim. 2020, 139, 2951–2963. https://doi.org/10.1007/s10973-019-08734-3.Search in Google Scholar
3. Mokhena, T. C., John, M. J. Cellulose nanomaterials: new generation materials for solving global issues. Cellulose 2020, 27, 1149–1194. https://doi.org/10.1007/s10570-019-02889-w.Search in Google Scholar
4. Tyagi, V. V., Chopra, K., Sharma, R. K., Pandey, A. K., Tyagi, S. K., Ahmad, M. S., Sarı, A., Kothari, R. A comprehensive review on phase change materials for heat storage applications: development, characterization, thermal and chemical stability. Sol. Energy Mater. Sol. Cell. 2022, 234, 111392. https://doi.org/10.1016/j.solmat.2021.111392.Search in Google Scholar
5. Singh, P., Sharma, R. K., Ansu, A. K., Goyal, R., Sarı, A., Tyagi, V. V. A comprehensive review on development of eutectic organic phase change materials and their composites for low and medium range thermal energy storage applications. Sol. Energy Mater. Sol. Cells 2021, 223, 110955. https://doi.org/10.1016/j.solmat.2020.110955.Search in Google Scholar
6. Pandey, A. K., Hossain, M. S., Tyagi, V. V., Rahim, N., Selvaraj, J. A. L., Sari, A. Novel approaches and recent developments on potential applications of phase change materials in solar energy. Renew. Sustain. Energy Rev. 2018, 82, 281–323. https://doi.org/10.1016/j.rser.2017.09.043.Search in Google Scholar
7. Al-Ahmed, A., Mazumder, M. A. J., Salhi, B., Sari, A., Afzaal, M., Al-Sulaiman, F. A. Effects of carbon-based fillers on thermal properties of fatty acids and their eutectics as phase change materials used for thermal energy storage: a review. Renew. Sustain. Energy Rev. 2021, 35, 102329. https://doi.org/10.1016/j.est.2021.102329.Search in Google Scholar
8. Lin, Y., Alva, G., Fang, G. Review on thermal performances and applications of thermal energy storage systems with inorganic phase change materials. Energy 2018, 165, 685–708. https://doi.org/10.1016/j.energy.2018.09.128.Search in Google Scholar
9. Qureshi, Z. A., Ali, H. M., Khushnood, S. Recent advances on thermal conductivity enhancement of phase change materials for energy storage system: review. Int. J. Heat Mass Tran. 2018, 127, 838–856. https://doi.org/10.1016/j.ijheatmasstransfer.2018.08.049.Search in Google Scholar
10. Latibari, S. T., Sadrameli, S. M. Carbon based material included-shaped stabilized phase change materials for sunlight-driven energy conversion and storage: an extensive review. Sol. Energy 2018, 170, 1130–1161. https://doi.org/10.1016/j.solener.2018.05.007.Search in Google Scholar
11. Kahwaji, S., Johnson, M. B., Kheirabadi, A. C., Groulx, D., White, M. A. A comprehensive study of properties of paraffin phase change materials for solar thermal energy storage and thermal management applications. Energy 2018, 162, 1169–1182. https://doi.org/10.1016/j.energy.2018.08.068.Search in Google Scholar
12. Ushak, S., Marín, P., Galazutdinova, Y., Cabeza, L. F., Farid, M. M., Grágeda, M. Compatibility of materials for macroencapsulation of inorganic phase change materials: experimental corrosion study. Appl. Therm. Eng. 2016, 107, 410–419. https://doi.org/10.1016/j.applthermaleng.2016.06.171.Search in Google Scholar
13. Pielichowska, K., Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. https://doi.org/10.1016/j.pmatsci.2014.03.005.Search in Google Scholar
14. Mohamed, S. A., Al-Sulaiman, F. A., Ibrahim, N. I., Zahir, M. H., Al-Ahmed, A., Saidur, R., Yılbaş, B. S., Sahin, A. Z. A review on current status and challenges of inorganic phase change materials for thermal energy storage systems. Renew. Sustain. Energy Rev. 2017, 70, 1072–1089.https://doi.org/10.1016/j.rser.2016.12.012.Search in Google Scholar
15. Milián, Y. E., Gutiérrez, A., Grágeda, M., Ushak, S. A review on encapsulation techniques for inorganic phase change materials and the influence on their thermophysical properties. Renew. Sustain. Energy Rev. 2017, 73, 983–999.10.1016/j.rser.2017.01.159Search in Google Scholar
16. Fan, L., Khodadadi, J. M. Thermal conductivity enhancement of phase change materials for thermal energy storage: a review. Renew. Sustain. Energy Rev. 2011, 15, 24–46. https://doi.org/10.1016/j.rser.2010.08.007.Search in Google Scholar
17. Lin, Y., Jia, Y., Alva, G., Fang, G. Review on thermal conductivity enhancement, thermal properties and applications of phase change materials in thermal energy storage. Renew. Sustain. Energy Rev. 2018, 82, 2730–2742. https://doi.org/10.1016/j.rser.2017.10.002.Search in Google Scholar
18. Sarı, A., Biçer, A., Hekimoğlu, G. Effects of carbon nanotubes additive on thermal conductivity and thermal energy storage properties of a novel composite phase change material. J. Compos. Mater. 2018, 53, 2967–2980.10.1177/0021998318808357Search in Google Scholar
19. Mochane, M. J., Luyt, A. S. The effect of expanded graphite on the thermal stability, latent heat, and flammability properties of EVA/wax phase change blends. Polym. Eng. Sci. 2015, 55, 1255–1262. https://doi.org/10.1002/pen.24063.Search in Google Scholar
20. Mochane, M. J., Luyt, A. S. The effect of expanded graphite on the flammability and thermal conductivity properties of phase change material based on PP/wax blends. Polym. Bull. 2015, 72, 2263–2283. https://doi.org/10.1007/s00289-015-1401-9.Search in Google Scholar
21. Shah, K. W. A review on enhancement of phase change materials - a nanomaterials perspective. Energy Build. 2018, 175, 57–68. https://doi.org/10.1016/j.enbuild.2018.06.043.Search in Google Scholar
22. Al-Ahmed, A., Sarı, A., Mazumder, M. A. J., Hekimoğlu, G., Al-Sulaiman, F. A., Inamuddin. Thermal energy storage and thermal conductivity properties of octadecanol-MWCNT composite PCMs as promising organic heat storage materials. Sci. Rep. 2020, 10, 9168. https://doi.org/10.1038/s41598-020-64149-3.Search in Google Scholar PubMed PubMed Central
23. Al-Ahmed, A., Sarı, A., Mazumder, M. A. J., Salhi, B., Hekimoğlu, G., Al-Sulaiman, F. A., Inamuddin. Thermal energy storage and thermal conductivity properties of fatty acid/fatty acid-grafted-CNTs and fatty acid/CNTs as novel composite phase change materials. Sci. Rep. 2020, 10, 15388. https://doi.org/10.1038/s41598-020-71891-1.Search in Google Scholar PubMed PubMed Central
24. Deka, P. P., Ansu, A. K., Sharma, R. K., Tyagi, V. V., Sarı, A. Development and characterization of form-stable porous TiO2/tetradecanoic acid based composite PCM with long-term stability as solar thermal energy storage material. Int. J. Energy Res. 2020, 44, 10044–10057. https://doi.org/10.1002/er.5615.Search in Google Scholar
25. Ouikhalfan, M., Sari, A., Chehouani, H., Benhamou, B., Biçer, A. Preparation and characterization of nano-enhanced myristic acid using metal oxide nanoparticles for thermal energy storage. Int. J. Energy Res. 2019, 43, 8592–8607.10.1002/er.4856Search in Google Scholar
26. Nomura, T., Tabuchi, K., Zhu, C., Sheng, N., Wang, S., Akiyama, T. High thermal conductivity phase change composite with percolating carbon fiber network. Appl. Energy 2015, 154, 678–685. https://doi.org/10.1016/j.apenergy.2015.05.042.Search in Google Scholar
27. Xu, T., Li, Y., Chen, J., Liu, J. Preparation and thermal energy storage properties of LiNO3-KCl-NaNO3/expanded graphite composite phase change material. Sol. Energy Mater. Sol. Cells 2017, 169, 215–221. https://doi.org/10.1016/j.solmat.2017.05.035.Search in Google Scholar
28. Huang, Z., Luo, Z., Gao, X., Fang, X., Fang, Y., Zhang, Z. Preparation and thermal property analysis of Wood’s alloy/expanded graphite composite as highly conductive form-stable phase change material for electronic thermal management. Appl. Therm. Eng. 2017, 122, 322–329. https://doi.org/10.1016/j.applthermaleng.2017.04.154.Search in Google Scholar
29. Ji, H., Sellan, D. P., Pettes, M. T., Kong, X., Ji, J., Shi, L., Ruoff, R. S. Enhanced thermal conductivity of phase change materials with ultrathin-graphite foams for thermal energy storage. Energy Environ. Sci. 2014, 7, 1185–1192. https://doi.org/10.1039/c3ee42573h.Search in Google Scholar
30. Fan, L. W., Fang, X., Wang, X., Zeng, Y., Xiao, Y. Q., Yu, Z. T., Xu, X., Hu, Y. C., Cen, K. F. Effects of various carbon nanofillers on the thermal conductivity and energy storage properties of paraffin-based nanocomposite phase change materials. Appl. Energy 2013, 110, 163–172. https://doi.org/10.1016/j.apenergy.2013.04.043.Search in Google Scholar
31. Ren, Y., Xu, C., Yuan, M., Ye, F., Ju, X., Du, X. Ca(NO3)2-NaNO3/expanded graphite composite as a novel shape-stable phase change material for mid- to high-temperature thermal energy storage. Energy Convers. Manag. 2018, 163, 50–58. https://doi.org/10.1016/j.enconman.2018.02.057.Search in Google Scholar
32. Mukwada, L. T., Mochane, M. J., Motaung, T. E., Motloung, S. V., Koao, L. F. Effect of sodium dodecylbenzene sulphonate modifier and PP-g-MA on the morphology and thermal conductivity of PP/EG composites. Plast., Rubber Compos. 2017, 46, 469–475. https://doi.org/10.1080/14658011.2017.1392713.Search in Google Scholar
33. Wu, Y., Yan, X., Meng, P., Sun, P., Cheng, G., Zheng, R. Carbon black/octadecane composites for room temperature electrical and thermal regulation. Carbon 2015, 94, 417–423. https://doi.org/10.1016/j.carbon.2015.06.037.Search in Google Scholar
34. Sun, P. C., Wu, Y. L., Gao, J. W., Cheng, G. A., Chen, G., Zheng, R. T. Room temperature electrical and thermal switching CNT/hexadecane composites. Adv. Mater. 2013, 25, 4938–4943. https://doi.org/10.1002/adma.201302165.Search in Google Scholar PubMed
35. Frühauf, F., Breitenstein, O. DLIT- versus ILIT-based efficiency imaging of solar cells. Sol. Energy Mater. Sol. Cells 2017, 169, 195–202.10.1016/j.solmat.2017.05.015Search in Google Scholar
36. Guo, C. X., Ma, X. L., Yang, L. PCM/graphite foam composite for thermal energy storage device. IOP Conf. Ser.: Conf. Ser. Mater. Sci. Eng. 2015, 87, 012014. https://doi.org/10.1088/1757-899x/87/1/012014.Search in Google Scholar
37. Chen, L., Zou, R., Xia, W., Liu, Z., Shang, Y., Zhu, J., Wang, Y., Lin, J., Xia, D., Cao, A. Electro- and photodriven phase change composites based on wax-infiltrated carbon nanotube sponges. ACS Nano 2012, 6, 10884–10892. https://doi.org/10.1021/nn304310n.Search in Google Scholar PubMed
38. Motahar, S., Nikkam, N., Alemrajabi, A. A., Khodabandeh, R., Toprak, M. S., Muhammed, M. A novel phase change material containing mesoporous silica nanoparticles for thermal storage: a study on thermal conductivity and viscosity. Int. Commun. Heat Mass. 2014, 56, 114–120. https://doi.org/10.1016/j.icheatmasstransfer.2014.06.005.Search in Google Scholar
39. Zhao, Y. J., Wang, R. Z., Wang, L. W., Yu, N. Development of highly conductive KNO3/NaNO3 composite for TES (thermal energy storage). Energy 2014, 70, 272–277. https://doi.org/10.1016/j.energy.2014.03.127.Search in Google Scholar
40. Xiao, X., Zhang, P., Li, M. Preparation and thermal characterization of paraffin/metal foam composite phase change material. Appl. Energy 2013, 112, 1357–1366. https://doi.org/10.1016/j.apenergy.2013.04.050.Search in Google Scholar
41. Shi, J. N., Ger, M. D., Liu, Y. M., Fan, Y. C., Wen, N. T., Lin, C. K., Pu, N. W. Improving the thermal conductivity and shape-stabilization of phase change materials using nanographite additives. Carbon 2013, 51, 365–372. https://doi.org/10.1016/j.carbon.2012.08.068.Search in Google Scholar
42. Chen, Y. J., Nguyen, D. D., Shen, M. Y., Yip, M. C., Tai, N. H. Thermal characterizations of the graphite nanosheets reinforced paraffin phase-change composites. Compos. Part A 2013, 44, 40–46. https://doi.org/10.1016/j.compositesa.2012.08.010.Search in Google Scholar
43. Mhike, W., Focke, W. W., Mackenzie, J., Mills, E. J., Badenhorst, H. Stearyl alcohol/palm triple pressed acid-graphite nanocomposites as phase change materials. Thermochim. Acta 2018, 663, 77–84. https://doi.org/10.1016/j.tca.2018.03.014.Search in Google Scholar
44. Ramakrishnan, S., Wang, X., Sanjayan, J., Wilson, J. Heat transfer performance enhancement of paraffin/expanded perlite phase change composites with graphene nano-platelets. Energy Proc. 2017, 105, 4866–4871. https://doi.org/10.1016/j.egypro.2017.03.964.Search in Google Scholar
45. Zhang, Q., Liu, J. Sebacic acid/CNT sponge phase change material with excellent thermal conductivity and photo-thermal performance. Sol. Energy Mater. Sol. Cells 2018, 179, 217–222. https://doi.org/10.1016/j.solmat.2017.11.019.Search in Google Scholar
46. Jiang, Z., Ouyang, T., Yang, Y., Chen, L., Fan, X., Chen, Y., Li, W., Fei, Y. Thermal conductivity enhancement of phase change materials with form-stable carbon bonded carbon fiber network. Mater. Des. 2018, 143, 177–184. https://doi.org/10.1016/j.matdes.2018.01.052.Search in Google Scholar
47. Liang, W., Wang, L., Zhu, H., Pan, Y., Zhu, Z., Sun, H., Ma, C., Li, A. Enhanced thermal conductivity of phase change material nanocomposites based on MnO2 nanowires and nanotubes for energy storage. Sol. Energy Mater. Sol. Cells 2018, 180, 158–167. https://doi.org/10.1016/j.solmat.2018.03.005.Search in Google Scholar
48. Venkitaraj, K. P., Suresh, S., Praveen, B., Venugopal, A., Nair, S. C. Pentaerythritol with alumina nano additives for thermal energy storage applications. J. Energy Storage 2017, 13, 359–377.10.1016/j.est.2017.08.002Search in Google Scholar
49. Liu, J., Xie, M., Ling, Z., Fang, X., Zhang, Z. Novel MgCl2-KCl/expanded graphite/graphite paper composite phase change blocks with high thermal conductivity and large latent heat. Sol. Energy 2018, 159, 226–233. https://doi.org/10.1016/j.solener.2017.10.083.Search in Google Scholar
50. Zhang, Q., Luo, Z., Guo, Q., Wu, G. Preparation and thermal properties of short carbon fibers/erythritol phase change materials. Energy Convers. Manag. 2017, 136, 220–228. https://doi.org/10.1016/j.enconman.2017.01.023.Search in Google Scholar
51. Zhao, Y., Thapa, S., Weiss, L., Lvov, Y. Phase change insulation for energy efficiency based on wax-halloysite composites. IOP Conference Ser. Mater. Sci. Eng. 2014, 64, 012045. https://doi.org/10.1088/1757-899x/64/1/012045.Search in Google Scholar
52. Nomura, T., Zhu, C., Sheng, N., Saito, G., Akiyama, T. Microencapsulation of metal-based phase change material for high-temperature thermal energy storage. Sci. Rep. 2015, 5, 9117. https://doi.org/10.1038/srep09117.Search in Google Scholar PubMed PubMed Central
53. Leng, G., Qiao, G., Xu, G., Vidal, T., Ding, Y. Erythritol-vermiculite form-stable phase change materials for thermal energy storage. Energy Proc. 2017, 142, 3363–3368. https://doi.org/10.1016/j.egypro.2017.12.471.Search in Google Scholar
54. Kim, T., Singh, D., Zhao, W., Yua, W. France, D. M. An investigation on the effects of phase change material on material components used for high temperature thermal energy storage system. Proc. AIP Conf. 2016, 1734, 050023. https://doi.org/10.1063/1.4949121.Search in Google Scholar
55. Mehrali, M., Latibari, S. T., Mehrali, M., Metselaar, H. S. C., Silakhori, M. Shape-stabilized phase change materials with high thermal conductivity based on paraffin/graphene oxide composite. Energy Convers. Manag. 2013, 67, 275–282. https://doi.org/10.1016/j.enconman.2012.11.023.Search in Google Scholar
56. Chen, Y., Luo, W., Wang, J., Huang, J. Enhanced thermal conductivity and durability of a paraffin wax nanocomposite based on carbon-coated aluminum nanoparticles. J. Phys. Chem. C 2017, 121, 12603–12609. https://doi.org/10.1021/acs.jpcc.7b02651.Search in Google Scholar
57. Liu, H., Wang, X., Wu, D. Fabrication of graphene/TiO2/paraffin composite phase change materials for enhancement of solar energy efficiency in photocatalysis and latent heat storage. ACS Sustainable Chem. Eng. 2017, 5, 4906–4915. https://doi.org/10.1021/acssuschemeng.7b00321.Search in Google Scholar
58. Nomura, T., Zhu, C., Nan, S., Tabuchi, K., Wang, S., Akiyama, T. High thermal conductivity phase change composite with a metal-stabilized carbon-fiber network. Appl. Energy 2016, 179, 1–6. https://doi.org/10.1016/j.apenergy.2016.04.070.Search in Google Scholar
59. Zhao, Y., Min, X., Huang, Z., Liu, Y. G., Wu, X., Fang, M. Honeycomb-like structured biological porous carbon encapsulating PEG: a shape-stable phase change material with enhanced thermal conductivity for thermal energy storage. Energy Build. 2018, 158, 1049–1062. https://doi.org/10.1016/j.enbuild.2017.10.078.Search in Google Scholar
60. Şahan, N., Fois, M., Paksoy, H. Improving thermal conductivity phase change materials—a study of paraffin nanomagnetite composites. Sol. Energy Mater. Sol. Cells 2015, 137, 61–67.10.1016/j.solmat.2015.01.027Search in Google Scholar
61. Mehrali, M., Latibari, S. T., Mehrali, M., Indra Mahlia, T. M., Metselaar, H. S. C., Naghavi, M. S., Sadeghinezhad, E., Akhiani, A. R. Preparation and characterization of palmitic acid/graphene nanoplatelets composite with remarkable thermal conductivity as a novel shape-stabilized phase change material. Appl. Therm. Eng. 2013, 61, 633–640. https://doi.org/10.1016/j.applthermaleng.2013.08.035.Search in Google Scholar
62. Liu, M., Ma, Y., Wu, H., Wang, R. Y. Metal matrix–metal nanoparticle composites with tunable melting temperature and high thermal conductivity for phase-change thermal storage. ACS Nano 2015, 9, 1341–1351. https://doi.org/10.1021/nn505328j.Search in Google Scholar PubMed
63. Qian, T., Li, J. Octadecane/C-decorated diatomite composite phase change material with enhanced thermal conductivity as aggregate for developing structural–functional integrated cement for thermal energy storage. Energy 2018, 142, 234–249. https://doi.org/10.1016/j.energy.2017.10.021.Search in Google Scholar
64. Deng, Y., Li, J., Nian, H. Polyethylene glycol-enwrapped silicon carbide nanowires network/expanded vermiculite composite phase change materials: form-stabilization, thermal energy storage behavior and thermal conductivity enhancement. Sol. Energy Mater. Sol. Cells 2018, 174, 283–291. https://doi.org/10.1016/j.solmat.2017.09.013.Search in Google Scholar
65. Wu, S., Zhu, D., Zhang, X., Huang, J. Preparation and melting/freezing characteristics of Cu/paraffin nanofluid as phase-change material (PCM). Energy Fuel. 2010, 24, 1894–1898. https://doi.org/10.1021/ef9013967.Search in Google Scholar
66. Li, Y., Li, J., Deng, Y., Guan, W., Wang, X., Qian, T. Preparation of paraffin/porous TiO2 foams with enhanced thermal conductivity as PCM, by covering the TiO2 surface with a carbon layer. Appl. Energy 2016, 171, 37–45. https://doi.org/10.1016/j.apenergy.2016.03.010.Search in Google Scholar
67. Xie, B., Cheng, W. L., Xu, Z. M. Studies on the effect of shape-stabilized PCM filled aluminum honeycomb composite material on thermal control. Int. J. Heat Mass Tran. 2015, 91, 135–143. https://doi.org/10.1016/j.ijheatmasstransfer.2015.07.108.Search in Google Scholar
68. Yavari, F., Fard, H. R., Pashayi, K., Rafiee, M. A., Zamiri, A., Yu, Z., Ozisik, R., Borca-Tasciuc, T., Koratkar, N. Enhanced thermal conductivity in a nanostructured phase change composite due to low concentration graphene additives. J. Phys. Chem. C 2011, 115, 8753–8758. https://doi.org/10.1021/jp200838s.Search in Google Scholar
69. Sami, S., Etesami, N. Improving thermal characteristics and stability of phase change material containing TiO2 nanoparticles after thermal cycles for energy storage. Appl. Therm. Eng. 2017, 124, 346–352. https://doi.org/10.1016/j.applthermaleng.2017.06.023.Search in Google Scholar
70. Liu, Z. P., Yang, R. Synergistically-enhanced thermal conductivity of shape-stabilized phase change materials by expanded graphite and carbon nanotube. Appl. Sci. 2017, 7, 1–12. https://doi.org/10.3390/app7060574.Search in Google Scholar
71. Al Ghossein, R. M., Hossain, M. S., Khodadadi, J. M. Experimental determination of temperature-dependent thermal conductivity of solid eicosane-based silver nanostructure-enhanced phase change materials for thermal energy storage. Int. J. Heat Mass Tran. 2017, 107, 697–711. https://doi.org/10.1016/j.ijheatmasstransfer.2016.11.059.Search in Google Scholar
72. Fang, X., Fan, L. W., Ding, Q., Wang, X., Yao, X. L., Hou, J. F., Yu, Z. T., Cheng, G. H., Hu, Y. C., Cen, K. F. Increased thermal conductivity of eicosane-based composite phase change materials in the presence of graphene nanoplatelets. Energy Fuel. 2013, 27, 4041–4047. https://doi.org/10.1021/ef400702a.Search in Google Scholar
© 2022 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Material properties
- Effect of nanodiamond particles on the structure, mechanical, and thermal properties of polymer embedded ND/PMMA composites
- A comparative investigation on wear characteristics of polymer and biopolymer gears
- Unsaturated polyester resin modified with a novel reactive flame retardant: effects on thermal stability and flammability
- Recent progress on the morphology and thermal cycle of phase change materials (PCMs)/conductive filler composites: a mini review
- Effect of tiny amount of DMC on thermal, mechanical, optical, and water resistance properties of poly(vinyl alcohol)
- Vibration and tribological properties of epoxy-granite composites used as novel foundations for machine elements
- Effect of lyocell fiber cross-sectional shape on structure and properties of lyocell/PLA composites
- Engineering and processing
- Quality prediction and control of thin-walled shell injection molding based on GWO-PSO, ACO-BP, and NSGA-II
- Doubly modified MWCNTs embedded in polyethersulfone (PES) ultrafiltration membrane and its anti-fouling performance
- Solid-state extrusion of polymers using simple shear deformation
- Molding process and properties of polyimide-fiber-fabric-reinforced polyether ether ketone composites
Articles in the same Issue
- Frontmatter
- Material properties
- Effect of nanodiamond particles on the structure, mechanical, and thermal properties of polymer embedded ND/PMMA composites
- A comparative investigation on wear characteristics of polymer and biopolymer gears
- Unsaturated polyester resin modified with a novel reactive flame retardant: effects on thermal stability and flammability
- Recent progress on the morphology and thermal cycle of phase change materials (PCMs)/conductive filler composites: a mini review
- Effect of tiny amount of DMC on thermal, mechanical, optical, and water resistance properties of poly(vinyl alcohol)
- Vibration and tribological properties of epoxy-granite composites used as novel foundations for machine elements
- Effect of lyocell fiber cross-sectional shape on structure and properties of lyocell/PLA composites
- Engineering and processing
- Quality prediction and control of thin-walled shell injection molding based on GWO-PSO, ACO-BP, and NSGA-II
- Doubly modified MWCNTs embedded in polyethersulfone (PES) ultrafiltration membrane and its anti-fouling performance
- Solid-state extrusion of polymers using simple shear deformation
- Molding process and properties of polyimide-fiber-fabric-reinforced polyether ether ketone composites

