Abstract
This study presents the preparation and flammability characteristics of polymeric composites based on the epoxy resin Epidian® 601. The triethylenetetramine (TETA) and commercial curing agents based on polyamines (IDA and PAC) were used as crosslinking compounds. Moreover, two flame retardant compounds were added to this composition, the commercial Fire Retardant (FR) and triphenyl phosphate (TP). The chemical structure of the composites and the course of curing processes were confirmed by the ATR/FT-IR (Attenuated Total Reflection–Fourier Transform Infrared) analysis. The influence of different amounts of FR or TP on the flammability and thermal resistance was discussed in detail. After the flammability test the samples were also studied to assess their thermal destruction. In addition, the composites were subjected to the swelling tests, solvent resistance, and microscopic observations. The DSC curves revealed that all materials were characterized by good thermal properties. All materials were temperature resistant up to 300 °C. Furthermore, the measurements of the hardness of the composite demonstrated that the material EP601 + TETA + 10 %FR is the hardest. The addition of FR and RP influenced the flammability of the composites increasing the thermal resistance. The ageing tests in methanol, acetone, hydrochloric acid, and potassium hydroxide were also carried out.
Introduction
The majority of organic compounds, including polymers, will burn readily in air or oxygen. The flammability of polymers is a serious issue and limits their applications severely. The flame retardant additives are intended to inhibit or to stop the polymer combustion process acting either physically (such as cooling, fuel dilution, formation of a protective layer) or chemically (reaction in the solid or gaseous phase, the addition of flame retardant) [1]. As follows from the industrial practice, the phosphorous-containing flame retardants are used as an alternative to the commonly applied toxic halogenated flame retardants. These compounds can suppress fire in a polymer in two ways. The first mechanism proceeds through the thermal degradation of phosphorus flame retardants into phosphoric acid which converts the polymer into carbon, whereas the other one is based on migration into the vapour phase and quenching the radicals [2, 3]. Organophosphorous compounds are good flame retardants for polymeric materials [4]. There are several reports on the thermal or thermo-oxidative degradation kinetics of flame-retarded polymeric materials [5], [6], [7], [8], [9], [10]. The phosphorus-based flame retardants burn more intensively, especially when the conversion of both polymer and flame retardant into char decreases the formation of gaseous phase degradation products [1].
Epoxide resins (EP) are one of the most popular types of matrices used for the preparation of compositions. EP has attracted great attention, due to its outstanding properties, such as high transparency, excellent adhesion to substrates and superior mechanical performances [11]. The addition of an additive-type flame retardant, such as phosphorus, nitrogen, and boron based flame retardants, is a facile method for improving the flame retardancy of epoxy compounds without incorporating halogenated flame retardants [12, 13]. Phosphorus is one of the most used elements in flame retarded EP systems, owing to its high flame retardant efficiency and low toxicity. Additionally, the P–H bond of hydrogen phosphonates or phosphinates can react with the epoxide group and the formed covalent bonds between the additive and the polymer chains render the epoxy resins fire resistant [14]. Phosphorus-containing flame retardants are important components of halogen-free flame retardants which possesses excellent properties such as low smoke emission, low toxicity, and the formation of a stable carbonized layer after burning [15], [16], [17].
Miturska et al. have prepared the adhesive epoxide compositions based on Epidian 5 and Epidian 53 with the different modifying agent (organic montmorillonite, ground chalk, activated carbon powder and other). The curing agent was an aminomethyl group, where curing occurred through the Mannich reaction. The examinations described the impact of the fillers used on strength properties. The type of filler affects the strength of the tested compositions over the storage time. Based on the research, it can be seen that the type of resin used in the adhesive composition also affects the elongation of the composition [18]. In another article, Wang et al. synthesised a novel phosphorous-containing compound via Atherton–Todd reaction between 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) and trihydroxymethylphosphine oxide (THPO) and then employed as flame retardant for diglycidyl ether of bisphenol A/4-4 diaminodiphenylmethane epoxy resin. After the combustion of modified resin, more compact and intumescent char residues are observed for the flame retarded EP [19]. In the work by Bratychak et al. Epidian-6 epoxy resin and methacrylic acid a monomethacrylic derivative of Epidian-6 with free epoxy and methacrylic groups (MMADER) has been synthesized. The cross-linking of unfilled epoxy-oligomeric mixtures and mixtures filled with CaCO3 has been studied in the presence of MMADER. It was established that the simultaneous introduction of the synthesized MMADER and CaCO3 into the mentioned mixtures significantly increases both the gel-fraction content and film hardness [20].
Teng et al. have prepared a hyperbranched flame retardant (HBFR) with a rigid backbone structure. They incorporated HBFR to diglycidyl ether of bisphenol A. The addition of only 0.24 wt% exhibited flame retardancy. Furthermore, the modified epoxy resin demonstrated superior toughness, indicated by the more than 96 % increment in impact strength both at 25 and −196 °C, due to the enlarged free volume originating from hyperbranched structures [21]. In the paper by Zou et al., a novel P/N/S-containing high-efficiency flame retardant was designed and synthesized from DOPO and heterocyclic compounds containing sulfur and nitrogen elements. Although thermogravimetry revealed that the degradation temperature of epoxy resin decreased slightly, the degradation rate of the resin decreased and fire safety was greatly improved. When the phosphorus content was only 0.48 %, the level of oxygen index value dramatically increased from 22.8 % to 30.8 %. In addition, the introduction of flame retardant endowed the substrate with smoke-suppression properties and improved the char residue [22].
Of the polymer systems, epoxy resins are used to develop a large array of high-performance products where, following the typical requirements, severe fire safety standards and regulations must be observed [23, 24]. Commonly used epoxy formulations are obtained by mixing oligomers based on diglycidyl ether of bisphenol A (DGEBA) and using aromatic or aliphatic amines as a curing agent (for example triethylenetetramine [TETA]). These formulations exhibit a broad range of thermal and mechanical properties depending on the molecular weights of the epoxy oligomers in the backbone, the functionality of the hardener, as well as on the curing conditions [25]. The use of phosphorous-based flame retardants, blended with epoxy resins, has been extensively reported since the seventies [26], [27], [28], [29], [30], [31]. These compounds, not being chemically bound to the network, can migrate toward the surface of molded components before cross-linking, especially at elevated temperatures, and can reduce the glass transition temperature of the cured resin acting as a plasticizer [32]. Therefore a special attention was paid, therefore to the development of novel epoxy systems prepared by the reaction of phosphorous-containing compounds with different epoxy resins, such as the grafting of organo-phosphorous compounds on the oligomer backbone [25, 33, 34]. Aromatic phosphates, such as triphenyl phosphate and bisphenol A bis(diphenyl phosphate) are common commercial phosphorus flame retardants [35]. However, the viscous liquid phosphates can cause some problems in processing [36]. Light-weight, high-performance polymeric materials offer many advantages in these applications over the conventional metallic and ceramic materials but the fire risk is considerably increased because of the flammability of the plastic materials and the possible release of toxic by-products [37]. Therefore, the research on epoxy resin flame retardancy, due to its very wide application in the construction industry, is a very important issue.
The main aim of the article was the synthesis of polymer composites based on epoxy resins (Epidian® 601) with different additives and the evaluation of their flammability, thermal, and mechanical properties. Three curing agents: two commercially available (PAC and IDA) and one chemical compound (TETA) were applied. In order to the reduction of flammability the samples were modified with two flame retardants: commercial Fire Retardant (FR) and triphenyl phosphate (FR). The resistance of obtained materials and flame retardants’ effects on the properties of composites were assessed. Due to the fact that many commercial materials (including everyday items) are based on epoxy resins, including derivatives of bisphenol A, it is important to keep them flame-retardant. Additionally, the ageing test in different solvents for 9 weeks was carried out. The detailed physico-chemical study was carried out to show the effects of the addition of flame retardants and different crosslinkers on the resistance of the composite.
Materials and methods
Chemicals
The epoxy resin Epidian® 601 (EP601) as well as, curing agent PAC and IDA were purchased from CIECH Resin Sp.z.o.o. (Nowa Sarzyna, Poland). Epidian® 601 is a clear, viscous liquid of light colour, epoxy number: 0.500–0.550 mol/100 g, viscosity at 25 °C: 700–1100 mPas, density at 20 °C: 1.140–1.170 g/cm3. Triphenyl phosphate (TP) was purchased from Merck (Darmstadt, Germany). The triethylenetetramine (TETA) (99 %) was obtained from Avantor Performance Materials Poland S.A. (Gliwice, Poland). Fire Retardant 421 (FR) was from West System (Romsey Hampshire, United Kingdom). The solvents: acetone (99.5 %), methanol (99.8 %), hydrochloric acid (35 %), potassium hydroxide (40 %) came from Merck (Darmstadt, Germany). The distilled water was obtained from Millipore (UMCS Lublin, Poland). The curing agent PAC contains: fatty acids, C18-unsaturated compounds, dimers, polymers, reaction products with triethylenetetramine. The curing agent IDA contains: 4,4′-isopropylidenedophenol, oligomeric reaction product with 1-chloro-2,3-epoxypropane, reaction product with 3-aminomethyl-3,5,5-trimethylcyclohexylamine, isophorone diamine.
Methods/analyses
The attenuated total reflection (ATR) was recorded based on the Fourier transform infrared (ATR/FT-IR) spectroscopy using a TENSOR 27 Bruker (Bruker GmbH, Mannheim, Germany) spectrometer equipped with a diamond crystal (Bruker Optics, Ettlingen, Germany). The spectra were recorded in the range of 4000–600 cm−1 with 64 scans per spectrum at a resolution of 4 cm−1. The analysis was preceded by the background spectrum measurements.
Differential scanning calorimetry (DSC) curves were obtained with the use of a DSC Netzsch 204 calorimeter (Netzsch, Günzbung, Germany). The measurements were made in the aluminum pans with a pierced lid with the sample mass approximately 10 mg in the nitrogen atmosphere (30 mL/min). Dynamic scans were performed at a heating rate of 10 °C/min in the temperature range −20–500 °C. An empty aluminum crucible was used as the reference.
The flammability tests were carried out in the laboratory of the Department of Technology and Polymer Processing, Lublin University of Technology. The flammability testing device was equipped with a combustion chamber, a ventilation system, and a thermal imaging camera. During the test the samples were fixed to a tripod, then the burner was brought closer to the sample for 20 s (and 30 s) at an angle of 45°. After that time, the samples burnt freely for another 30 s. The flammability tests were made in a horizontal burning test according to the PN-EN 60695-11-10 – method A. During the burning process, observations were made using a V-20 thermovision camera model ER005-25 (Vigo System, Ożarów Mazowiecki, Poland) in the temperature measurement range from 0 to 250 °C. During the tests, photos were taken.
The hardness of the materials was measured based on the Shore D method using a 7206/H04 analog hardness testing apparatus (Zwick, Ulm, Germany) at 20 °C. Readings were taken after 15 s.
The swelling coefficients (B) were determined using equilibrium swelling in acetone, methanol, hydrochloric acid, potassium hydroxide and distilled water. B is expressed:
where Vs is the volume of a sample after swelling and Vd is the volume of a dry sample.
The ageing tests were carried out in traditional vials. The cut 10 mm × 10 mm composite pieces were poured over with the solvent (the same as used in swelling tests). Weight changes were recorded using the scales.
Synthesis of composites
All composites were prepared using the cross-linking technique. An appropriate amount of the resin EP601 was added to the curing agent (TETA or PAC or IDA). The chemicals were stirred at room temperature until a homogenous solution was obtained. Then the flame retardant (FR or TP) was added. The whole content was stirred to obtain a homogeneous mixture and next put into the oven (for 10 min at 50 °C). The beaker contents were poured into the glass molds (10 mm × 8 mm × 2 mm) and polymerized for 24 h at room temperature. The samples were heated at 80 °C for 60 min after being tested. The detailed information regarding the reagents and their amounts is presented in Table 1. Figure 1 shows the structure of the monomers and the proposed scheme of the composite structure.
Amounts of chemicals.
| Name of sample | Epidian® 601 [g] | PAC [g] | IDA [g] | TETA [g] | FR [%] | TP [%] |
|---|---|---|---|---|---|---|
| EP601 + PAC | 12 | 7.2 | 0 | 0 | 0 | 0 |
| EP601 + PAC + 10 %FR | 12 | 7.2 | 0 | 0 | 10 | 0 |
| EP601 + PAC + 20 %FR | 12 | 7.2 | 0 | 0 | 20 | 0 |
| EP601 + PAC + 10 %TP | 12 | 7.2 | 0 | 0 | 0 | 10 |
| EP601 + PAC + 20 %TP | 12 | 7.2 | 0 | 0 | 0 | 20 |
|
|
||||||
| EP601 + TETA | 14 | 0 | 0 | 1.4 | 0 | 0 |
| EP601 + TETA + 10 %FR | 14 | 0 | 0 | 1.4 | 10 | 0 |
| EP601 + TETA + 20 %FR | 14 | 0 | 0 | 1.4 | 20 | 0 |
| EP601 + TETA + 10 %TP | 14 | 0 | 0 | 1.4 | 0 | 10 |
| EP601 + TETA + 20 %TP | 14 | 0 | 0 | 1.4 | 0 | 20 |
|
|
||||||
| EP601 + IDA | 12 | 0 | 6 | 0 | 0 | 0 |
| EP601 + IDA + 10 %FR | 12 | 0 | 6 | 0 | 10 | 0 |
| EP601 + IDA + 20 %FR | 12 | 0 | 6 | 0 | 20 | 0 |
| EP601 + IDA + 10 %TP | 12 | 0 | 6 | 0 | 0 | 10 |
| EP601 + IDA + 20 %TP | 12 | 0 | 6 | 0 | 0 | 20 |

Fragment of composite structure.
Results and discussion
As a result of the cross-linking reaction of the epoxy resin EP601 with various hardeners (TETA, IDA or PAC) and flame retardants (FR and TP), fifteen different polymeric materials were obtained. All of them obtained were subjected to the detailed physico-chemical tests. Analysing the photos (Fig. 2), it can be seen that the system based on Epidian® 601 + PAC is yellow, and those based on EP601 + TETA and EP601 + IDA are transparent. The addition of 20 %FR resulted in the greatest colour change. In any layout, this sample is the darkest. We managed to get all the compositions in the solid form. During the preparation of the composites, it was found that the best solubility of the substrates was associated with the system EP601 + TETA, and the worst with EP601 + IDA. The samples containing 20 %TP will take longer to reach hardness. Particularly the EP601 + IDA + 20 %TP sample reached the full rigidity after about 2 weeks. Then the samples were cut into strips (10 × 80 × 3 mm) for the flammability tests and for ageing into the squares of (10 × 10 × 3 mm) dimensions.

Photos of all systems.
ATR-FTIR analysis
Characterization of chemical structure by the spectroscopic analysis ATR/FT-IR was made for the all obtained materials. Figure S1(a–c) shows the spectra of the samples (Supplementary material) and Table 2 presents the wavelength values with the attributed intramolecular vibrations. Generally, the spectra of all obtained systems have a similar course. The epoxy resin is the fundamental component of the analyzed samples. Thus, characteristic bands of these reactants are pointed out on the spectra. In the 3332–3293 cm−1 range wide bands derived from the stretching vibrations of –OH groups are found. Moreover, this signal can be more intensive due to the presence of amine (TETA) and strong tendency towards water absorption of these molecules. In the modified composites by IDA and PAC these signals become weaker. Characteristic bands of methyl and methylene groups are visible as two signals, from one 2959 to 2923 cm−1 and the other in the range of 1459–1450 cm−1. These peaks correspond to the symmetrical and asymmetric stretching vibrations of both types of the groups. This effect is visible for each composite. There is a doublet of bands at 1509–1508 cm−1 corresponding to the symmetrical and asymmetric stretching vibrations of aromatic rings. Multiple bands ranging from 1509 cm−1 to 1508 cm−1 can be associated with the vibrations of C–H and C=C bonds related to the benzene rings and aromatic skeletons ones. They come from both the aromatic Epidian 601 part. Additionally, in the range 828–827 cm−1, the signal of deformation vibrations of Ar and Ar-H is notable. In the range from 1295 to 1293 cm−1, a band derived from the stretching vibrations of oxygen atoms associated with aromatic carbon atoms can be seen in the spectra. The bands at 1036–1034 cm−1 are associated with the stretching vibrations of hydroxyl groups. Deformation vibrations of the mentioned groups –CH3, –CH2– are visible in the range from 1459 to 1458 cm−1. The presence of the TETA-derived amine groups is confirmed by the valence band of stretching vibrations in the N–H bonds. This signal is observed from 638 to 617 cm−1. In summary, the addition of a flame retardant and triphenyl phosphate does not drastically change the course of the ATR-FTIR curves of the copolymers, however, it does affect the intensity of the signals.
Sample wavelength values with the attributed intramolecular vibrations.
| Name of sample | –OH | –CH3, –CH2– | C=C | C–O arom. | C–C | C–O aliph. | Ar | –NH– |
|---|---|---|---|---|---|---|---|---|
| EP601 + PAC | 3332 | 2923 1459 |
1509 | 1294 | 1244 | 1035 | 827 | – |
| EP601 + PAC + 10 %FR | 3366 | 2923 1450 |
1509 | 1294 | 1244 | 1035 | 828 | – |
| EP601 + PAC + 20 %FR | 3367 | 2924 1460 |
1508 | 1294 | 1244 | 1035 | 828 | 617 |
| EP601 + PAC + 10 %TP | 3338 | 2924 1459 |
1509 | 1294 | 1244 | 1036 | 828 | 637 |
| EP601 + PAC + 20 %TP | 3293 | 2923 | 1509 | 1294 | 1244 | 1036 | 828 | 613 |
|
|
||||||||
| EP601 + TETA | 3373 | 2925 1458 |
1508 | 1294 | 1242 | 1034 | 828 | 636 |
| EP601 + TETA + 10 %FR | 3372 | 2926 1459 |
1508 | 1294 | 1242 | 1034 | 828 | 637 |
| EP601 + TETA + 20 %FR | 3367 | 2925 1459 |
1508 | 1294 | 1242 | 1034 | 828 | 638 |
| EP601 + TETA + 10 %TP | 3361 | 2926 1459 |
1508 | 1294 | 1241 | 1034 | 828 | 637 |
| EP601 + TETA + 20 %TP | 3362 | 2926 1458 |
1509 | 1295 | 1242 | 1033 | 828 | 637 |
|
|
||||||||
| EP601 + IDA | 3346 | 2924 1458 |
1509 | 1295 | 1244 | 1036 | 827 | 617 |
| EP601 + IDA + 10 %FR | 3370 | 2925 1458 |
1509 | 1295 | 1244 | 1035 | 827 | 616 |
| EP601 + IDA + 20 %FR | 3371 | 2925 1458 |
1508 | 1295 | 1241 | 1034 | 828 | 618 |
| EP601 + IDA + 10 %TP | 3367 | 2925 1458 |
1509 | 1294 | 1242 | 1035 | 828 | 618 |
| EP601 + IDA + 20 %TP | 3372 | 2959 1458 |
1509 | 1293 | 1244 | 1035 | 828 | 619 |
DSC analysis
DSC measurements were carried out in the course of the heating cycle at temperature ranging from 0 to 500 °C. The numerical data of the DSC analysis is presented in Table 3.
DSC data.
| Samples | Tg [°C] | Texo [°C] | Ten1 [°C] | Ten2 [°C] | Ten3 [°C] |
|---|---|---|---|---|---|
| EP601 + PAC | 57/78 | 250 | 278 | 358 | 445 |
| EP601 + PAC + 10 %FR | 55 | 357 | 296 | – | 407 |
| EP601 + PAC + 20 %FR | 53 | 361 | 293 | – | 416 |
| EP601 + PAC + 10 %TP | 55/76 | 337 | 379 | – | 425 |
| EP601 + PAC + 20 %TP | 35/78 | 327 | 372 | – | 457 |
|
|
|||||
| EP601 + IDA | 110 | 364 | 311 | 395 | – |
| EP601 + IDA + 10 %FR | 94 | 361 | 320 | 383 | – |
| EP601 + IDA + 20 %FR | 80 | 340 | 320 | 389 | – |
| EP601 + IDA + 10 %TP | 65/85 | 327 | 280 | 360 | – |
| EP601 + IDA + 20 %TP | 67/85 | 240/321 | 260 | 353 | – |
|
|
|||||
| EP601 + TETA | – | – | 379 | – | – |
| EP601 + TETA + 10 %FR | 85 | 357 | 310 | 377 | – |
| EP601 + TETA + 20 %FR | 74/89 | 359 | 311 | 387 | – |
| EP601 + TETA + 10 %TP | 60/85 | 329 | 273 | 358 | – |
| EP601 + TETA + 20 %TP | 50 | 210/320 | 250 | 350 | – |
The thermal behaviour of the obtained composites was studied by means of the DSC analysis. The DSC curves of these materials are presented in Fig. 3(a–c). The composite consists of an epoxy resin, a crosslinker, and a flame retardant additive. All these reagents influence the thermodynamic phenomena occurring in the sample during the DSC analysis. On the DSC curves, there can be seen several endo and exo effects, their presence indicates the composites heterogeneity. The EP601 + PAC system can be seen in Fig. 1(a). With the addition of flame retardants, the glass transition temperature changed from 57 °C to 35 °C. On the curve of the parent EP601 + PAC system, the exothermic effects (250 °C) associated with the additional crosslinking are visible. For the other samples exothermic effects probably related to the opening of the remaining epoxy rings in the range 327–361 °C are observed. For the systems with the flame retardant additives, two endothermic effects related to the degradation of the sample are observed. For the initial sample, this decomposition is three-stage (278, 358, 445 °C).

DSC curves. (a) EP601 + PAC. (b) EP601 + IDA. (c) EP601 + TETA.
In the case of the EP601 + IDA systems, the studied DSC curves have different courses. The glass transition temperature changed from 110 °C to 65 °C with the addition of flame retardants. An exoenergetic effect characteristic of epoxy resins around 321–364 °C is also observed. The effects connected with the degradation of samples occur at about 260–320 and 353–395 °C. The last analyzed system contains TETA as a resin curing agent. This amine is used in the smallest (10 wt%) amount compared to the other commercial hardeners (IDA, PAC). The curve courses are similar to those for the samples with IDA. In general, it can be concluded that the influence of the additive on the temperature effects in the analyzed samples.
As can be seen in each graph, the DSC curves from the heating cycle reveal both endo- and exothermic changes. Endothermic peaks for the EP601 + PAC system (in the range of 35–78 °C) are responsible for the glass transition of the crystalline form. For the second system based on EP601 + IDA, these effects occur in the range of 65–110 °C. For the system, EP601 + TETA glass transition effect is visible only for modifications (50–89 °C). Another endothermic effect occurs in temperature higher than 250 °C. Values are higher for EP601 + PAC + 10 %TP (379 °C), and lower for EP601 + TETA + 20 %TP (250 °C). Next, the endothermic effect above 400 °C is visible for the EP601 + PAC system only. The exothermic peaks for the materials (pure and 10 %/20 %FR) are responsible for the cross-linking of materials.
In Supplementary materials one can see Figure S2 with the DSC curve of pure Fire Retardant.
Flammability tests
The parent samples as well as those with the largest content of FR and TP were selected for the flammability tests. Figure S3 (Supplementary materials) shows the burning compositions. The photos were taken 20 s after the burner had been put off. As follows from the photos the composites without any additives burnt the most intensively. This became apparent in the form of the brightest and greatest flame. The addition of Fire Retardant and Triphenyl Phosphate reduces the flame height and intensity. For the systems with TETA and PAC as curing agents, the greatest reduction in the flame height and intensity was observed for commercial FR. In the case of the IDA curing agent, a better effect was found for triphenyl phosphate. The flame was shorter and less intense. The temperatures of burning from the thermographic camera show that composites based on TETA (22–23, 227–249 °C) and PAC (29–21, 199–249 °C) as curing agents have lower temperatures than composites based on IDA (22–25, 231–249 °C). These values are affected by the structural constructions of curing agents. Amine curing agent IDA is based on modified cycloaliphatic polyamine. It is moisture-resistant. Polyamide curing agent – polyaminoamide C – PAC is produced by polycondensation of polyamine with dimers of unsaturated fatty acid methyl esters. The use of this curing agent results in higher impact resistance and elasticity of adhesive compounds. IDA has an amine number in the range of 200–350 mg KOH/g, although PAC has between 290 and 360 mg KOH/g. This proves that PAC binds the epoxy resin more intensively with the modifier. Therefore, during burning, the flame is smaller [38, 39].
Figure S4 (Supplementary materials) shows the composites after burning. All the tested samples were burnt with a bright flame, after burning the samples were charred with no visible blisters or cavities. Nevertheless, the additives affected the appearance, shape, and size of the flame, the temperature in the centre area of the burning sample, and the rate at which the fire was absorbed by the sample section. From Figure S4 it can be concluded that the sample b (EP601 + PAC + 20 %FR) burnt to the smallest extent, and the sample g (EP601 + IDA) to the largest extent. The addition of flame retardants affected the length of sample charring.
Table 4 includes the temperature data from the thermographic camera. Additionally, in Figure S5 (Supplementary materials) the sample thermograms are presented. The analysis of the temperature field in the area of the burning sample includes the determination of changes in the maximum temperature in the entire recorded thermal image after 20 and 30 s from turning on the ignition source and the temperature recorded along a straight line running through the longitudinal axis of the sample. The data presented in the paper were obtained after 30 s of burning. Analyzing the data in Table 3 and the thermograms in Figure S7 (Supplementary materials), it can be concluded that the highest flame temperatures were achieved by pure composites without additives. Virtually all three systems reached their maxima at 250 °C. The addition of 20 % Fire Retardant reduced the temperature in the EP601 + PAC system most effectively.
The data from the thermographic camera.
| Composite | Temperature min [°C] | Temperature max [°C] |
|---|---|---|
| EP601 + PAC | 20.24 | 249.9 |
| EP601 + PAC + 20 %FR | 19.91 | 199.7 |
| EP601 + PAC + 20 %TP | 21.46 | 213.58 |
|
|
||
| EP601 + TETA | 23.35 | 249.9 |
| EP601 + TETA + 20 %FR | 22.31 | 227.68 |
| EP601 + TETA + 20 %TP | 22.13 | 235.05 |
|
|
||
| EP601 + IDA | 25.14 | 249.9 |
| EP601 + IDA + 20 %FR | 23.51 | 241.15 |
| EP601 + IDA + 20 %TP | 22.90 | 231.16 |
In Supplementary materials, there is Figure S6 (it shows the temperature graphs after 20 and 30 s of burning) and Figure S7 (histograms). Figure 4 presents the proposed scheme of decomposition.

Proposal scheme of decomposition.
Hardness test
Measurements of hardness consisted in vertical immersion of the indenter into the composite surface. The numerical values of these parameters are expressed in the D scale in Table 5. For the system based on EP601 + PAC, the composites with 20 % Fire Retardant reached the greatest value. The composites without the modifier addition were characterized by the smallest hardness. Analyzing the obtained results one can conclude that the addition of modificatory reduces slightly the composites hardness by about 1.3–1.9 Sh. For the system based on EP601 + TETA the addition of 10 % of Fire Retardant caused the hardness growth. The composites hardness was in the range of 76.7–82.4 units. The smallest hardness was observed for the sample with 20 % of Triphenyl Phosphate. For the latter system based on EP601 + IDA the highest value of hardness was characteristic of the pure composite. The composite with 20 % of Triphenyl Phosphate had the smallest value, it was soft, and its addition prevented complete crosslinking. The range of this parameter for the materials was 39.8–76.2 units in the D scale.
Medium hardness in the Shore scale [°Sh].
| EP601 + PAC | EP601 + PAC + 10 %FR | EP601 + PAC + 20 %FR | EP601 + PAC + 10 %TP | EP601 + PAC + 20 %TP | EP601 + TETA | EP601 + TETA + 10 %FR | EP601 + TETA + 20 %FR | EP601 + TETA + 10 %TP | EP601 + TETA + 20 %TP | EP601 + IDA | EP601 + IDA + 10 %FR | EP601 + IDA + 20 %FR | EP601 + IDA + 10 %TP | EP601 + IDA + 20 %TP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average | 77.6 | 78.9 | 78.9 | 79.5 | 78.4 | 82.3 | 82.4 | 80.8 | 81.0 | 76.7 | 76.2 | 66.1 | 70.6 | 61.5 | 39.8 |
| Measurement | 78 78 78 77 77 |
79.5 79 79 79 78 |
80 80 79 79 79.5 |
78.5 79 79 79 79 |
79 79 78 78 78 |
82.5 82 83 82 82 |
81.5 82 82.5 83 83 |
80.5 82 81 81.5 80 |
82 80 81 81 80 |
77 76.5 77.5 77.5 75 |
76.5 78 77 75 74.5 |
68 66.5 67 64 65 |
61 62.5 60 62 62 |
72 70 70 70 71 |
40 38 42 40 39 |
| Standard deviation | 0.49 | 0.49 | 0.45 | 0.2 | 0.49 | 0.4 | 0.58 | 0.5 | 0.56 | 0.93 | 1.29 | 1.43 | 0.8 | 0.64 | 1.76 |
Swelling test
Swellabillity is a factor that defines accessibility of the internal chemical structure of cross-linked polymers to the solvent molecules’ penetration. This factor provides information how the resulting materials will behave under varying environmental conditions (in different solvents). The swellability tests in hydrochloric acid (0.1 M), potassium hydroxide (0.1 M), methanol, water, and acetone were carried out. A 1 cm × 1 cm sample was cut from each composite. Initially, the samples were weighed and placed in solvents. After 24 h they were taken and reweighed. Based on the results presented below, it can be concluded that the greatest weight changes were obtained for acetone. The increase in the FR/TP content in the material during tests caused a slight increase in swelling. The highest swelling coefficient was found in the case of EP601 + TETA in acetone and the lowest EP601 + IDA + 20 %TP in potassium hydroxide. The additives influenced the extent of the swelling. Both Triphenyl phosphate and Fire Retardant increased the factors. Supplementary materials in Figure S8 show the swelling test. Table 6 shows the exact values of the swellability factor.
Swelling test. Results are given in swelling coefficient B [%].
| Sample | Water | Hydrochloric acid | Potassium hydroxide | Acetone | Methanol |
|---|---|---|---|---|---|
| EP601 + PAC | 0.73 | 0.53 | 0.65 | 13.31 | 9.10 |
| EP601 + PAC + 10 %FR | 0.53 | 6.76 | 0.39 | 12.18 | 8.83 |
| EP601 + PAC + 20 %FR | 0.25 | 0.75 | 0.86 | 16.01 | 12.25 |
| EP601 + PAC + 10 %TP | 0.54 | 0.50 | 0.60 | 11.42 | 7.53 |
| EP601 + PAC + 20 %TP | 1.92 | 3.06 | 2.39 | 22.29 | 24.93 |
|
|
|||||
| EP601 + TETA | 0.79 | 0.68 | 0.51 | 27.53 | 6.28 |
| EP601 + TETA + 10 %FR | 0.19 | 0.39 | 0.54 | 24.33 | 6.40 |
| EP601 + TETA + 20 %FR | 0.91 | 0.66 | 0.57 | 24.33 | 8.29 |
| EP601 + TETA + 10 %TP | 0.43 | 0.81 | 0.58 | 18.66 | 4.31 |
| EP601 + TETA + 20 %TP | 0.49 | 1.30 | 0.32 | 14.59 | 9.16 |
|
|
|||||
| EP601 + IDA | 0.77 | 0.69 | 0.54 | 24.36 | 11.18 |
| EP601 + IDA + 10 %FR | 0.98 | 1.16 | 0.79 | 22.80 | 13.10 |
| EP601 + IDA + 20 %FR | 0.98 | 0.60 | 0.55 | 24.77 | 8.34 |
| EP601 + IDA + 10 %TP | 1.29 | 1.60 | 0.53 | 23.47 | 7.66 |
| EP601 + IDA + 20 %TP | 0.40 | 1.44 | 0.11 | 14.88 | 9.77 |
Ageing test and ATR-FTIR analysis after the ageing
In order to assess the resistance to solvents action to the ageing tests were carried. The measurements were made weekly for 9 weeks in glass tubes just like the swelling tests. Before each weighing, the samples were dried for 1 h at 70 °C to remove the adsorbed solvent. The results are given as the difference between the largest and smallest measurements are summarized in Table 7. The measurements included the determination of the mass after each week that the composite has been flooded with the solvent. Based on the results presented below, one can conclude that the highest resistance to methanol action has EP601 + IDA, acetone action EP601 + PAC + 20 %TP, potassium hydroxide action EP601 + TETA + 20 %FR, hydrochloric acid action EP601 + TETA + 10 %FR. The differences in weight gain are the smallest. Water has the lowest tendency to penetrate composite structures which proves that obtained composites are less resistant to organic solvents than water. The results obtained are a consequence of the chemical nature of the additives and resins used.
Ageing tests in the solvents.
| Sample | Water | Hydrochloric acid | Potassium hydroxide | Acetone | Methanol |
|---|---|---|---|---|---|
| EP601 + PAC | 0.3390 ± 0.0112 | 0.3399 ± 0.0117 | 0.3348 ± 0.0074 | 0.3706 ± 0.0630 | 0.3587 ± 0.0557 |
| EP601 + PAC + 10 %FR | 0.3553 ± 0.0118 | 0.3560 ± 0.0099 | 0.3602 ± 0.0067 | 0.3989 ± 0.0590 | 0.3856 ± 0.0602 |
| EP601 + PAC + 20 %FR | 0.3526 ± 0.0081 | 0.3640 ± 0.0061 | 0.3477 ± 0.0054 | 0.3954 ± 0.0509 | 0.3790 ± 0.0520 |
| EP601 + PAC + 10 %TP | 0.3196 ± 0.0100 | 0.3466 ± 0.0123 | 0.3357 ± 0.0080 | 0.3594 ± 0.0343 | 0.3558 ± 0.0487 |
| EP601 + PAC + 20 %TP | 0.3604 ± 0.0245 | 0.3551 ± 0.0448 | 0.3560 ± 0.0240 | 0.4013 ± 0.0282 | 0.4109 ± 0.0532 |
| EP601 + TETA | 0.3650 ± 0.0067 | 0.3669 ± 0.0060 | 0.3588 ± 0.0145 | 0.4053 ± 0.0299 | 0.3855 ± 0.0709 |
| EP601 + TETA + 10 %FR | 0.3766 ± 0.0083 | 0.3863 ± 0.0056 | 0.3837 ± 0.0054 | 0.3991 ± 0.0515 | 0.3951 ± 0.0658 |
| EP601 + TETA + 20 %FR | 0.3988 ± 0.0081 | 0.4077 ± 0.0067 | 0.3983 ± 0.0046 | 0.4222 ± 0.0628 | 0.4115 ± 0.0568 |
| EP601 + TETA + 10 %TP | 0.3620 ± 0.0082 | 0.3642 ± 0.0059 | 0.3653 ± 0.0060 | 0.3677 ± 0.0584 | 0.3823 ± 0.0498 |
| EP601 + TETA + 20 %TP | 0.3678 ± 0.0064 | 0.3712 ± 0.0086 | 0.3710 ± 0.0065 | 0.3554 ± 0.0537 | 0.3916 ± 0.0199 |
| EP601 + IDA | 0.3517 ± 0.0123 | 0.3487 ± 0.0076 | 0.3457 ± 0.0071 | 0.3733 ± 0.0551 | 0.3797 ± 0.0017 |
| EP601 + IDA + 10 %FR | 0.3781 ± 0.0098 | 0.3815 ± 0.0358 | 0.3787 ± 0.0075 | 0.4173 ± 0.0470 | 0.4128 ± 0.0042 |
| EP601 + IDA + 20 %FR | 0.3947 ± 0.0132 | 0.3887 ± 0.0415 | 0.3930 ± 0.0073 | 0.3945 ± 0.1089 | 0.3394 ± 0.0550 |
| EP601 + IDA + 10 %TP | 03823 ± 0.0090 | 0.3990 ± 0.0349 | 0.3859 ± 0.0229 | 0.4160 ± 0.0351 | 0.3950 ± 0.0283 |
| EP601 + IDA + 20 %TP | 0.3840 ± 0.0050 | 0.3870 ± 0.0177 | 0.3920 ± 0.0163 | 0.4105 ± 0.0617 | 0.4005 ± 0.0210 |
Figure 5 shows the exemplary samples for the PAC system. One can see that solvents 1, 2, and 3 did not change at all, i.e., water, hydrochloric acid, and potassium hydroxide did not degrade the composites after 9 weeks. However, such solvents as acetone and methanol caused slight damage to the materials. The greatest damage was observed for acetone and methanol for the compositions with the 20%TP addition as the flame retardant. In summary, the composites after 9 weeks of ageing under light conditions were in good shape and showed adequate resistance to such an environment.
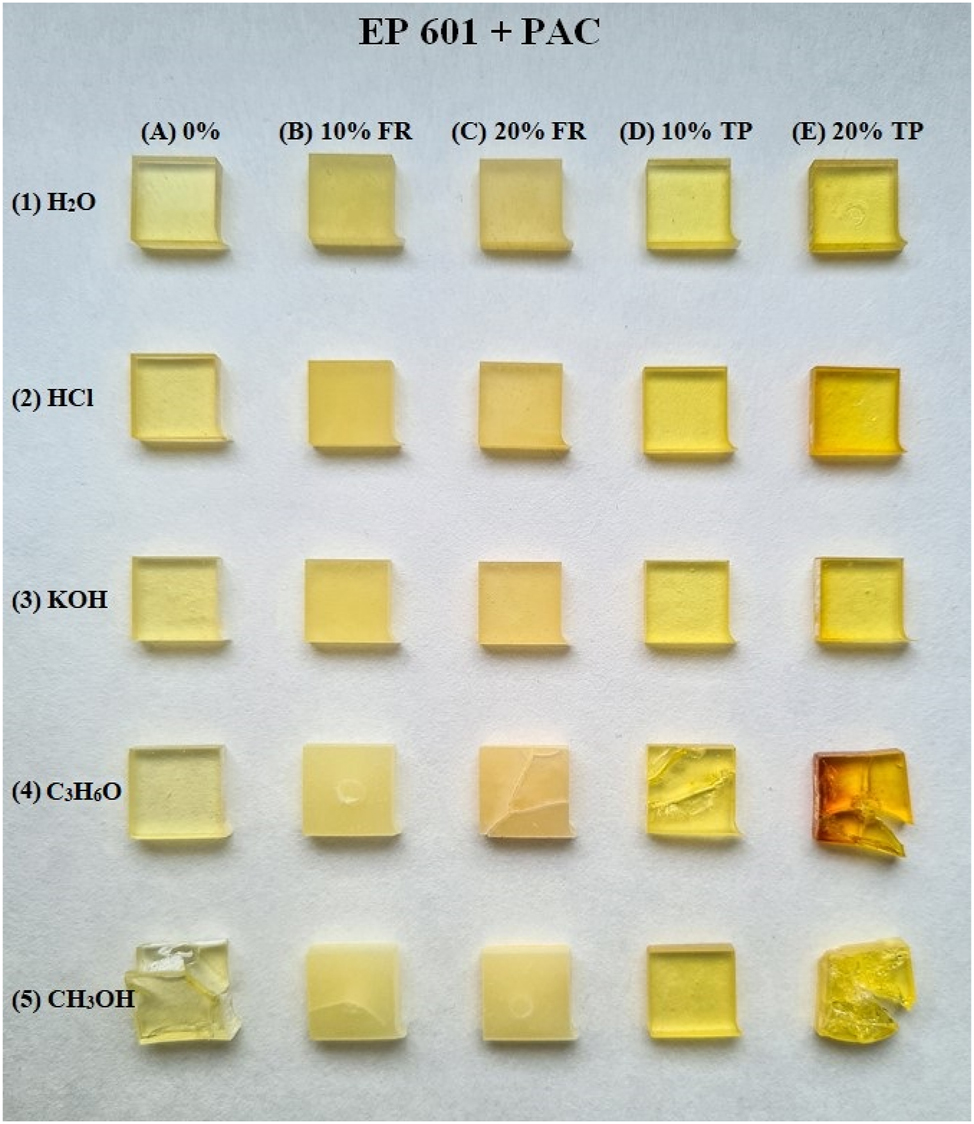
Photo of the PAC system after ageing.
The ATR/FT(IR) spectra were made in order to evaluate the changes occurring in the composite structure after degradation in the solvents. Chemical structure characterization was made for the PAC system. In the range of 3372–3293 cm−1, broad bands derived from the stretching vibrations of the residual –OH groups or adsorbed water are present. Their intensity changed only in the 0 % and 10 % systems of the flame retardant after ageing in HCl. In each of the five composites in the solvent acetone, a separate band appeared at about 1700 cm−1. The most intense bands in the data range come from the solvents: methanol, acetone or potassium hydroxide. Figures S9–13 show the spectra after ageing tests (Supplementary materials).
Conclusions
The new composites based on the epoxy resin Epidian® 601 and three different crosslinkers (PAC/IDA/TETA) with the functional additives were successfully obtained. The commercial FR and triphenyl phosphate at 10 and 20 wt% were used as flame retardant compounds. The applied hardeners as well as the flame retardants influence on the physicochemical properties of the composites in different ways. The spectroscopy analysis (ATR/FT-IR) confirmed the presence of characteristic groups in the composite structures such as methyl and methylene groups, aromatic rings as well as amine groups. The data obtained during the DSC analysis proved that the amount of FR or TP affects the thermal properties of the samples. The samples without modification additives were characterized by lower thermal stability. The addition of FR caused the increase of the endothermic effect and the TP additive resulted in the exothermic effect at above 300 °C (in each system). According to the hardness tests it can be assumed that the modification type contributes to the changing hardness of the obtained materials (the highest result was for EP601 + TETA + 10 %FR and the lowest for EP601 + IDA + 20 %TP). The results of solvents tests show that the EP601 + IDA + 20 %FR sample was the most sensitive to acetone and samples were stable in water, 0.1 M KOH, and 0.1 M HCl.
The influence of the addition of FR/TP on the thermal resistance and combustibility of the obtained composites was evaluated. According to the flammability tests the addition of FR and TP affects the burning behaviour of the samples. The materials without additives burnt the fastest and most intensively. The addition of flame retardants to the PAC system reduced the flame temperature by 50 °C for FR and 36 °C for TP compounds. The tests confirmed the flame retardant effect of additives on various cross-linked systems based on the epoxy resin.
Funding source: Polish Ministry of Science and Higher Education
Award Identifier / Grant number: 030/RID/2018/19
-
Research funding: The project/research was financed in the framework of the project Lublin University of Technology – Regional Excellence Initiative, funded by the Polish Ministry of Science and Higher Education (contract no. 030/RID/2018/19).
References
[1] B. Podkościelna, K. Wnuczek, M. Goliszek, T. Klepka, K. Dziuba. Molecules 25, 5947 (2020), https://doi.org/10.3390/molecules25245947.Search in Google Scholar PubMed PubMed Central
[2] E. D. Weil, M. Lewin. Mechanisms and modes of action in flame retardancy of polymers. in Fire Retardant Materials, A. R. Horrocks, D. Price (Eds.), pp. 31–68, CRC Press, Cambridge (2006).Search in Google Scholar
[3] C. Hobbs. Polymers 11, 224 (2019), https://doi.org/10.3390/polym11020224.Search in Google Scholar PubMed PubMed Central
[4] E. Feyz, Y. Jahani, M. Esfandeh. Macromol. Symp. 298, 130 (2010), https://doi.org/10.1002/masy.201000029.Search in Google Scholar
[5] G. J. Van Esch. in Flame Retardants: A General Introduction, Report in WHO Library Cataloguing in Publication Data, Geneva (1997).Search in Google Scholar
[6] H. Liang, W. Shi, M. Gong. Polym. Degrad. Stab. 90, 1 (2005), https://doi.org/10.1016/j.polymdegradstab.2005.01.022.Search in Google Scholar
[7] X. Almeras, F. Dabrowski, M. Le Bras, F. Poutch, S. Bourbigot, G. Marosi. Polym. Degrad. Stab. 77, 305 (2002), https://doi.org/10.1016/s0141-3910(02)00068-x.Search in Google Scholar
[8] H. Demir, E. Arkis, D. Balkose, S. Ulku. Polym. Degrad. Stab. 89, 478 (2005), https://doi.org/10.1016/j.polymdegradstab.2005.01.028.Search in Google Scholar
[9] P. Anna, G. Marosi, S. Bourbigot, M. Le Bras, R. Delobel. Polym. Degrad. Stab. 77, 243 (2002), https://doi.org/10.1016/s0141-3910(02)00040-x.Search in Google Scholar
[10] Y. Chen, Y. Liu, Q. Wang, H. Yin, N. Aelmans, R. Kierkels. Polym. Degrad. Stab. 81, 215 (2003), https://doi.org/10.1016/s0141-3910(03)00091-0.Search in Google Scholar
[11] E. Ciecierska, A. Boczkowska, M. Kubis, P. Chabera, T. Wisniewski. Przem. Chem. 94, 2033 (2005).Search in Google Scholar
[12] A. Battig, J. C. Markwart, F. R. Wurm, B. Schartel. Polym. Chem. 10, 4346 (2019), https://doi.org/10.1039/c9py00737g.Search in Google Scholar
[13] W. J. Lee, S. H. Cha, D. H. Kim. Polymers 14, 5205 (2022), https://doi.org/10.3390/polym14235205.Search in Google Scholar PubMed PubMed Central
[14] J. Zhang, X. Mi, S. Chen, Z. Xu, D. Zhang, M. Miao, J. Wang. Chem. Eng. J. 381, 122719 (2020), https://doi.org/10.1016/j.cej.2019.122719.Search in Google Scholar
[15] V. Der Veen, I. J. De Boer. Chemosphere 88, 1119 (2012), https://doi.org/10.1016/j.chemosphere.2012.03.067.Search in Google Scholar PubMed
[16] Q. Liu, D. Wang, Z. Li, Z. Li, X. Peng, C. Liu, Y. Zhang, P. Zheng. Materials 13, 2145 (2020), https://doi.org/10.3390/ma13092145.Search in Google Scholar PubMed PubMed Central
[17] E. R. Rada, H. Vahabib, A. Rios de Andad, M. R. Saebb, S. Thomase. Prog. Org. Coat. 135, 608 (2019), https://doi.org/10.1016/j.porgcoat.2019.05.046.Search in Google Scholar
[18] I. Miturska, A. Rudawska, M. Müller, P. Valášek. Materials 13, 291 (2020), https://doi.org/10.3390/ma13020291.Search in Google Scholar PubMed PubMed Central
[19] J. Wang, C. Ma, P. Wang, S. Qiu, W. Cai, Y. Hu. Polym. Degrad. Stab. 10, 1016 (2018), https://doi.org/10.1016/j.polymdegradstab.2018.01.024.Search in Google Scholar
[20] M. Bratychak, O. Astakhova, O. Shyshchak, M. Sienkiewicz, J. Kucinska-Lipka. Chem. Chem. Technol. 14, 343 (2020), https://doi.org/10.23939/chcht14.03.343.Search in Google Scholar
[21] N. Teng, J. Dai, S. Wang, J. Hu, X. Liu. Chem. Eng. J. 428, 131226 (2020), https://doi.org/10.1016/j.cej.2021.131226.Search in Google Scholar
[22] J. Zou, H. Duan, Y. Chen, S. Ji, J. Cao, H. Ma. Composites 199, 108228 (2020), https://doi.org/10.1016/j.compositesb.2020.108228.Search in Google Scholar
[23] V. Venezia, S. Matta, S. Lehner, G. Vitiello, A. Costantini, S. Gaan, G. Malucelli, F. Branda, G. Luciani, A. Bifulco. ACS Appl. Polym. Mater. 3, 5969 (2021), https://doi.org/10.1021/acsapm.1c01240.Search in Google Scholar
[24] J. C. Markwart, A. Battig, L. Zimmermann, M. Wagner, J. Fischer, B. Schartel, F. R. Wurm. ACS Appl. Polym. Mater. 1, 1118 (2019), https://doi.org/10.1021/acsapm.9b00129.Search in Google Scholar
[25] M. Frigione, A. Maffezzoli, P. Finocchiaro, S. Failla. Adv. Polym. Technol. 224, 329 (2003), https://doi.org/10.1002/adv.10060.Search in Google Scholar
[26] F. J. Martin, K. R. Price. J. Appl. Polym. Sci. 12, 143 (1968), https://doi.org/10.1002/app.1968.070120114.Search in Google Scholar
[27] J. W. Lyons. The Chemistry and Uses of Fire Retardants, Wiley-Interscience, New York (1970).Search in Google Scholar
[28] A. H. Landrock. Handbook of Plastics Flammability and Combustion Toxicology, Noyes Publications, Park Ridge, New York (1983).Search in Google Scholar
[29] J. A. Mikroyannidis. J. Polym. Sci. Part A: Polym. Chem. 26, 1885 (1988), https://doi.org/10.1002/pola.1988.080260221.Search in Google Scholar
[30] J. A. Green. J. Fire Sci. 14, 353 (1996), https://doi.org/10.1177/073490419601400504.Search in Google Scholar
[31] A. A. Kettrup, D. Lenoir, W. Thumm, K. Kampke-Thiel, B. Beck. Polym. Degrad. Stab. 54, 175 (1996), https://doi.org/10.1016/s0141-3910(96)00041-9.Search in Google Scholar
[32] W. C. Kuryla, A. J. Papa. Flame Retardancy of Polymeric Materials, Marcel Dekker, Inc, New York, Vol. 1 (1973).Search in Google Scholar
[33] S. P. Bhuniya, S. Maiti. Eur. Polym. J. 38, 195 (2002), https://doi.org/10.1016/s0014-3057(01)00163-x.Search in Google Scholar
[34] R. J. Jeng, S. M. Shau, J. J. Lin, W. C. Su, Y. S. Chiu. Eur. Polym. J. 38, 683 (2002), https://doi.org/10.1016/s0014-3057(01)00246-4.Search in Google Scholar
[35] S. V. Levchik, E. D. Weil. J. Fire Sci. 242, 137 (2006), https://doi.org/10.1177/0734904106055997.Search in Google Scholar
[36] S. V. Levchik, E. D. Weil. Polym. Int. 54, 981 (2005), https://doi.org/10.1002/pi.1806.Search in Google Scholar
[37] M. Kilinc, G. O. Cakal, G. Bayram, I. Eroglu, S. Özkar. J. Appl. Polym. Sci. 132, 22 (2015), https://doi.org/10.1002/app.42016.Search in Google Scholar
[38] A. Rudawska. Polymers 11, 804 (2019), https://doi.org/10.3390/polym11050804.Search in Google Scholar PubMed PubMed Central
[39] A. Rudawska. Appl. Mech. 2, 108 (2021), https://doi.org/10.3390/applmech2010007.Search in Google Scholar
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/pac-2022-1102).
© 2023 IUPAC & De Gruyter. This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. For more information, please visit: http://creativecommons.org/licenses/by-nc-nd/4.0/
Articles in the same Issue
- Frontmatter
- In this issue
- Preface
- Preface for special issue of ICGC-9 in Athens, Greece
- Conference papers
- HOME-Chemistry: hydrazone as organo-metallic equivalent
- Towards lignin valorization: lignin as a UV-protective bio-additive for polymer coatings
- How the physio-chemical properties of char from the pyrolysis of Automotive Shredder Residue (ASR) influences its future uses
- Plant based fabrication of CuO/NiO nanocomposite: a green approach for low-level quantification of vanillin in food samples
- Synthesis and characterization of new polycarbonates free of bisphenol A components (BPA-free) based on dimethyl/diphenyl carbonate and diphenylmethane derivative
- Adsorption capacity of biocarbons from residue of supercritical extraction of raw plants
- Cd(II) and As(V) removal from the multicomponent solutions in the presence of ionic polymers using carbonaceous adsorbents obtained from herbs
- Synthesis and thermal characterization of composites based on Epidian 601 with flame retardants compounds
- A green sorptive extraction method (HiSorb-TD-GC-MS) for determining the extra virgin olive oil (EVOO) aroma profile
- Synthesis, aging and antimicrobial tests of (di)acrylate composites
Articles in the same Issue
- Frontmatter
- In this issue
- Preface
- Preface for special issue of ICGC-9 in Athens, Greece
- Conference papers
- HOME-Chemistry: hydrazone as organo-metallic equivalent
- Towards lignin valorization: lignin as a UV-protective bio-additive for polymer coatings
- How the physio-chemical properties of char from the pyrolysis of Automotive Shredder Residue (ASR) influences its future uses
- Plant based fabrication of CuO/NiO nanocomposite: a green approach for low-level quantification of vanillin in food samples
- Synthesis and characterization of new polycarbonates free of bisphenol A components (BPA-free) based on dimethyl/diphenyl carbonate and diphenylmethane derivative
- Adsorption capacity of biocarbons from residue of supercritical extraction of raw plants
- Cd(II) and As(V) removal from the multicomponent solutions in the presence of ionic polymers using carbonaceous adsorbents obtained from herbs
- Synthesis and thermal characterization of composites based on Epidian 601 with flame retardants compounds
- A green sorptive extraction method (HiSorb-TD-GC-MS) for determining the extra virgin olive oil (EVOO) aroma profile
- Synthesis, aging and antimicrobial tests of (di)acrylate composites

