Abstract
The synthesis and characteristics of composites based on bisphenol A diacrylate with the addition of 2-ethylhexyl acrylate, butyl acrylate, or methyl methacrylate were carried out. Benzethonium chloride and zinc oxide were used as special additives in amounts of 1, 2, and 5 % by weight of monomers. These are compounds that exhibit antimicrobial properties. The composites were produced by the UV polymerization method with the addition of Irgacure 651 as a photoinitiator. Aging and swelling tests were carried out in water, solutions of hydrochloric acid and sodium hydroxide, and acetone. To confirm the changes in the structure of the composites, ATR/FT-IR spectra were performed before and after the degradation process. The hardness of the composites was also tested. The antimicrobial properties against Escherichia coli, Staphylococcus aureus, Aspergillus niger, and Candida albicans were checked. The conducted study also showed the significant changes in the Cerrena unicolor fungus secretome caused by the presence of the composites.
Introduction
Polymer composites are materials that contain two or more phases with different chemical compositions and properties. The components interact with each other but do not dissolve into each other. Polymer composites are very easy to synthesize and can be used in many fields of science. Among their properties are high mechanical strength, X-ray permeability, and low specific gravity [1–4]. Polymer composites are commonly used in medicine as components of prostheses, in mechanics, they are used to construct bridges and airplanes. They are used to manufacture automotive parts, machine housings, and chemical apparatus [5–7].
The fact that antibiotics are increasingly widely used has caused many strains of bacteria to become resistant to their effects. Increasingly, scientists are looking for compounds that exhibit antimicrobial activity. Examples include zinc oxide and benzethonium chloride, which is a quaternary ammonium salt that is synthetically derived. Synthesis of composites with potential antimicrobial properties may be the answer to the problem of bacterial resistance to antibiotics [8–10].
Zinc oxide has antibacterial and antifungal properties. Due to the physicochemical properties of ZnO, is widely used in many scientific and industrial fields. It is applied as an ingredient in salves and ointments (pharmaceutical industry), as well as in toothpaste. In combination with eugenol, a filling for dental cavities is applied. Zinc white is a white pigment used in the paint industry. It can also be used as a catalyst for organic synthesis, for the production of semiconductors, sensors, or optoelectronics. ZnO exhibits strong antibacterial activity for a wide range of bacterial types. The mechanism of its action is not fully understood. It is assumed that it may be the photocatalytic generation of reactive oxygen species. The inhibition of bacterial growth may be due to the penetration and disorganization of the cell membrane by the interaction of ZnO nanoparticles [11–13].
Benzethonium chloride is otherwise known as hyamine. It has surfactants with antiseptic, and anti-infective properties. It is used as an antibacterial agent in disinfectant fluids. It is found in the composition of cosmetic and toilet products (soap. mouthwash. disinfectant wipes). Its properties allow it to use as a food preservative and disinfectant of hard surfaces [14–17].
Activities aimed at the more frequent use of compounds with antimicrobial properties are very important in the SARS-CoV-2 virus pandemic. It is very important to systematically disinfect surfaces – tables, doorknobs, handrails, or containers for disinfectant liquids that are made of plastic. An interesting idea is the synthesis of composites, which in their structure contain a protective agent against microorganisms.
The main objective of this work was to synthesize new composites with potential antimicrobial properties. The composites consist of bisphenol A glycerolate diacrylate as the main monomer. Zinc oxide and benzethonium chloride were used as additives with antimicrobial properties. In the chemical part of the conducted research, the thermal properties of the obtained composites were investigated using the DSC technique. The swelling coefficient of the materials was also determined. Aging studies of the obtained materials in organic and inorganic solvents were conducted. The structure of the obtained material before and after the aging process was investigated using ATR-FT/IR spectroscopy. In the biological part of the research, the antimicrobial effect of the composites was studied using the modified disk-diffusion method. The next stage of the composites biological activity research was the analysis of the effect of composites on selected metabolic parameters of the white rot fungus C. unicolor well known for numerous biotechnological applications, including biodegradation processes and biomedical [18].
Materials and methods
Materials
Chemical materials
Bisphenol A glycerolate diacrylate (BPA.DA), 2-ethylhexyl acrylate (AEH), methyl methacrylate (MMA), butyl acrylate (BA), zinc oxide (ZnO) and 2.2-dimethoxy-2-phenylacetophenone (IQ) were obtained from Sigma-Aldrich. Benzethonium chloride (BEN) was obtained from Lonzagard®.
Biological materials
The following bacterial and fungal strains were used as biological material in the work: Escherichia coli ATCC 25,922, Staphylococcus aureus ATCC 25923, C. albicans ATCC 10,231, Aspergillus niger (accession number G13) and C. unicolor (accession number DQ056858). The biological material used in the work came from the Department of Biochemistry and Biotechnology UMCS strain collection.
Preparation of composites
Appropriate amounts of BPA.DA and one of three active diluents (AEH, MMA, BA) were placed in a 50 mL glass beaker. The weight ratio of BPA.DA to the diluents was 7:3. The monomer mixture was placed in a heating chamber at 65 °C for venting. Appropriate amounts of filler (ZnO and BEN) were added to the mixture which was 1, 2, and 5 % by weight. A composite containing no filler was also prepared for comparison. The contents of the beakers were mixed and the UV initiator, which was IQ in the amount of 1 % by weight concerning the weight of monomers was added. The mixture was transferred into a 12 × 10 × 0.2 cm glass mold, which consisted of two glass plates coated with a non-stick agent between which a Teflon spacer was placed. The molds were placed in a chamber equipped with 160 W mercury lamps for 30 min. Then the composites were transferred to the heating chamber to be crosslinked (for 4 h at 85 °C). Composites containing ZnO, with increasing filler content, became more and more white and were less transparent. The addition of BEN did not affect the color of the composites. Thus, 21 polymer composites were obtained [19, 20]. The chemical structure of the monomers is shown in Fig. 1. The experimental parameters of the synthesis are summarized in Table 1.

Chemical structures of monomers.
Experimental parameters of the synthesis.
| Composite | BPA.DA (g) | Active diluent (g) | Filler (%w/w) | Filler (g) | IQ (g) |
|---|---|---|---|---|---|
| AEH | 10 | 4.29 | 0 | 0 | 0.1429 |
| AEH + 1 % ZnO | 10 | 4.29 | 1 | 0.1429 | 0.1429 |
| AEH + 2 % ZnO | 10 | 4.29 | 2 | 0.2858 | 0.1429 |
| AEH + 5 % ZnO | 10 | 4.29 | 5 | 0.7145 | 0.1429 |
| MMA | 10 | 4.29 | 0 | 0 | 0.1429 |
| MMA + 1 % ZnO | 10 | 4.29 | 1 | 0.1429 | 0.1429 |
| MMA + 2 % ZnO | 10 | 4.29 | 2 | 0.2858 | 0.1429 |
| MMA + 5 % ZnO | 10 | 4.29 | 5 | 0.7145 | 0.1429 |
| BA | 10 | 4.29 | 0 | 0 | 0.1429 |
| BA + 1 % ZnO | 10 | 4.29 | 1 | 0.1429 | 0.1429 |
| BA + 2 % ZnO | 10 | 4.29 | 2 | 0.2858 | 0.1429 |
| BA + 5 % ZnO | 10 | 4.29 | 5 | 0.7145 | 0.1429 |
| AEH + 1 % BEN | 10 | 4.29 | 1 | 0.1429 | 0.1429 |
| AEH + 2 % BEN | 10 | 4.29 | 2 | 0.2858 | 0.1429 |
| AEH + 5 % BEN | 10 | 4.29 | 5 | 0.7145 | 0.1429 |
| MMA + 1 % BEN | 10 | 4.29 | 1 | 0.1429 | 0.1429 |
| MMA + 2 % BEN | 10 | 4.29 | 2 | 0.2858 | 0.1429 |
| MMA + 5 % BEN | 10 | 4.29 | 5 | 0.7145 | 0.1429 |
| BA + 1 % BEN | 10 | 4.29 | 1 | 0.1429 | 0.1429 |
| BA + 2 % BEN | 10 | 4.29 | 2 | 0.2858 | 0.1429 |
| BA + 5 % BEN | 10 | 4.29 | 5 | 0.7145 | 0.1429 |
Methods
Chemical methods
ATR/FT-IR and DSC analysis
FTIR spectra were recorded using a Bruker Tensor 27 FTIR spectrometer (Germany) and the attenuated total reflection (ATR) technique. The spectra were obtained in the range of 600–4000 cm−1 in transmittance mode. The spectral resolution is 4 cm−1 and 32 scans were taken for each spectrum. Before calorimetric measurement, the samples were heated for 2 h at 70 °C to remove moisture. Using a Netzch DSC 204 calorimeter (Selb, Germany), calorimetric measurements were made. The scan was conducted in 10 °C/min heating mode in the temperature range of 20 °C–500 °C under a nitrogen atmosphere (30 cm3/min). An empty aluminum crucible was used as a reference. The hardness of the materials was measured using the Shore method. An analog hardness measuring apparatus Zwick 7206/H04 (Germany) at 23 °C was used. Readings were taken after 10s.
Aging tests
For aging tests, the composites were cut into 15 × 10 mm specimens, their thickness was 2 mm. They were placed in tightly capped tubes, which were filled with solvents such as water, acetone, 1 M solution of sodium hydroxide, and 1 M solution of hydrochloric acid. The aging process was carried out for 6 months at ambient temperature. The samples were also exposed to sunlight. To evaluate the changes, the samples were weighed weekly for 12 weeks, then at 16, 20, and 24 weeks. Before weighing, the samples were dried at 85 °C for 1 h to remove adsorbed solvents. After each weighing, photographs were taken of the samples to visually assess the changes occurring in the structure.
The swelling coefficient (B%) was determined for all the composites obtained from the formula:
where: mk – a mass of the swollen polymer; m0 – a mass of the dry polymer.
Biological tests
Determination of antimicrobial activity
In this step, a modification of the disk-diffusion method on Mueller–Hinton agar plates for bacterial and fungal reference strains was used. 20 mL of broth (Mueller–Hinton agar, for fungal studies glucose was also added) was placed in a culture plate and allowed to solidify. A suspension of the young fungal or bacterial culture corresponding to 0.5 McFarland units was prepared. 200 μL of this suspension were transferred to the plate and spread over the surface with a spatula. The composites were placed on the surface of the medium inoculated with microorganisms. Cultures were run for 24 h for bacteria and 48 h for fungi at 37 °C. After this time, the composites were removed and the pathogen growth zones around the composite fragments were measured. The same process was carried out for composites containing no filler to compare the results.
The influence of composites on selected metabolic markers of the fungus C. unicolor (detection of proteins, phenolic compounds, the activity of laccase, the relative level of free radicals and antioxidant properties)
An analysis of the effect of composites on selected metabolic parameters of the fungus C. unicolor, known for its numerous biotechnological applications, including biomedical ones, was carried out. For this purpose, the mycelium was cultured in the presence of composite samples for 10 days on a liquid medium under constant aeration and temperature conditions (26 °C). The liquid medium was Lindeberg Holm with the composition: glucose 10 g/L, L-asparagine 1.5 g/L, MgSO4 × 7 H2O, 0.5 g/L, KH2PO4 0.47 g/L, Na2HPO4 × 12 H2O 0.48 g/L, yeast extract 0.1 g/L, Mn(CH3COO)2 × 4 H2O 12 mg/L, Zn(NO3)2 × 6 H2O 3.14 mg/L, CuSO4 × 5 H2O 3.9 mg/L, Ca(NO3)2 × 4 H2O 50 mg/L, FeCl3 × 6 H2O 3.2 mg/L, thiamine 50 μg/L. Samples cultured without composites were used as a control. The resulting post-culture fluid was served as a source of the test material. It was used to determine the level of protein, phenolic substances, the relative level of superoxide anion radical, antioxidant properties, and the activity of the laccase. Protein concentration was determined according to the Bradford method. It uses the reaction of Coomassie brilliant blue (G-250) with proteins, which forms a blue complex in an acidic medium. Its color is directly proportional to the concentration of the protein in the solution. Absorbance was measured at 595 nm, and the result is presented in µg/mL [21]. Syringaldazine (4-hydroxy 3,5-dimethoxybenzaldehyde) was used as a substrate for the reaction enabling the determination of laccase activity. During the course of the reaction, the substrate is oxidized. Appearing pink colored reaction product and its intensity depends on the laccase activity. The measurement was carried out at 525 nm in 50 mM buffer at pH 5.2. The result is presented in nkat/mg [22]. The DASA method (the coupling reaction of phenolic compounds with sulfanilamide), was used to determine the level of phenolic compounds. The measurement was carried out at 500 nm, and the result is presented in mM [23]. A method with NBT(nitrotetrazolium blue), was used to determine the relative level of free radicals. A 1 M NaOH solution was used, and the measurement was carried out at 560 nm [24]. The method with ABTS allows the determination of antioxidant properties. ABTS are radicals that are formed during chemical reactions. In this method, the ability to scavenge them is tested. The measurement is carried out at 734 nm [25].
Results and discussion
DSC analysis
The DSC curves of the individual composites are shown in Figs. 2–4 and the data are additionally summarized in Table 2. Each of the graphs is characterized by a similar pattern, where one distinct endothermic peak is observed corresponding to the thermal decomposition of the compositions in the range of temperatures 381.8–413.5 °C. The glass temperatures (Tg) are not clearly visible (∼140–150 °C) due to the crosslinking structure of the materials. The MMA-derived composites proved to be the most thermal resistant. The DSC curves show an endothermic effect related to the decomposition of the pure sample at 405.4 °C and for 5 % additives of benzethonium chloride at 413.5 °C. The thermal resistance of AEH and BA derivatives is at a similar level and ranges from 382 to 402 °C. The addition of modifiers reduces the degradation temperature by about 20 °C in the case of zinc oxide. This effect is less pronounced for benzethonium chloride (c.a. 10 °C). On curves with the addition of BEN a small endothermic effect c.a. 200 °C connected with the degradation of BEN is visible.

DSC curves of composites containing AEH.

DSC curves of composites containing MMA.

DSC curves of composites containing BA.
DSC data of the composites.
| Composite | Td [°C] | DSC [mW/mg] |
|---|---|---|
| AEH | 401.7 | −0.5959 |
| AEH + 5 % ZnO | 381.8 | −0.6781 |
| AEH + 5 % BEN | 390.4 | −0.4274 |
| MMA | 405.4 | −0.7602 |
| MMA + 5 % ZnO | 382.9 | −0.6225 |
| MMA + 5 % BEN | 413.5 | −0.5642 |
| BA | 401.3 | −0.6649 |
| BA + 5 % ZnO | 387.8 | −0.6160 |
| BA + 5 % BEN | 397.9 | −0.5064 |
Shore hardness
The polymer composite AEH for HCl and H2O after aging showed higher or the same hardness value. In NaOH, its hardness decreased. For the composite with 1 % ZnO, the hardness index was lower only for HCl, while for 2 % ZnO for NaOH. The other hardness values in solvents were higher. In the composite containing 5 % ZnO for NaOH, it was not possible to measure the hardness as the composite had degraded. The values for HCl and H2O were lower than before aging. For MMA without ZnO addition, as well as with addition, all hardness values were higher (the exception being the addition of 5 % ZnO during aging in NaOH). Composites composed of bisphenol A diacrylate and butyl acrylate without and with zinc oxide addition had higher hardness values after aging than before aging. The exception is BA + 5 % ZnO, which showed a lower hardness value when 2 and 5 wt% zinc oxide was added. The obtained trend may be because, during the aging process, the fillers leach out from the composites, and what remains is a stiff copolymer that shows quite a high hardness in comparison with the fillers (in powder form). In composites where benzethonium chloride was the filler, all hardness values were higher. The only exception was a composite composed of bisphenol A diacrylate and 2-ethylhexyl acrylate, which obtained a lower hardness value when aged in sodium hydroxide (Table 3).
The hardness of the composites.
| Composite | Before [°Sh] | After NaOH [°Sh] | After HCl [°Sh] | After water [°Sh] |
|---|---|---|---|---|
| AEH | 78 | 76.4 | 79.8 | 78 |
| AEH + 1 % ZnO | 69.6 | 76.6 | 65.6 | 74.6 |
| AEH + 2 % ZnO | 69 | 68.4 | 83.6 | 70 |
| AEH + 5 % ZnO | 50.2 | – | 41 | 41.2 |
| MMA | 70.8 | 84.4 | 85.2 | 85.8 |
| MMA + 1 % ZnO | 73 | 80.2 | 76.6 | 83.6 |
| MMA + 2 % ZnO | 74.2 | 85 | 84.4 | 81.6 |
| MMA + 5 % ZnO | 71.2 | 60 | 75.2 | 83.8 |
| BA | 67.4 | 78.2 | 73.2 | 79 |
| BA + 1 % ZnO | 67.8 | 80 | 83.2 | 78.2 |
| BA + 2 % ZnO | 75 | 81.6 | 79.4 | 75.2 |
| BA + 5 % ZnO | 71.6 | 76.8 | 69.6 | 70.4 |
| AEH + 1 % BEN | 67.8 | 76.6 | 76.2 | 78.6 |
| AEH + 2 % BEN | 68.6 | 74 | 79.4 | 77.6 |
| AEH + 5 % BEN | 71 | 77 | 78 | 76.2 |
| MMA + 1 % BEN | 74.6 | 84.2 | 86 | 84.4 |
| MMA + 2 % BEN | 79.6 | 82.6 | 84.6 | 83.6 |
| MMA + 5 % BEN | 76.4 | 83.2 | 83.8 | 85.8 |
| BA + 1 % BEN | 76.8 | 78.8 | 78.8 | 82.6 |
| BA + 2 % BEN | 75 | 77 | 80.2 | 83.6 |
| BA + 5 % BEN | 73 | 75.6 | 80.8 | 81.8 |
Swellability
The values of the swelling coefficients (B) are shown in Tables 4 and 5.
Swelling coefficients of ZnO-containing composites in water.
| Week/composite | AEH | MMA | BA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 % | 1 % ZnO | 2 % ZnO | 5 % ZnO | 0 % | 1 % ZnO | 2 % ZnO | 5 % ZnO | 0 % | 1 % ZnO | 2 % ZnO | 5 % ZnO | |
| 1 | 1.21 % | 1.11 % | 1.39 % | 1.15 % | 0.98 % | 1.07 % | 0.90 % | 1.14 % | 1.09 % | 1.02 % | 1.06 % | 1.01 % |
| 2 | 1.12 % | 1.30 % | 1.48 % | 1.20 % | 1.34 % | 1.32 % | 1.62 % | 1.78 % | 1.81 % | 1.66 % | 1.36 % | 1.31 % |
| 3 | 0.78 % | 1.20 % | 1.44 % | 1.44 % | 1.19 % | 1.40 % | 1.56 % | 1.61 % | 1.71 % | 1.46 % | 1.29 % | 1.50 % |
| 4 | 0.65 % | 1.37 % | 1.40 % | 1.64 % | 1.29 % | 1.28 % | 1.48 % | 1.40 % | 1.49 % | 1.36 % | 1.29 % | 1.25 % |
| 5 | 0.94 % | 1.23 % | 1.14 % | 1.79 % | 1.59 % | 1.46 % | 1.75 % | 1.54 % | 1.55 % | 1.56 % | 1.59 % | 1.25 % |
| 6 | 0.87 % | 0.99 % | 1.64 % | 1.35 % | 1.05 % | 1.22 % | 1.35 % | 1.04 % | 1.44 % | 1.44 % | 1.63 % | 1.21 % |
| 7 | 0.87 % | 1.04 % | 1.22 % | 1.39 % | 0.93 % | 1.10 % | 1.39 % | 0.97 % | 0.95 % | 1.24 % | 1.56 % | 1.11 % |
| 8 | 0.82 % | 0.97 % | 1.22 % | 1.13 % | 1.12 % | 0.93 % | 1.12 % | 1.09 % | 1.09 % | 1.14 % | 1.02 % | 1.40 % |
| 9 | 0.72 % | 0.78 % | 0.89 % | 0.76 % | 1.02 % | 0.96 % | 1.04 % | 0.80 % | 1.14 % | 0.98 % | 0.98 % | 1.11 % |
| 10 | 1.11 % | 1.18 % | 1.46 % | 1.46 % | 1.11 % | 1.33 % | 1.53 % | 1.20 % | 1.30 % | 1.34 % | 1.25 % | 1.31 % |
| 11 | 0.79 % | 1.36 % | 1.78 % | 1.04 % | 0.95 % | 1.16 % | 1.45 % | 1.11 % | 1.19 % | 1.16 % | 1.03 % | 1.18 % |
| 12 | 0.94 % | 1.15 % | 1.72 % | 1.20 % | 1.09 % | 1.10 % | 1.39 % | 1.60 % | 1.19 % | 1.46 % | 1.23 % | 1.26 % |
| 16 | 1.33 % | 1.96 % | 1.89 % | 1.33 % | 1.11 % | 1.12 % | 2.00 % | 1.72 % | 1.94 % | 2.44 % | 1.88 % | 2.89 % |
| 20 | 1.79 % | 1.32 % | 1.35 % | 1.83 % | 1.41 % | 1.52 % | 2.41 % | 1.89 % | 1.68 % | 1.88 % | 1.90 % | 1.76 % |
| 24 | 0.96 % | 1.06 % | 1.57 % | 1.81 % | 1.29 % | 1.66 % | 1.28 % | 1.24 % | 1.30 % | 1.95 % | 1.34 % | 1.36 % |
Swelling coefficients of BEN-containing composites in water.
| Week/composite | AEH | MMA | BA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 % | 1 % BEN | 2 % BEN | 5 % BEN | 0 % | 1 % BEN | 2 % BEN | 5 % BEN | 0 % | 1 % BEN | 2 % BEN | 5 % BEN | |
| 1 | 1.21 % | 0.54 % | 1.27 % | 1.43 % | 0.98 % | 1.12 % | 1.05 % | 1.33 % | 1.09 % | 1.39 % | 1.74 % | 1.86 % |
| 2 | 1.12 % | 1.22 % | 1.19 % | 1.24 % | 1.34 % | 1.21 % | 1.11 % | 1.25 % | 1.81 % | 1.18 % | 1.67 % | 1.45 % |
| 3 | 0.78 % | 1.24 % | 3.00 % | 2.02 % | 1.19 % | 0.89 % | 1.00 % | 0.86 % | 1.71 % | 0.85 % | 1.71 % | 1.30 % |
| 4 | 0.65 % | 1.33% | 0.74 % | 0.94 % | 1.29 % | 1.15 % | 1.00 % | 1.22 % | 1.49 % | 1.11 % | 2.43 % | 1.27 % |
| 5 | 0.94 % | 1.12 % | 1.13 % | 1.36 % | 1.59 % | 1.08 % | 1.34 % | 1.19 % | 1.55 % | 1.25 % | 1.33 % | 1.27 % |
| 6 | 0.87 % | 0.79 % | 0.86 % | 1.10 % | 1.05 % | 0.86 % | 1.09 % | 1.00 % | 1.44 % | 1.03 % | 1.33 % | 0.77 % |
| 7 | 0.87 % | 0.91 % | 0.56 % | 0.87 % | 0.93 % | 0.90 % | 1.09 % | 1.68 % | 0.95 % | 0.91 % | 1.42 % | 1.14 % |
| 8 | 0.82 % | 0.86 % | 0.81 % | 1.38 % | 1.12 % | 1.20 % | 1.26 % | 1.03 % | 1.09 % | 1.11 % | 1.31 % | 1.32 % |
| 9 | 0.72 % | 1.26 % | 1.43 % | 1.84 % | 1.02 % | 1.47 % | 1.24 % | 1.91 % | 1.14 % | 1.56 % | 1.86 % | 1.37 % |
| 10 | 1.11 % | 1.14 % | 1.27 % | 1.39 % | 1.11 % | 1.30 % | 1.12 % | 1.14 % | 1.30 % | 1.11 % | 1.31 % | 1.27 % |
| 11 | 0.79 % | 1.33 % | 1.65 % | 1.75 % | 0.95 % | 1.40 % | 1.40 % | 1.32 % | 1.19 % | 1.44 % | 2.21 % | 1.52 % |
| 12 | 0.94 % | 0.98 % | 1.08 % | 1.26 % | 1.09 % | 1.37 % | 1.44 % | 1.34 % | 1.19 % | 1.48 % | 1.69 % | 1.22 % |
| 16 | 1.33 % | 1.61 % | 1.29 % | 1.58 % | 1.11 % | 1.74 % | 1.38 % | 1.81 % | 1.94 % | 1.96 % | 2.38 % | 1.87 % |
| 20 | 1.79 % | 1.16 % | 1.32 % | 1.40 % | 1.41 % | 1.17 % | 1.05 % | 1.77 % | 1.68 % | 1.15 % | 1.63 % | 1.46 % |
| 24 | 0.96 % | 1.26 % | 1.59 % | 1.51 % | 1.29 % | 1.32 % | 1.23 % | 1.33 % | 1.30 % | 1.26 % | 1.77 % | 1.82 % |
In the case of swelling in water, no large changes in the swelling coefficient were observed. The highest ratio was recorded for BA + 5 % ZnO (2.89 %) and AEH + 2 % BEN (3.00 %). The lowest swelling coefficients were obtained for AEH (0.65 %) and AEH + 1 % BEN (0.54 %) (Table 4). This suggests that the 1 % addition of benzethonium chloride reduces water penetration into the composite structure the most. Swelling coefficients of less than 3 % indicate that the composites are limited in their ability to absorb water into their structure. This has positive implications for their potential use. The penetration of solvents into the composite structure will limit their mechanical strength, which will contribute directly to their durability. Composites with benzethonium chloride generally have lower swelling ratios than composites containing zinc oxide. This means that, concerning degradation in water, composites containing BEN will be more durable.
Swelling tests were conducted in the presence of acetone (Tables S1 and S2). Samples containing zinc oxide degraded in week 3 of testing, and samples with benzethonium chloride degraded in week 1 of testing. Swelling coefficients for ZnO ranged from 8.3 % to 36.45 %, indicating that the composites absorbed acetone. For the composites with BEN, B% takes on values ranging from 11.22 % to 23.23 %. The action of acetone on the surface of the composite resulted in complete degradation of the composite, so the study was stopped early. It can be concluded that these composites should not be used for surfaces exposed to acetone, as the coating will be damaged in a very short time.
In the presence of sodium hydroxide (Tables S3 and S4), the highest swelling coefficients were determined for AEH + 5 % ZnO (383.79 %) and AEH + 2 % BEN (6.16 %). The lowest swelling coefficients were obtained for AEH + 5 % ZnO (0.90 %) and MMA + 5 % ZnO (0.32 %). It can be seen that low swelling coefficients persist for composites containing no zinc oxide additive throughout the test cycle. A similar relationship is observed for materials with 1 % ZnO addition, but here the swelling rates in the following weeks are generally higher than for composites without the additive. Materials containing a 2 % ZnO additive are durable up to test week 8 (AEH + 2 % ZnO), week 11 (MMA + 2 % ZnO), and week 3 (BA + 2 % ZnO). This is when the swelling ratios take on values of less than 10 %. Hence, it can be seen that the composite containing MMA as an active diluent shows the highest durability, while the composite with butyl acrylate is the least durable. Concerning the composites with the highest, i.e. 5 % ZnO addition, the composite containing AEH shows the lowest durability (at week 3 the B% value is significantly higher than 10 %), while the composite with BA shows higher durability (at week 6 of the tests the swelling factor value increases dramatically). Referring to the swelling coefficients of materials containing benzethonium chloride as filler, it can be seen that the values remain below 10 % throughout the test cycle. This confirms that the addition of benzethonium chloride reduces solvent absorption. Therefore, it can be concluded that the use of composites containing benzethonium chloride as a coating for surfaces exposed to NaOH will be significantly better than composites with ZnO. The results indicate that exposing ZnO-containing composites to prolonged contact with sodium hydroxide causes the breakdown of the internal bonds and thus contributes to the degradation of the composites. Composites containing ZnO are much more susceptible to swelling in sodium hydroxide.
Swelling tests in the presence of hydrochloric acid were also carried out. The highest swelling coefficients were observed for MMA + 5 % ZnO (2.88 %) and BA + 1 %BEN (36.76 %). The lowest swelling coefficients were determined for AEH (0.47 %). All swelling coefficient values assume less than 10 % (BA + 1 % BEN is the exception). This means that hydrochloric acid is not absorbed by the composite. This allows it to retain its good service properties even when in prolonged contact with the solvent. Lower values were observed for composites containing ZnO, which means that they would be better materials for coating surfaces exposed to hydrochloric acid.
Aging study
ATR/FT-IR
Using the ATR/FT-IR method, spectra of the composites were taken before and after the aging process (Figs. 5–13). In this way, their structure and changes that occurred during aging were analyzed. On each of the spectra, a signal at 3450 cm−1 can be observed from the stretching vibration of the hydroxyl group. The signal corresponding to a wavelength of 2946 cm−1 comes from the stretching vibrations of the –CH group. The bending vibration of the –CH group is located at 1459 cm−1. The band at 1758 cm−1 is from the vibration of the carbonyl group C=O.

The ATR/FT-IR spectra of AEH composite before and after the aging test.

The ATR/FT-IR spectra of AEH + 5 % ZnO composite before and after the aging test.

The ATR/FT-IR spectra of AEH + 5 % BEN composite before and after the aging test.

AEH composites before and after aging in acetone.

MMA composites before and after aging in acetone.

BA composites before and after aging in acetone.

AEH composites before and after aging in sodium hydroxide.

Results obtained in the modified disk-diffusion method for AEH composites.
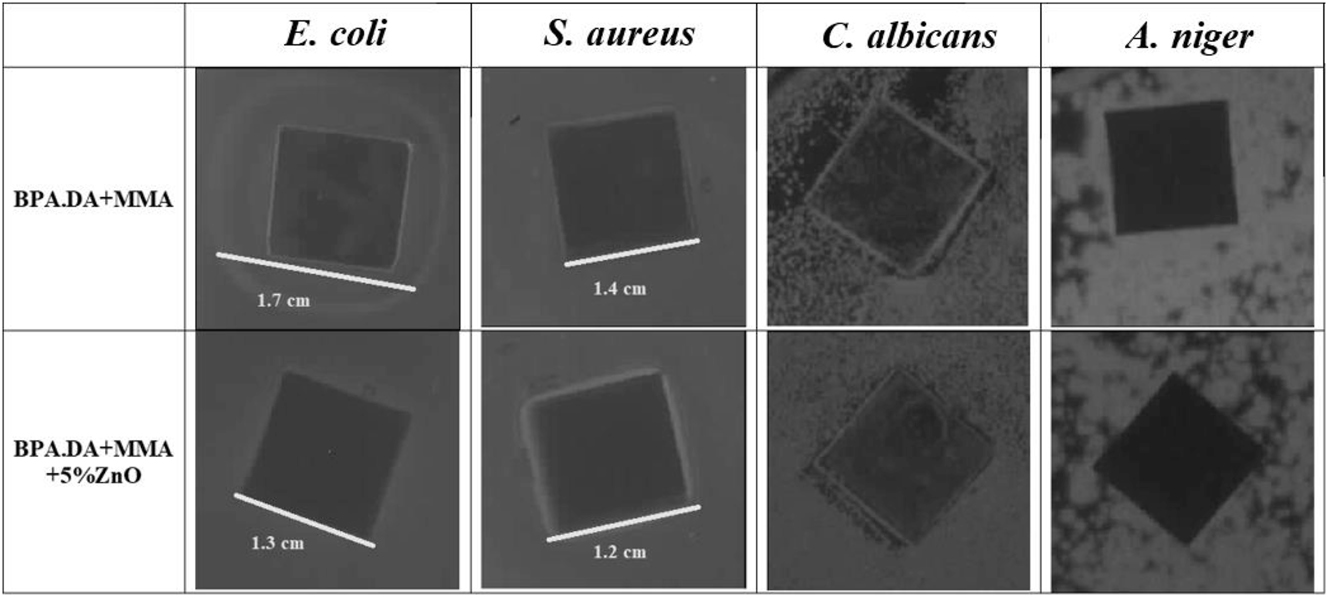
Results a obtained in the modified disk-diffusion method for MMA composites.
For the spectra of BPA.DA + AEH and BPA.DA + AEH composites containing benzethonium chloride an increase in signal intensity (especially –OH and C=O) after aging in all solvents is observed. On this basis, it can be concluded that these solvents cause a partial degradation of the composites. In aqueous media, hydrolysis of ester groups can occur, while in acetone, shortening of polymer chains is most likely.
BPA.DA + MMA composites degrade in sodium hydroxide, acetone, and hydrochloric acid, which is confirmed by changes in the intensity of the bands on the ATR/FT-IR spectra (Figs. S1–S3, Supplementary Material). They do not degrade in water as the spectra show a similar pattern. Composites filled with BEN undergo degradation in all solvents. Composites with zinc oxide do not degrade in HCl and H2O. The intensity of the bands after aging of the composites in sodium hydroxide and acetone decreased.
The spectrum of the BA composite (Figs. S4–S6) shows an increase in the intensity of the bands after the aging test in NaOH, H2O and HCl. The intensity after aging in acetone decreased, so it can be concluded that the polymer has partially degraded. On the spectrum of the BA + 5 % BEN composite, the intensity of the bands after the aging test decreased, which allows us to conclude that the addition of BEN decreases the durability of the composite and makes it less resistant to solvent-induced chemical degradation. For the BA + 5 % ZnO composite. it was observed that the effect of NaOH and acetone causes partial degradation of the polymer. An increase in the signal intensity of the –OH group was observed, which may indicate that hydrolysis is taking place.
No visible changes in appearance were observed in composites containing BEN and ZnO that were placed in hydrochloric acid and water, and the same situation was observed for composites containing BEN that were placed in sodium hydroxide. In the case of composites containing BEN in acetone, the tests were discontinued after one week (with the addition of ZnO after 3 weeks) because complete degradation of the samples occurred. These composites are not suitable for use in the presence of acetone. Figures 8–10 show the changes in the appearance of composites containing benzethonium chloride before and during the aging process in acetone.
Figure 11 shows composites containing zinc oxide as filler, which was placed in sodium hydroxide during the aging process. The study was stopped after 16 weeks due to the complete degradation of the AEH + 5 % ZnO composite. The first changes in the appearance of this composite were observed in week 3 of the study. Its surface started to become slimy and slippery. In each week, the composite decreased in weight. For the AEH + 2 % ZnO composite, changes in appearance were observed in week 12. As above, the composite began to have a slippery surface and lose mass. No significant changes in appearance were observed in the other samples, and for this reason, control images were presented initially after 6, 12, and 16 weeks to compare the de-activation of NaOH on the composites. For the both BEN- and ZnO-containing composites, no changes in external appearance were observed during aging in water and hydrochloric acid.
Determination of antimicrobial activity
It is known that a number of chemical substances may exhibit antimicrobial activity. On the other hand, bacteria and fungi are capable of creating natural antimicrobial resistance, which is why new substances with antimicrobial properties are being sought. For the composite containing AEH + 5 % ZnO, a zone of inhibition was observed for the bacterial strains used. For E. coli, the zone is larger (0.1 cm) for the composite that contained zinc, while for S. aureus the zone of inhibition is larger (0.4 cm) for the composition without the filler. For fungi, a visible effect was observed for A. niger. For the composite containing zinc, the zone of inhibition is visible for all microorganisms (Fig. 12). It can be observed that bacterial growth is the most inhibited when in contact with the composite material. No zones of bacterial growth are visible where the culture plate that was in contact with the composite. The situation is different for C. albicans, where fungal growth were visible under the surface of the both composites.
In the case of BPA.DA + MMA and BPA.DA + MMA + 5 % ZnO the antimicrobial activity against E. coli, S. aureus and A. niger was observed. The growth of C. albicans, was not completely inhibited. The zone of inhibition is larger (0.4 cm for E. coli and 0.2 cm for S. aureus) for the composite without zinc (MMA), which may indicate that the monomers show antimicrobial activity (Fig. 13). Similar to AEH-based composites, materials with MMA are capable of inhibiting microbial growth on contact. For all micro-organisms except C. albicans on the surface on which the composite plate was placed, no colony growth was observed. Zones of growth inhibition located outside the area where the tile was located may be due to diffusion of components that were not permanently bound in the composite.
For E. coli and S. aureus, composites containing butyl acrylate and 5 % zinc oxide have a greater zone of inhibition than composites without filler. The differences are 0.05–0.1 cm. With regard to the fungi tested, for the both C. albicans and A. niger, the composites without and with the special additive showed an antimicrobial effect only in contact with the microorganism (Fig. 14).

Results a obtained in the modified disk-diffusion method for BA composites.
It is difficult to find information relating to the modification of the diffusion-disc method for this type of material, as it is new. However, tests were carried out on paper discs impregnated with ZnO nanoparticles. They obtained results for different concentrations, but the zones of inhibition ranged from 10 to 16 mm [26].
Investigation of the influence of composites on selected biochemical parameters in secretome of white rot fungus C. unicolor
In order to study the biological activity of the tested composites, they were introduced into the cultures of a biotechnologically important fungus with a broad biodegradation potential, C. unicolor. The analysis of chosen biochemical parameters in the fungal secretome showed that the most significant difference between protein concentrations was observed for composites containing AEH as the active diluent. The addition of 5 % zinc oxide almost doubled the protein concentration (a difference of 10.9 μg/mL). For the composite containing MMA (16.8 μg/mL), 5 % zinc oxide addition increased the protein concentration to 18.2 μg/mL. In contrast, 5 % zinc oxide addition in composites with BA decreased the protein concentration by 3.4 μg/mL compared to the composite without filler addition. It can be seen that the protein concentration depended on the type of monomer used. MMA had the best effect, as both composites containing this diluent increased the protein concentration relative to the control sample by 4.2 μg/mL for BPA.DA + MMA and by 5.6 μg/mL for BPA.DA + MMA + 5 % ZnO (Fig. 15). The changes of protein concentration showed the response of the fungal cells to changes in external factors, such as the appearance of stress substances. The addition of composites increased the production of extracellular proteins. Similar results were obtained for composites containing modified lignin [27]. Changes resulting in increased protein production were also observed when factors such as light stress, starvation or oxidative stress were applied [28, 29].

Determination of protein concentration.
All composites stimulated laccase activity in the investigated cultures. The smallest differences were detected for the BPA.DA + AEH composite (2221.5 nkat/L) because the value of the laccase activity is practically the same as for the control sample (2205.6 nkat/L). For composites containing AEH and MMA, the addition of zinc oxide increases the laccase activity compared to the composite without filler. For AEH, the difference between the composites is 630.2 nkat/L, while for MMA it is 236.7 nkat/L. The composite with butyl acrylate and zinc oxide reduces the laccase activity by 215.5 nkat/L compared to the composite containing butyl acrylate only. It was observed that the introduction of modified polymers into C. unicolor cultures resulted in an increase in extracellular laccase activity. This may indicate that the fungal cells can use the composite components as effective inducers. This may also indicate that composites can be degraded by such fungi (Fig. 16). Similar results in which laccase activity was stimulated were obtained for lignin-modified composites [27]. Attempts have also been made to add industrial wastes to fungal cultures. These include wheat and rice, wheat and rice straw, wheat bran or maize cobs. For these materials, the stimulation of laccase activity was also observed [30, 31].

Determination of laccase activity.
Another step of investigations was the study of changes in phenolic substances in the tested mushroom cultures under the influence of composites. Compounds from this group are important elements of the metabolism of wood-degrading fungi, they also show antioxidant properties. It was detected that the presence of composites reduced the content of phenolic compounds in culture fluids, this is most evident for 2-ethylhexyl acrylate (reduction by 0.028 µM for the composite without ZnO and by 0.004 µM for BPA.DA + AEH + 5 % ZnO) and butyl acrylate (0.018 µM for BPA.DA + BA and 0.001 µM for BPA.DA + BA + 5 % ZnO). The composite with methyl methacrylate and the addition of zinc oxide (by 0.012 µM) reduces the phenolic compounds more than the composite without zinc (by 0.003 µM). C. unicolor has the ability to produce bioactive metabolites, among which are polysaccharides, low molecular weight compounds and polysaccharide-protein compounds. Among the metabolites, phenolic compounds, which possess specific medicinal and antioxidant properties, occupy a very important place. The addition of composites to the culture medium reduced the level of extracellular phenolic compounds. This may mean that the components of the composites restrict the production of phenolic compounds by C. unicolor (Fig. 17). In the results obtained by [27]; composites containing lignin increased the content of phenolic compounds in solution.

The level of phenolic compounds.
The presence of the composites increased the relative level of free radicals in solution, which is most evident in composites 3 (251.5 % increase relative to the control) and 5 (369.9 % increase relative to the control sample). Composite 2 has a 58.9 % greater effect on the presence of free radicals than composite 1 (Fig. 18). Free radicals are formed as a result of biochemical reactions taking place in cells. They are a by-product that, in excess, can damage the body’s cells. They can cause the oxidative stress phenomenon and cause inflammation. It is important to keep free radical levels relatively low. A study showed that in C. unicolor cultures, all composites stimulated the formation of free radicals, which means that these materials stimulate biochemical reactions of fungal cells. Similar conclusions were reached by Romero-Freire et al. [32], Garcia-Gomez et al. [33], who confirm that the presence of ZnO stimulates free radical production. The obtained results confirm in this case the oxidative stress-inducing effect of composites, however, from the point of view of stimulation, e.g. the activity of selected enzymes such as laccase, the inducing effect of oxidative stress has been previously confirmed [18, 34].

The relative level of free radicals.
The addition of composites containing zinc ions (composites 2 and 4) more effectively stimulated the antioxidant properties of the fungal samples tested relative to the control. For composite 2, an increment of 14.5 % was obtained relative to the control, while for composite 4 the increment was 12.8 %. Composite 1 increased the free radical scavenging capacity by 6.1 % and composite 3 by 5.0 %. For butyl acrylate composites, the presence of zinc reduces the radical scavenging properties. A decrease of 2.2 % was observed for composite 5 and 5.0 % for composite 6 relative to the control (Fig. 19). Antioxidant properties are of great importance as they aim to inactivate free radicals, which, as mentioned earlier, can interfere with basic metabolic reactions and also have deleterious effects. In the case of the fungal culture conducted for C. unicolor, the addition of composites to the culture medium increased the free radical scavenging properties compared to the control sample. Similar results were obtained in a study by [35]. indicate that the addition of zinc oxide increases scavenging capacity.

Antioxidant properties.
Conclusions
It was observed that the compositions consisting of bisphenol A glycerolate diacrylate (main crosslinking monomer), active diluents (2-ethylhexyl acrylate, methyl methacrylate, and butyl acrylate) by using UV-polymerization initiator were successfully obtained. To introduce antimicrobial properties special additives in the form of zinc oxide (ZnO) or benzethonium chloride (BEN) were added.
Aging tests of the obtained materials in water, acetone, 1 M solution of sodium hydroxide, and hydrochloric acid for 16 weeks were carried out. For composites containing BEN and ZnO placed in solutions: hydrochloric acid and sodium hydroxide as well as water, no visible changes on the surface of the composite were observed. The composites were unsuitable for use in the presence of acetone. DSC studies have shown that the addition of zinc oxide decreases the decomposition temperature. No clear relationship was observed between the effect of solvent and the hardness of composites containing ZnO as filler. For composites with benzethonium chloride, the obtained values after the aging process were higher.
The composites inhibited the growth of E. coli, S. aureus, and A. niger. They had a weak effect on inhibiting the growth of C. albicans. The composites induced the antioxidative potential of the C. unicolor culture fluid (the best effect was observed in the case of composites with 5 % zinc oxide). The presence of composites increased the level of free radicals in the solution, which was the most evident with BPA.DA + MMA and BPA.DA + BA. The BPA.DA + AEH + 5 % ZnO composite has a greater effect on the presence of free radicals than the BPA.DA + AEH composite. The addition of the composite to the culture medium with the fungus C. unicolor increased the protein concentration. This demonstrated the response of the fungus cells to changes in external factors, such as the appearance of stress substances. Composites with zinc and methyl methacrylate and 2-ethylhexyl acrylate stimulated the activity of laccase better than the other composites. Summary, it can be concluded that the tested composites, apart from their antimicrobial properties, can probably also be used as effective factors modifying the metabolism of microorganisms important from the biotechnological point of view.
References
[1] S. Doppalapudi, S. Katiyar, A. J. Domb, W. Khan. Expet Opin. Drug Deliv. 12, 45 (2019).Search in Google Scholar
[2] R. Naseem, C. Tzivelekis, M. J. German, P. Gentile, A. M. Ferreira, K. Dalgarno. Molecules 26, 992 (2021), https://doi.org/10.3390/molecules26040992.Search in Google Scholar PubMed PubMed Central
[3] C. H. Park, K. M. Woo. Adv. Exp. Med. Biol. 25, 255 (2018).Search in Google Scholar
[4] M. Ward. Mechanical and Structural Performance of Melt-Processable Bioresorbable Engineering Nanocomposites, University Nottingham, Nottingham (2018).Search in Google Scholar
[5] A. Aravamudhan, D. M. Ramos, A. A. Nada, S. G. Kumbar. Natural and Synthetic Biomedical Polymers, pp. 67–89, Elsevier, Burlington, MA, Vol. 6 (2014).10.1016/B978-0-12-396983-5.00004-1Search in Google Scholar
[6] S. G. FarahAnderson, R. Langer. Adv. Drug Deliv. Rev. 107, 367 (2016), https://doi.org/10.1016/j.addr.2016.06.012.Search in Google Scholar PubMed
[7] D. P. Gamliel, S. Du, G. M. Bollas, J. A. Valla. Bioresour. Technol. 119, 187 (2015), https://doi.org/10.1016/j.biortech.2015.04.129.Search in Google Scholar PubMed
[8] A. Badura, A. Krysiński, J. Nowaczyk, A. Buciński. J. Appl. Microbiol. 130, 40 (2021), https://doi.org/10.1111/jam.14763.Search in Google Scholar PubMed
[9] S. Imazato. Dent. Mat. 11, 449 (2003), https://doi.org/10.1016/s0109-5641(02)00102-1.Search in Google Scholar PubMed
[10] D. R. Monteiro, L. F. Gorup, A. S. Takamiya, A. C. Ruvollo-Fihlo, E. Rodrigues de Camargo, D. B. Barbosa. Int. J. Antimicrob. Agents 2, 103 (2009), https://doi.org/10.1016/j.ijantimicag.2009.01.017.Search in Google Scholar PubMed
[11] E. Dimapilis, C. Hsu, R. Mendoza, M. Lu. Sust. Environ. Res. 28, 47 (2017), https://doi.org/10.1016/j.serj.2017.10.001.Search in Google Scholar
[12] X. L. Guo, H. Tabata, T Kawai. J. Cryst. Growth 237, 544 (2002), https://doi.org/10.1016/s0022-0248(01)01974-1.Search in Google Scholar
[13] Y. Liu, J. Zhou, A. Larbot, M. Persin. J. Mater. Process. Technol. 189, 379 (2007), https://doi.org/10.1016/j.jmatprotec.2007.02.007.Search in Google Scholar
[14] H. Dao, C. Fricker, S. Nedorost. Dermatitis 23, 162 (2012), https://doi.org/10.1097/der.0b013e318260d78d.Search in Google Scholar PubMed
[15] J. Y. Maillard. J. Appl. Microbiol. 133, 3322 (2022), https://doi.org/10.1111/jam.15739.Search in Google Scholar PubMed PubMed Central
[16] G. Takeoka, L. Dao, R. Y. Wong, R. Lundin, N. Mahoney. J. Agric. Food Chem. 49, 3316 (2001), https://doi.org/10.1021/jf010222w.Search in Google Scholar PubMed
[17] S. Türker, F. Yarza, R. M. Torres Sánchez, S. Yapar. Colloids Surf., A 520, 817 (2017), https://doi.org/10.1016/j.colsurfa.2017.02.019.Search in Google Scholar
[18] M. Jaszek, M. Osińska-Jaroszuk, G. Janusz, A. Matuszewska, D. Stefaniuk, J. Sulej, J. Polak, M. Ruminowicz, K. Grzywnowicz, A. Jarosz-Wilkołazka. BioMed Res. Int. 2013, 1 (2013).10.1155/2013/497492Search in Google Scholar PubMed PubMed Central
[19] R. Łyszczek, B. Podkościelna, A. Lipke, A. Ostasz, A. Puszka. J. Therm. Anal. Calor. 138, 4463 (2019), https://doi.org/10.1007/s10973-019-08914-1.Search in Google Scholar
[20] B. Podkościelna. J. Therm. Anal. Calor. 116, 785 (2014), https://doi.org/10.1007/s10973-013-3602-5.Search in Google Scholar
[21] M. Bradford. Anal. Biochem. 72, 248 (1976), https://doi.org/10.1016/0003-2697(76)90527-3.Search in Google Scholar
[22] A. Leonowicz, K. Grzywnowicz. Enzyme. Microb. Technol. 3, 55 (1981), https://doi.org/10.1016/0141-0229(81)90036-3.Search in Google Scholar
[23] E. Malarczyk. Acta Microbiol. Pol. 38, 45 (1989).Search in Google Scholar
[24] M. Paździoch-Czochra, E. Malarczyk, J. Sielewiesiuk. Cell Biol. Int. 27, 325 (2003), https://doi.org/10.1016/s1065-6995(02)00282-2.Search in Google Scholar PubMed
[25] M. Ozgen, R. Neil Reese, A. J. Tulio, J. C. Scheerens, A. Raymond Miller. J. Agric. Food Chem. 4, 1151 (2006), https://doi.org/10.1021/jf051960d.Search in Google Scholar PubMed
[26] A. Gunalan, R. Sivaraj, V. Rajendran. Prog. Nat. Sci. Mater. Int. 22, 693 (2012).10.1016/j.pnsc.2012.11.015Search in Google Scholar
[27] B. Podkościelna, A. Matuszewska, D. Stefaniuk, M. Ruminowicz-Stefaniuk, B. Ciołek, M. Jaszek. Ind. Crops Prod. 186, 115 (2022), https://doi.org/10.1016/j.indcrop.2022.115125.Search in Google Scholar
[28] M. Jaszek, K. Kos, A. Matuszewska, M. Grąz, D. Stefaniuk, M. Osińska-Jaroszuk, M. Prendecka, E. Jóźwik, K. Grzywnowicz. Appl. Biochem. Biotechnol. 174, 644 (2014), https://doi.org/10.1007/s12010-014-1064-2.Search in Google Scholar PubMed PubMed Central
[29] A. Pawlik, M. Jaszek, D. Stefaniuk, U. Swiderska-Burek, A. Mazur, J. Wielbo, P. Koper, K. Zebracki, G. Janusz. Int. J. Mol. Sci. 21, 1678 (2020), https://doi.org/10.3390/ijms21051678.Search in Google Scholar PubMed PubMed Central
[30] M. Jaszek, J. Miłek, J. Zuchowski, D. Stefaniuk, M. Prendecka. Acta Biochim. Pol. 63, 549 (2016), https://doi.org/10.18388/abp.2015_1227.Search in Google Scholar PubMed
[31] Y. P. Teoh, M. D. Mashitah. J. Appl. Sci. 10, 1036 (2010), https://doi.org/10.3923/jas.2010.1036.1043.Search in Google Scholar
[32] A. Romero-Freire, S. Lofts, F. J. Martin Peinado, C. A. M. van Gestel. Environ. Toxicol. 36, 137 (2016).10.1002/etc.3512Search in Google Scholar PubMed
[33] C. García-Gómez, M. Babin, A. Obrador, J. M. Álvarez, M. D. Fernández. Environ. Sci. Pollut. Res. 22, 16803 (2015).10.1007/s11356-015-4867-ySearch in Google Scholar PubMed
[34] M. Mizerska-Dudka, M. Jaszek, A. Błachowicz, T. P. Rejczak, A. Matuszewska, M. Osińska-Jaroszuk, D. Stefaniuk, G. Janusz, J. Sulej, M. Kandefer-Szerszeń. Int. J. Biol. Macromol. 79, 459 (2015), https://doi.org/10.1016/j.ijbiomac.2015.05.015.Search in Google Scholar PubMed
[35] H. A. Rudayni, M. H. Shemy, M. Aladwani, L. M. Alneghery, G. M. Abu-Taweel, A. A. Allam, M. R. Abukhadra, S. Bellucci. J. Funct. Biomater. 14, 198 (2023), https://doi.org/10.3390/jfb14040198.Search in Google Scholar PubMed PubMed Central
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/pac-2023-0109).
© 2023 IUPAC & De Gruyter. This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. For more information, please visit: http://creativecommons.org/licenses/by-nc-nd/4.0/
Articles in the same Issue
- Frontmatter
- In this issue
- Preface
- Preface for special issue of ICGC-9 in Athens, Greece
- Conference papers
- HOME-Chemistry: hydrazone as organo-metallic equivalent
- Towards lignin valorization: lignin as a UV-protective bio-additive for polymer coatings
- How the physio-chemical properties of char from the pyrolysis of Automotive Shredder Residue (ASR) influences its future uses
- Plant based fabrication of CuO/NiO nanocomposite: a green approach for low-level quantification of vanillin in food samples
- Synthesis and characterization of new polycarbonates free of bisphenol A components (BPA-free) based on dimethyl/diphenyl carbonate and diphenylmethane derivative
- Adsorption capacity of biocarbons from residue of supercritical extraction of raw plants
- Cd(II) and As(V) removal from the multicomponent solutions in the presence of ionic polymers using carbonaceous adsorbents obtained from herbs
- Synthesis and thermal characterization of composites based on Epidian 601 with flame retardants compounds
- A green sorptive extraction method (HiSorb-TD-GC-MS) for determining the extra virgin olive oil (EVOO) aroma profile
- Synthesis, aging and antimicrobial tests of (di)acrylate composites
Articles in the same Issue
- Frontmatter
- In this issue
- Preface
- Preface for special issue of ICGC-9 in Athens, Greece
- Conference papers
- HOME-Chemistry: hydrazone as organo-metallic equivalent
- Towards lignin valorization: lignin as a UV-protective bio-additive for polymer coatings
- How the physio-chemical properties of char from the pyrolysis of Automotive Shredder Residue (ASR) influences its future uses
- Plant based fabrication of CuO/NiO nanocomposite: a green approach for low-level quantification of vanillin in food samples
- Synthesis and characterization of new polycarbonates free of bisphenol A components (BPA-free) based on dimethyl/diphenyl carbonate and diphenylmethane derivative
- Adsorption capacity of biocarbons from residue of supercritical extraction of raw plants
- Cd(II) and As(V) removal from the multicomponent solutions in the presence of ionic polymers using carbonaceous adsorbents obtained from herbs
- Synthesis and thermal characterization of composites based on Epidian 601 with flame retardants compounds
- A green sorptive extraction method (HiSorb-TD-GC-MS) for determining the extra virgin olive oil (EVOO) aroma profile
- Synthesis, aging and antimicrobial tests of (di)acrylate composites

