Polylactide-based stent coatings: biodegradable polymeric coatings capable of maintaining sustained release of the thrombolytic enzyme streptokinase
-
A. G. Kolmakov
, S. V. Gudkov
Abstract
The paper describes synthesis and testing of novel biodegradable polylactide-based polymer membranes with desired mechanical properties, which are capable of sustained and directed release of biomacromolecules with high molecular weight (in particular, streptokinase; m.w. 47 kDa). Streptokinase is a pharmaceutical agent, possessing a pronounced thrombolytic activity. The membranes synthesized had a percentage elongation of 2–11% and tensile strength of 25–85 MPa. They were biodegradable – yet being stored in aqueous media in the absence of biological objects, would be dissolved by no more than 10% in 6 months. The synthesized membranes were capable of controlled release of streptokinase into the intercellular space, with the enzyme retaining more than 90% of its initial activity. The rate of streptokinase release from the membranes varied from 0.01 to 0.04 mg/day per cm2 of membrane surface. The membrane samples tested in the work did not have any short-term toxic effects on the cells growing de novo on the membrane surface. The mitotic index of those cells was approximately 1.5%, and the number of non-viable cells on the surface of the polymer films did not exceed 3–4% of their total amount. The implantation of the synthesized polymers – as both individual films and coatings of nitinol stents – was not accompanied by any postoperative complications. The subsequent histological examination revealed no abnormalities. Two months after the implantation of polymer films, only traces of polylactide were found in the implant-surrounding tissues. The implantation of stents coated with streptokinase-containing polymers resulted in the formation of a mature and thick connective-tissue capsules. Thus, the polylactide membranes synthesized and tested in this work are biodegradable, possess the necessary mechanical properties and are capable of sustained and directed release of streptokinase macromolecules.
Introduction
The systems of controlled drug delivery (CDD systems) based on the use of biodegradable polymeric membranes are a promising and rapidly developing area of chemical technology [1]. One of their possible applications is the design of coatings for stent-type devices, which are used to aid healing and relieve obstruction of narrowed hollow organs of the human body (ducts, canals or blood vessels). The local release of medicinal agents from biodegradable coatings can solve a number of postoperative complications at the sites of stent implantation: formation of restenoses, inflammatory reactions, thrombosis etc. [2]. The postoperative complications can make it necessary to repeat surgery and replace the stent, creating more risks for the patient. Correspondingly, a lot of ongoing studies is focused on testing various materials as a prospective basis for biodegradable stent coatings suitable for local drug delivery [2]. The main advantage of CDD systems is their ability to maintain – steadily and for a long time – the required level of a pharmaceutical preparation in the tissue or biological fluid [3]. The efficiency of CDD systems is largely determined by the properties of the coating materials [4]. The materials should be non-invasive, biocompatible and biodegradable, as well as possess a number of certain physical, chemical and mechanical features. Polylactide – a polymer of lactic acid, an aliphatic polyester – is considered to be a promising material for CDD systems [5]. It can be synthesized by two methods: polycondensation of lactic acid and lactide polymerization. The industrial synthesis of polylactide often combines these two approaches. The first approach (polycondensation of lactic acid) only yields polylactide of low molecular weight. This limitation pertains to the fact that water released during polycondensation terminates the growth of polymer chains, and it is difficult to remove it from the reaction medium. The low-molecular polylactide synthesized in the reaction of polycondensation is further depolymerized to the dimer of lactic acid, lactide. Lactide, on the other hand, can then be re-polymerized at a high temperature into high-molecular polylactide [6]. High-molecular polylactide is biodegradable, biocompatible and thermoplastic. An obvious advantage of this polymer is also the fact that it is produced from renewable resources (silage crops). Polylactide has been used in medicine for the manufacture of surgical sutures [7], subcutaneous implants [8] and nail coatings [9]. The polymer has not been thoroughly tested as a material for the production of CDD systems, yet a theoretical possibility of using it for this purpose exists [10] – as does the possibility of encapsulating enzymes in polylactide matrices [11].
5wWe have developed a number of technological solutions for the production of biodegradable polylactide-based membranes and coatings with desirable mechanical properties. In this work, we describe a method for the synthesis of polylactide stent coatings that are capable of sustained release of streptokinase. Streptokinase, an antithrombotic enzyme which was first isolated from β-hemolytic streptococcus in 1933 [12], is an effective, cheap and widely available agent for thrombolytic therapy [13], [14]. The novelty of our study is the synthesis and testing of a range of biodegradable polylactide-based membranes with desirable mechanical properties (a high durability and the capability of directional release of biomacromolecules with high molecular weight).
Synthesis of polylactide membranes
The preliminary experiments showed that polylactide solutions with the concentration of the polymer 2.8–3.2% were the most promising for membrane synthesis. The solutions of 3% polylactide (Nature Work, USA) in chloroform (Irea 2000, Russia) were prepared by stirring the suspensions for 1 h at 57 °C (to a homogeneous state). To make membranes which would be infused with the molecules of streptokinase, the homogeneous 3% solution of polylactide was supplemented with the solution of streptokinase in phosphate-buffered saline (15 mM; pH = 7.4). The resulting mixture was poured into 12-ml plastic molds (85 mm in diameter). Polylactide membranes were obtained by the method of two-step solvent evaporation. The first evaporation step was conducted for 3 h at the atmospheric pressure of 50 Torr; the second, for 5 h at 3 Torr.
Examination of mechanical properties of polylactide films
The tensile strength of polylactide films (pure and drug-infused) was assessed on a universal testing machine INSTRON 3382 at the loading rate of 10 mm/min. Film samples (100 µm thick; in the shape of a double spade) were fixed in the grips of the testing machine, which were tightened evenly to ensure that samples did not slip during tests. The morphology of the sample surface was examined using a scanning electron microscope (SEM) TESCAN VEGA II SBU (TESCAN, Czech Republic).
Assay for streptokinase fibrinolytic activity
The fibrinolytic activity of streptokinase was assessed by its ability to lyse a bovine blood clot according to Christensen’s method [15]. The stock enzyme solution was diluted and sampled into assay tubes, followed by the addition of fibrinogen and thrombin. When streptokinase was used as an activating agent for plasminogen, 1000 units of the enzyme were added to the assay tubes prior to the addition of thrombin. A fibrisolytic unit was defined as the least amount of the enzyme necessary to lyse a blood clot in 30 min under the test conditions.
Release of streptokinase from polylactide films and dissolution of the polymer
The optical spectra of polylactide and streptokinase aqueous solutions differ substantially. This fact was used as a basis for the spectroscopic monitoring of changes in the streptokinase and polylactide concentrations in aqueous systems. The changes were comparatively monitored in the systems of “enzyme + polymer + water” vs. “polymer + water” and “polymer + water” versus “water” [16]. Absorbance of the solutions was measured using a spectroscopic system Ocean Optics USB 2000. Streptokinase absorbance was measured at 280 nm; it varied in the range of 0.1–2.5, with the mass absorption coefficient of the enzyme being equal to 0.19 L/g. In addition to being measured spectroscopically, the dissolution of polylactide was also assessed by weighing the dry residue after evaporation of samples. The measurements of streptokinase release were accompanied by the measurements of the enzyme activity.
Cell culture studies
The studies of the polymer biocompatibility were performed using standard in vitro test systems. A culture of human neuroblastoma cells (SH-SY5Y) was used as a standard experimental model. The SH-SY5Y cell line was subcloned from the line SK-N-SH, which was isolated from the bone marrow material of a 4-year old neuroblastoma patient [17]. The SH-SY5Y cell line is a classical model for neuronal functions and differentiation in vitro. In tissue cultures, SH-SY5Y cells usually develop in two different ways, which makes them useful in the study of cell differentiation. Additionally, cells of the SH-SY5Y line are interesting by their ability to form not only monolayers, but also aggregates, able to adhere to the substrate. Moreover, when growing in vitro, SH-SY5Y cells can spontaneously switch between two phenotypes: neuroblastomic and epithelial [18].
SH-SY5Y cells were cultivated in a DMEM medium (Biolot, Russia) supplemented with 10% fetal calf serum (Gibco, USA) and 30 ug/mL gentamicin in a CO2 incubator (Binder, Germany) at 37 °C and 5% CO2. Samples of polylactide films (20 × 20 mm) were placed in 35-mm Petri dishes (1 sample per dish), followed by inoculation with SH-SY5Y cells (104 cells/cm2; 3 mL per dish). After cultivation for 3 days, the cells growing on the surface of the polymer samples were stained with the fluorescent dyes Hoechst 33342 (Sigma, USA; 2 ug/mL) and propidium iodide (Sigma, USA; 2 ug/mL). Hoechst 33342 stains both alive and dead cells. Propidium iodide practically does not stain alive cells within the time frame of the staining procedure (about 10 min); it only penetrates into the cells whose plasma membrane is damaged, which is a characteristic feature of dead cells. After staining the polymer samples, the number of alive and dead cells on their surface was counted using an imaging system Leica DMI6000 (Leica, Germany). At least 500 cells in total was counted per each sample [19].
The number of cells undergoing mitosis was counted under a fluorescence microscope using the technique of vital staining with Hoechst 33342 (Sigma, USA). Mitotic cells were identified by the distribution of chromatin characteristic of prophase (P), metaphase (M), anaphase (A) and telophase (T). At least 500 cells in total was counted per each sample. The mitotic index (MI) was calculated using the formula MI = (P + M + A + T)/N × 100%, where (P + M + A + T) is the number of cells at different mitotic phases and N is the total number of analyzed cells [20].
Experimental animal studies
In the experiments, male Wistar rats (180–200 g) were used . The animals were kept under standard conditions (temperature, 22 ± 2 °C; humidity, 30–70%; 12-h light period; feed and water ad libitum). All animal manipulations were approved by the Bioethics Committee of the Institute of Theoretical and Experimental Biophysics of the Russian Academy of Sciences. At least five animals were used to study each material sample.
Implantation tests
For the assessment of biocompatibility of polylactide films, a rat model of subcutaneous biomaterial implantation was used [21]. During implantation, we used polylactide films with a molecular weight of 45, 90, and 180 kDa containing 1 or 2% streptokinase. In tests on cell cultures, polylactide films did not prove to be the best, so we did not study them using implantation. Films for implantation were made with a width of 5 mm, a length of 10 mm, and a thickness of 10 μm. The marker thread was glued on top using thermal extrusion of polylactide of the required molecular weight not containing streptokinase. To sterilize the film surface and prevent the primary acute reaction of the recipient organism, the polymer samples were conditioned for 4 h at 37 °C in a nutrient medium containing 10% cattle serum, a mixture of antibiotics and an antimycotic. Samples were labeled with colored silk threads and implanted subcutaneously by blunt dissection through a cut in the lower part of the back using a trocar. The samples were implanted along the back line of the animal (three samples per rat; spaced by 3 cm from each other). The surgery was carried out under Zoletil/xylazine anesthesia (6/12 mg per 1 kg of body weight). The operated animals were sacrificed 60 days after the implantation. For histological studies fragments of material were explanted along with the surrounding tissues and fixed in a neutral formaline solution (10%) at 4 °С for 18 h. Histology was done based on standard staining with hematoxylin (Gill’s) and eosin [22].
Results
As shown in Fig. 1, the molecular weight of polylactide, as well as the content of streptokinase in the polymer film, substantially affected the value of percentage elongation of the film. The incorporation of streptokinase into a polylactide film reduced its ability to elongate. In general, elongation of the polylactide films examined linearly depended on the content of streptokinase. Raising the concentration of streptokinase by 1% reduced the value of the film percentage elongation by 20–30%.
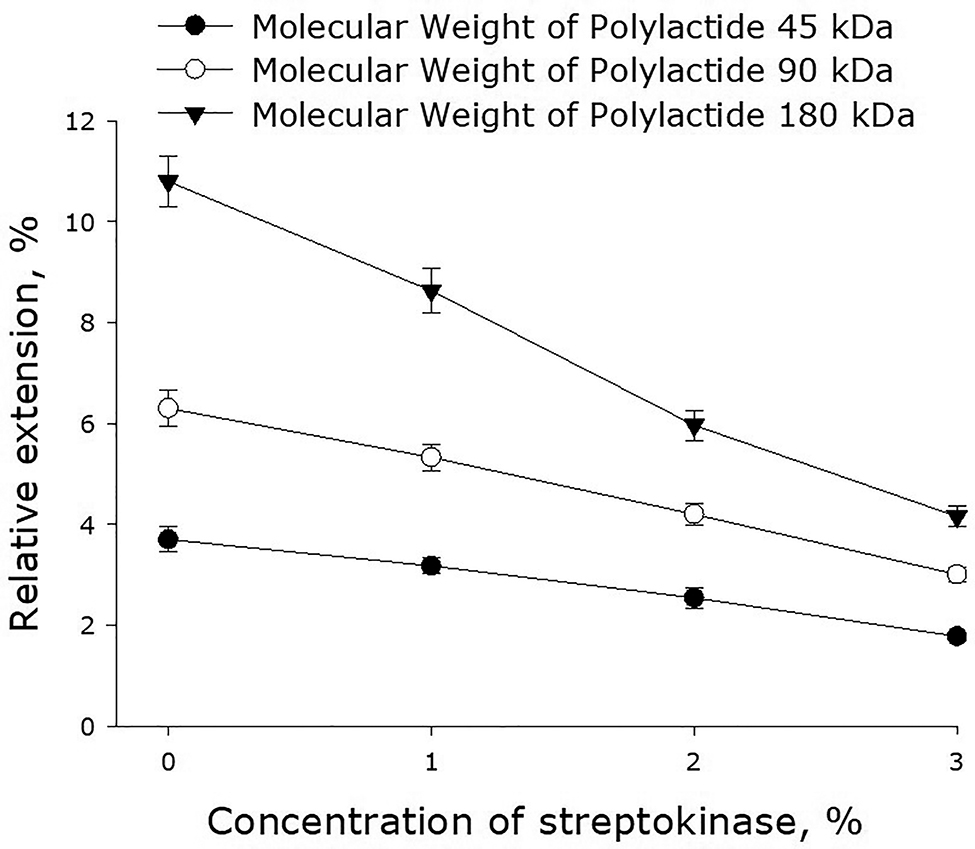
Effect of streptokinase on the elongation of polymer films made of polylactide of different molecular weight. Mean values and their standard errors calculated from the data of three independent experiments are given.
Figure 2 shows the effect of streptokinase on the tensile strength of polymer films made of polylactide of different molecular weight. As it turned out, the introduction of streptokinase in the polymer films decreased their tensile strength. Generally, tensile strength of the films depended linearly on the concentration of streptokinase. A 1% increase in the concentration of streptokinase diminished tensile strength of a polylactide film by 15–20%. The use of high-molecular polylactide increased the ultimate strength of the polymer films. For example, the ultimate strength of the films made of polylactide with the molecular mass 45 kDa less than half as that of the films made of 90-kDa polylactide. The latter films were, in their turn, 30% less strong than the films made of 180-kDa polylactide.
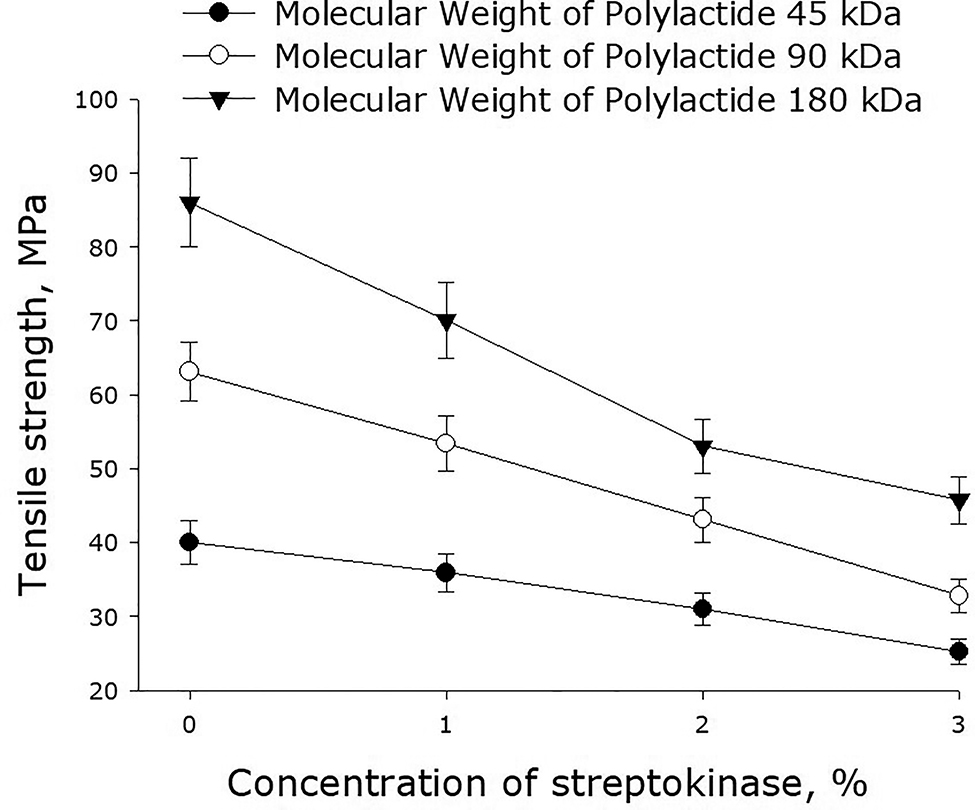
Effect of streptokinase on the tensile strength of polymer films made of polylactide of different molecular weight. Mean values and their standard errors calculated from the data of three independent experiments are given.
Figure 3 shows the yield strength of polylactide films versus the concentration of streptokinase. One can see that the higher is the molecular weight of polylactide, the higher is the yield strength of the polymer film. Incorporation of streptokinase into a film results in the reduction of its yield strength.
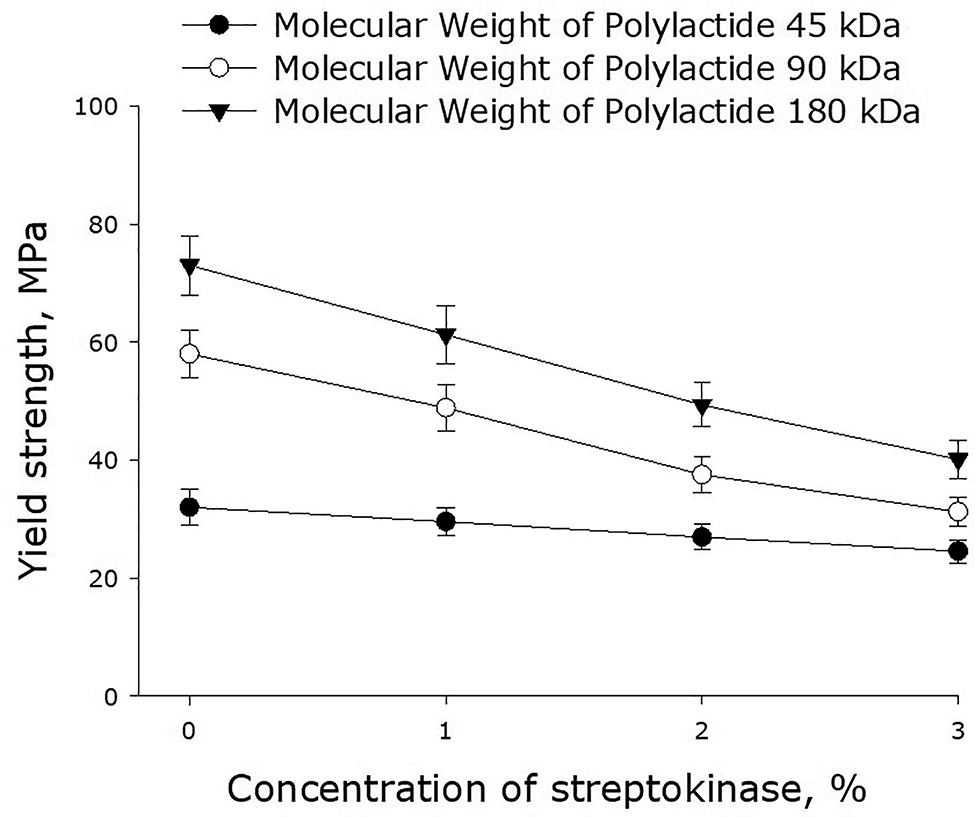
Effect of streptokinase on the yield strength of polymer films made of polylactide of different molecular weight. Mean values and their standard errors calculated from the data of three independent experiments are given.
Represented in Fig. 4 are micrographs of the surface of polylactide films, either with or without streptokinase at different concentrations. The images demonstrate that the technology we used yields films with smooth surfaces, which are free of any defects. At higher concentrations of streptokinase (2–3%), there might be protein aggregates and dried droplets of saline on the surface of films, which was quite a rare occurrence observed in some film batches. Such protein aggregates mostly appeared on the surface of films made of polylactide with the molecular mass of 45 kDa. For the films made of high-molecular polylactides, that was not a problem.
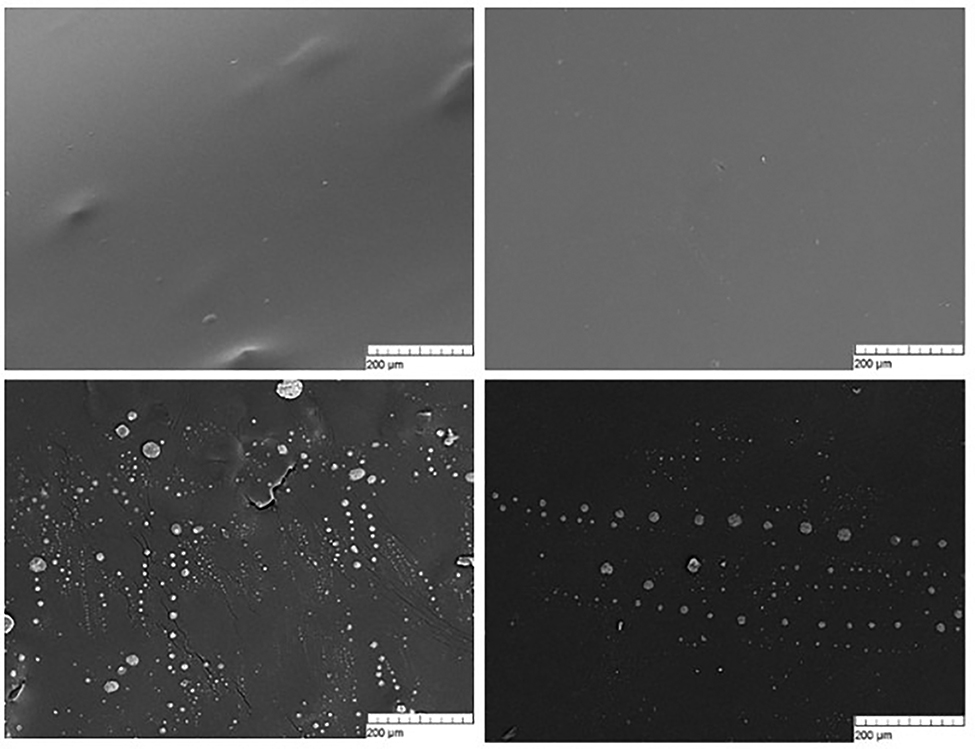
Micrographs of the surface of 45-kDa polylactide films without streptokinase (a) and with 1% (b), 2% (c) and 3% (d) streptokinase. The micrographs were obtained using a scanning electron microscope, at a magnification of 300 × 1.
Figure 5 shows the dependence of the rate of decomposition of polylactide films on the molecular mass of the polymer. One can see that the lower is the molecular mass of polylactide, the slower is the degradation of films made of this polymer. The degradation of polylactide was found to occur in two phases. The first phase takes about 1 month from the moment a film was put into a saline solution for storage and is characterized by a relatively high rate of degradation. During the second phase, which begins after storing a film for about a month, the rate of film degradation drops substantially. For the films made of 45-kDa polylactide, the degradation rate at the first phase was about 0.08% of polymer weight per day – and it became four times as low during the second phase (0.02% of polymer weight per day). For 90-kDa polylactide films, the values were 0.15 and 0.03% of polymer weight per day respectively; for 180-kDa films, they were 0.21 and 0.04% – five times as lower at the second phase in both cases. In a number of experiments, the degradation of polylactide films was monitored in phosphate buffers of different capacities and pH. As it turned out, both these parameters: buffer capacity and pH (7.0–8.0) – did not have a particular effect on the rate of film degradation, which was about the same as in saline. The presence of streptokinase in the polymer films increased the rate of their degradation by 10–20%, yet the dynamics of degradation was very close to that of films without streptokinase.
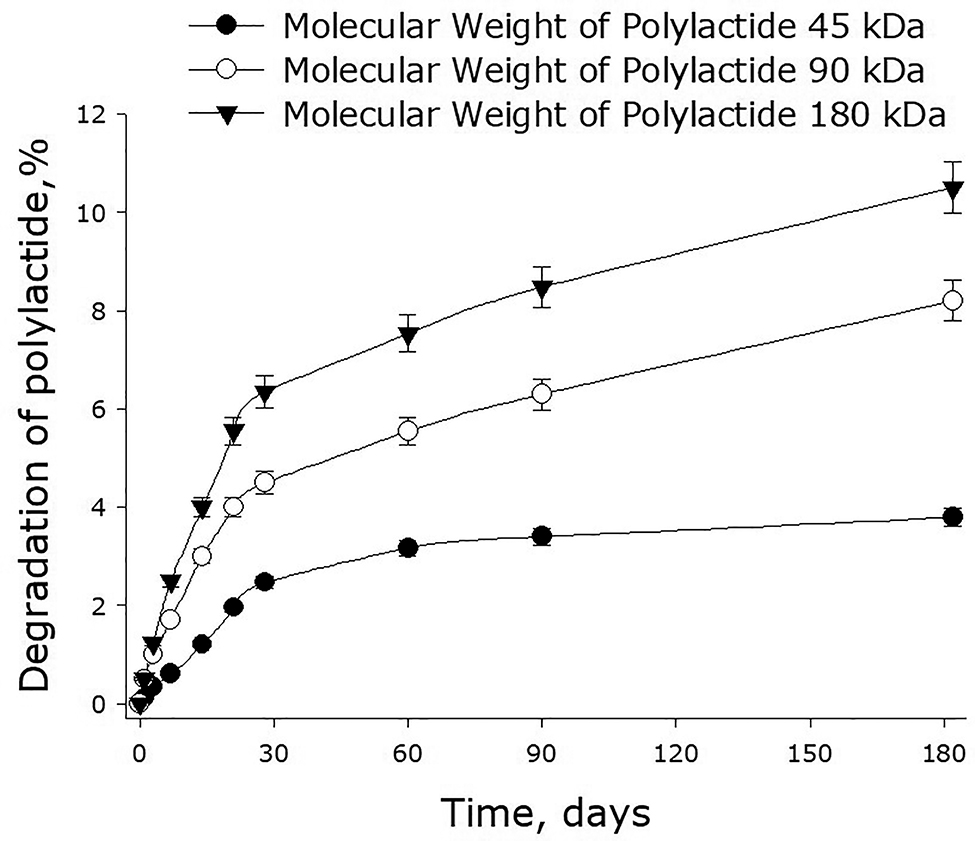
The rate of decomposition of polylactide films depending on the molecular mass of the polymer. Mean values and their standard errors calculated from the data of three independent experiments are given.
Our experiments showed that streptokinase was slowly released from 45-kDa polylactide films (Fig. 6). The rate of the release depended on the initial concentration of the enzyme in the film. The higher was the concentration, the faster the enzyme diffused in the medium. For the films containing 1, 2 and 3% streptokinase, the initial rates of the enzyme release were about 0.013, 0.020 and 0.041 mg/day respectively. In other words, the initial rate of streptokinase release was proportional to its concentration in the film.
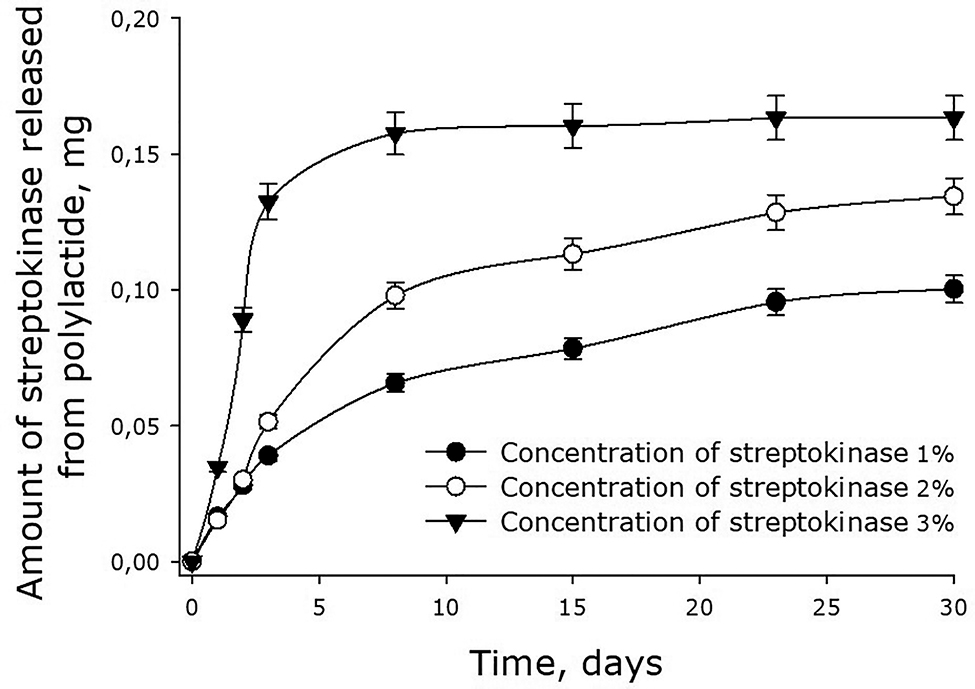
Dynamics of streptokinase release from 45-kDa polylactide films. Mean values and their standard errors calculated from the data of three independent experiments are given.
After 3 days, the rate of streptokinase release from polylactide films dropped substantially. When the initial concentration of the enzyme was 3%, the release of the enzyme practically stopped by the 4th day. At that point, the enzyme molecules remaining in the film (approximately 50% of the initial amount) would be released in parallel with the dissolution of the film. For the films with the initial concentration of streptokinase 1 and 2%, the rates of the enzyme release also dropped – to the levels of 0.003–0.004 mg/day. By the 30th day, films with the initial content of streptokinase 1% had only 10% of the protein remained (i.e., 90% out it had already been released). The molecules of streptokinase released from polylactide films retained over 90% of their enzymatic activity, indicating preservation of the native protein packing in the films.
In the experiments with 90-kDa films, we also observed a slow release of streptokinase from the membrane (Fig. 7). The rate of the release depended on the initial concentration of the enzyme in the film. The higher was the concentration, the faster the enzyme diffused in the medium. For the films containing 1, 2 and 3% streptokinase, the initial rates of the enzyme release were about 0.023, 0.031 and 0.043 mg/day respectively. In other words, the initial rate of streptokinase release was proportional to its concentration in the film. After 3 days, the rate of streptokinase release from polylactide films dropped substantially. When the initial concentration of the enzyme was 3%, the release of the enzyme practically stopped by the 4th day. At that point, the enzyme molecules remaining in the film (approximately 50% of the initial amount) would be released in parallel with the dissolution of the film.
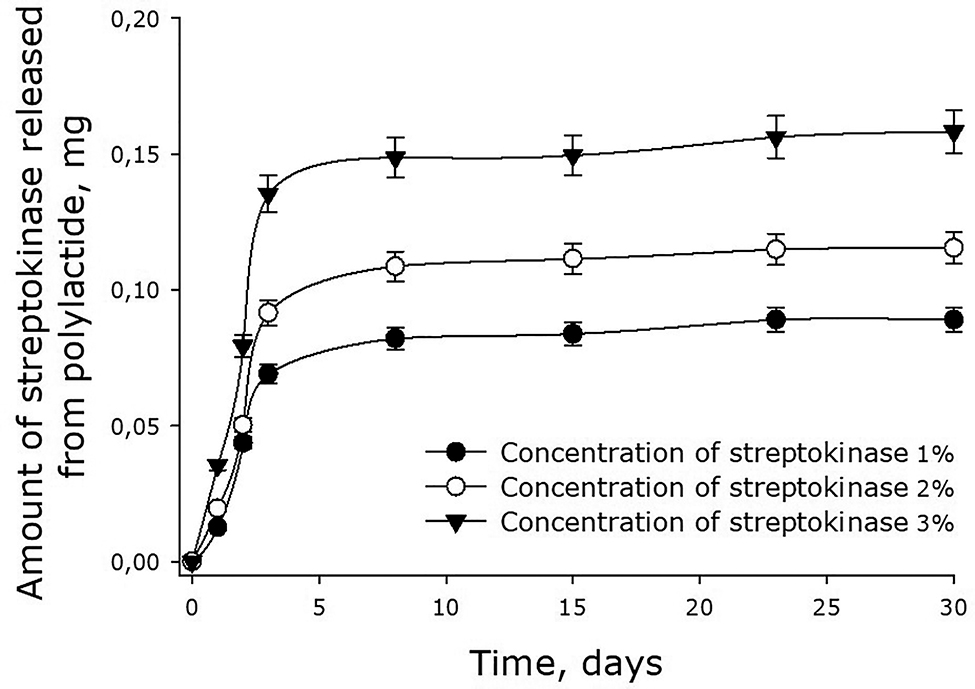
Dynamics of streptokinase release from 90-kDa polylactide films. Mean values and their standard errors calculated from the data of three independent experiments are given.
Streptokinase was also found to diffuse from 180-kDa polylactide films (Fig. 8). The dynamics of the enzyme release was similar to that observed with the films made of 45- and 90-kDa polylactide. The initial rates of streptokinase release were about 0.023, 0.031 and 0.043 mg/day in case of films containing 1, 2 and 3% streptokinase respectively. After 7 days, the rate of streptokinase release decreased substantially. When the initial concentration of streptokinase was 3%, the release of the enzyme by the 8th day dropped to 0.001 mg/day. The release of the remaining 70% of streptokinase would, therefore, go in parallel with the dissolution of the film.
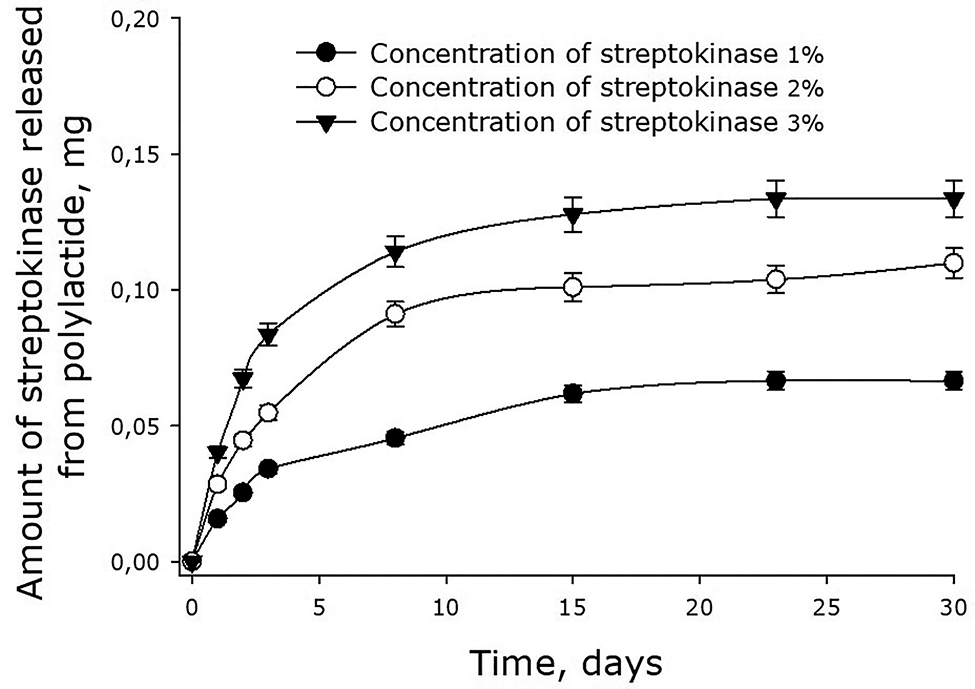
Dynamics of streptokinase release from 180-kDa polylactide films. Mean values and their standard errors calculated from the data of three independent experiments are given.
Figure 9 shows the effect of polylactide films on the viability of cell cultures. The number of nonviable cells on the surface of streptokinase-free polylactide films did not exceed 3–4%, which was even lower than the number of nonviable cells on the dish surface (5.5% ± 1.2). On the surface of streptokinase-containing films, the number of nonviable cells was a bit higher, yet the differences with control samples were statistically significant only for the films made of 45-kDa polylactide and containing 3% streptokinase. In all the other cases, the films did not have any short-term toxic effects on the cells growing de novo on the sample surfaces.
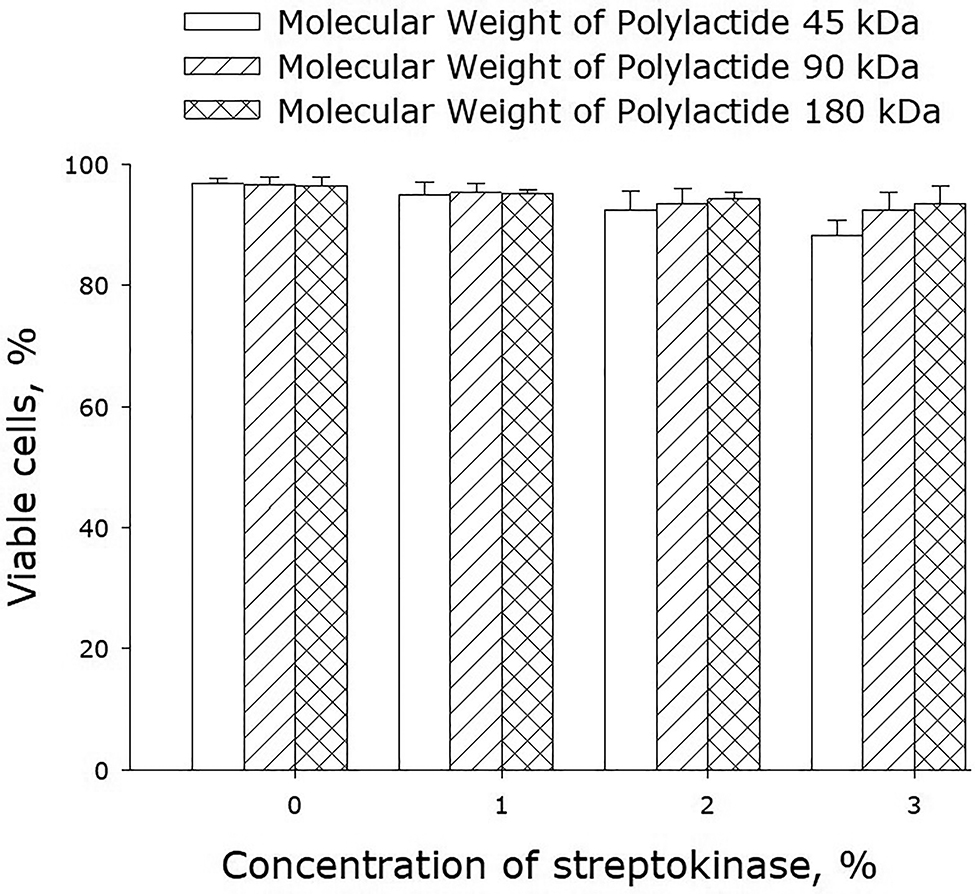
Effect of streptokinase-containing polylactide films on the viability of cell cultures. Mean values calculated from the data of three independent experiments are given.
To analyze the mitotic activity of cells, we determined their mitotic index (MI) when the cells were in the phase of logarithmic growth (3rd day since the start of cultivation; Fig. 10). MI of cells growing on the surface of streptokinase-free films was approximately 1.5%. Incorporation of streptokinase (1–2%) into the films did not change MI much. When the concentration of streptokinase in the polymer was increased to 3%, MI dropped to about 0.8%.
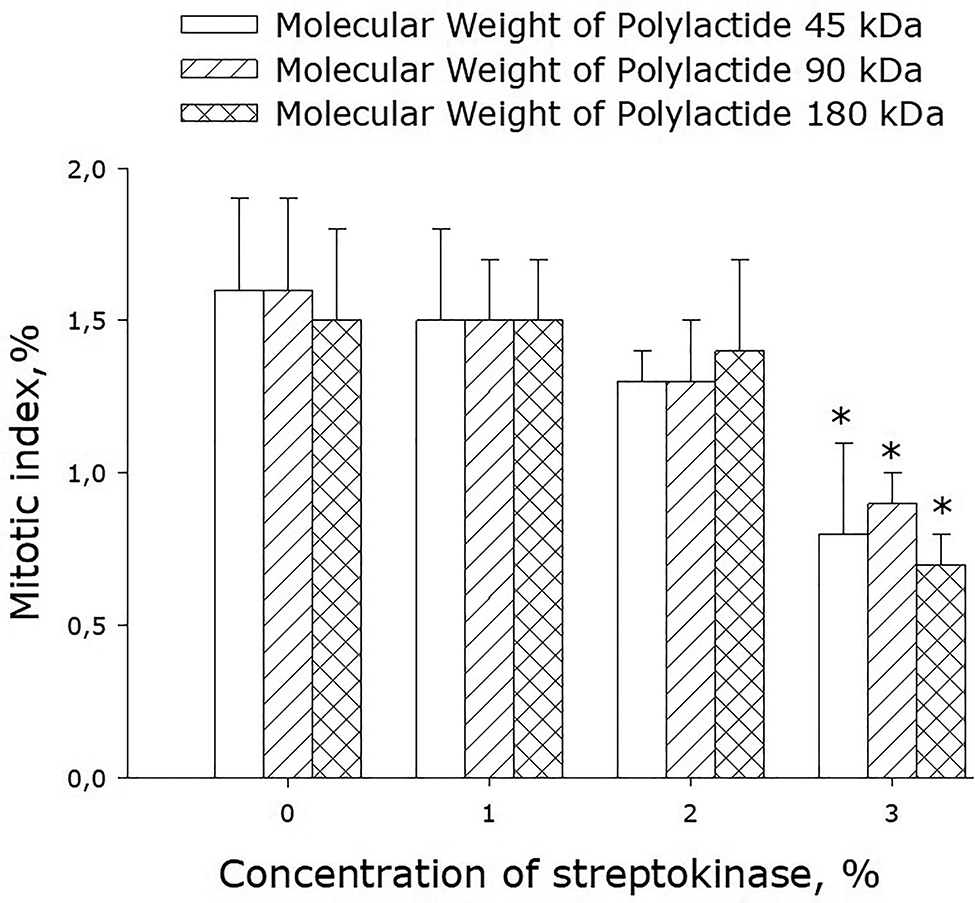
Effect of streptokinase-containing polylactide films on the mitotic index of cell cultures. Mean values calculated from the data of three independent experiments are given.
Figure 11 shows data on the spread of cells over available polymer surfaces by the 3rd day of cultivation. On streptokinase-free films, approximately 35% of their area remained unpopulated – just the same as on glass surfaces in control experiments. The same picture was observed when the films were infused with 1–2% streptokinase. When the concentration of streptokinase was increased to 3%, the area populated by cells was reduced as compared to control samples.
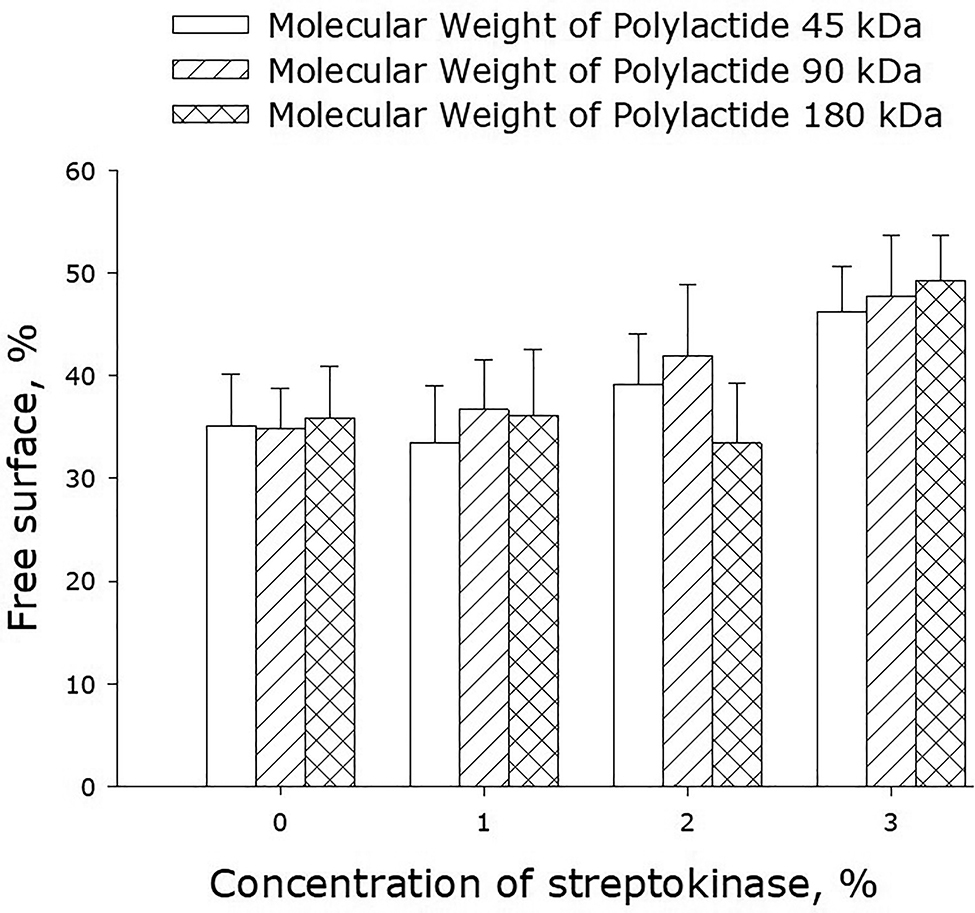
Populating surfaces of polylactide films with cells depending on the molecular weight of the polymer and concentration of streptokinase. The averaged data of three independent experiments are given.
Biocompatibility of streptokinase-containing polylactide films was assessed in the experiments in vivo, in which films were implanted subcutaneously into the spinal muscle tissues of rats. In the postoperative period, the condition of all the animals was satisfactory, and they were removed from the experiment 60 days after the surgery. The tissues surrounding implants were examined microscopically. The examination showed that tissue samples had no traces of streptokinase-containing polylactide (Fig. 12a, b). In some cases, there were channels, which were left by the threads used to label the implants and flag the postoperative topology (Fig. 12c, d).
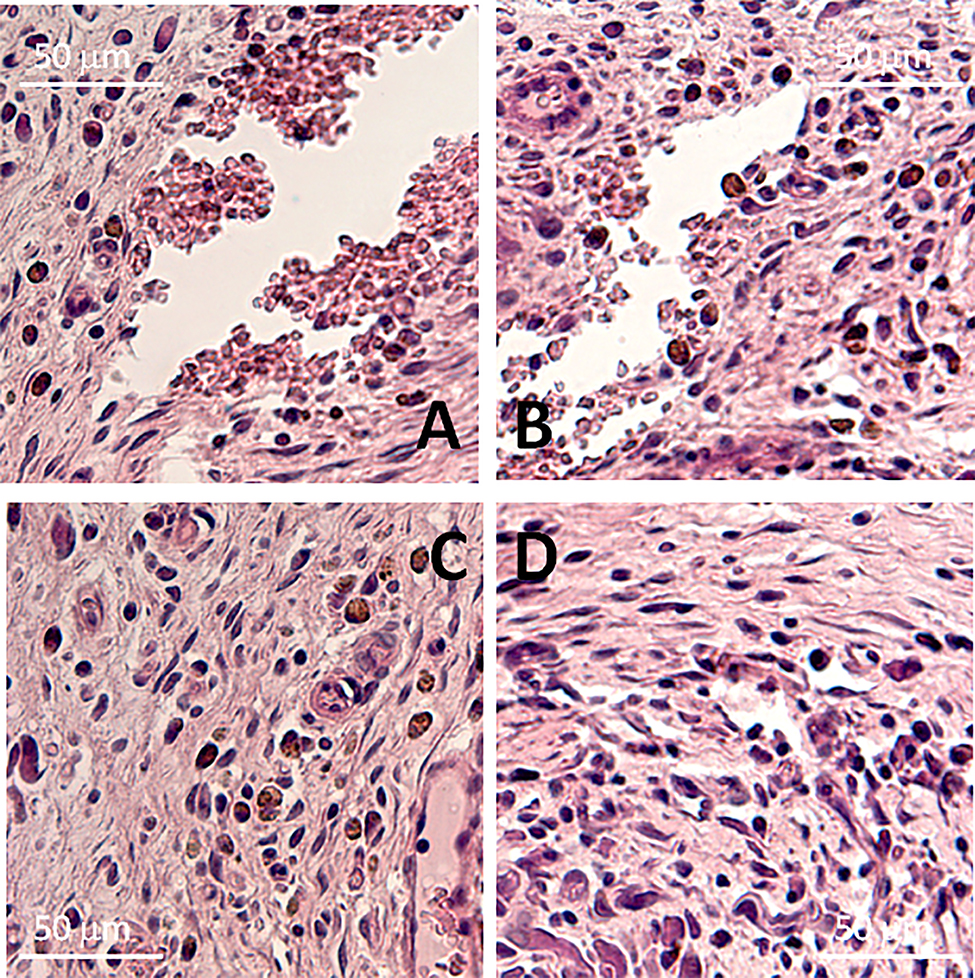
Sections of the tissues surrounding the streptokinase-containing films made of polylactide of different molecular mass. The sections were obtained 8 weeks after the subcutaneous implantation of films in rats. Polymers with a molecular weight of 45, 90, and 180 kDa containing 1 or 2% streptokinase were studied. Representative micrographs are presented. a, с – streptokinase-containing polylactide films (1%, 90 kDa), b, d – streptokinase-containing polylactide films (2%, 90 kDa).
Discussion
Currently, a large number of polymer materials and unique solutions based on them have been created [23], [24], [25], [26]. In this work, we have tested a number of streptokinase-containing polylactide coatings and established their basic mechanical properties. In particular, we have tested the dependence of percentage elongation of polymer films made of polylactides of different molecular weight on the concentration on streptokinase (Fig. 1). This dependence can be generally described by the equation y = 6 − bx, where y is the elongation of the film, x is the concentration of the enzyme used in the procedure of film formation, and b is a proportionality coefficient. At the concentrations of streptokinase 1, 2 and 3%, b is equal to 0.6, 1.1 and 2.2 respectively. The change of the coefficient b is proportional to the change of the molecular weight of polylactide. The obtained formula allows one to predict the values of percentage elongation of films made of polylactides of different molecular weight, as well as estimate the average molecular weight of polylactides synthesized by the described technology. Using the theory of elasticity of rubber, one considers a polymer chain in a cross-linked network as an entropy spring. When the chain stretches, entropy decreases with a large margin because fewer conformations are available [27]. It can be assumed that the presence of streptokinase can affect the conformations of chains, because if they are more stretched in undeformed material, their ability to stretch is lower.
A number of other mechanical properties of polylactide films are illustrated in Figs. 2, 3, and 4. It should be noted that the polylactide films we synthesized have much higher yield strength as compared to other polylactide polymers [28], [29]. It shown that with increasing streptokinase concentration in the polymer, the ultimate tensile strength decreases. This may be due to mechanical weakness forming on the streptokinase-PLA interface. With an increase in the molecular weight of the polymer, the ultimate tensile strength increases. This behavior could be accounted for by the increase of the number of entanglements with the increase of molar mass. The films described in this paper degrade over time in aqueous solutions – even in the absence of biological objects (Fig. 5). The lower is the molecular weight of polylactide, the slower is its degradation. The degradation of the polymer occurs in two phases. The first, rapid, phase is probably associated with the swelling of the polymer and depolymerization of bonds between the primary particles. The second phase is apparently related to the destruction of the polymer backbone. Similar processes are observed upon thermal degradation of polylactide [30].
The kinetics of streptokinase release from polylactide membranes has been shown to depend on the concentration of the immobilized enzyme and the molecular weight of polylactide (Figs. 6, 7, and 8). It should be noted that about 30% of the enzyme molecules remain encapsulated in the membrane, whereas 70% of the molecules diffuse into the outer medium. The biodegradable polymeric membranes tested in this paper are, therefore, suitable for the production of stent and prosthesis coatings capable of sustained and controlled release of drugs into the surrounding tissues. Over the past 20 years, there have been many attempts to make drug-infused coatings [31], [32], [33], yet none of those drugs have their molecular weight greater than 10 kDa [34]. In the present work, we have managed to create systems with the controlled release of compounds with the molecular weight of 47 kDa.
Using in vitro models, we have studied biocompatibility of the synthesized polylactide films (Figs. 9–11). As it turned out, the surface of polylactide films is even more suited for cell growth than the culture glass. In general, there is no doubt about high biocompatibility of polylactide [35], [36]. The results obtained on cell cultures have been confirmed by experiments on animal models (Fig. 12). It has been known for a long time (over 70 years) that streptokinase is toxic at concentrations exceeding 5000 units/g [37]. In our experiments, this toxicity manifested itself as a drop of MI and increase in the number of nonviable cells when the concentration of streptokinase in the polymer films was raised to 3%. When administered at high doses, streptokinase can cause bradycardia, fibrillation, ventricular fibrillation, less fever and severe hypotension [38]. The development of allergic and immune reactions is also possible [39]. None of these adverse effects were observed in our experiments on animal models. Furthermore, 60 days after the operation, no traces of streptokinase-containing polylactide were found in the animal tissues, and only traces of polylactide molecules were found on the surface of stents. The implantation data indicate that all the materials used are biocompatible and not toxic to the recipient organism. Thus, the polylactide membranes synthesized and tested in this work are biodegradable, possess the necessary mechanical properties, and are capable of sustained and directed release of biomacromolecules with high molecular weight.
However, the question may be, which polymer is the best? What molecular weight polymer? Polymer with what concentration of streptokinase? The last question is easy to answer. A polymer containing 3% of the enzyme is toxic to cells. Polymers with lower concentrations are not toxic, but will differ in antithrombotic action. As for molecular weight, the polylactide with the highest molecular weight has the best mechanical characteristics, but it also has the highest degradation rate and the lowest release rate of streptokinase. Low molecular polymers have significantly worse mechanical properties, but degrade longer and release the enzyme better. In this regard, depending on the tasks, both low and high molecular weight polylactide polymer may be the best.
References
[1] J. Li, C. Cai, J. Li, J. Li, J. Li, T. Sun, L. Wang, H. Wu, G. Yu. Molecules 23, 2661 (2018).10.3390/molecules23102661Suche in Google Scholar PubMed PubMed Central
[2] D. Arai, A. Ishii, H. Ikeda, Y. Abekura, H. Nishi, S. Miyamoto, Y. Tabata. J. Biomed. Mater. Res. Part B Appl. Biomater. 107, 2185–2194 (2019).10.1002/jbm.b.34314Suche in Google Scholar PubMed
[3] S. W. K. Raphael M. Ottenbrite (Eds.), Polymeric Drugs and Drug Delivery Systems - Google Books, CRC Press, London (2002). https://books.google.ru/books?hl=ru&lr=&id=hvy34dryFQoC&oi=fnd&pg=PR13&dq=controlled+release+of+drugs&ots=LVNoj0gkTM&sig=lin0L41GB3J46J4qvAndCoKbaEY&redir_esc=y#v=onepage&q=controlled release of drugs&f=false.Suche in Google Scholar
[4] Agis F. Kydonieus (Ed.), Controlled Release Technologies: Methods, Theory, and Applications, CRC Press, London (2019). https://books.google.ru/books?hl=ru&lr=&id=AxemDwAAQBAJ&oi=fnd&pg=PA1&dq=controlled+release+of+drugs,+material&ots=82DiIGN1e0&sig=756cDM0go5IedrtusAC_b0cgpwI&redir_esc=y#v=onepage&q=controlled release of drugs%2C material&f=false.Suche in Google Scholar
[5] A. J. R. Lasprilla, G. A. R. Martinez, B. H. Lunelli, A. L. Jardini, R. M. Filho. Biotechnol. Adv. 30, 321–328 (2012).10.1016/j.biotechadv.2011.06.019Suche in Google Scholar PubMed
[6] S. Jacobsen, H. G. Fritz, P. Degée, P. Dubois, R. Jérôme. Polym. Eng. Sci. 39, 1311–1319 (1999).10.1002/pen.11518Suche in Google Scholar
[7] P. Saini, M. Arora, M. N. V. R. Kumar. Adv. Drug Deliv. Rev. 107, 47–59 (2016).10.1016/j.addr.2016.06.014Suche in Google Scholar PubMed
[8] W. Ezzat, G. Keller. Facial Plast. Surg. 27, 503–509 (2011).10.1055/s-0031-1298782Suche in Google Scholar PubMed
[9] L. Shue, Z. Yufeng, U. Mony. Biomatter 2, 271–277 (2012).10.4161/biom.22948Suche in Google Scholar PubMed PubMed Central
[10] L. Quiles-Carrillo, N. Montanes, J. Lagaron, R. Balart, S. Torres-Giner. Appl. Sci. 9, 533 (2019).10.3390/app9030533Suche in Google Scholar
[11] S. Boi, E. Dellacasa, P. Bianchini, P. Petrini, L. Pastorino, O. Monticelli. Colloids Surfaces B Biointerfaces 179, 190–198 (2019).10.1016/j.colsurfb.2019.03.071Suche in Google Scholar PubMed
[12] W. S. Tillett, R. L. Garner. J. Exp. Med. 58, 485–502 (1933).10.1084/jem.58.4.485Suche in Google Scholar PubMed PubMed Central
[13] M. A. Zia. Protein Pept. Lett. 26 (2019). https://doi.org/10.2174/0929866526666191014150408.Suche in Google Scholar
[14] A. Arshad, M. A. Zia, M. Asgher, F. A. Joyia, M. Arif. Pak. J. Pharm. Sci. 31, 1597–1602 (2018). http://www.ncbi.nlm.nih.gov/pubmed/30058554 (accessed Oct 21, 2019).Suche in Google Scholar
[15] L. R. Christensen. J. Clin. Invest. 28, 163–72 (1949).10.1172/JCI102045Suche in Google Scholar
[16] M. A. Sevost’yanov, A. Y. Fedotov, E. O. Nasakina, A. Y. Teterina, A. S. Baikin, K. V. Sergienko, A. G. Kolmakov, V. S. Komlev, V. E. Ivanov, O. E. Karp, S. V. Gudkov, S. M. Barinov. Dokl. Chem. 465, 278–280 (2015).10.1134/S001250081511004XSuche in Google Scholar
[17] J.L. Biedler, S. Roffler-Tarlov, M. Schachner, L. S. Freedman. Cancer Res. 38, 3751–3757 (1978). http://www.ncbi.nlm.nih.gov/pubmed/29704 (accessed Oct 21, 2019).Suche in Google Scholar
[18] M. P. La Quaglia, K. M. Manchester. J. Pediatr. Surg. 31, 315–318 (1996).10.1016/S0022-3468(96)90025-1Suche in Google Scholar
[19] S. A. Garmash, V. S. Smirnova, O. E. Karp, A. M. Usacheva, A. V. Berezhnov, V. E. Ivanov, A. V. Chernikov, V. I. Bruskov, S. V. Gudkov. J. Environ. Radioact. 127, 163–170 (2014).10.1016/j.jenvrad.2012.12.009Suche in Google Scholar
[20] M. A. Sevostyanov, A. S. Baikin, K. V. Sergienko, L. A. Shatova, A. A. Kirsankin, I. V. Baymler, A. V. Shkirin, S.V . Gudkov. React. Funct. Polym. 150, 104550 (2020).10.1016/j.reactfunctpolym.2020.104550Suche in Google Scholar
[21] C.-H. Lee, N. Vyavahare, R. Zand, H. Kruth, F. J. Schoen, R. Bianco, R. J. Levy. J. Biomed. Mater. Res. 42, 30–37 (1998).10.1002/(SICI)1097-4636(199810)42:1<30::AID-JBM5>3.0.CO;2-PSuche in Google Scholar
[22] M. A. Sevost’yanov, E. O. Nasakina, A. S. Baikin, K. V. Sergienko, S. V. Konushkin, M. A. Kaplan, A. V. Seregin, A. V. Leonov, V. A. Kozlov, A. V. Shkirin, N. F. Bunkin, A. G. Kolmakov, S. V. Simakov, S. V. Gudkov. J. Mater. Sci. Mater. Med. 29, 33 (2018).10.1007/s10856-018-6039-3Suche in Google Scholar
[23] B. Guo, P. X. Ma. Sci. China Chem. 57, 490 (2014).10.1007/s11426-014-5086-ySuche in Google Scholar
[24] Y. Wu, L. Wang, X. Zhao, S. Hou, B. Guo, P. X. Ma. Biomaterials 104, 18 (2016).10.1016/j.biomaterials.2016.07.011Suche in Google Scholar
[25] J. Chen, M. Yu, B. Guo, P. X. Ma, Z. Yin. J. Colloid Interface Sci. 514, 517 (2018).10.1016/j.jcis.2017.12.062Suche in Google Scholar
[26] J. Chen, J. Ge, B. Guo, K. Gaoc, P. X. Ma. J. Mater. Chem. B. 4, 2477 (2016).10.1039/C5TB02703ASuche in Google Scholar
[27] L. R. G. Treloar. Physics of Rubber Elasticity, Oxford University Press, ISBN 9780198570271 (1975).Suche in Google Scholar
[28] A. S. Baikin, A. G. Kolmakov, L. A. Shatova, E. O. Nasakina, M. G. Sharapov, I. V. Baymler, S. V. Gudkov, M. A. Sevostyanov. Materials 12, 4107 (2019).10.3390/ma12244107Suche in Google Scholar
[29] A. S. Baikin, M. A. Sevostyanov, E. O. Nasakina, K. V. Sergienko, M. A. Kaplan, S. V. Konushkin, A. A. Kolmakova, A. D. Yakubov, A. G. Kolmakov. IOP Conf. Ser. Mater. Sci. Eng. 347, 012026 (2018).10.1088/1757-899X/347/1/012026Suche in Google Scholar
[30] E. Ozdemir, J. Hacaloglu. J. Anal. Appl. Pyrolysis. 129, 181–188 (2018).10.1016/j.jaap.2017.11.014Suche in Google Scholar
[31] P. Le Corre, P. Le Guevello, V. Gajan, F. Chevanne, R. Le Verge. Int. J. Pharm. 107, 41–49 (1994).10.1016/0378-5173(94)90300-XSuche in Google Scholar
[32] N. Mhlanga, S. S. Ray. Int. J. Biol. Macromol. 72, 1301–1307 (2015).10.1016/j.ijbiomac.2014.10.038Suche in Google Scholar
[33] Y. Hu, X. Jiang, Y. Ding, L. Zhang, C. Yang, J. Zhang, J. Chen, Y. Yang. Biomaterials, 24, 2395–2404 (2003).10.1016/S0142-9612(03)00021-8Suche in Google Scholar
[34] M. Brzeziński, M. Socka, B. Kost. Polym. Int. 68, 997–1014 (2019).10.1002/pi.5753Suche in Google Scholar
[35] D. Pappalardo, T. Mathisen, A. Finne-Wistrand. Biomacromolecules, 20, 1465–1477 (2019).10.1021/acs.biomac.9b00159Suche in Google Scholar PubMed
[36] A. Siiki, J. Sand, J. Laukkarinen. Eur. J. Gastroenterol. Hepatol. 30, 813–818 (2018).10.1097/MEG.0000000000001167Suche in Google Scholar
[37] J. W. Smillie. Am. J. Ophthalmol. 37, 911–917 (1954).10.1016/0002-9394(54)91932-7Suche in Google Scholar
[38] H.-P. Klöcking. in Fibrinolytics and Antifibrinolytics, pp. 151–177, Springer Berlin Heidelberg, Berlin, Heidelberg (1978).10.1007/978-3-642-66863-0_6Suche in Google Scholar
[39] Y. Birnbaum, D. Hasdai, B. Stahl. Int. J. Cardiol. 46, 1–6 (1994).10.1016/0167-5273(94)90110-4Suche in Google Scholar
© 2020 IUPAC & De Gruyter. This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. For more information, please visit: http://creativecommons.org/licenses/by-nc-nd/4.0/
Artikel in diesem Heft
- Frontmatter
- In this issue
- Conference papers of the 21st Mendeleev Congress on General and Applied Chemistry
- Recent achievements in copper catalysis for C–N bond formation
- Novel selective anticancer agents based on Sn and Au complexes. Mini-review
- Novel colchicine conjugate with unusual effect on the microtubules of cancer cells
- Surface chemistry of structural materials subjected to corrosion
- Redox-conducting polymers based on metal-salen complexes for energy storage applications
- Thermodynamic approach for prediction of oxide materials properties at high temperatures
- Competitive ways for three-component cyclization of polyfluoroalkyl-3-oxo esters, methyl ketones and amino alcohols
- Fragment-based approach to novel bioactive purine derivatives
- Reaction of 1,3-dimethylimidazolium dimethylphosphate with elemental sulfur
- Synthetic models of hydrogenases based on framework structures containing coordinating P, N-atoms as hydrogen energy electrocatalysts – from molecules to materials
- Macrokinetic investigation of the interaction mechanism of the pyrophoric iron nanopowder compacts with air
- Polylactide-based stent coatings: biodegradable polymeric coatings capable of maintaining sustained release of the thrombolytic enzyme streptokinase
- 3D printing in analytical chemistry: current state and future
- High purity substances – prototypes of elements of Periodic Table
- Pd(0)-catalyzed amination in the synthesis of chiral derivatives of BINAM and their evaluation as fluorescent enantioselective detectors
Artikel in diesem Heft
- Frontmatter
- In this issue
- Conference papers of the 21st Mendeleev Congress on General and Applied Chemistry
- Recent achievements in copper catalysis for C–N bond formation
- Novel selective anticancer agents based on Sn and Au complexes. Mini-review
- Novel colchicine conjugate with unusual effect on the microtubules of cancer cells
- Surface chemistry of structural materials subjected to corrosion
- Redox-conducting polymers based on metal-salen complexes for energy storage applications
- Thermodynamic approach for prediction of oxide materials properties at high temperatures
- Competitive ways for three-component cyclization of polyfluoroalkyl-3-oxo esters, methyl ketones and amino alcohols
- Fragment-based approach to novel bioactive purine derivatives
- Reaction of 1,3-dimethylimidazolium dimethylphosphate with elemental sulfur
- Synthetic models of hydrogenases based on framework structures containing coordinating P, N-atoms as hydrogen energy electrocatalysts – from molecules to materials
- Macrokinetic investigation of the interaction mechanism of the pyrophoric iron nanopowder compacts with air
- Polylactide-based stent coatings: biodegradable polymeric coatings capable of maintaining sustained release of the thrombolytic enzyme streptokinase
- 3D printing in analytical chemistry: current state and future
- High purity substances – prototypes of elements of Periodic Table
- Pd(0)-catalyzed amination in the synthesis of chiral derivatives of BINAM and their evaluation as fluorescent enantioselective detectors

