Abstract
Sebaceous carcinoma (SC) is an aggressive cancer arising from sebaceous glands, rarely occurring in the oral cavity (IOSC). With 21 documented cases, it typically affects the buccal and labial mucosa, tongue, and palate. The cause remains unclear, but Fordyce granules, sebaceous structures in oral mucosa, may play a role in malignancy. IOSC often presents as a painless lump, leading to delayed diagnosis and increased risks of recurrence and metastasis. Early detection and treatment are crucial. Wide local excision (WLE) is the primary treatment, while radiotherapy (RT) is an option for patients unfit for surgery, unwilling to undergo it, or requiring palliative care for advanced cases. RT may also be used pre- or postoperatively to reduce recurrence risks. Cryotherapy and chemotherapy are not standard treatments for IOSC. Regular follow-up is essential for long-term management and to monitor for potential recurrence. The current review provides an updated overview of IOSC, focusing on its clinical presentation, risk factors, pathogenesis, diagnostic approaches, treatment strategies, and prognosis, aims to emphasize the importance of early intervention and a multidisciplinary approach to optimize outcomes.
Introduction
Sebaceous carcinoma (SC) is a rare, aggressive cancer originating from the sebaceous glands of the skin, with approximately 400 cases documented in the literature. Due to its low incidence and the absence of a universally accepted histopathological classification, diagnosing SC remains challenging [1]. The overall incidence of SC in the United States was estimated at two cases per million for whites, one per million for Asian/Pacific Islanders, and 0.5 per million for Blacks [2]. Age-adjusted incidence rate (to the 2000–2025 WHO World Standard Population) ranged from 0.08 to 0.18 per 100,000 person-years and was not higher in Taiwanese than in white populations [3]. About 39 % of the cases occurred on the eyelid, followed by the skin of other unspecified parts of the face (26.8 %).
Although SC predominantly affects the head and neck region, especially the eyelids, it can develop anywhere on the body, including rare locations like the oral cavity, salivary glands, palate, and lips [1], [4], [5], [6], [7]. SC is frequently misdiagnosed as sebaceous adenoma or sebaceous hyperplasia. In advanced stages, it may also mimic basal cell carcinoma (BCC), squamous cell carcinoma (SCC), or metastatic adenocarcinoma, complicating accurate diagnosis. Commonly, it is categorized based on the location as either ocular or extraocular, with the latter further divided into extraoral or intraoral SC (IOSC). Ocular SC typically originates from the meibomian glands of the eyelid or the glands of Zeis in the cutaneous hair follicles [8]. While the origin of extra-ocular SC remains uncertain due to limited evidence of carcinoma arising from pre-existing sebaceous glands, it is believed that extra-ocular SC may originate from sebaceous glands located outside the ocular adnexa, which are found throughout the body in areas rich in sebaceous glands [8]. Ocular SC represents 75 % of all SC cases [9]. In-situ lesions of SC are commonly identified within the sebaceous glands of Zeis [10], yet the pathogenesis of extra-ocular SC is still unclear as no in-situ lesions have been identified in extra-ocular sebaceous glands [11]. Thus, the etiology of extra-ocular SC is still under research.
Unlike SC in the eyelid or other areas, sebaceous glands in the oral mucosa often contain “Fordyce granules,” found in approximately 80 % of adults and possibly represent a precancerous state for IOSC [12]. The incidence of Fordyce granules increases with age, from childhood to adulthood, and is slightly more common in men [13]. These granules are most frequently found on the vermilion border of the lips and the buccal mucosa. Histologically, Fordyce granules resemble cutaneous sebaceous glands, and pathological alterations are rare. There is no significant association between Fordyce granules and systemic diseases. Fordyce granules, small asymptomatic yellow-white papules, are likely produced by sebaceous cells present in 10–40 % of the parotid glands and 6–10 % of the submandibular glands [13], [14], [15]. Both sebaceous cells and the granular content play significant roles in malignant sebocytic transformation, though the exact reasons remain unclear [16]. Due to their common presence in salivary glands, SC appears more frequently in the salivary glands than in other oral areas [16].
This review discusses the epidemiology, probable pathogenesis, and risk factors associated with IOSC and evaluates the differential diagnoses, diagnostic challenges, histological features, and treatment options for SC based on currently reported cases. Our search was conducted in literature databases including PubMed, Cochrane, Google Scholar, and Scopus using the search string “Sebaceous carcinoma”, “Oral”, and “Intra-oral”. Additional research and review articles were manually searched. No restrictions were applied regarding language or publication year.
The prevalence and Incidence of Intraoral SC (IOSC): age, sex and location
Damm et al. reported the first case of IOSC in 1991 [17]. Since then, an additional 20 cases have been documented between 1996 and 2024 (Table 1). IOSC commonly affects areas such as the lips, gums, tongue, floor of the mouth, and other oral structures. The buccal mucosa was the most frequently affected site (6 out of 21 cases, 28.57 %), followed by the labial mucosa (7/21, 33.3 %), the anterior floor of the mouth (2/21, 9.5 %), gingiva (2/21, 9.5 %), palate (2/21, 9.5 %), and tongue (2/21, 9.5 %) [17].
Reported cases of primary intraoral sebaceous carcinoma since 1991.
| No. | Case | Year | Country/region | Sex | Age | Smoker | Site | Size | Extension | Treatment | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Damm et al. [17] | 1991 | USA | M | 53 | Unknown | Buccal mucosa (PG) | 3 cm | No | Excision | 5 years |
| 2 | Abuzeid et al. [18] | 1996 | KSA | F | 11 | No | Buccal mucosa | 3 cm | Submandibular LN | Excision | 2 years |
| 3 | Liu et al. [19] | 1997 | Taiwan | M | 68 | Unknown | Buccal mucosa | 2.5 cm | No | Excision | 3 years |
| 4 | Li et al. [20] | 1997 | Japan | M | 78 | Yes | Buccal mucosa | 3.5 cm | Surrounding muscle | Excision | 6 years |
| 5 | Handschel et al. [5] | 2003 | Germany | F | 80 | No | Floor of the mouth | 1.5 cm | No | Excision | 1 year |
| 6 | Alawi et al. [4] | 2005 | USA | M | 66 | Yes | Upper lip | 1.5 cm | No | Excision | 1 year |
| 7 | Innocenzi et al. [21] | 2005 | Italy | F | 68 | No | Upper lip | 2 cm | No | Excision | 3 years |
| 8 | Gomes et al. [22] | 2007 | Brazil | M | 55 | Yes | Floor of mouth | Unknown | Mandible body and ramus+Masseter | Excision, CT+RT | 1 year |
| 9 | Wang et al. [23] | 2010 | USA | M | 50 | No | Buccal mucosa | 4.6 cm | No | Excision+RT | <1 year |
| 10 | Oshiro et al. [24] | 2010 | Japan | M | 66 | Unknown | Tongue and dorsum | 2.5 cm | Bilateral cervical LN, lung | CT+RT | Died in 17 m |
| 11 | Somashekara et al. [25] | 2011 | UK | F | 25 | No | Hard palate | 1.5 cm | No | Excision | Unknown |
| 12 | Rowe et al. [26] | 2016 | USA | M | 76 | Yes | Gingiva | 3 cm | Skin of thigh, buttocks, lungs | Excision+CT | <1 year |
| 13 | Arai et al. [27] | 2015 | Japan | M | 61 | Yes | Lower lip | 1.5 cm | No | Excision | 2 years |
| 14 | Greenall et al. [28] | 2015 | UK | M | 81 | Unknown | Upper lip | Unknown | Buccal space, soft tissues of the right upper lip, retromolar trigone, pterygopalatine fossa, and apex of the infratemporal fossa | Palliative radiotherapy | (N/A) |
| 15 | Wetzel et al. [29] | 2015 | USA | M | 75 | Yes | Gingiva | Unknown | No | Excision | N/A |
| 16 | Jawanda et al. [30] | 2018 | India | F | 40 | No | Buccal mucosa | 5 cm | Mandible and tragus | Excision | N/A |
| 17 | Lu et al. [16] | 2021 | China | F | 62 | Unknown | Soft palate | 2 cm | No | Excision | N/A |
| 18 | Di Cosola et al. [7] | 2021 | Italy | M | 71 | Yes | Upper lip | 1.8 cm | No | Excision | 3 years |
| 19 | Benedict et al. [31] | 2022 | USA | F | 7 | No | Upper lip | 1.2 cm | No | Excision | 1.5 years |
| 20 | Cunha et al. [32] | 2023 | Brazil | M | 59 | Yes | Tongue | Unknown | Bilateral cervical LN | Excision+CT+RT | 1 year |
| 21 | Katib et al. [33] | 2024 | KSA | M | 47 | Yes | Upper lip | 1 cm | No | Excision+RT | 1 year |
-
USA, United States of America; KSA, Kingdom of Saudi Arabia; M, male; F, female; UK, United Kingdom; PG, parotid gland; LN, lymph node; CT, chemotherapy; RT, radiotherapy; m, months; N/A, not applicable.
IOSC appears to occur more often in men, with patients ranging from 7 to 81 years old (mean age of 57). Lesions ranged in size from 1 to 5 cm (mean size 2.34 cm), with 33 % (8/21 cases) showing local spread or metastasis to lymph nodes or lungs. Smoking history is notable, with 42.86 % (9/21) of cases being smokers (Table 1). Seven of the 21 cases reported involved the lips between 2005 and 2024 (Table 1). Most cases involved male smokers in their 70s, with no documented occurrences of tumor recurrence. One case occurred in a child with squamous cell carcinoma (SCC) in the upper lip accompanied by a significant SC component [31].
Possible pathogenesis and underlying mechanisms of SC development
While Fordyce granules are considered the most likely risk factor or potential precancerous condition for SC, other pathogenic mechanisms may also be considered as other risk factors. Smoking may contribute to IOSC by inducing chronic inflammation, DNA damage, immune suppression, and altering sebaceous gland environments, promoting malignant transformation, especially when combined with other factors [34]. It has been also suggested that some extra-ocular SCs might originate from intraepidermal pluripotent neoplastic cells [12]. Intraepidermal pluripotent neoplastic cells, often presenting as pagetoid spread within the epidermis, are a significant feature in extra-ocular SC. These malignant cells can act as precursors or coexist with invasive SC, contributing to local recurrence and tumor progression. Their presence is associated with poorly differentiated, multicentric disease and increased aggressiveness [35]. Additionally, factors such as subcutaneous injections and sebaceous differentiation during inflammation seem to contribute to a specific anatomical predisposition for SC in the eyelids [13], [14], [15, 17]. Subcutaneous injections or inflammation can trigger oxidative stress, DNA damage, and the activation of pro-inflammatory pathways such as NF-κB, which may contribute to carcinogenesis [36]. Persistent inflammation can cause sebaceous gland hyperplasia, disrupted differentiation, and local immune suppression, creating conditions conducive to malignant transformation. Abnormal changes in sebaceous cells, coupled with mutations in critical genes like tumor suppressor-53 (TP53), can drive the development of tumors [36].
Although these theories are touched upon in the literature, the exact pathogenesis of SC still remains unclear, with possible contributions from other factors. Environmental and genetic influences may also play essential roles in the development of ocular and extra-ocular SC. Genetic mutations, for example, can drive unchecked growth of sebaceous gland cells. SC has been linked to inherited mutations associated with Muir-Torre Syndrome, a subtype of Lynch syndrome (HNPCC), which affects DNA mismatch repair genes (MSH2, MSH6, MLH1), and causes microsatellite instability, leading to genomic errors, promoting SC development alongside other malignancies [37]. Individuals with these genetic conditions face higher risks of developing both precancerous skin lesions and cancers of the reproductive and digestive systems [38]. The Mayo Muir-Torre syndrome algorithm assesses sebaceous neoplasms by evaluating factors like age, number of lesions, and personal/family history of Lynch syndrome-related cancers. A score ≥2 predicts Muir-Torre syndrome, aiding SC diagnosis by identifying patients with DNA mismatch repair mutations requiring genetic testing and cancer screening [39]. Evaluation using the Mayo Muir-Torre syndrome algorithm, as shown in Table 2, is necessary in cases with SC or suspected SC conditions. Some studies further suggest that mutations in specific genes, such as Patched-1 (PTCH1) and TP53, may contribute to SC development [40], as these genetic changes can impair normal cell function and promote tumor formation.
The Mayo Muir–Torre syndrome risk score.
| Risk factor | Scorea |
|---|---|
| Age at diagnosis of first sebaceous neoplasm | |
|
|
|
| ≥60 | 0 |
| <60 | 1 |
|
|
|
| Number of sebaceous neoplasms | |
|
|
|
| <2 | 0 |
| ≥2 | 2 |
|
|
|
| Personal history of HNPCC-related cancer | |
|
|
|
| No | 0 |
| Yes | 1 |
|
|
|
| Family history of HNPCC-related cancer | |
|
|
|
| No | 0 |
| Yes | 1 |
| Total MTS risk scorea | |
-
aA risk score of >2 is highly predictive of MTS.
Jayaraj et al. summarized the molecular landscape of SC, which provides essential insights into its aggressive behavior and potential therapeutic targets [41]. While they predominantly focus on ocular SC, the involvement of extra-ocular SC warrants consideration. However, due to the rarity of IOSC, a comprehensive review of its molecular characteristics remains challenging. Genetic alterations in key pathways, such as β-catenin, hedgehog signaling, cytochrome oxidase-2 (COX-2), epithelial growth factor receptor (EGFR), and TP53, contribute to SC progression and poor prognosis. Unlike other skin cancers, SC lacks ultraviolet (UV) signature mutations in TP53, suggesting unique mutational mechanisms [42]. Next-generation sequencing has identified actionable mutations in genes like Phosphatase and Tensin Homolog (PTEN), Erb-B2 Receptor Tyrosine Kinase 2 (ERBB2), and Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), enabling personalized therapeutic approaches [43], 44]. Epigenetic modifications, including DNA promoter methylation of genes like E-cadherin and Cyclin-Dependent Kinase Inhibitor 2A (CDKN2A), and microRNA dysregulation play pivotal roles in SC pathogenesis by influencing cell adhesion, signaling, and metastasis [45]. Epithelial-mesenchymal transition (EMT), driven by markers such as E-cadherin, vimentin, and Zinc Finger E-Box Binding Homeobox 2 (ZEB2), further underscores the tumor’s aggressiveness and metastatic potential [46].
Molecular chaperones like X-linked inhibitor of apoptosis protein (XIAP) and Bcl-2-Associated Athanogene 3 (BAG3) are implicated in promoting tumor survival and proliferation [47], 48]. Their proteins promote SC survival, proliferation, and metastasis by inhibiting apoptosis, enhancing stress response via autophagy, stabilizing anti-apoptotic proteins, and regulating cytoskeletal dynamics, contributing to chemoresistance and aggressive tumor behavior. Moreover, cancer stem cell markers (e.g. CD44, CD33) are associated with poor prognosis in SC [40]. CD33 and CD44 are driving SC growth, metastasis, and resistance to therapies. While CD33 aids immune evasion and enhances proliferation, CD44 supports cell adhesion, migration, and tumor relapse.
Studies reveal that SC may also exhibit PD-L1 expression on tumor cells and PD-1 on tumor-infiltrating lymphocytes (TILs) in 46 % of cases, indicating immune evasion within an active yet suppressed tumor microenvironment [41].
Prolonged exposure to sunlight and ultraviolet radiation (UVR) are well-established risk factors for skin cancers and have occasionally been associated with SC. In the United States, approximately 78 % of SC cases have been reported in areas with high sun exposure, with 85 % of these cases diagnosed in individuals of white race. Population-level analyses have shown a link between elevated ambient UVR and increased SC risk in both individuals with and without Muir–Torre syndrome [49], 50]. However, this correlation is less evident in other racial and ethnic groups [49]. Sun exposure and UVR are more frequently associated with extraoral SC than IOSC. Current evidence does not support a direct association between UVR and the development of SC in the oral cavity. The rarity of oral SC and the protective environment of the oral cavity against UV exposure may not contribute to its pathogenesis. Additionally, individuals who received radiotherapy (RT) for retinoblastoma were found to have a second cancer within 15 years [50], highlighting the need for careful monitoring for SC in patients with RT history. Despite various theories and mechanisms, the precise cause of IOSC remains uncertain due to the limited number of reported cases.
Clinical diagnosis of IOSC
The diagnosis of IOSC is mainly made through a physical examination. Lesion detection may arise from patient self-observation, often as a slow-growing, chronic lesion, or during a clinician’s routine exam. Clinically, IOSC commonly presents as an ulcerated, exophytic, irregularly shaped, and indurated mass within the oral cavity or nearby areas, including the lips. Although typically painless, patients frequently seek medical attention as the mass enlarges, often due to cosmetic concerns. The lesion’s specific features help differentiate between benign-appearing and malignant growths. However, IOSC may sometimes be misdiagnosed as benign conditions, such as sebaceous adenoma or hyperplasia, necessitating additional diagnostic methods. Unfortunately, imaging techniques are not consistently helpful in diagnosis, except for orbital and peri-orbital/ocular lesions. Patients presenting with periorbital SC should undergo computed tomography (CT) or magnetic resonance imaging (MRI) scans. Imaging is also valuable for assessing lymph node status for potential extension and for ruling out distant metastasis [31], 51]. In patients with confirmed nodal involvement or suspected Muir–Torre syndrome, CT–Positron emission tomography (PET) has been identified as a reliable imaging modality for detecting any metastasis [52]. Because IOSC might be clinically diagnosed as another type of skin cancer such as BCC or SCC, a biopsy from the lesion is always recommended. The choice of biopsy technique is determined by factors, including the size, location, and clinical judgment of the surgeon or clinician. Excisional and punch biopsy or local resection are common clinical procedures used by most surgeons [53]. These procedures are preferred for their ability to provide an accurate diagnosis, achieve complete tumor removal, and preserve surrounding healthy tissues. However, they have limitations, including potential recurrence, missed metastases, cosmetic or functional issues, misdiagnosis from small samples, and the need for additional interventions to ensure comprehensive management [54].
The efficacy of sentinel lymph node biopsy (SLNB) in SC remains uncertain due to limited data. Studies show a modest yield, with 7.5 % positivity in a 2023 analysis of 149 cases, suggesting its potential utility in detecting nodal metastasis [55], 56]. However, false-negative results and cases of recurrence despite negative SLNB highlight its limitations. While SLNB may identify metastases not detectable via imaging, it cannot guarantee the absence of future nodal involvement.
Histopathological features of IOSC
The typical histopathological features of IOSC include nodules or sheets of neoplastic cells within a fibrovascular stroma, infiltrating deeply and demonstrating sebaceous and squamous differentiation. Histologically, SCs are classified as well-differentiated, moderately differentiated, or poorly differentiated (Figure 1) [5]. Well-differentiated tumors resemble sebocytes, featuring vacuolated, foamy cytoplasm [18]. Moderate differentiation shows anaplastic cells interspersed with areas of differentiation, while poorly differentiated lesions lack lobules and display sebaceous and squamous differentiation, with large cytoplasm, prominent nucleoli, and mitotic figures [57], 58]. SCs can occur within sebaceous neoplasms linked to visceral carcinomas in Muir-Torre syndrome, as illustrated in (Table 2) [7]. SC must be differentiated from BCC with sebaceous differentiation (BCCSD) and SCC with hydropic degeneration. BCCSD shows superficial, plate-like basaloid or squamoid cell proliferation attached to the epidermis [5], 31], without significant atypia or atypical mitoses. In these lesions, clusters of mature cells are abruptly interspersed among basaloid nests [8], 57]. Differentiating the two conditions is challenging, as sebaceous differentiation patterns may overlap [7]. Diagnosis can be supported by lipophilic stains on frozen sections or immunostains for epithelial membrane antigen (EMA) and S-100 (Table 3). SC typically shows diffuse EMA positivity, while BCCSD reacts only in sebaceous-differentiated areas [59], [60], [61]. Additionally, androgen receptors (ARs) are noted to be a more reliable marker of sebaceous differentiation, with strong nuclear AR immunoreactivity in basaloid and sebaceous cells, as well as distinct EMA reactivity [62]. Other studies report that SC may test negative for Periodic Acid Schiff (PAS) and S100 but positive for EMA, aiding in differentiation [22], 62]. Pronounced cytological atypia and atypical mitoses are also observed, with neoplastic cells testing positive for cytokeratin (CK) and EMA but negative for S-100 [60], 61], 63]. A study by Plaza et al. assessed the efficacy of IHC in diagnosing SC using a broad panel of markers, analyzing 27 SC cases and controls (21 BCC and 22 SCC cases) [64]. Adipophilin emerged as a highly sensitive and reliable marker, expressed in all SC cases but absent in controls, making it valuable for distinguishing SC. Other markers like PGRMC1, SQS, and ABHD5 showed lower sensitivity. Ber-EP4 was also found to be common in BCC but negative in SC [64]. IOSC also should be differentiated from adenoid cystic carcinoma and melanoma. Adenoid cystic carcinoma exhibits cribriform architecture and is smooth-muscle active (SMA) positive while melanoma shows atypical melanocytes and is positive for S100 and HMB-45. It also lacks sebaceous differentiation.
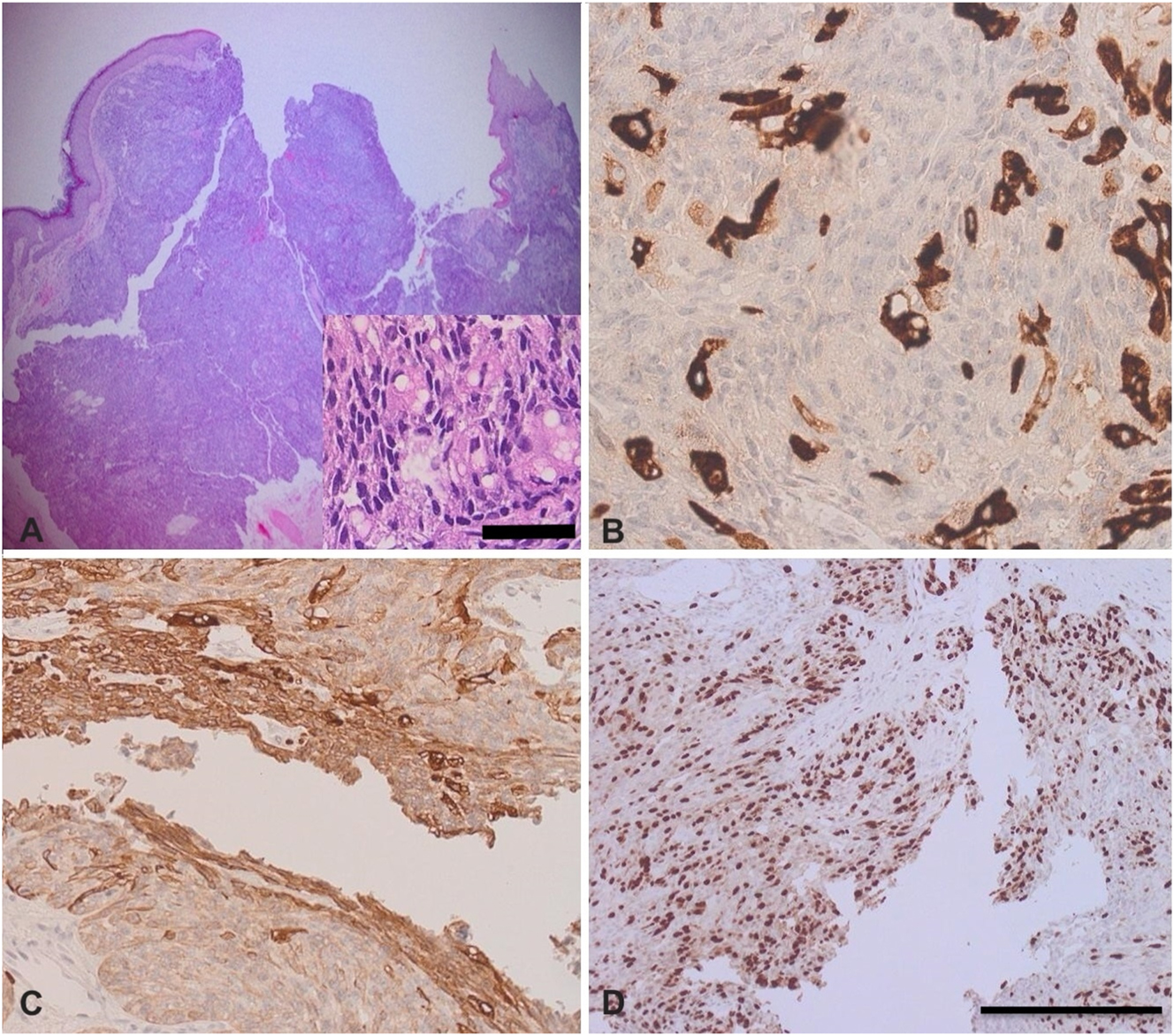
The histological features of sebaceous carcinoma (SC). (A, B) H&E-stained sections showing a dermal-based malignant sebocytic neoplasm arranged in nodules and sheets-like pattern; (B) SC with cytokeratin stain; (C) SC with epithelial membrane antigen (EMA) stain; (D) SC with Ki-67 proliferative index shows high proliferation rate. This is an original image provided by the corresponding author (Yousef Katib) (scale bar for the inserted image=25 μm, scale bar for other images=40 μm).
Three commonly used IHC markers in the differential diagnosis of SC.
| EMA | Ber-Ep4 | AR | Adipophilin | p53 | ERBB2 | |
|---|---|---|---|---|---|---|
| Sebaceous carcinoma (SC) | + | ± | + | + | + | + |
| Basal cell carcinoma (BCC) | – | + | ± | – | ± | – |
| Squamous cell carcinoma (SCC) | + | – | – | – | ± | – |
-
EMA, epithelial membrane antigen; AR, androgen receptor; ERBB2, erythroblastic leukemia viral oncogene homolog.
Post-diagnosis clinical staging and treatment approaches
After diagnosing IOSC, treatment planning should consider patient-specific factors such as age, overall health, tumor location, and cancer stage. Accurate staging is essential, as it assesses the cancer’s extent, guides treatment choices, and provides prognostic insights. SC staging primarily focuses on ocular and periocular locations, with different systems based on anatomical sites; the American Joint Committee on Cancer (AJCC) stages SC in the head and neck region similarly to skin SCC [35]. Staging considers tumor size, local invasion, nodal involvement, and metastasis (TNM system). Accurate staging guides treatment decisions, balancing surgery, RT, and systemic therapies based on disease extent, ensuring optimal outcomes while minimizing morbidity. Treatment options for SC range from wide local excision (WLE) to pre- and postoperative RT, with or without cryotherapy/chemotherapy [35], 53]. WLE is commonly used and effective; a study involving 14 SC patients showed a 36 % local recurrence rate with 1–3 mm margins, but no recurrence with 5 mm margins [65]. Although WLE has been a standard approach for SC management, Mohs micrographic surgery (MMS) offers more advantages such as complete margin assessment and tissue preservation. MMS is a precise, tissue-sparing technique for SC, removing cancer layer by layer with microscopic examination, ensuring complete tumor removal [53]. A systematic review by Yadlapati et al. analyzed 70 studies published in the literature and found that WLE has been preferred in 32 studies, MMS in 29, and both in nine [66]. Recurrence rates were lower with MMS: local (7 vs. 24 %), regional (4 vs. 13 %), and distant (4.5 vs. 11 %). Patients treated with MMS were significantly less likely to experience recurrence. RT serves as an alternative for patients who decline surgery, though reports of its postoperative use in IOSC are limited. RT cannot be considered as a primary treatment for SC. Positive responses to RT in ocular SC have been observed in initial studies [67]. For example, Yen et al. reported successful responses in two SC patients treated with over 55 Gy of radiation, with no recurrences during 40–45 months of follow-up [68]. However, some studies note a significant recurrence rate following RT [67]. RT is frequently used as an adjuvant therapy for advanced-stage cancer or for palliative purposes.
The standard treatment for IOSC typically involves WLE with appropriate margins, with or without regional lymph node dissection. The use of postoperative RT or chemotherapy remains a topic of debate [1], 69], 70]. While WLE is effective, it poses challenges in the oral cavity due to its complex anatomy, where achieving adequate margins without functional or cosmetic impairment can be difficult. Careful surgical planning is essential to balance tumor clearance with functional preservation [35]. A study comparing MMS and WLE for SC demonstrated that MMS for IOSC significantly lowers recurrence rates [71]. Local recurrence was around 15 % with MMS vs. 40 % with WLE, while regional and distant metastasis rates were also lower with MMS compared to WLE. Despite its advantages, MMS requires specialized expertise and may encounter challenges with SC due to “skip lesions” or discontinuous tumor spread, which can complicate margin evaluation. Nevertheless, MMS remains highly effective for local control and tissue preservation in managing oral SC.
Cryotherapy and chemotherapy, combined with standard surgical approaches, have been explored as treatment options for SC. A retrospective study by Kaliki et al. demonstrated systemic chemotherapy’s safety, achieving an average tumor reduction of more than 70 % [11]. Immunotherapy has also shown promise, as evidenced by a case where pembrolizumab, a PD-1 inhibitor, led to positive clinical and radiographic responses [72]. In metastatic SC, agents like mitomycin C, cisplatin, and 5-fluorouracil have been utilized [73]. Additionally, cetuximab, targeting the EGFR, has shown potential for unresectable SC [73]. Nevertheless, the efficacy of cryotherapy and chemotherapy in IOSC has not been explored yet.
Prognosis and outcomes
Regardless of tumor location, SC is considered an aggressive malignancy with risks of metastasis and fatality, making early diagnosis and timely treatment essential to prevent spread [69], 70]. Prognosis is affected by factors such as tumor location, size, clinical stage, and the treatment approach used [70]. Studies indicate that orbital SC is generally less aggressive than extra-ocular or IOSC, with a rare occurrence of metastasis [70]. In a study of over 2,400 SC cases, none of the orbital tumors metastasized to locoregional lymph nodes, while two out of five extra-ocular SC cases did [69]. A 2021 systematic review and meta-analysis by Desiato et al. examined 1,333 cases of SC affecting the eyelid [74]. The study reported recurrence in 16 %, metastasis in 12 %, and tumor-related mortality in 6 %. Notably, the average delay in diagnosing SC was around 15 months. Incomplete assessment of circumferential peripheral and deep margins, along with larger lesion size, were identified as independent risk factors for recurrence, while anatomic subtype and Muir-Torre syndrome status were not significant predictors [75].
For IOSC cases, post-surgical follow-up typically involves biannual visits for three years, followed by annual checkups [53].
Conclusions
Diagnosing IOSC is challenging due to its resemblance to other sebaceous lesions and limited understanding of its development. Fordyce granules in the oral mucosa may indicate a precancerous condition for IOSC and genetic association is commonly documented in SC. Diagnosis relies on clinical evaluation, histopathology, biopsy, and IHC staining to distinguish it from similar lesions. Imaging aids in staging and assessing metastasis, especially in ocular cases. Treatment typically involves wide local excision, with RT or CT in advanced cases. Early detection, regular follow-up, and further research are essential to improve patient outcomes. Further studies including wide genomic testing are advised to explore the genetic mutation associated with this condition.
Acknowledgments
Special thanks to the deanship of scientific research and innovation, ministry of education, Saudi Arabia.
-
Research ethics: Not applicable.
-
Informed consent: Not required.
-
Author contributions: Yousef Katib has critically reviewed and approved the final draft and is responsible for the content and similarity index of the manuscript.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interests: The corresponding author has no relevant conflict of interest to disclose.
-
Research funding: None.
-
Data availability: Not applicable.
References
1. Bailet, JW, Zimmerman, MC, Arnstein, DP, Wollman, JS, Mickel, RA. Sebaceous carcinoma of the head and neck. Case report and literature review. Arch Otolaryngol Head Neck Surg 1992;118:1245–9. https://doi.org/10.1001/archotol.1992.01880110113020.Suche in Google Scholar PubMed
2. Dasgupta, T, Wilson, LD, Yu, JB. A retrospective review of 1349 cases of sebaceous carcinoma. Cancer 2009;115:158–65. https://doi.org/10.1002/cncr.23952.Suche in Google Scholar PubMed
3. Wu, A, Rajak, SN, Chiang, CJ, Lee, WC, Huilgol, SC, Selva, D. Epidemiology of cutaneous sebaceous carcinoma. Australas J Dermatol 2021;62:57–9. https://doi.org/10.1111/ajd.13387.Suche in Google Scholar PubMed
4. Alawi, F, Siddiqui, A. Sebaceous carcinoma of the oral mucosa: case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;99:79–84. https://doi.org/10.1016/j.tripleo.2004.05.007.Suche in Google Scholar PubMed
5. Handschel, J, Herbst, H, Brand, B, Meyer, U, Piffko, J. Intraoral sebaceous carcinoma. Br J Oral Maxillofac Surg 2003;41:84–7. https://doi.org/10.1016/s0266-4356(03)00036-6.Suche in Google Scholar PubMed
6. Esnal Leal, F, García-Rostan y Pérez, GM, Garatea Crelgo, J, Gorriarán Terreros, M, Arzoz Sainz de Murieta, E. [Sebaceous carcinoma of salivary gland. Report of two cases of infrequent location]. An Otorrinolaringol Ibero Am 1997;24:401–13 [Article in Spanish].Suche in Google Scholar
7. Di Cosola, M, Spirito, F, Ambrosino, M, Somma, P, Santarelli, A, Staibano, S, et al.. Sebaceous carcinoma of the lip: a case report and review of the literature. J Med Case Rep 2022;16:241. https://doi.org/10.1186/s13256-022-03435-2.Suche in Google Scholar PubMed PubMed Central
8. Lee, MJ, Kim, YC, Lew, W. A case of superficial epithelioma with sebaceous differentiation. Yonsei Med J 2003;44:347–50. https://doi.org/10.3349/ymj.2003.44.2.347.Suche in Google Scholar PubMed
9. Tripathi, R, Chen, Z, Li, L, Bordeaux, JS. Incidence and survival of sebaceous carcinoma in the United States. J Am Acad Dermatol 2016;75:1210–5. https://doi.org/10.1016/j.jaad.2016.07.046.Suche in Google Scholar PubMed
10. Owen, JL, Kibbi, N, Worley, B, Kelm, RC, Wang, JV, Barker, CA, et al.. Sebaceous carcinoma: evidence-based clinical practice guidelines. Lancet Oncol 2019;20:e699–714. https://doi.org/10.1016/s1470-2045(19)30673-4.Suche in Google Scholar
11. Kaliki, S, Morawala, A, Krishnamurthy, A, Jajapuram, SD, Mohamedet, A. Sebaceous gland carcinoma: influence of age at presentation on outcomes. Ophthalmic Plast Reconstr Surg 2021;37:341–5. https://doi.org/10.1097/iop.0000000000001863.Suche in Google Scholar
12. Halperin, V, Kolas, S, Jefferis, KR, Huddleston, SO, Robinson, HB. The occurrence of Fordyce spots, benign migratory glossitis, median rhomboid glossitis, and fissured tongue in 2,478 dental patients. Oral Surg Oral Med Oral Pathol 1953;6:1072–7. https://doi.org/10.1016/0030-4220(53)90220-5.Suche in Google Scholar PubMed
13. Kyllo, RL, Brady, KL, Hurst, EA. Sebaceous carcinoma: review of the literature. Dermatol Surg 2015;41:1–15. https://doi.org/10.1097/dss.0000000000000152.Suche in Google Scholar PubMed
14. Gnepp, DR, Brannon, R. Sebaceous neoplasms of salivary gland origin. Report of 21 cases. Cancer 1984;53:2155–70. https://doi.org/10.1002/1097-0142(19840515)53:10<2155::aid-cncr2820531026>3.0.co;2-f.10.1002/1097-0142(19840515)53:10<2155::AID-CNCR2820531026>3.0.CO;2-FSuche in Google Scholar
15. Carlson, ER, Schlieve, T. Salivary gland malignancies. Oral Maxillofac Surg Clin North Am 2019;31:125–44. https://doi.org/10.1016/j.coms.2018.08.007.Suche in Google Scholar
16. Lu, Q, Fu, XY, Huang, Y. Sebaceous carcinoma of the right palate: case report and literature review. Gland Surg 2021;10:1819–25. https://doi.org/10.21037/gs-21-218.Suche in Google Scholar
17. Damm, DD, O’Connor, WN, White, DK, Drummond, JF, Morrow, LW, Kenady, DE. Intraoral sebaceous carcinoma. Oral Surg Oral Med Oral Pathol 1991;72:709–11. https://doi.org/10.1016/0030-4220(91)90016-6.Suche in Google Scholar
18. Abuzeid, M, Gangopadhyay, K, Rayappa, CS, Antonios, JI. Intraoral sebaceous carcinoma. J Laryngol Otol 1996;110:500–2. https://doi.org/10.1017/s0022215100134103.Suche in Google Scholar
19. Liu, CJ, Chang, KW, Chang, RC. Sebaceous carcinoma of buccal mucosa. Report of a case. Int J Oral Maxillofac Surg 1997;26:293–4. https://doi.org/10.1016/s0901-5027(97)80873-9.Suche in Google Scholar
20. Li, TJ, Kitano, M, Mukai, H, Yamashita, S. Oral sebaceous carcinoma: report of a case. J Oral Maxillofac Surg 1997;55:751–4. https://doi.org/10.1016/s0278-2391(97)90592-9.Suche in Google Scholar
21. Innocenzi, D, Balzani, A, Lupi, F, Panetta, C, Skroza, N, Cantoresi, F, et al.. Morpheaform extra-ocular sebaceous carcinoma. J Surg Oncol 2005;92:344–6. https://doi.org/10.1002/jso.20383.Suche in Google Scholar
22. Gomes, CC, Lacerda, JC, Pimenta, FJ, do Carmo, MA, Gomez, RS. Intraoral sebaceous carcinoma. Eur Arch Oto-Rhino-Laryngol 2007;264:829–32. https://doi.org/10.1007/s00405-007-0248-6.Suche in Google Scholar PubMed
23. Wang, H, Yao, J, Solomon, M, Axiotis, CA. Sebaceous carcinoma of the oral cavity: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;110:e37–40. https://doi.org/10.1016/j.tripleo.2010.04.027.Suche in Google Scholar PubMed
24. Oshiro, H, Iwai, T, Hirota, M, Mitsudo, K, Tohnai, I, Minamimoto, R, et al.. Primary sebaceous carcinoma of the tongue. Med Mol Morphol 2010;43:246–52. https://doi.org/10.1007/s00795-010-0521-4.Suche in Google Scholar PubMed
25. Somashekara, KG, Lakshmi, S, Priya, NS. A rare case of sebaceous adenoma of the palate, with literature review. J Laryngol Otol 2011;125:750–2. https://doi.org/10.1017/s0022215111000582.Suche in Google Scholar
26. Rowe, ME, Khorsandi, AS, Urken, GR, Wenig, BM. Intraoral sebaceous carcinoma metastatic to the lung and subcutis: case report and discussion of the literature. Head Neck 2016;38:E20–4. https://doi.org/10.1002/hed.24091.Suche in Google Scholar PubMed
27. Arai, N, Tomihara, K, Atakei, R, Noto, Z, Wada, S, Noguchi, M. A case of sebaceous carcinoma of the lower lip. Jpn J Oral Maxillofac Surg 2015;61:217–21. https://doi.org/10.5794/jjoms.61.217.Suche in Google Scholar
28. Greenall, CJ, Drage, NA. Sebaceous carcinoma of the lip: comparing normal lip and cheek anatomy with the imaging features of a rare cutaneous malignancy. Ultrasound 2015;23:126–9. https://doi.org/10.1177/1742271x15569295.Suche in Google Scholar
29. Wetzel, S, Pacelli, P, Reich, R, Freedman, P. Sebaceous carcinoma of the maxillary gingival: first reported case involving the gingiva. Oral Surg Oral Med Oral Pathol Oral Radiol 2015;120:e1–3. https://doi.org/10.1016/j.oooo.2014.10.018.Suche in Google Scholar PubMed
30. Jawanda, MK, Subramanyam, RV, Grewal, H, Anandani, C, Narula, R. Intraoral sebaceous carcinoma: case report of a rare tumor emphasizing the histopathological differential diagnosis. Case Rep Dent 2018;2018:3054931–6. https://doi.org/10.1155/2018/3054931.Suche in Google Scholar PubMed PubMed Central
31. Benedict, K, Al Hmada, Y, Gordon, C, Hoppe, I. Squamous cell carcinoma admixed with sebaceous carcinoma of upper lip in a 7-year-old female. Ped Hem Onco J 2022;7:126–9. https://doi.org/10.1016/j.phoj.2022.08.002.Suche in Google Scholar
32. Cunha, JLS, de Almeida, OP, de Carvalho, MGF, Soares, CD. Intraoral sebaceous carcinoma: a rare presentation on the tongue and review of the literature. Oral Oncol 2024;148:106647. https://doi.org/10.1016/j.oraloncology.2023.106647.Suche in Google Scholar PubMed
33. Katib, Y, Essatari, M. Case report: a rare case of oral spaceous carcinoma in the upper lip. Pathol Oncol Res 2024;30:1611968. https://doi.org/10.3389/pore.2024.1611968.Suche in Google Scholar PubMed PubMed Central
34. Tolkachjov, SN, Schmitt, AR, Muzic, JG, Weaver, AL, Baum, CL. Incidence and clinical features of rare cutaneous malignancies in Olmsted County, Minnesota, 2000 to 2010. Dermatol Surg 2017;43:116–24. https://doi.org/10.1097/dss.0000000000000936.Suche in Google Scholar
35. Dowell-Esquivel, C, Lee, R, DiCaprio, RC3rd, Nouri, K. Sebaceous carcinoma: an updated review of pathogenesis, diagnosis, and treatment options. Arch Dermatol Res 2023;316:55. https://doi.org/10.1007/s00403-023-02747-7.Suche in Google Scholar PubMed
36. Zouboulis, CC, Coenye, T, He, L, Kabashima, K, Kobayashi, T, Niemann, C, et al.. Sebaceous immunobiology – skin homeostasis, pathophysiology, coordination of innate immunity and inflammatory response and disease associations. Front Immunol 2022;13:1029818. https://doi.org/10.3389/fimmu.2022.1029818.Suche in Google Scholar PubMed PubMed Central
37. Everett, JN, Raymond, VM, Dandapani, M, Marvin, M, Kohlmann, W, Chittenden, A, et al.. Screening for germline mismatch repair mutations following diagnosis of sebaceous neoplasm. JAMA Dermatol 2014;150:1315–21. https://doi.org/10.1001/jamadermatol.2014.1217.Suche in Google Scholar PubMed PubMed Central
38. Roberts, ME, Riegert-Johnson, DL, Thomas, BC, Rumilla, KM, Thomas, CS, Heckman, MG, et al.. A clinical scoring system to identify patients with sebaceous neoplasms at risk for the Muir-Torre variant of Lynch syndrome. Genet Med 2014;16:711–6. https://doi.org/10.1038/gim.2014.19.Suche in Google Scholar PubMed
39. Eiger-Moscovich, M, Eagle, RCJr, Shields, CL, Racher, H, Lally, SE, Silkiss, RZ, et al.. Muir-torre syndrome associated periocular sebaceous neoplasms: screening patterns in the literature and in clinical practice. Ocul Oncol Pathol 2020;6:226–37. https://doi.org/10.1159/000504984.Suche in Google Scholar PubMed PubMed Central
40. Kim, YS, Park, GS, Chung, YJ, Lee, JH. Whole-exome sequencing of secondary tumors arising from nevus sebaceous revealed additional genomic alterations besides RAS mutations. J Dermatol 2023;50:1072–5. https://doi.org/10.1111/1346-8138.16784.Suche in Google Scholar PubMed
41. Jayaraj, P, Ray, D, Goel, K, Singh, A, Kant, N, Sen, S. Molecular landscape of eyelid sebaceous gland carcinoma: a comprehensive review. Indian J Ophthalmol 2024;72:1393–403. https://doi.org/10.4103/ijo.ijo_167_24.Suche in Google Scholar
42. Hussain, RM, Matthews, JL, Dubovy, SR, Thompson, JM, Wang, G. UV-independent p53 mutations in sebaceous carcinoma of the eyelid. Ophthal Plast Reconstr Surg 2014;30:392–5. https://doi.org/10.1097/iop.0000000000000121.Suche in Google Scholar
43. Peterson, C, Moore, R, Hicks, JL, Morsberger, LA, De Marzo, AM, Zou, Y, et al.. NGS analysis confirms common TP53 and RB1 mutations, and suggests MYC amplification in ocular adnexal sebaceous carcinomas. Int J Mol Sci 2021;22:8454. https://doi.org/10.3390/ijms22168454.Suche in Google Scholar PubMed PubMed Central
44. Na, HY, Park, JH, Shin, SA, Lee, S, Lee, H, Chae, H, et al.. Targeted sequencing revealed distinct mutational profiles of ocular and extraocular sebaceous carcinomas. Cancers 2021;13:4810. https://doi.org/10.3390/cancers13194810.Suche in Google Scholar PubMed PubMed Central
45. Liau, JY, Liao, SL, Hsiao, CH, Lin, MC, Chang, HC, Kuo, KT. Hypermethylation of the CDKN2A gene promoter is a frequent epigenetic change in periocular sebaceous carcinoma and is associated with younger patient age. Hum Pathol 2014;45:533–9. https://doi.org/10.1016/j.humpath.2013.10.019.Suche in Google Scholar PubMed
46. Ribatti, D, Tamma, R, Annese, T. Epithelial-mesenchymal transition in cancer: a historical overview. Transl Oncol 2020;13:100773. https://doi.org/10.1016/j.tranon.2020.100773.Suche in Google Scholar PubMed PubMed Central
47. Takeda, H, Lyle, S, Lazar, AJF, Zouboulis, CC, Smyth, I, Watt, FM. Human sebaceous tumours harbor inactivating mutations in LEF1. Nat Med 2006;12:395–7. https://doi.org/10.1038/nm1386.Suche in Google Scholar PubMed
48. Xu, S, Moss, TJ, Laura Rubin, M, Ning, J, Eterovic, K, Yu, H, et al.. Whole-exome sequencing for ocular adnexal sebaceous carcinoma suggests PCDH15 as a novel mutation associated with metastasis. Mod Pathol 2020;33:1256–63. https://doi.org/10.1038/s41379-020-0454-y.Suche in Google Scholar PubMed
49. Sargen, MR, Mai, ZM, Engels, EA, Goldstein, AM, Tucker, MA, Pfeifferet, RM, et al.. Ambient ultraviolet radiation and sebaceous carcinoma incidence in the United States, 2000–2016. JNCI Cancer Spectr 2020;4:pkaa020. https://doi.org/10.1093/jncics/pkaa020.Suche in Google Scholar PubMed PubMed Central
50. Kivelä, T, Asko-Seljavaara, S, Pihkala, U, Hovi, L, Heikkonen, J. Sebaceous carcinoma of the eyelid associated with retinoblastoma. Ophthalmology 2001;108:1124–8. https://doi.org/10.1016/s0161-6420(01)00555-3.Suche in Google Scholar PubMed
51. Connor, M, Droll, L, Ivan, D, Cutlan, J, Weber, RS, Frank, SJ, et al.. Management of perineural invasion in sebaceous carcinoma of the eyelid. Ophthalmic Plast Reconstr Surg 2011;27:356–9. https://doi.org/10.1097/iop.0b013e3182163653.Suche in Google Scholar PubMed
52. Ishiguro, Y, Homma, S, Yoshida, T, Ohno, Y, Ichikawa, N, Kawamura, H, et al.. Usefulness of PET/CT for early detection of internal malignancies in patients with Muir-Torre syndrome: report of two cases. Surg Case Rep 2017;3:71. https://doi.org/10.1186/s40792-017-0346-7.Suche in Google Scholar PubMed PubMed Central
53. Takeuchi, D, Ishida, M, Yasuda, E, Ueda, K, Hirose, Y. Ocular and extraocular sebaceous carcinomas: a retrospective study with emphasis on the presence of in situ lesion and discussion and review of the histogenesis of extraocular sebaceous carcinoma. Oncol Lett 2023;26:337. https://doi.org/10.3892/ol.2023.13923.Suche in Google Scholar PubMed PubMed Central
54. Wang, S, Yang, M, Li, R, Bai, J. Current advances in noninvasive methods for the diagnosis of oral squamous cell carcinoma: a review. Eur J Med Res 2023;28:53. https://doi.org/10.1186/s40001-022-00916-4.Suche in Google Scholar PubMed PubMed Central
55. Maloney, NJ, Nguyen, KA, So, NA, Zaba, LC. Yield of sentinel lymph node biopsy in sebaceous carcinoma and predictors of advanced disease: a retrospective analysis of the National Cancer Database. J Am Acad Dermatol 2023;88:504–6. https://doi.org/10.1016/j.jaad.2022.07.015.Suche in Google Scholar PubMed
56. Ho, VH, Ross, MI, Prieto, VG, Khaleeq, A, Kim, S, Esmaeli, B. Sentinel lymph node biopsy for sebaceous cell carcinoma and melanoma of the ocular adnexa. Arch Otolaryngol Head Neck Surg 2007;133:820–6. https://doi.org/10.1001/archotol.133.8.820.Suche in Google Scholar PubMed
57. Pereira, PR, Odashiro, AN, Rodrigues-Reyes, AA, Correa, ZMS, de Souza Filho, JP, Burnier, MNJr. Histopathological review of sebaceous carcinoma of the eyelid. J Cutan Pathol 2005;32:496–501. https://doi.org/10.1111/j.0303-6987.2005.00371.x.Suche in Google Scholar PubMed
58. Muqit, MM, Roberts, F, Lee, WR, Kemp, E. Improved survival rates in sebaceous carcinoma of the eyelid. Eye 2004;18:49–53. https://doi.org/10.1038/sj.eye.6700523.Suche in Google Scholar PubMed
59. Friedman, KJ, Boudreau, S, Farmer, ER. Superficial epithelioma with sebaceous differentiation. J Cutan Pathol 1987;14:193–7. https://doi.org/10.1111/j.1600-0560.1987.tb01331.x.Suche in Google Scholar PubMed
60. Ohara, N, Taguchi, K, Yamamoto, M, Nagano, T, Akagi, T. Sebaceous carcinoma of the submandibular gland with high-grade malignancy: report of a case. Pathol Int 1998;48:287–91. https://doi.org/10.1111/j.1440-1827.1998.tb03907.x.Suche in Google Scholar PubMed
61. Ansai, S, Hashimoto, H, Aoki, T, Hozumi, Y, Aso, K. A histochemical and immunohistochemical study of extra-ocular sebaceous carcinoma. Histopathology 1993;22:127–33. https://doi.org/10.1111/j.1365-2559.1993.tb00090.x.Suche in Google Scholar PubMed
62. Mulay, K, White, VA, Shah, SJ, Honavar, SG. Sebaceous carcinoma: clinicopathologic features and diagnostic role of immunohistochemistry (including androgen receptor). Can J Ophthalmol 2014;49:326–32. https://doi.org/10.1016/j.jcjo.2014.04.004.Suche in Google Scholar PubMed
63. Siriwardena, BSMS, Tilakaratne, WM, Rajapakshe, RMSK. A case of sebaceous carcinoma of the parotid gland. J Oral Pathol Med 2003;32:121–3. https://doi.org/10.1034/j.1600-0714.2003.00037.x.Suche in Google Scholar PubMed
64. Plaza, JA, Mackinnon, A, Carrillo, L, Prieto, VG, Sangueza, M, Suster, S. Role of immunohistochemistry in the diagnosis of sebaceous carcinoma: a clinicopathologic and immunohistochemical study. Am J Dermatopathol 2015;37:809–21. https://doi.org/10.1097/dad.0000000000000255.Suche in Google Scholar PubMed
65. Dogru, M, Matsuo, H, Inoue, M, Okubo, K, Yamamoto, M. Management of eyelid sebaceous carcinomas. Ophthalmologica 1997;211:40–3. https://doi.org/10.1159/000310872.Suche in Google Scholar PubMed
66. Yadlapati, S, Rosa-Nieves, PM, Mehta, N, Merritt, BG, Carrasquillo, OY. Treatment of sebaceous carcinoma with Mohs micrographic surgery versus wide local excision: a systematic review. Int J Dermatol 2024;63:1357–62. https://doi.org/10.1111/ijd.17283.Suche in Google Scholar PubMed
67. Belaid, A, Nasr, C, Benna, M, Cherif, A, Jmour, O, Bouguila, H, et al.. Radiation therapy for primary eyelid cancers in Tunisia. Asian Pac J Cancer Prev 2016;17:3643–6.Suche in Google Scholar
68. Yen, MT, Tse, DT, Wu, X, Wolfson, AH. Radiation therapy for local control of eyelid sebaceous cell carcinoma: report of two cases and review of the literature. Ophthalmic Plast Reconstr Surg 2000;16:211–5. https://doi.org/10.1097/00002341-200005000-00008.Suche in Google Scholar PubMed
69. Bassetto, F, Baraziol, R, Sottosanti, MV, Scarpa, C, Montesco, M. Biological behavior of the sebaceous carcinoma of the head. Dermatol Surg 2004;30:472–6. https://doi.org/10.1097/00042728-200403000-00035.Suche in Google Scholar
70. Duman, DG, Ceyhan, BB, Celikel, T, Ahiskali, R, Duman, D. Extraorbital sebaceous carcinoma with rapidly developing visceral metastases. Dermatol Surg 2003;29:987–9. https://doi.org/10.1097/00042728-200309000-00026.Suche in Google Scholar
71. Zhou, C, Wu, F, Chai, P, Shi, Y, Ye, J, Shi, X, et al.. Mohs micrographic surgery for eyelid sebaceous carcinoma: a multicenter cohort of 360 patients. J Am Acad Dermatol 2019;80:1608–17.e1. https://doi.org/10.1016/j.jaad.2018.12.053.Suche in Google Scholar PubMed
72. Domingo-Musibay, E, Murugan, P, Giubellino, A, Sharma, S, Steinberger, D, Yuan, J, et al.. Near complete response to Pembrolizumab in microsatellite-stable metastatic sebaceous carcinoma. J Immunother Cancer 2018;6:58. https://doi.org/10.1186/s40425-018-0357-3.Suche in Google Scholar PubMed PubMed Central
73. Jung, YH, Woo, IS, Kim, MY, Han, CW, Rha, EY. Palliative 5-fluorouracil and cisplatin chemotherapy in recurrent metastatic sebaceous carcinoma: case report and literature review. Asia Pac J Clin Oncol 2016;12:e189–93. https://doi.org/10.1111/ajco.12088.Suche in Google Scholar PubMed
74. Desiato, VM, Byun, YJ, Nguyen, SA, Thiers, BH, Day, TA. Sebaceous carcinoma of the eyelid: a systematic review and meta-analysis. Dermatol Surg 2021;47:104–10. https://doi.org/10.1097/dss.0000000000002660.Suche in Google Scholar PubMed
75. Kibbi, N, Petric, UB, El-Banna, G, Beaulieu, DM, Rajan, N, Srivastava, D, et al.. Clinical outcomes in sebaceous carcinoma: a retrospective two-center cohort study. Dermatol Surg 2023;49:1122–7. https://doi.org/10.1097/dss.0000000000004016.Suche in Google Scholar
© 2025 the author(s), published by De Gruyter on behalf of Tech Science Press (TSP)
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Review Articles
- Targeting HER2 in metastatic urothelial carcinoma: a contemporary review
- Advances in research on the impact of exosomal miRNAs on liver metastasis of colorectal cancer
- Sebaceous carcinoma of the intraoral origin: a literature review
- Role of emerging theranostic technologies in precision oncology: revolutionizing cancer diagnosis and treatment
- Research Articles
- Half-body irradiation with dose escalation in the era of advanced systemic therapies: unveiling new therapeutic opportunities
- Characteristics of MMR protein expression in colorectal cancer and MMR gene variations in Vietnamese patients with Lynch syndrome
- BMPR1A promotes the proliferation of colorectal cancer cells through the activation of Smad1
- Construction and validation of a diagnostic model for cholangiocarcinoma based on tumor-educated platelet RNA expression profiles
- Prognostic implications of PCSK9 expression in HER2-positive breast cancer
- Rapid Communication
- Potential impact of hormone replacement therapy on the risk of hepatocellular carcinoma in women of the PLCO cohort
- Article Commentary
- “Plant-based and ketogenic diets as diverging paths to address cancer”: a commentary concerning the supposed superiority of a plant-based diet
- Novel insights into molecular landscape of advanced renal cell carcinoma
- Corrigendum
- Corrigendum to “Y27632 induces tongue squamous cell carcinoma cell apoptosis through MAPK-ERK/JNK signal”
Artikel in diesem Heft
- Frontmatter
- Review Articles
- Targeting HER2 in metastatic urothelial carcinoma: a contemporary review
- Advances in research on the impact of exosomal miRNAs on liver metastasis of colorectal cancer
- Sebaceous carcinoma of the intraoral origin: a literature review
- Role of emerging theranostic technologies in precision oncology: revolutionizing cancer diagnosis and treatment
- Research Articles
- Half-body irradiation with dose escalation in the era of advanced systemic therapies: unveiling new therapeutic opportunities
- Characteristics of MMR protein expression in colorectal cancer and MMR gene variations in Vietnamese patients with Lynch syndrome
- BMPR1A promotes the proliferation of colorectal cancer cells through the activation of Smad1
- Construction and validation of a diagnostic model for cholangiocarcinoma based on tumor-educated platelet RNA expression profiles
- Prognostic implications of PCSK9 expression in HER2-positive breast cancer
- Rapid Communication
- Potential impact of hormone replacement therapy on the risk of hepatocellular carcinoma in women of the PLCO cohort
- Article Commentary
- “Plant-based and ketogenic diets as diverging paths to address cancer”: a commentary concerning the supposed superiority of a plant-based diet
- Novel insights into molecular landscape of advanced renal cell carcinoma
- Corrigendum
- Corrigendum to “Y27632 induces tongue squamous cell carcinoma cell apoptosis through MAPK-ERK/JNK signal”

