Abstract
Metastatic urothelial carcinoma (mUC) is considered an incurable malignancy; however, patient outcomes are improving with the adoption of novel treatments. A significant portion of patients with UC have HER2 overexpression measured by immunohistochemistry (IHC) and/or fluorescence in situ hybridization (FISH) or HER2 genomic amplifications and/or mutations detected on next-generation sequencing, making HER2 an attractive therapeutic target. Several clinical trials have assessed the efficacy of HER2-targeted therapies in UC, and trastuzumab deruxtecan has recently been approved as a tumor-agnostic systemic therapy for metastatic solid tumors, including mUC, that are HER2-positive by IHC 3+ expression. Nonetheless, clinical trials of HER2-targeting therapies in mUC have used different assays for the measurement of HER2 positivity, which could affect results. This review summarizes past experiences and delves into the controversies and potential of HER2-targeting therapies, including antibodies, tyrosine kinase inhibitors, and antibody-drug conjugates, in mUC.
Introduction
Metastatic urothelial carcinoma (mUC) is an aggressive malignancy, with a reported five-year survival rate of under 10 % when distant metastases are present [1]. The classical treatment paradigm was based on frontline platinum-based chemotherapy [2]. Within the last decade, pembrolizumab and atezolizumab have also been approved as frontline therapy for platinum-ineligible patients, and avelumab switch maintenance was approved for patients with disease control on platinum-based chemotherapy [2]. The frontline mUC treatment paradigm has recently shifted, with improved survival outcomes with the combination of frontline enfortumab vedotin (EV) with pembrolizumab against platinum-based chemotherapy in the phase 3 EV-302 trial [3]. A median overall survival (OS) of 31.5 months was reported in EV-302, which represents a major advance for patients with mUC. However, this survival could be potentially further improved with more treatment options.
One potential therapeutic target is HER2, a member of the human epidermal growth factor receptor (HER) family [4]. Several HER2-guided therapies have been developed and approved for multiple cancers. These include monoclonal antibodies, tyrosine kinase inhibitors, and most recently antibody-drug conjugates (ADCs) [5] (Figure 1). Trastuzumab (the first approved HER2-targeting agent), pertuzumab, and margetuximab are all regulatory-approved antibodies targeting extracellular domains of HER2 to prevent com- and heterodimerization of receptors. Lapatinib, afatinib, and neratinib are tyrosine kinase inhibitors targeting the intracellular domain to block phosphorylation and the signaling cascade. More recently, ADCs, including trastuzumab emtansine (T-DM1), trastuzumab deruxtecan (T-DXd), and disitamab vedotin (DV) have provided promising data and have improved outcomes in HER2-positive cancers. In April 2024, T-DXd was granted accelerated approval by the US Food and Drug Administration (USFDA) for unresectable or metastatic HER2-positive by immunohistochemistry (IHC) 3+ solid tumors who have received prior systemic treatment without satisfactory alternative treatment options, making it the first tumor-agnostic HER2-directed approval [6].
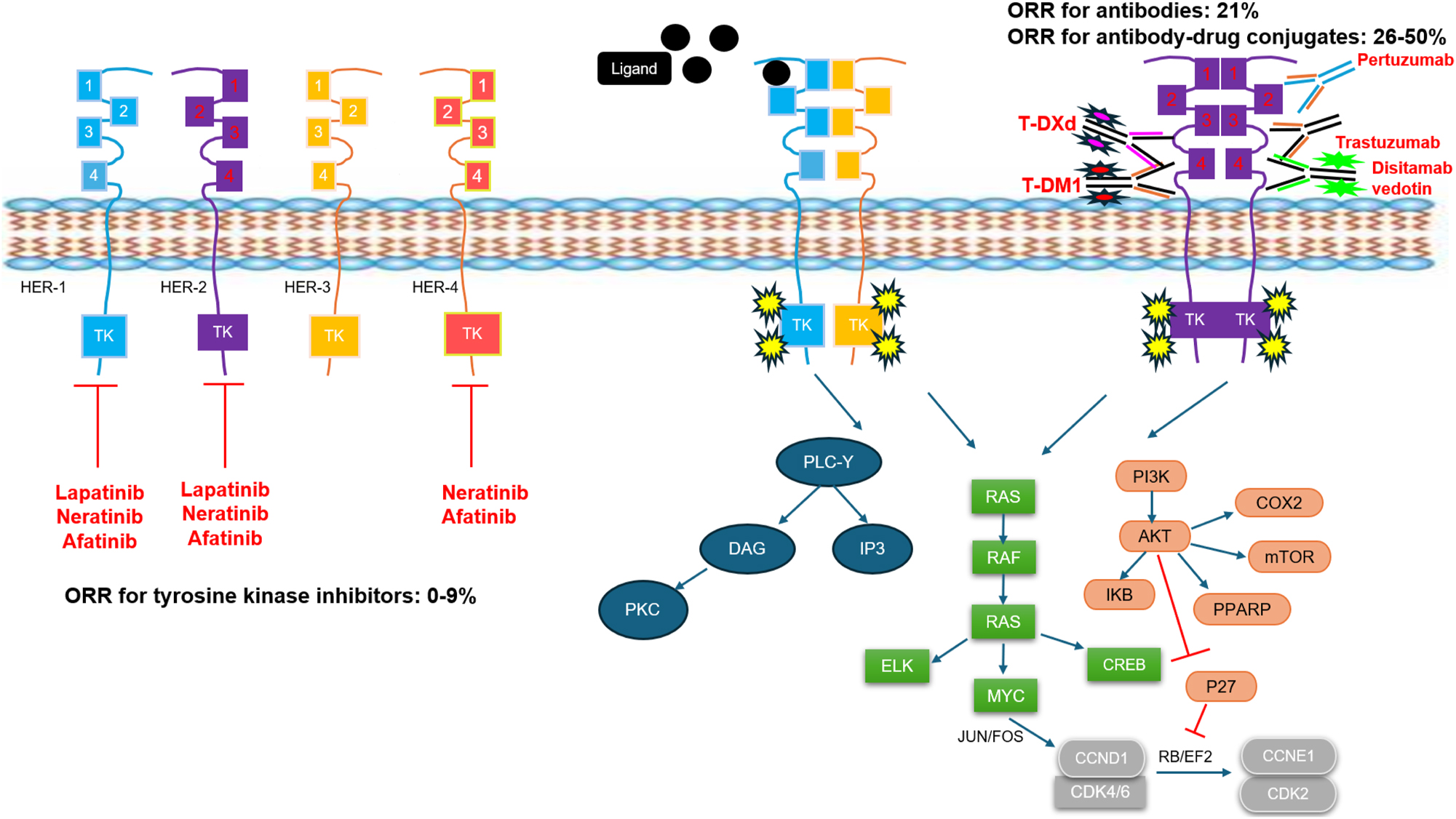
Diagram of the HER family signaling pathway, with the mechanisms of action for different HER2-targeting agents that have been evaluated in clinical trials for patients with metastatic urothelial carcinoma (mUC). Ranges for objective response rates (ORR) in clinical trials for mUC are listed for tyrosine kinase inhibitors, antibodies, and antibody-drug conjugates. All ORR ranges are for monotherapy, except for trastuzumab in combination with pertuzumab. Overall, antibody-drug conjugates have demonstrated the highest ORR.
In this review article, we discuss the various methods of determining HER2 positivity in UC, along with related controversies. We summarize the clinical trials involving HER2-targeted therapies for mUC. These trials have had varying degrees of success, from the early studies on trastuzumab to tyrosine kinase inhibitors and most recently HER2-targeting ADCs. We delve into the importance of standardizing the identification of HER2 positivity for patients with mUC, and we discuss where HER2-targeting agents may be sequenced amongst other therapies for patients with mUC. Finally, we provide an overview of novel options that may soon become available for these patients.
HER2 biology
The human epidermal growth factor receptor (HER), also known as ErbB, family is a subclass of receptor tyrosine kinase proteins that consists of four members: HER1 (ErbB1), HER2 (ErbB2), HER3 (ErbB3), and HER4 (ErbB4) [4]. This HER family of proteins comprises a cysteine-rich extracellular ligand-binding site and an intracellular domain with tyrosine kinase activity, connected by a single-chain transmembrane lipophilic component. The HER family signaling pathways lead to several downstream actions including cell proliferation, protein synthesis, cell cycle progression, transcription factor activation, and migration.
HER2, unlike its counterparts, does not bind to any direct ligand; instead, it heterodimerizes with HER1, HER3, and HER4 [4]. HER2 overexpression is attributed to somatic amplifications within the HER2 gene [7]. The most common mechanism of overexpression is through transcriptional upregulation leading to DNA amplification, transcription, and translation. When overexpressed, HER2 can also form homodimers. Dimerization of HER proteins leads to tyrosine kinase autophosphorylation and transphosphorylation at the C-terminal of both HER proteins, except for HER3, as it lacks an ATP binding site at the tyrosine kinase receptor. The signaling function is mediated through the kinase of the heterodimeric receptor with other HER families. The phosphorylated tyrosine kinase further activates downstream signaling, leading to increased cell proliferation and invasion through rat sarcoma/mitogen-activated protein kinase/extracellular signal-related kinases (RAS/MEK/ERK), phosphoinositide 3-kinase/protein kinase B (PI3K/AKT), phospholipase C/protein kinase C (PLC/PKC), signal transducer and activator of transcription (STAT), and the Par6-atypical PKC pathway (Figure 1) [4], 8]. Upregulation of PI3K and MAPK pathways from HER2 overexpression induces cyclin D1 for cell cycle progression in the S phase [9]. Furthermore, the PI3K/AKT pathway upregulates both downstream cyclooxygenase-2 (COX2), which promotes angiogenesis, and CXCR4, which promotes invasion, angiogenesis, and metastasis [10], [11], [12]. Overexpression of HER2 also leads to downregulation of pro-apoptotic transcription factors by ubiquitination of p53 [13]. Evasion of apoptosis is also maintained by HER2 by inhibiting the cGAS-STING signaling pathway which normally causes cells to enter senescence [14].
Besides HER2 amplification, HER2 mutations and fusions can also drive tumorigenic effects. In a pan-cancer analysis amongst 200,000 cases of 25 different cancers, HER2 mutations were found in over 2 % of cases, and more frequently in the bladder (8.3 %), compared to other organs [15]. This analysis further noted that the most frequent mutations were in the tyrosine kinase domain (58 %), followed by extracellular domains (37 %), and transmembrane domain (5 %). These mutations at the HER2 kinase domain allow for increased kinase activity for tumorigenicity [16], 17]. Fusion of HER2 is relatively uncommon and often coexists with HER2 amplification. In a retrospective analysis of the HER2 function genomic profile by next-generation sequencing (NGS), HER2-kinase domain fusion was encoded by exons 18–24 with TP53, APC, and CDK4 being the top three co-mutant genes. Furthermore, HER2 amplification combined with fusion had a worse prognosis compared to HER2 amplification alone [18].
Methods of HER2 testing
To test for HER2 expression in cancer cells, there are two approved methods by the American Society of Clinical Oncology (ASCO) and the College of American Pathologists (CAP) [19]. Immunohistochemistry (IHC) assigns scores based on the protein staining intensity and percentage of cancer cells stained by IHC and are reported as 3+ (HER2 positive), 2+ (HER2 equivocal), 1+ (HER2 negative), and 0 (HER2 negative) [20]. Fluorescence in situ hybridization (FISH) is used to help further classify a tumor sample that has IHC 2+ expression as HER2 positive or HER2 negative. FISH uses fluorescent probes to bind to chromosomes, in this instance focusing on chromosome 17, where the HER2 proto-oncogene is located. Based on expert consensus, the ratio of HER2 signals to chromosome enumeration probe 17 (CEP17) signals per cell determines HER2 positivity by FISH. HER2 positivity by FISH is defined as ≥6.0 HER2 signals per cell or HER2:CEP17 ratio ≥2.0 with an average of HER2 signals per cell >4.0 [20]. In addition to HER2 positive, an additional categorization is HER2 low, which is defined as IHC 1+ or IHC 2+ with negative FISH [21].
Guidelines for HER2 scoring have been clearly standardized for breast and gastric cancers, but there is no consensus guideline for UC, although gastric scoring is typically favored. Though IHC testing has been the clinical standard, substantial discordance amongst IHC 1/2+ scoring in real-world settings has been noted. One retrospective study observed <1 % agreement for 1+ and 3.6 % agreement for 2+ HER2 IHC staining between licensed pathologists [22]. Other studies have also reported intermediate IHC categories to be less reproducible compared to 0 and 3+ scoring [23], [24], [25], which is a potential concern for HER2-low categorization.
An alternative measurement of HER2 expression is by NGS, analyzing genetic changes in HER2, including mutations, amplifications, and fusions [26], 27]. One study evaluated HER2 amplification for breast and gastroesophageal cancer and found 98.4 % overall concordance with IHC/FISH and NGS [27]. In patients with HER2 IHC negative but FISH positive breast cancer, NGS demonstrated HER2 amplification concordant with FISH. In this study, some discrepancies in equivocal HER2 scoring by IHC and FISH that were not resolved by NGS were attributed to low tumor content or HER2 heterogeneity [28]. NGS has limitations given the inability to discern heterogeneity and inclusion of stromal cells in the analyzed specimens. NGS also has difficulty detecting low-level copy number gains and/or high-level amplification in samples with low neoplastic cell percentages [29].
HER2 expression in UC
Testing for HER2 expression of UC had not traditionally been performed in clinical practice. Frequently cited HER2 criteria in studies examining HER2 positivity for UC are extrapolated from the ASCO/CAP guidelines for breast and gastric cancers. However, many clinical studies defined their own criteria for HER2 positivity, such as using only IHC 3+ only or including IHC 2+ without confirmatory FISH testing, and various antibodies were used to stain for HER2 [30]. This induces some variability in the HER2-positive definition and HER2-positivity rates for UC.
Because of the subtle differences in classification by study, comparing the prevalence of HER2 expression between non-metastatic UC and mUC is difficult to determine [30]. A comparison of molecular profiling between bladder vs. non-bladder mUC suggests bladder UC may have a higher frequency of abnormal HER2 protein expression (p=0.04) and increased HER2 amplification (p=0.06) compared to non-bladder UC [31]. Overall, HER2 amplification and overexpression comprise a sizeable subset of all patients with UC with reported positivity rates of approximately 10–15 %. This rate is comparable to breast and gastric cancers, underlying the importance of targeted therapies for patients with HER2-expressing UC [30], 32]. Similarly, data comparing the concordance between HER2 overexpression by IHC and gene amplification by NGS or ISH/FISH for UC is lacking based on few studies that compared them directly [30]. From limited published studies, there is some concordance but wide variability, ranging from 68 to 91 %. Some patient tumors without HER2 overexpression have gene amplification and vice versa, suggesting the importance of both tests for assessing HER2 status [31], 33], 34].
Notably, HER2 staining patterns vary across tumor types. Comparing HER2-positive breast vs. gastric cancer tissue, gastric tumors have a higher incidence of tumor heterogeneity (<30 % of tumor cells) [35], and the gastric tumor cells tend to show incomplete, basolateral, or lateral staining in addition to incomplete membrane staining [36]. Like HER2-positive gastric cancer, HER2 protein expression in UC also appears to be heterogeneous. One study, which defined HER2 heterogeneity by IHC 2/3+ in 10–90 % of tumor cells, found 15 of 27 primary tumors (55.5 %) to be HER2-positive [37]. The HER2 staining pattern was noted to include expression in both the cell membrane and the cytoplasm for some UC samples (30 %, 13/43), while most samples had membranous staining only (70 %, 30/43).
HER2 protein overexpression has generally been linked to a worse prognosis in UC [38]. In a systematic review, HER2 overexpression was associated with more aggressive features of UC including carcinoma in situ, multifocal tumor, large tumor size, high tumor stage and grade, lymph node metastases, cancer progression, and recurrence [39]. However, the association between HER2-positive UC and decreased overall survival compared to HER2-negative UC was not demonstrated [39].
Compared to HER2 overexpression, HER2 mutations in UC have been less studied. In the Cancer Genome Atlas analysis, a comprehensive genetic analysis of 131 high-grade muscle-invasive bladder cancer (MIBC) tumors showed approximately 8 % had HER2 mutations [40]. In another comprehensive genomic profiling of 295 cases of mUC arising from the bladder, 49 cases exhibited HER2 alterations (17 %), including 29 cases with HER2 mutations, 29 cases with HER2 amplifications, and 5 cases containing both HER2 amplifications and mutations [41]. HER2 mutations in the extracellular domain were noted to be enriched in micropapillary histology (p<0.0001). Enrichment of HER2 mutations in the extracellular domain in micropapillary variant UC has been previously noted in another study [42]. There is currently no evidence to date correlating the overall prognosis of patients with UC with HER2 mutations.
HER2-targeting antibodies
Trastuzumab and pertuzumab
Trastuzumab was the first HER2-directed therapy approved by the USFDA in 1998 and European Medicines Agency in 2000 [43]. It binds to the extracellular domain IV of HER2 to prevent homodimerization [44]. It is regulatory-approved for HER2-positive breast, gastroesophageal, and colorectal cancers. Trastuzumab has been evaluated in combination with either chemotherapy or pertuzumab in patients with UC. Pertuzumab is an antibody that binds to extracellular domain II of HER2, preventing HER2 from forming heterodimers with other HER family receptors [45]. The trials enrolling patients with UC with these antibodies are summarized in Table 1.
Clinical trials involving HER2-targeting antibodies.
| Trial | Phase | Setting | HER2 positivity criteria | Number of patients with UC (number of HER2-positive patients) | Intervention | Notable results |
|---|---|---|---|---|---|---|
| NCT00238420 (RTOG 0524) [46], 47] | 1/2 | Patients with muscle-invasive bladder cancer not candidates for cystectomy | IHC 2/3+ | 66 (20) | Radiation and paclitaxel with trastuzumab for HER2-positive cohort vs. radiation and paclitaxel for HER2-negative cohort | Median OS 2.8 years (95 % CI, 1.1–4.4) for HER2-positive cohort vs. 2.0 years (95 % CI, 1.1–8.5) for HER2-negative cohort |
| Hussain et al. [48] | 2 | Frontline systemic | At least one of the following: IHC 2/3+, FISH positive on primary or metastatic tissue, or elevated serum HER2 extracellular domain | 44 (44) | Trastuzumab, carboplatin, paclitaxel, and gemcitabine | ORR 70 % median PFS 9.3 months Median OS 14.1 months |
| NCT01828736 (CVH-CT02) [49] | 2 | Frontline systemic | Both IHC 2/3+ and FISH+ | 61 (61) | Trastuzumab, cisplatin/carboplatin and gemcitabine vs. cisplatin/carboplatin and gemcitabine | ORR 65.5 vs. 53.2 % (p=0.39) median PFS 10.2 vs. 8.2 months (p=0.689) Median OS 15.7 vs. 14.1 months (p=0.684) |
| NCT02091141 (MyPathway HER-2 positive basket study) [50], 51] | 2 | Metastatic disease with no prior HER2-targeted therapy | Amplification, overexpression, or mutation determined by FISH, NGS, or IHC 3+ | 32 (32) 22 with HER2 amplified or overexpressed 10 with HER2 mutations |
Trastuzumab and pertuzumab | For HER2 amplified or overexpressed: ORR 21.1 % median PFS 3.4 months Median OS 8.6 months |
-
IHC, immunohistochemistry; XRT, radiation; FISH, fluorescent in situ hybridization; ORR, objective response rate; median PFS, median progression-free survival; median OS, median overall survival.
A phase 1/2 trial (NCT00238420) enrolled patients with MIBC, who were not cystectomy candidates, to receive daily radiation and concurrent weekly paclitaxel for seven weeks, with or without trastuzumab [46], 47]. Patients were stratified by HER2 positivity, with group 1 enrolling 20 patients with HER2 IHC 2/3+ tumors and group 2 enrolling 46 patients with HER2 IHC 0/1+ tumors. Patients in group 1 received paclitaxel and trastuzumab, whereas patients in group 2 received paclitaxel alone. The primary endpoint of treatment-related toxicity was similar between groups, with 35 % in group 1 and 30.4 % in group 2. The complete response (CR) rate for the two groups was also similar at 73 and 68 %, respectively. Median OS was 2.8 years (95 % CI, 1.1–4.4) for the first group and 2.0 years (95 % CI, 1.1–8.5) for the second group. The study concluded patients with HER2-positive UC could potentially benefit from trastuzumab without much added toxicity.
Trastuzumab has also been evaluated for mUC treatment. One phase 2 trial investigated the safety and efficacy of trastuzumab in combination with carboplatin, paclitaxel, and gemcitabine for HER2-positive patients with mUC as frontline systemic therapy. The primary endpoint was cardiac toxicity [48]. Of 109 patients screened, 57 patients were HER2-positive as defined by at least one of the following criteria: IHC 2/3+, FISH positive on either primary or metastatic tissue, or elevated serum HER2 extracellular domain. Notably, HER2-positive patients trended towards more liver and bone metastases, with a significantly greater median number of metastases (p=0.014). Amongst the 44 HER2-positive patients who were treated with combination therapy, the objective response rate (ORR) was 70 % (31/44) including 5 CR (11 %) median progression-free survival (PFS) was 9.3 months, and the median OS was 14.1 months. Ten patients (22.7 %) experienced cardiac toxicity, including two grade 3 events, which was acceptable by predetermined criteria. Two patients died from therapy-related events.
Furthermore, the efficacy of trastuzumab and platinum-based chemotherapy was assessed in a phase 2 randomized trial (NCT01828736) that evaluated cisplatin/carboplatin and gemcitabine with or without trastuzumab for HER2-positive mUC as frontline systemic therapy [49]. HER2 positivity was defined as having both IHC 2/3+ and FISH+. There were 75 of 563 screened patients (13.3 %) who met HER2+ criteria, and 29 patients were randomized into the chemotherapy arm and 32 patients into the trastuzumab with the chemotherapy arm. Between the two groups, there was no significant difference in the primary endpoint of median PFS (10.2 vs. 8.2 months, p=0.689), or in the secondary endpoints of median OS (15.7 vs. 14.1 months, p=0.684) or the ORR (65.5 vs. 53.2 %, p=0.39). In an exploratory analysis, patients who received trastuzumab with a cisplatin-based regimen demonstrated favorable median PFS (10.6 vs. 8.0 months) and median OS (33.1 vs. 9.5 months) than trastuzumab with a carboplatin-based regimen. In the trastuzumab combination arm, one patient had grade 3 cardiotoxicity, and one patient died from febrile neutropenia. The authors concluded the HER2 positivity rate was lower than expected due to stringent criteria, and a larger trial would have been necessary to establish benefit with the addition of trastuzumab.
Trastuzumab was evaluated in combination with pertuzumab in the tissue-agnostic MyPathway HER2-positive phase 2 basket study (NCT02091141) [50], 51]. HER2 positivity, which included amplification, overexpression, and mutations in this study, was determined by FISH, NGS, or IHC 3+. Overall, 346 patients were HER2-positive, including 22 HER2-amplified/overexpressed and 10 HER2-mutated UC. For 19 patients with HER2-amplified/overexpressed and KRAS wild-type UC, the ORR was 21.1 % (4/19, including 2 CR), median PFS was 3.4 months (95 % CI, 2.5–7.6), and median OS was 8.6 months (95 % CI, 3.4–28.1). For all patients in the trial with only HER2 mutations, the ORR was 6.0 % (5/83). Data for HER2 mutations in UC was not reported. The study overall showed this double HER2 antibody combination could be effective across different HER2-amplified/overexpressed tumor types, but less so for HER2 mutations alone.
HER2-targeting tyrosine kinase inhibitors
HER2-targeting tyrosine kinase inhibitors have been evaluated in patients with UC. These include lapatinib, afatinib, and neratinib, which have been regulatory approved for other cancers including lung and breast. The trials enrolling patients with UC with these tyrosine kinase inhibitors are summarized in Table 2.
Clinical trials involving HER2-targeting tyrosine kinase inhibitors.
| Trial | Phase | Setting | HER2 positivity criteria | Number of patients with UC (number of HER2-positive patients) | Intervention | Notable results |
|---|---|---|---|---|---|---|
| Wülfing et al. [52] | 2 | Second-line systemic | IHC 1/2/3+ | 59 (43) | Lapatinib | ORR 3 % for HER1/2+ median OS 30.3 weeks for HER1/2 IHC 2/3+ vs. 10.6 weeks for HER1/2 IHC 0/1+ (p=0.0001) |
| NCT00447226 [53] | 2 | Prior systemic therapy allowed | FISH probe ratio >2.0 | 9 (9) | Lapatinib | ORR 0 % |
| NCT00623064 (EORTC 30061) [54] | 1 | Frontline systemic | N/A | 17 | Lapatinib, gemcitabine, and cisplatin | ORR 59 % |
| NCT00949455 (LaMB) [55] | 3 | Maintenance therapy after frontline systemic chemotherapy | IHC 2/3+ | 232 (42) | Lapatinib vs. placebo | Median PFS 4.5 vs. 5.1 months (p=0.63) for HER1/2+ Median OS 12.6 vs. 12.0 months (p=0.80) for HER1/2+ |
| NCT01382706 (USC trial 4B-10-4) [56] | 2 | Second-line systemic | N/A | 15 | Lapatinib and docetaxel | ORR 8 % median PFS 2.0 months Median OS 6.3 months |
| Choudhury et al. [57] | 2 | Second-line systemic | N/A | 23 | Afatinib | ORR 8.6 % median PFS 1.4 months Median OS 5.3 months |
| NCT02780687 (LUX-Bladder 1 trial) [58] | 2 | Second-line systemic | Amplification by FISH, or mutation | 42 (8 with mutation, 20 with amplification, not mutually exclusive) | Afatinib | Median PFS 12.6 weeks (95 % CI, 7.1–22.7) for HER2 amplification cohort and 9.2 weeks (95 % CI, 6.4–16.1) for HER2 mutation cohort |
| NCT02465060 (EAY131) [59] | 2 | Prior systemic therapy allowed | Mutation | 40 (4) | Afatinib | ORR 0 % |
| NCT01953926 (SUMMIT) [60] | 2 | Prior systemic therapy allowed | Mutation | 18 (16) | Neratinib | ORR 0 % median PFS 1.8 months |
-
IHC, immunohistochemistry; FISH, fluorescent in situ hybridization; ORR, objective response rate; median PFS, median progression-free survival; median OS, median overall survival.
Lapatinib
Lapatinib is a tyrosine kinase inhibitor that targets the intracellular domains of both HER1 and HER2 kinase. Lapatinib was evaluated in several settings for mUC, including as monotherapy after progression on frontline chemotherapy, as maintenance monotherapy following frontline chemotherapy, and as a combination with chemotherapy.
A phase 2 trial first evaluated lapatinib as second-line therapy for mUC with prior progression on platinum-based chemotherapy for patients with either HER1 or HER2 expression [52]. HER1 and HER2 positivity were determined by IHC expression of ≥1+ of either receptor. A total of 59 patients were enrolled, of which 34 were evaluated for response. The primary endpoint of ORR ≥10 % was not met, with just 3 % partial response and 31 % stable disease reported. Patients with tumors that overexpressed (IHC 2/3+) either HER1 or HER2 had longer median OS compared to patients with tumors with lower expression (IHC 0/1+) (30.3 vs. 10.6 weeks, p=0.0001). This prompted lapatinib to be further evaluated as a therapy for UC.
A phase 2 basket trial (NCT00447226) for HER2-amplified (FISH+) treatment-refractory solid tumors treated patients with lapatinib for 12 weeks, and patients with stable disease were subsequently randomized evenly to either continue lapatinib or receive placebo [53]. Accrual was poor. Of 141 patients screened, 32 patients were enrolled, including 9 with mUC. Amongst patients with mUC, the ORR was 0 %, with only 33 % of patients achieving stable disease (SD) as the best response, indicating modest activity of lapatinib in non-breast tumors.
EORTC 30061 (NCT00623064) was a phase 1 trial of lapatinib combined with gemcitabine and cisplatin for mUC as frontline systemic therapy [54]. Overall, 17 patients regardless of HER1/2 expression were treated. The ORR was 59 % (1 CR and 9 partial responses [PR]), and four patients had SD. The study concluded the feasibility and tolerability of lapatinib in combination with gemcitabine/cisplatin.
LaMB was a phase 3, double-blind, randomized trial (NCT00949455) of lapatinib compared to placebo for maintenance following frontline chemotherapy for patients with both HER1 and HER2-positive mUC based on IHC 2/3+ [55]. Of 464 patients who were screened for HER1 or HER2 positivity, 232 patients were enrolled. The study produced negative results, with median PFS 4.5 vs. 5.1 months (HR 1.07; 95 % CI, 0.81–1.43; p=0.63) and median OS 12.6 months vs. 12.0 months (HR 0.96; 95 % CI, 0.70–1.31; p=0.80) for lapatinib vs. placebo, respectively. Subanalysis of these results also suggested that IHC determination of HER1/2 expression was not prognostic.
USC trial 4B-10-4 (NCT01382706) was a phase 2 trial that evaluated lapatinib combined with docetaxel as second-line chemotherapy for patients with mUC regardless of HER2 expression [56]. The trial was terminated prematurely due to missing the predetermined efficacy cutoff following the accrual of 15 patients. The ORR was 8 %, although one patient achieved CR. The median PFS and OS were 2.0 and 6.3 months, respectively. The combination of lapatinib and docetaxel was not recommended as mUC treatment based on these results.
Afatinib
Afatinib is an irreversible tyrosine kinase inhibitor of HER1, HER2, and HER4. Afatinib has been evaluated as monotherapy for patients with mUC refractory to chemotherapy.
A phase 2 trial evaluated afatinib monotherapy for 23 patients with refractory mUC irrespective of HER-positive status [57]. The study failed to meet its endpoint at interim analysis based on a 3-month PFS of 21.7 % (just five patients). The median PFS was 1.4 months and the median OS was 5.3 months. The ORR was 8.6 %, with 30.4 % of patients achieving SD. On subanalysis, the five patients without progression at 3 months exhibited either HER2 or HER3 amplification by FISH. The median PFS for the six patients with HER2/3 alterations was 6.6 months, compared to 1.4 months for patients without HER2/3 alterations (p<0.001). HER2 PCR and FISH assessments demonstrated high concordance, but IHC was poorly concordant with PCR and FISH.
The phase 2 LUX-Bladder 1 trial (NCT02780687) cohort A enrolled 34 patients with HER2/3 mutated or HER2 amplified mUC refractory to platinum chemotherapy to receive afatinib [58]. This cohort had an ORR of 5.9 %, and 44.1 % patients with SD. Both patients with PR exhibited HER2 amplification. The median PFS was 9.8 weeks, and the median OS was 30.1 weeks. On subanalysis, median PFS for patients with HER2 amplification was 12.6 weeks (95 % CI, 7.1–22.7), while the median PFS for patients with HER2 mutation was 9.2 weeks (95 % CI, 6.4–16.1). Overall, the study did not meet its prespecified endpoint, but afatinib demonstrated some efficacy for treatment of HER2 amplified mUC.
Subprotocol EAY131-B of the phase 2 NCI-MATCH (NCT02465060) precision medicine umbrella study enrolled 40 patients with HER2 single-nucleotide variants or insertions/deletions without evidence of gene amplification to receive afatinib [59]. The primary endpoint was to evaluate the ORR for patients with HER2 mutations treated with afatinib, with a threshold of 16 %. There were four patients with mUC, with two patients achieving SD and two patients with PD. For the entire cohort of 37 analyzable patients, the ORR was 2.7 %, and the 6-month PFS was 12.0 % (90 % CI, 5.6–25.8). Afatinib did not meet its prespecified threshold for antitumor activity in this study.
Neratinib
Neratinib is an irreversible HER1, HER2, and HER4 inhibitor. SUMMIT (NCT01953926) was a phase 2 basket trial evaluating neratinib for HER2/3-mutated cancers, and 141 patients were analyzed including 18 patients with mUC (16 with HER2 mutations, 2 with HER3 mutations) [60]. For the 16 patients with mUC with HER2 mutations, the ORR was 0 %, although three patients had SD. For patients with mUC, the median PFS was 1.8 months. Following these negative results, no expanded enrollment occurred.
HER2-targeting antibody drug conjugates
In contrast to the underwhelming efficacy of HER2-targeting antibodies and HER2-targeting tyrosine kinase inhibitors for the treatment of mUC, even for HER2-positive tumors, ADCs are becoming a very promising option for patients with mUC. With demonstrated efficacy in HER2-positive tumors, there is potential, as demonstrated in other cancers, for mUC patients without HER2-positive tumors. The trials enrolling patients with UC with these ADCs are summarized in Table 3.
Clinical trials involving HER2-targeting antibody-drug conjugates.
| Trial | Phase | Setting | HER2 positivity criteria | Number of patients with UC (number of HER2-positive patients) | Intervention | Notable results |
|---|---|---|---|---|---|---|
| NCT02999672 (KAMELEON) [61] | 2 | Metastatic disease with no standard therapies available | IHC 3+ in ≥30 % of tumor cells | 13 (13) | Trastuzumab emtansine | ORR 38.5 % median PFS 2.2 months (95 % CI, 1.2–4.3) Median OS 7.0 months (95 % CI, 3.8-NR) |
| NCT02277717 [62] | 1 | Metastatic disease with no standard therapies available | IHC 1/2/3+ | 16 (16) | Trastuzumab duocarmazine | ORR 25 % median PFS 4.0 months (95 % CI, 1.3–NR) |
| NCT04482309 (DESTINY-PanTumor02) [63] | 2 | Metastatic disease with at least one prior systemic therapy | IHC 2/3+ | 41 (41) | Trastuzumab deruxtecan | ORR 39 % median PFS 7.0 months (95 % CI, 4.2–9.7) Median OS 12.8 months (95 % CI, 11.2–15.1) |
| NCT04639219 (DESTINY-PanTumor01) [64] | 2 | Metastatic disease with at least one prior systemic therapy | Mutation | 7 (7) | Trastuzumab deruxtecan | ORR 28.6 % |
| NCT03523572 (DS8201-A-U105) [65] | 1 | Second-line systemic | IHC 2/3+ and IHC 1+ (low) | 30 for IHC 2/3+, 4 for IHC 1+ | Trastuzumab deruxtecan and nivolumab | ORR 36.7 %, median PFS 6.9 months (95 % CI, 2.7–14.4), and median OS 11.0 months (95 % CI, 7.2–NR) for IHC 2/3+ |
| NCT02881190 [66] | 1 | Metastatic disease with no standard therapies available | IHC 2/3+ | 4 (4) | Disitamab vedotin | ORR 50 % |
| NCT03507166 (RC48-C005) and NCT03809013 (RC48-C009) [67] | 2 | Metastatic disease with at least one prior systemic therapy | IHC 2/3+ | 107 (107) | Disitamab vedotin | ORR 50.5 % median PFS 5.8 months (95 % CI, 4.3–6.9) Median OS 14.2 months (95 % CI, 9.7–18.8) |
| NCT04073602 [68] | 2 | Metastatic disease with at least one prior systemic therapy | IHC 0/1+ | 19 | Disitamab vedotin | ORR 26.3 % median PFS 5.5 months (95 % CI, 3.9–6.8) Median OS 16.4 months (95 % CI, 7.1–21.7) |
| NCT04264936 [69] | 1/2 | Metastatic disease | N/A | 41 | Disitamab vedotin and toripalimab | ORR 73.2 % median PFS 9.2 months (95 % CI, 5.7–10.3) 2-year OS rate 63.2 % |
| NCT04879329 (cohort C) [70] | 2 | Metastatic disease with no prior systemic therapy | IHC 1/2/3+ | 20 | Disitamab vedotin and pembrolizumab | ORR 75 % |
-
IHC, immunohistochemistry; FISH, fluorescent in situ hybridization; ORR, objective response rate; median PFS, median progression-free survival; median OS, median overall survival.
Trastuzumab emtansine (T-DM1)
T-DM1 is the first regulatory-approved HER2-directed ADC, with DM1, an anti-microtubule agent, as cytotoxic payload and a drug-to-antibody ratio of 3.5 [71]. In preclinical studies, it was more effective than trastuzumab in killing HER2-overexpressed bladder cancer cells, and it significantly inhibited tumor growth of a cisplatin-resistant bladder cancer xenograft model compared to control IgG or trastuzumab [72].
There are two active basket trials for T-DM1. The phase 2 trial KAMELEON (NCT02999672) screened 284 patients with either UC, pancreatic cancer, or cholangiocarcinoma for HER2 positivity, defined by HER2 IHC 3+ in ≥30 % of tumor cells [61]. The trial ultimately enrolled 20 patients, including 13 with UC. The ORR for UC was 38.5 %, with a median duration of response (DoR) of 3.4 months (95 % CI, 2.8–5.5). Of patients who responded, heterogeneous HER2 expression was observed in two patients and homogeneous HER2 expression in three patients. The median PFS was 2.2 months (95 % CI, 1.2–4.3), and the median OS was 7.0 months (95 % CI, 3.8–not reached [NR]). Seven patients (53.8 %) had an adverse event reported as at least grade 3. KAMELEON closed early due to poor accrual, but it provided insight into the prevalence of HER2 positivity and patterns in HER2 expression. Another phase 2 trial (NCT02675829) enrolled patients with metastatic HER2-positive cancers, defined by amplification determined by NGS with confirmation on tissue by FISH and IHC. So far, results from the subset of patients with UC have not yet been reported.
T-DM1 is being evaluated in combination with other agents in the MyTACTIC (NCT04632992) phase 2 trial, which is enrolling patients with metastatic solid tumors with targetable genomic alterations or protein expression patterns. Arm F enrolled patients, including those with mUC, with HER2 mutations or amplification without known high tumor mutational burden or high microsatellite instability/deficient mismatch repair. Patients on this arm received the combination of T-DM1 and atezolizumab. Preliminary results have reported that the first 25 patients enrolled in Arm F had a confirmed ORR of 12 % (n=3), although none were patients with mUC [73].
Trastuzumab duocarmazine
Trastuzumab duocarmazine is an ADC that contains a duocarmycin prodrug, a DNA-binding alkylating agent, cytotoxic payload with a cleavable linker bound to trastuzumab at a 2.8:1 drug-to-antibody ratio [74], 75]. In epithelial ovarian cancer cell lines and ovarian cancer xenografts, trastuzumab duocarmazine was shown to be more potent than T-DM1, including activity against moderate/low and heterogeneous HER2 expression. Activity in low expression is likely because of the cleavage of the duocarmycin from its linker both inside tumor cells as well as extracellularly, which T-DM1 is unable to due to its non-cleavable linker, to induce a potent bystander effect [76].
The first in-human phase 1 dose-escalation and dose-expansion trial (NCT02277717) enrolled 16 patients with mUC refractory to standard of care therapies and expressing HER2 1+ and above on IHC [62]. For mUC, the mean treatment duration was 3.5 months, the ORR was 25 %, and the median PFS was 4.0 months (95 % CI, 1.3–NR). Trastuzumab duocarmazine showed clinical activity in heavily pretreated patients, including in those with HER2-low tumors, warranting further investigation.
Trastuzumab deruxtecan (T-DXd)
T-DXd, another ADC, comprises a topoisomerase I drug payload conjugated to trastuzumab via a cleavable linker, with a drug-to-antibody ratio of 8:1 [77]. The cleavable antibody-drug linker, like the linker for trastuzumab duocarmazine, allows T-DXd to exhibit tumor bystander effects, making it an effective therapy for HER2-low cancer.
DESTINY-PanTumor02 (NCT04482309) is a phase 2 trial evaluating T-DXd at 5.4 mg/kg once every 3 weeks for HER2-positive (IHC 2/3+) metastatic solid tumors, refractory to at least one prior systemic therapy [63]. There were 41 patients with mUC enrolled. Amongst these patients, the ORR was 39.0 % including one with CR. There were notable differences in patient numbers between HER2 IHC status at enrollment eligibility and centrally confirmed HER2 IHC status. The ORR was 56.3 % for centrally confirmed IHC 3+ tumors and 35.0 % for centrally confirmed IHC 2+ tumors. The median DoR was 8.7 months (95 % CI, 4.3–11.8). The overall median PFS for mUC was 7.0 months (95 % CI, 4.2–9.7). Median PFS was 7.4 months (95 % CI, 3.0–11.9) and 7.8 months (95 % CI, 2.6–11.6) for patients with centrally confirmed IHC 3+ and 2+ tumors, respectively. The median OS for mUC patients was 12.8 months (95 % CI, 11.2–15.1) overall. Median OS was 13.4 months (95 % CI, 6.7–19.8) and 13.1 months (11.0–19.9) for patients with centrally confirmed IHC 3+ and 2+ tumors, respectively. Notably, amongst all patients, grade ≥3 drug-related adverse events were reported in 40.8 % of patients. Importantly, 10.5 % of patients experienced interstitial lung disease (ILD), including 3 deaths from ILD. This trial supports the use of T-DXd as a tumor-agnostic therapy for patients with HER2-positive solid tumors. In April 2024, the USFDA granted T-DXd accelerated approval for patients with previously treated metastatic HER2-positive IHC 3+ solid tumors, including mUC [6].
A similar study, DESTINY-PanTumor01 (NCT04639219) is a phase 2 trial evaluating T-DXd for HER2-mutated metastatic solid tumors [64]. There were 102 patients who received T-DXd, including seven patients with mUC. For patients with mUC, the reported ORR was 28.6 %. For the entire cohort of patients in the trial, the median PFS was 5.4 months (95 % CI, 2.7–7.1), and the median OS was 10.9 months (95 % CI, 8.3–14.9). Notably, there were 51 % of patients who had grade ≥3 treatment-emergent adverse events, including 2 deaths (both due to ILD/pneumonitis). HER2 expression levels were not concordant with HER2 mutation, as IHC 3+ was observed in 10 %, IHC 2+ in 21 %, IHC 1+ in 6 % and IHC 0 in 36 % of patients. These preliminary results show potential efficacy in patients with HER2-mutated metastatic tumors as well.
DS8201-A-U105 (NCT03523572) is a phase 1b trial assessing the safety and efficacy of T-DXd plus nivolumab for patients with HER2-positive mUC with progression on prior platinum-based chemotherapy [65]. This study consists of a high expression, HER2 IHC 2/3+, cohort and a low expression, HER2 IHC 1+, cohort. For the 30 patients enrolled in the HER2 high expression cohort, the ORR was 36.7 % with a 13.3 % CR rate. The median DoR was 13.1 months (95 % CI, 4.1–NR). The median PFS was 6.9 months (95 % CI, 2.7–14.4), and the median OS was 11.0 months (95 % CI, 7.2–NR). Amongst both cohorts (n=34), 73.5 % of patients had grade ≥3 drug-related adverse events, including one death from ILD/pneumonitis. The combination of T-DXd and nivolumab showed good antitumor activity for HER2-positive mUC with a tolerable safety profile.
Disitamab vedotin (DV)
DV is another ADC with the HER2-targeting antibody hertuzumab conjugated to monomethyl auristatin E, a microtubule inhibitor, via a cleavable linker, and a drug-to-antibody ratio of 4:1. There has been reported bystander effect [78]. Hertuzumab has a stronger affinity to HER2 than trastuzumab, as well as more potent antibody-dependent cell-mediated cytotoxicity based on in vitro studies [79]. A HER2-positive breast cancer lung metastasis mouse model found DV to be more efficacious against tumors compared to both T-DM1 and T-DXd [80]. DV is currently being evaluated in clinical trials for several HER2-positive metastatic solid tumors including UC.
A phase 1 trial (NCT02881190) enrolled 57 patients to evaluate DV with HER2 overexpression by IHC 2/3+ regardless of FISH status [66]. This study included four patients with mUC. Amongst these patients, two achieved PR and two had SD, for an ORR of 50 % and disease control rate (DCR) of 100 %.
Two phase 2 trials, RC48-C005 (NCT03507166) and RC48-C009 (NCT03809013), enrolled patients with IHC 2/3+ HER2-positive mUC with progression on prior chemotherapy [67]. The primary endpoint of both studies was ORR. Overall, there were 304 patients screened, with 107 patients enrolled in these two trials. In a combined analysis of both trials, the ORR was 50.5 %, including two patients with CR, and the DCR was 82.2 %. The median DoR was 7.3 months (95 % CI, 5.7–10.8). In subgroup analyses, the ORR for upper tract UC and bladder UC were both 50.0 %. ORR for higher HER2 expression (HER2 IHC 2+ and FISH-positive or IHC 3+) was 62.2 %, while the ORR for lower HER2 expression (HER2 IHC 2+ and FISH-negative) was lower at 39.6 %. The overall median PFS was 5.8 months (95 % CI, 4.3–6.9), and median OS was 14.2 months (95 % CI, 9.7–18.8). Overall treatment-related adverse events were manageable, with peripheral neuropathy as the most common reason for drug discontinuation in 11.2 % of cases.
DV monotherapy was evaluated in another phase 2 trial (NCT04073602) for patients with mUC with HER2 negative/low (IHC 0/1+) disease with progression on prior systemic therapy [68]. Preliminary data was presented on 19 patients. The ORR was 26.3 %, the DCR was 94.7 %, the median PFS was 5.5 months (95 % CI, 3.9–6.8), and the median OS was 16.4 months (95 % CI, 7.1–21.7). On subgroup analysis, the six patients with IHC 0 all had SD, while the 13 patients with IHC 1+ had an ORR of 38.4 % (5/13). The study suggested that DV monotherapy for patients with both HER2-low and HER2-absent mUC was feasible.
The combination of DV with toripalimab, an anti-PD-1 immune checkpoint inhibitor, has been evaluated in a phase 1 b/2 trial (NCT04264936) for 41 patients with mUC regardless of HER2 expression or prior lines of therapy [69]. There was HER2 IHC 2/3+ expression in 59 % of patients. Overall, the ORR was 73.2 % including 9.8 % CR, and the DCR was 90.2 %. The median PFS was 9.2 months (95 % CI, 5.7–10.3), and the 2-year OS rate was 63.2 %. On subgroup analysis, the ORR was 76.0 % for treatment-naive patients. For HER2 IHC 2/3+, 1+, and 0, the ORR was 83.3 , 64.3, and 33.3 %, respectively. Grade ≥3 adverse events occurred in 43.9 % of patients, which were most commonly immune-related. This study suggested the potential efficacy of the combination of DV and ICI for patients with mUC regardless of HER2 expression.
As all the aforementioned trials with DV were performed in China, this ADC has not yet been fully approved in the United States or in Europe. RC48G001 (NCT04879329) is an ongoing phase 2 multinational trial evaluating the efficacy and safety of DV as either monotherapy or in combination with pembrolizumab for patients with HER2-low and HER2-positive mUC. Preliminary results of RC48G001 cohort C part 1, which enrolled 20 patients with HER2-positive (defined as IHC ≥1+) treatment-naïve mUC receiving DV with pembrolizumab, revealed an ORR of 75 % in all patients, including 78.6 % in the HER2-low group (IHC 2+ and FISH-negative or IHC 1+) and 66.7 % in the HER2-positive group (IHC 2+ and FISH-positive or IHC3+) [70]. DV-001 (NCT05911295) is a phase 3 trial for previously untreated HER2-positive mUC randomized to receive either the combination of DV and pembrolizumab or platinum-based chemotherapy followed by avelumab maintenance [81]. DV is also being evaluated in other stages of UC, including for non-muscle invasive bladder cancer (NMIBC) and MIBC.
Future directions
While studies have shown promise for HER2-directed therapy in the treatment of mUC, especially ADCs, several questions have arisen that could impact its widespread adoption. One potential issue is how HER2 is measured and how HER2 positivity is determined. Discrepancies between IHC, FISH, and NGS are well documented [28], [82], [83], [84]. A standardized classification system of HER2 positivity status for UC would be beneficial, although gastric scoring has largely been adopted. Moreover, whether the conventional HER2 assay is sensitive enough to distinguish low ranges of HER2 expression needs to be further explored, as it is becoming increasingly important in the era of ADCs, with benefits in other tumors such as breast cancer, for patients with HER2-low and possibly HER2-ultra-low expression of tumor cells. High sensitivity assays are being developed that can identify very low levels of HER2 expression, challenging the standard HER2 assays currently being used [85].
Similarly to other tumors, including breast and lung cancer, the role of HER2-targeting therapies in HER2-low or HER2-mutated mUC needs further investigation. The pan-tumor approval for T-DXd was restricted to IHC 3+, yet some patients with IHC 2+ tumors demonstrated response in the DESTINY-PanTumor02 study, indicating potential benefit in this population. The role of mutated HER2 in mUC also needs to be better understood, as this does not always lead to overexpression and could play a different role in tumorigenesis and therefore necessitate alternative targeting strategies.
While much can be extrapolated from studies in other tumors, therapies that exhibited benefit in other tumors have not been efficacious in clinical trials for mUC. For example, traditional HER2-targeting therapies like the antibodies trastuzumab and pertuzumab, and the tyrosine kinase inhibitors lapatinib, neratinib, and afatinib, have not been successful for mUC. There are likely several underlying biological differences in UC compared to other HER2-positive cancers such as breast and gastric cancers, and with variable HER2 expression and no established consensus in measuring overexpression, these traditional agents of antibodies and tyrosine kinase inhibitors are not as effective in treating UC. Therefore, analysis specific to UC and further granularity in HER2 scoring are necessary in the future.
HER2-directed therapies have also been explored in combination with standard of care mUC treatment; however, potential synergy needs to be better understood. HER2 may be an unreliable prognostic or predictive biomarker for UC. In a recent single-center study, HER2 alterations have not been shown to be predictive of better outcomes with platinum-based therapy or ICIs [86], 87]. Conversely, patients with HER2 alterations in NMIBC were noted to have a significantly shorter time to progression to MIBC or metastatic disease [86]. Moreover, more investigation into how HER2 interacts with the microenvironment, especially in consideration of combination with immune checkpoint inhibition, could better elucidate the utility of potential interactions. Preliminary results from the RC48G001 cohort C suggest that UC tumors having low expression of HER2 may derive as much benefit from the combination of DV and pembrolizumab compared to UC tumors with high HER2 expression [70].
Determining the sequence of therapies for HER2-positive mUC will be challenging. The recent adoption of the combination of EV and pembrolizumab as frontline therapy for mUC means that the optimal second line and beyond therapies are uncertain [88]. For patients who exhibit HER2 positivity either by IHC or NGS, HER2 targeting could be an attractive option for being a second-line agent after progression on EV and pembrolizumab, if there are no other targetable alterations such as FGFR3. Nonetheless, HER2-targeting agents, whether used as monotherapy or in combination with other agents, have not yet demonstrated head-to-head superiority over other options. Moreover, in the front-line setting, clinical trials with comparison to established standard of care would be necessary to determine the superiority of HER2-targeting treatment combinations prior to its widespread adoption.
Many novel immune-stimulating agents are being evaluated in phase 1 clinical trials enrolling patients with HER2-positive metastatic and refractory solid tumors (Table 4). Several chimeric antigen receptor (CAR)-designed immune cells are being tested for mUC, including anti-HER2 CAR T cells, CAR macrophages, and CAR NK (natural killer) cells. HER2-bispecific molecules are being evaluated in early-phase clinical trials for other HER2-expressing metastatic cancers [89], 90] and will likely be assessed in patients with mUC soon.
Notable active and recruiting trials involving patients with UC evaluating HER2-targeting novel agents.
| Trial | Phase | Setting | HER2 positivity criteria | Total planned enrollment | Intervention | Mechanism of action | Primary endpoint |
|---|---|---|---|---|---|---|---|
| NCT03740256 | 1 | Metastatic disease with no standard therapies available | IHC 2/3+ | 45 | CAdVEC | Oncolytic adenovirus with HER2-specific CAR T cell | DLT |
| NCT04660929 | 1 | Metastatic disease with no standard therapies available | IHC 2/3+ | 48 | CT-0508 | HER2-specific CAR-macrophage | Safety, tolerability |
| NCT06293898 | 1 | Metastatic disease with ≥2 lines of standard therapy | IHC 1/2/3+ | 280 | BL-M07D1 | HER2 antibody-drug conjugate | Safety, MTD |
| NCT04143711 | 1/2 | Prior chemotherapy and immunotherapy | IHC 1/2/3+ | 378 | DF1001 with or without nivolumab | Tri-specific, NK cell engager | Safety, tolerability, ORR |
| NCT04278144 | 1/2 | Metastatic disease with no standard therapies available | IHC 2/3+ | 390 | BDC-1001 with or without nivolumab | HER2-targeting immune-stimulating antibody conjugate | Safety, tolerability, ORR |
| NCT05754853 | 3 | Prior chemotherapy and immunotherapy | IHC 2/3+ | 290 | MRG002 vs. chemotherapy | HER2 antibody-drug conjugate | OS, PFS |
-
ORR, objective response rate; DLT, dose limiting toxicity; AE, adverse events; RP2D, recommended phase 2 dose; MTD, maximum tolerated dose; OS, overall survival; PFS, progression-free survival.
Conclusions
HER2 is an important therapeutic target in many cancers and has been widely studied in mUC. The present and future for targeting HER2 in mUC is bright, especially with the results of trials with HER2-targeting ADCs. T-DXd, with its recent approval for HER2 IHC 3+ metastatic solid tumors, has ushered in a new era. With the successes of T-DXd for HER2-low positive breast cancer [77] and HER2-mutated non-small cell lung cancer [91], 92] (which have achieved regulatory approval in these settings for these cancers), as well as the potential of DV for UC [67], 70], HER2-targeting ADCs will likely impact treatment paradigms. Further treatments, including bispecific molecules, are promising as well. Yet, several open questions remain including how to incorporate these ADCs, whether as monotherapy or in combination, and how to sequence them with current therapies. Determination of patients who would benefit remains unclear, given discrepancies in the results of scoring assays as well as multiple ways of measuring HER2 positivity. Moreover, the impact of HER2 overexpression on treatment response with ADCs remains subject of debate, given the evidence of HER2-low-expressing tumors responding to ADCs in other malignancies. The role of HER2 as a therapeutic target will be further elucidated with insights gained from translational studies and clinical trials in the future.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: Albert Jang: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Visualization; Writing – original draft; Writing – review & editing. Nirmala Ghimirey: Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Visualization; Writing – original draft; Writing – review & editing. Hamsa L.S. Kumar: Writing – original draft; Writing – review & editing. Prateek Mendiratta: Writing – original draft; Writing – review & editing. Santosh Rao: Writing – original draft; Writing – review & editing. Iris Y. Sheng: Writing – original draft; Writing – review & editing. Pedro C. Barata: Writing – original draft; Writing – review & editing. Jorge A. Garcia: Writing – original draft; Writing – review & editing. Jason R. Brown: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Visualization; Writing – original draft; Writing – review & editing. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None.
-
Conflict of interest: Albert Jang: Personal fees from RealTimeCase. Prateek Mendiratta: Consulting or Advisory Role in Pfizer; Cardinal Health Care Speakers’ Bureau in Pfizer, Astellas, and AstraZeneca. Pedro C. Barata: Honoraria from UroToday; Consulting or Advisory Role in Bayer, BMS, Pfizer, EMD Serono, Eisai, Caris Life Sciences, Dendreon (Inst), AstraZeneca, Exelixis, AVEO, Merck, Ipson. Jason R. Brown: Consulting or Advisory Role in Pfizer, EMD Serono, Janssen; Speakers’ Bureau in EMD Serono.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Bladder Cancer [Online]. Available from: https://seer.cancer.gov/statfacts/html/urinb.html [Accessed 21 Jan 2025].Search in Google Scholar
2. Roviello, G, Santoni, M, Sonpavde, GP, Catalano, M. The evolving treatment landscape of metastatic urothelial cancer. Nat Rev Urol 2024;21:580–92. https://doi.org/10.1038/s41585-024-00872-0.Search in Google Scholar PubMed
3. Powles, T, Valderrama, BP, Gupta, S, Bedke, J, Kikuchi, E, Hoffman-Censits, J, et al.. Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. N Engl J Med 2024;390:875–88. https://doi.org/10.1056/nejmoa2312117.Search in Google Scholar PubMed
4. Cheng, X. A comprehensive review of HER2 in cancer biology and therapeutics. Genes 2024;15:903. https://doi.org/10.3390/genes15070903.Search in Google Scholar PubMed PubMed Central
5. Swain, SM, Shastry, M, Hamilton, E. Targeting HER2-positive breast cancer: advances and future directions. Nat Rev Drug Discov 2023;22:101–26. https://doi.org/10.1038/s41573-022-00579-0.Search in Google Scholar PubMed PubMed Central
6. U.S. Food & Drug Administration. FDA grants accelerated approval to fam-trastuzumab deruxtecan-nxki for unresectable or metastatic HER2-positive solid tumors [Online]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-fam-trastuzumab-deruxtecan-nxki-unresectable-or-metastatic-her2 [Accessed 21 Jan 2025].Search in Google Scholar
7. Galogre, M, Rodin, D, Pyatnitskiy, M, Mackelprang, M, Koman, I. A review of HER2 overexpression and somatic mutations in cancers. Crit Rev Oncol Hematol 2023;186:103997. https://doi.org/10.1016/j.critrevonc.2023.103997.Search in Google Scholar PubMed
8. Peckys, DB, Hirsch, D, Gaiser, T, de Jonge, N. Visualisation of HER2 homodimers in single cells from HER2 overexpressing primary formalin fixed paraffin embedded tumour tissue. Mol Med 2019;25:42. https://doi.org/10.1186/s10020-019-0108-z.Search in Google Scholar PubMed PubMed Central
9. Koirala, N, Dey, N, Aske, J, De, P. Targeting cell cycle progression in HER2+ breast cancer: an emerging treatment opportunity. Int J Mol Sci 2022;23:6547. https://doi.org/10.3390/ijms23126547.Search in Google Scholar PubMed PubMed Central
10. Subbaramaiah, K, Norton, L, Gerald, W, Dannenberg, AJ. Cyclooxygenase-2 is overexpressed in HER-2/neu-positive breast cancer: evidence for involvement of AP-1 and PEA3. J Biol Chem 2002;277:18649–57. https://doi.org/10.1074/jbc.m111415200.Search in Google Scholar
11. Chi, F, Wu, R, Jin, X, Jiang, M, Zhu, X. HER2 induces cell proliferation and invasion of non-small-cell lung cancer by upregulating COX-2 expression via MEK/ERK signaling pathway. Oncol Targets Ther 2016;9:2709–16. https://doi.org/10.2147/ott.s96197.Search in Google Scholar
12. Moasser, MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 2007;26:6469–87. https://doi.org/10.1038/sj.onc.1210477.Search in Google Scholar PubMed PubMed Central
13. Zhou, BP, Liao, Y, Xia, W, Zou, Y, Spohn, B, Hung, MC. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol 2001;3:973–82. https://doi.org/10.1038/ncb1101-973.Search in Google Scholar PubMed
14. Chen, Q, Sun, L, Chen, ZJ. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol 2016;17:1142–9. https://doi.org/10.1038/ni.3558.Search in Google Scholar PubMed
15. Robichaux, JP, Elamin, YY, Vijayan, RSK, Nilsson, MB, Hu, L, He, J, et al.. Pan-cancer landscape and analysis of ERBB2 mutations identifies poziotinib as a clinically active inhibitor and enhancer of T-DM1 activity. Cancer Cell 2019;36:444–57.e7. https://doi.org/10.1016/j.ccell.2019.09.001.Search in Google Scholar PubMed PubMed Central
16. Iqbal, N, Iqbal, N. Human epidermal growth factor receptor 2 (HER2) in cancers: overexpression and therapeutic implications. Mol Biol Int 2014;2014:852748–9. https://doi.org/10.1155/2014/852748.Search in Google Scholar PubMed PubMed Central
17. Wang, SE, Narasanna, A, Perez-Torres, M, Xiang, B, Wu, FY, Yang, S, et al.. HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell 2006;10:25–38. https://doi.org/10.1016/j.ccr.2006.05.023.Search in Google Scholar PubMed
18. Guan, Y, Wang, Y, Li, H, Meng, J, You, X, Zhu, X, et al.. Molecular and clinicopathological characteristics of ERBB2 gene fusions in 32,131 Chinese patients with solid tumors. Front Oncol 2022;12:986674. https://doi.org/10.3389/fonc.2022.986674.Search in Google Scholar PubMed PubMed Central
19. Wolff, AC, Hammond, ME, Hicks, DG, Dowsett, M, McShane, LM, Allison, KH, et al.. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997–4013. https://doi.org/10.1200/jco.2013.50.9984.Search in Google Scholar PubMed
20. Wolff, AC, Hammond, MEH, Allison, KH, Harvey, BE, Mangu, PB, Bartlett, JMS, et al.. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol 2018;36:2105–22. https://doi.org/10.1200/jco.2018.77.8738.Search in Google Scholar
21. Tarantino, P, Hamilton, E, Tolaney, SM, Cortes, J, Morganti, S, Ferraro, E, et al.. HER2-low breast cancer: pathological and clinical landscape. J Clin Oncol 2020;38:1951–62. https://doi.org/10.1200/jco.19.02488.Search in Google Scholar PubMed
22. Robbins, CJ, Fernandez, AI, Han, G, Wong, S, Harigopal, M, Podoll, M, et al.. Multi-institutional assessment of pathologist scoring HER2 immunohistochemistry. Mod Pathol 2023;36:100032. https://doi.org/10.1016/j.modpat.2022.100032.Search in Google Scholar PubMed PubMed Central
23. Casterá, C, Bernet, L. HER2 immunohistochemistry inter-observer reproducibility in 205 cases of invasive breast carcinoma additionally tested by ISH. Ann Diagn Pathol 2020;45:151451. https://doi.org/10.1016/j.anndiagpath.2019.151451.Search in Google Scholar PubMed
24. Lambein, K, Van Bockstal, M, Vandemaele, L, Geenen, S, Rottiers, I, Nuyts, A, et al.. Distinguishing score 0 from score 1+ in HER2 immunohistochemistry-negative breast cancer: clinical and pathobiological relevance. Am J Clin Pathol 2013;140:561–6. https://doi.org/10.1309/ajcp4a7ktayhzsoe.Search in Google Scholar PubMed
25. McCormick, SR, Lillemoe, TJ, Beneke, J, Schrauth, J, Reinartz, J. HER2 assessment by immunohistochemical analysis and fluorescence in situ hybridization: comparison of HercepTest and PathVysion commercial assays. Am J Clin Pathol 2002;117:935–43. https://doi.org/10.1309/3643-f955-7q6b-ewwl.Search in Google Scholar
26. Jung, J, Kim, ST, Ko, J, Hong, JY, Park, JO, Ha, SY, et al.. Clinical implication of HER2 aberration in patients with metastatic cancer using next-generation sequencing: a pan-tumor analysis. JCO Precis Oncol 2023;7:e2200537. https://doi.org/10.1200/PO.22.00537.Search in Google Scholar PubMed
27. Ross, DS, Zehir, A, Cheng, DT, Benayed, R, Nafa, K, Hechtman, JF, et al.. Next-generation assessment of human epidermal growth factor receptor 2 (ERBB2) amplification status: clinical validation in the context of a hybrid capture-based, comprehensive solid tumor genomic profiling assay. J Mol Diagn 2017;19:244–54. https://doi.org/10.1016/j.jmoldx.2016.09.010.Search in Google Scholar PubMed PubMed Central
28. Morsberger, L, Pallavajjala, A, Long, P, Hardy, M, Park, R, Parish, R, et al.. HER2 amplification by next-generation sequencing to identify HER2-positive invasive breast cancer with negative HER2 immunohistochemistry. Cancer Cell Int 2022;22:350. https://doi.org/10.1186/s12935-022-02761-1.Search in Google Scholar PubMed PubMed Central
29. Eijkelenboom, A, Tops, BBJ, van den Berg, A, van den Brule, AJC, Dinjens, WNM, Dubbink, HJ, et al.. Recommendations for the clinical interpretation and reporting of copy number gains using gene panel NGS analysis in routine diagnostics. Virchows Arch 2019;474:673–80. https://doi.org/10.1007/s00428-019-02555-3.Search in Google Scholar PubMed PubMed Central
30. Scherrer, E, Kang, A, Bloudek, LM, Koshkin, VS. HER2 expression in urothelial carcinoma, a systematic literature review. Front Oncol 2022;12:1011885. https://doi.org/10.3389/fonc.2022.1011885.Search in Google Scholar PubMed PubMed Central
31. Millis, SZ, Bryant, D, Basu, G, Bender, R, Vranic, S, Gatalica, Z, et al.. Molecular profiling of infiltrating urothelial carcinoma of bladder and nonbladder origin. Clin Genitourin Cancer 2015;13:e37–49. https://doi.org/10.1016/j.clgc.2014.07.010.Search in Google Scholar PubMed
32. Yan, M, Schwaederle, M, Arguello, D, Millis, SZ, Gatalica, Z, Kurzrock, R. HER2 expression status in diverse cancers: review of results from 37,992 patients. Cancer Metastasis Rev 2015;34:157–64. https://doi.org/10.1007/s10555-015-9552-6.Search in Google Scholar PubMed PubMed Central
33. Cimpean, AM, Tarlui, V, Cumpănaş, AA, Bolintineanu, S, Cumpănaş, A, Raica, M. Critical overview of HER2 assessement in bladder cancer: what is missing for a better therapeutic approach? Anticancer Res 2017;37:4935–42. https://doi.org/10.21873/anticanres.11903.Search in Google Scholar PubMed
34. Nedjadi, T, Al-Maghrabi, J, Assidi, M, Dallol, A, Al-Kattabi, H, Chaudhary, A, et al.. Prognostic value of HER2 status in bladder transitional cell carcinoma revealed by both IHC and BDISH techniques. BMC Cancer 2016;16:653. https://doi.org/10.1186/s12885-016-2703-5.Search in Google Scholar PubMed PubMed Central
35. Hofmann, M, Stoss, O, Shi, D, Büttner, R, van de Vijver, M, Kim, W, et al.. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology 2008;52:797–805. https://doi.org/10.1111/j.1365-2559.2008.03028.x.Search in Google Scholar PubMed
36. Rüschoff, J, Hanna, W, Bilous, M, Hofmann, M, Osamura, RY, Penault-Llorca, F, et al.. HER2 testing in gastric cancer: a practical approach. Mod Pathol 2012;25:637–50. https://doi.org/10.1038/modpathol.2011.198.Search in Google Scholar PubMed
37. Lei, H, Ling, Y, Yuan, P, Yan, X, Wang, L, Shi, Y, et al.. Assessment of the expression pattern of HER2 and its correlation with HER2-targeting antibody-drug conjugate therapy in urothelial cancer. J Natl Cancer Cent 2023;3:121–8. https://doi.org/10.1016/j.jncc.2023.02.003.Search in Google Scholar PubMed PubMed Central
38. Sanguedolce, F, Zanelli, M, Palicelli, A, Bisagni, A, Zizzo, M, Ascani, S, et al.. HER2 expression in bladder cancer: a focused view on its diagnostic, prognostic, and predictive role. Int J Mol Sci 2023;24:3720. https://doi.org/10.3390/ijms24043720.Search in Google Scholar PubMed PubMed Central
39. Gan, K, Gao, Y, Liu, K, Xu, B, Qin, W. The clinical significance and prognostic value of HER2 expression in bladder cancer: a meta-analysis and a bioinformatic analysis. Front Oncol 2021;11:653491. https://doi.org/10.3389/fonc.2021.653491.Search in Google Scholar PubMed PubMed Central
40. The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014;507:315–22. https://doi.org/10.1038/nature12965.Search in Google Scholar PubMed PubMed Central
41. Ross, JS, Wang, K, Khaira, D, Ali, SM, Fisher, HA, Mian, B, et al.. Comprehensive genomic profiling of 295 cases of clinically advanced urothelial carcinoma of the urinary bladder reveals a high frequency of clinically relevant genomic alterations. Cancer 2016;122:702–11. https://doi.org/10.1002/cncr.29826.Search in Google Scholar PubMed
42. Ross, JS, Wang, K, Gay, LM, Al-Rohil, RN, Nazeer, T, Sheehan, CE, et al.. A high frequency of activating extracellular domain ERBB2 (HER2) mutation in micropapillary urothelial carcinoma. Clin Cancer Res 2014;20:68–75. https://doi.org/10.1158/1078-0432.ccr-13-1992.Search in Google Scholar
43. Maadi, H, Soheilifar, MH, Choi, WS, Moshtaghian, A, Wang, Z. Trastuzumab mechanism of action; 20 years of research to unravel a dilemma. Cancers 2021;13:3540. https://doi.org/10.3390/cancers13143540.Search in Google Scholar PubMed PubMed Central
44. Nami, B, Maadi, H, Wang, Z. Mechanisms underlying the action and synergism of trastuzumab and pertuzumab in targeting HER2-positive breast cancer. Cancers 2018;10:342. https://doi.org/10.3390/cancers10100342.Search in Google Scholar PubMed PubMed Central
45. Barthélémy, P, Leblanc, J, Goldbarg, V, Wendling, F, Kurtz, JE. Pertuzumab: development beyond breast cancer. Anticancer Res 2014;34:1483–91.Search in Google Scholar
46. Michaelson, MD, Hu, C, Pham, HT, Dahl, DM, Lee-Wu, C, Swanson, GP, et al.. A phase 1/2 trial of a combination of paclitaxel and trastuzumab with daily irradiation or paclitaxel alone with daily irradiation after transurethral surgery for noncystectomy candidates with muscle-invasive bladder cancer (Trial NRG Oncology RTOG 0524). Int J Radiat Oncol Biol Phys 2017;97:995–1001. https://doi.org/10.1016/j.ijrobp.2016.12.018.Search in Google Scholar PubMed PubMed Central
47. Dahl, DM, Karrison, TG, Michaelson, MD, Pham, HT, Wu, CL, Swanson, GP, et al.. Long-term outcomes of chemoradiation for muscle-invasive bladder cancer in noncystectomy candidates. Final results of NRG Oncology RTOG 0524-A phase 1/2 trial of paclitaxel + trastuzumab with daily radiation or paclitaxel alone with daily irradiation. Eur Urol Oncol 2024;7:83–90. https://doi.org/10.1016/j.euo.2023.05.013.Search in Google Scholar PubMed PubMed Central
48. Hussain, MH, MacVicar, GR, Petrylak, DP, Dunn, RL, Vaishampayan, U, Lara, PNJr, et al.. Trastuzumab, paclitaxel, carboplatin, and gemcitabine in advanced human epidermal growth factor receptor-2/neu-positive urothelial carcinoma: results of a multicenter phase II National Cancer Institute trial. J Clin Oncol 2007;25:2218–24. https://doi.org/10.1200/jco.2006.08.0994.Search in Google Scholar PubMed
49. Oudard, S, Culine, S, Vano, Y, Goldwasser, F, Théodore, C, Nguyen, T, et al.. Multicentre randomised phase II trial of gemcitabine+platinum, with or without trastuzumab, in advanced or metastatic urothelial carcinoma overexpressing Her2. Eur J Cancer 2015;51:45–54. https://doi.org/10.1016/j.ejca.2014.10.009.Search in Google Scholar PubMed
50. Hainsworth, JD, Meric-Bernstam, F, Swanton, C, Hurwitz, H, Spigel, DR, Sweeney, C, et al.. Targeted therapy for advanced solid tumors on the basis of molecular profiles: results from MyPathway, an open-label, phase IIa multiple basket study. J Clin Oncol 2018;36:536–42. https://doi.org/10.1200/jco.2017.75.3780.Search in Google Scholar
51. Sweeney, CJ, Hainsworth, JD, Bose, R, Burris, HA, Kurzrock, R, Swanton, C, et al.. MyPathway human epidermal growth factor receptor 2 basket study: pertuzumab + trastuzumab treatment of a tissue-agnostic cohort of patients with human epidermal growth factor receptor 2-altered advanced solid tumors. J Clin Oncol 2024;42:258–65. https://doi.org/10.1200/jco.22.02636.Search in Google Scholar
52. Wülfing, C, Machiels, JP, Richel, DJ, Grimm, MO, Treiber, U, De Groot, MR, et al.. A single-arm, multicenter, open-label phase 2 study of lapatinib as the second-line treatment of patients with locally advanced or metastatic transitional cell carcinoma. Cancer 2009;115:2881–90. https://doi.org/10.1002/cncr.24337.Search in Google Scholar PubMed
53. Galsky, MD, Von Hoff, DD, Neubauer, M, Anderson, T, Fleming, M, Nagarwala, Y, et al.. Target-specific, histology-independent, randomized discontinuation study of lapatinib in patients with HER2-amplified solid tumors. Invest New Drugs 2012;30:695–701. https://doi.org/10.1007/s10637-010-9541-0.Search in Google Scholar PubMed
54. Cerbone, L, Sternberg, CN, Sengeløv, L, Agerbaek, M, Van Herpen, C, Marreaud, S, et al.. Results from a phase I study of lapatinib with gemcitabine and cisplatin in advanced or metastatic bladder cancer: EORTC trial 30061. Oncology 2016;90:21–8. https://doi.org/10.1159/000440959.Search in Google Scholar PubMed
55. Powles, T, Huddart, RA, Elliott, T, Sarker, SJ, Ackerman, C, Jones, R, et al.. Phase III, double-blind, randomized trial that compared maintenance lapatinib versus placebo after first-line chemotherapy in patients with human epidermal growth factor receptor 1/2-positive metastatic bladder cancer. J Clin Oncol 2017;35:48–55. https://doi.org/10.1200/jco.2015.66.3468.Search in Google Scholar PubMed
56. Tang, S, Dorff, TB, Tsao-Wei, DD, Massopust, K, Ketchens, C, Hu, J, et al.. Single arm phase II study of docetaxel and lapatinib in metastatic urothelial cancer: USC trial 4B-10-4. J Clin Oncol 2016;34:424. https://doi.org/10.1200/jco.2016.34.2_suppl.424.Search in Google Scholar
57. Choudhury, NJ, Campanile, A, Antic, T, Yap, KL, Fitzpatrick, CA, Wade, JL3rd, et al.. Afatinib activity in platinum-refractory metastatic urothelial carcinoma in patients with ERBB alterations. J Clin Oncol 2016;34:2165–71. https://doi.org/10.1200/jco.2015.66.3047.Search in Google Scholar PubMed PubMed Central
58. Font, A, Mellado, B, Climent, MA, Virizuela, JA, Oudard, S, Puente, J, et al.. Phase II trial of afatinib in patients with advanced urothelial carcinoma with genetic alterations in ERBB1-3 (LUX-bladder 1). Br J Cancer 2024;130:434–41. https://doi.org/10.1038/s41416-023-02513-6.Search in Google Scholar PubMed PubMed Central
59. Bedard, PL, Li, S, Wisinski, KB, Yang, ES, Limaye, SA, Mitchell, EP, et al.. Phase II study of afatinib in patients with tumors with human epidermal growth factor receptor 2-activating mutations: results from the National Cancer Institute-molecular analysis for therapy choice ECOG-ACRIN trial (EAY131) subprotocol EAY131-B. JCO Precis Oncol 2022;6:e2200165. https://doi.org/10.1200/PO.22.00165.Search in Google Scholar PubMed PubMed Central
60. Hyman, DM, Piha-Paul, SA, Won, H, Rodon, J, Saura, C, Shapiro, GI, et al.. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 2018;554:189–94. https://doi.org/10.1038/nature25475.Search in Google Scholar PubMed PubMed Central
61. de Vries, EGE, Rüschoff, J, Lolkema, M, Tabernero, J, Gianni, L, Voest, E, et al.. Phase II study (KAMELEON) of single-agent T-DM1 in patients with HER2-positive advanced urothelial bladder cancer or pancreatic cancer/cholangiocarcinoma. Cancer Med 2023;12:12071–83. https://doi.org/10.1002/cam4.5893.Search in Google Scholar PubMed PubMed Central
62. Banerji, U, van Herpen, CML, Saura, C, Thistlethwaite, F, Lord, S, Moreno, V, et al.. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol 2019;20:1124–35. https://doi.org/10.1016/s1470-2045(19)30328-6.Search in Google Scholar PubMed
63. Meric-Bernstam, F, Makker, V, Oaknin, A, Oh, DY, Banerjee, S, González-Martín, A, et al.. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: primary results from the DESTINY-PanTumor02 phase II trial. J Clin Oncol 2024;42:47–58. https://doi.org/10.1200/jco.23.02005.Search in Google Scholar PubMed PubMed Central
64. Li, BT, Meric-Bernstam, F, Bardia, A, Naito, Y, Siena, S, Aftimos, P, et al.. Trastuzumab deruxtecan in patients with solid tumours harbouring specific activating HER2 mutations (DESTINY-PanTumor01): an international, phase 2 study. Lancet Oncol 2024;25:707–19. https://doi.org/10.1016/s1470-2045(24)00140-2.Search in Google Scholar PubMed
65. Galsky, MD, Conte, GD, Foti, S, Yu, EY, Machiels, J-PH, Doger, B, et al.. Primary analysis from DS8201-A-U105: a phase 1b, two-part, open-label study of trastuzumab deruxtecan (T-DXd) with nivolumab (nivo) in patients (pts) with HER2-expressing urothelial carcinoma (UC). J Clin Oncol 2022;40:438.10.1200/JCO.2022.40.6_suppl.438Search in Google Scholar
66. Xu, Y, Wang, Y, Gong, J, Zhang, X, Peng, Z, Sheng, X, et al.. Phase I study of the recombinant humanized anti-HER2 monoclonal antibody-MMAE conjugate RC48-ADC in patients with HER2-positive advanced solid tumors. Gastric Cancer 2021;24:913–25. https://doi.org/10.1007/s10120-021-01168-7.Search in Google Scholar PubMed PubMed Central
67. Sheng, X, Wang, L, He, Z, Shi, Y, Luo, H, Han, W, et al.. Efficacy and safety of disitamab vedotin in patients with human epidermal growth factor receptor 2-positive locally advanced or metastatic urothelial carcinoma: a combined analysis of two phase II clinical trials. J Clin Oncol 2024;42:1391–402. https://doi.org/10.1200/jco.22.02912.Search in Google Scholar PubMed PubMed Central
68. Xu, H, Sheng, X, Zhou, L, Yan, X, Li, S, Chi, Z, et al.. A phase II study of RC48-ADC in HER2-negative patients with locally advanced or metastatic urothelial carcinoma. J Clin Oncol 2022;40:4519. https://doi.org/10.1200/jco.2022.40.16_suppl.4519.Search in Google Scholar
69. Sheng, X, Zhou, L, He, Z, Guo, H, Yan, X, Li, S, et al.. Preliminary results of a phase Ib/II combination study of RC48-ADC, a novel humanized anti-HER2 antibody-drug conjugate (ADC) with toripalimab, a humanized IgG4 mAb against programmed death-1 (PD-1) in patients with locally advanced or metastatic urothelial carcinoma. J Clin Oncol 2022;40:4518. https://doi.org/10.1200/jco.2022.40.16_suppl.4518.Search in Google Scholar
70. Galsky, M, Koshkin, V, Campbell, M, Drakaki, A, Bowman, I, Rose, A, et al.. 1967MO Preliminary efficacy and safety of disitamab vedotin (DV) with pembrolizumab (P) in treatment (Tx)-naive HER2-expressing, locally advanced or metastatic urothelial carcinoma (la/mUC): RC48G001 cohort C. Ann Oncol 2024;35:S1138-S9.10.1016/j.annonc.2024.08.2052Search in Google Scholar
71. Lambert, JM, Chari, RV. Ado-trastuzumab emtansine (T-DM1): an antibody-drug conjugate (ADC) for HER2-positive breast cancer. J Med Chem 2014;57:6949–64. https://doi.org/10.1021/jm500766w.Search in Google Scholar PubMed
72. Hayashi, T, Seiler, R, Oo, HZ, Jäger, W, Moskalev, I, Awrey, S, et al.. Targeting HER2 with T-DM1, an antibody cytotoxic drug conjugate, is effective in HER2 over expressing bladder cancer. J Urol 2015;194:1120–31. https://doi.org/10.1016/j.juro.2015.05.087.Search in Google Scholar PubMed
73. Schwartzberg, LS, Spigel, DR, VanderWalde, A, Zuniga, RM, Hong, J, Szado, T, et al.. MyTACTIC: activity of targeted therapy in patients (pts) with advanced solid tumors harboring specific biomarkers. J Clin Oncol 2024;42:3100. https://doi.org/10.1200/jco.2024.42.16_suppl.3100.Search in Google Scholar
74. van der Lee, MM, Groothuis, PG, Ubink, R, van der Vleuten, MA, van Achterberg, TA, Loosveld, EM, et al.. The preclinical profile of the duocarmycin-based HER2-targeting ADC SYD985 predicts for clinical benefit in low HER2-expressing breast cancers. Mol Cancer Ther 2015;14:692–703. https://doi.org/10.1158/1535-7163.mct-14-0881-t.Search in Google Scholar
75. Black, J, Menderes, G, Bellone, S, Schwab, CL, Bonazzoli, E, Ferrari, F, et al.. SYD985, a novel duocarmycin-based HER2-targeting antibody-drug conjugate, shows antitumor activity in uterine serous carcinoma with HER2/neu expression. Mol Cancer Ther 2016;15:1900–9. https://doi.org/10.1158/1535-7163.mct-16-0163.Search in Google Scholar PubMed
76. Menderes, G, Bonazzoli, E, Bellone, S, Black, J, Altwerger, G, Masserdotti, A, et al.. SYD985, a novel duocarmycin-based HER2-targeting antibody-drug conjugate, shows promising antitumor activity in epithelial ovarian carcinoma with HER2/Neu expression. Gynecol Oncol 2017;146:179–86. https://doi.org/10.1016/j.ygyno.2017.04.023.Search in Google Scholar PubMed PubMed Central
77. Modi, S, Jacot, W, Yamashita, T, Sohn, J, Vidal, M, Tokunaga, E, et al.. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med 2022;387:9–20. https://doi.org/10.1056/nejmoa2203690.Search in Google Scholar PubMed PubMed Central
78. Shi, F, Liu, Y, Zhou, X, Shen, P, Xue, R, Zhang, M. Disitamab vedotin: a novel antibody-drug conjugates for cancer therapy. Drug Deliv 2022;29:1335–44. https://doi.org/10.1080/10717544.2022.2069883.Search in Google Scholar PubMed PubMed Central
79. Li, H, Yu, C, Jiang, J, Huang, C, Yao, X, Xu, Q, et al.. An anti-HER2 antibody conjugated with monomethyl auristatin E is highly effective in HER2-positive human gastric cancer. Cancer Biol Ther 2016;17:346–54. https://doi.org/10.1080/15384047.2016.1139248.Search in Google Scholar PubMed PubMed Central
80. Pourjamal, N, Yazdi, N, Halme, A, Joncour, VL, Laakkonen, P, Saharinen, P, et al.. Comparison of trastuzumab emtansine, trastuzumab deruxtecan, and disitamab vedotin in a multiresistant HER2-positive breast cancer lung metastasis model. Clin Exp Metastasis 2024;41:91–102. https://doi.org/10.1007/s10585-024-10278-2.Search in Google Scholar PubMed PubMed Central
81. Galsky, MD, Grande, E, Necchi, A, Koontz, MZ, Iyer, G, Campbell, MT, et al.. Phase 3 open-label, randomized, controlled study of disitamab vedotin with pembrolizumab versus chemotherapy in patients with previously untreated locally advanced or metastatic urothelial carcinoma that expresses HER2 (DV-001). J Clin Oncol 2024;42:TPS717–TPS. https://doi.org/10.1200/jco.2024.42.4_suppl.tps717.Search in Google Scholar
82. Agersborg, S, Mixon, C, Nguyen, T, Aithal, S, Sudarsanam, S, Blocker, F, et al.. Immunohistochemistry and alternative FISH testing in breast cancer with HER2 equivocal amplification. Breast Cancer Res Treat 2018;170:321–8. https://doi.org/10.1007/s10549-018-4755-5.Search in Google Scholar PubMed PubMed Central
83. Aznab, M, Izadi, B, Amirian, F, Khazaei, S, Madani, SH, Ramezani, M. Comparison of immunohistochemical methods (IHC) and fluorescent in situ hybridization (FISH) in the detection of HER 2/neu gene in Kurdish patients with breast cancer in Western Iran. Int J Hematol Oncol Stem Cell Res 2022;16:217–23. https://doi.org/10.18502/ijhoscr.v16i4.10879.Search in Google Scholar PubMed PubMed Central
84. DiPeri, TP, Kong, K, Varadarajan, K, Karp, DD, Ajani, JA, Pant, S, et al.. Discordance of HER2 expression and/or amplification on repeat testing. Mol Cancer Ther 2023;22:976–84. https://doi.org/10.1158/1535-7163.mct-22-0630.Search in Google Scholar PubMed PubMed Central
85. Moutafi, M, Robbins, CJ, Yaghoobi, V, Fernandez, AI, Martinez-Morilla, S, Xirou, V, et al.. Quantitative measurement of HER2 expression to subclassify ERBB2 unamplified breast cancer. Lab Invest 2022;102:1101–8. https://doi.org/10.1038/s41374-022-00804-9.Search in Google Scholar PubMed
86. Lattanzi, M, Niederhausern, A, Zheng, J, Bahadur, N, Nichols, C, Barton, L, et al.. Incidence and clinical outcomes of HER2-altered bladder cancer (BC) patients (pts). J Clin Oncol 2022;40:556. https://doi.org/10.1200/jco.2022.40.6_suppl.556.Search in Google Scholar
87. Aggen, DH, Shah, NJ, Zheng, J, Alam, SM, Balar, O, Niederhausern, A, et al.. HER2 and PD-L1 immunohistochemistry (IHC) expression, and HER2 genomic alterations: associations and clinical outcomes for advanced bladder cancer. J Clin Oncol 2024;42:538. https://doi.org/10.1200/jco.2024.42.4_suppl.538.Search in Google Scholar
88. Brown, JR, Koshkin, VS. Therapies after progression on enfortumab vedotin and pembrolizumab: navigating second-line options for metastatic urothelial carcinoma in the new treatment landscape. Eur Urol Focus 2024;10:231–3. https://doi.org/10.1016/j.euf.2024.05.011.Search in Google Scholar PubMed
89. Zhang, J, Ji, D, Cai, L, Yao, H, Yan, M, Wang, X, et al.. First-in-human HER2-targeted bispecific antibody KN026 for the treatment of patients with HER2-positive metastatic breast cancer: results from a phase I study. Clin Cancer Res 2022;28:618–28. https://doi.org/10.1158/1078-0432.ccr-21-2827.Search in Google Scholar
90. Meric-Bernstam, F, Beeram, M, Hamilton, E, Oh, DY, Hanna, DL, Kang, YK, et al.. Zanidatamab, a novel bispecific antibody, for the treatment of locally advanced or metastatic HER2-expressing or HER2-amplified cancers: a phase 1, dose-escalation and expansion study. Lancet Oncol 2022;23:1558–70. https://doi.org/10.1016/s1470-2045(22)00621-0.Search in Google Scholar
91. Li, BT, Smit, EF, Goto, Y, Nakagawa, K, Udagawa, H, Mazières, J, et al.. Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N Engl J Med 2022;386:241–51. https://doi.org/10.1056/nejmoa2112431.Search in Google Scholar PubMed PubMed Central
92. Goto, K, Goto, Y, Kubo, T, Ninomiya, K, Kim, SW, Planchard, D, et al.. Trastuzumab deruxtecan in patients with HER2-mutant metastatic non-small-cell lung cancer: primary results from the randomized, phase II DESTINY-lung02 trial. J Clin Oncol 2023;41:4852–63. https://doi.org/10.1200/jco.23.01361.Search in Google Scholar
© 2025 the author(s), published by De Gruyter on behalf of Tech Science Press (TSP)
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review Articles
- Targeting HER2 in metastatic urothelial carcinoma: a contemporary review
- Advances in research on the impact of exosomal miRNAs on liver metastasis of colorectal cancer
- Sebaceous carcinoma of the intraoral origin: a literature review
- Role of emerging theranostic technologies in precision oncology: revolutionizing cancer diagnosis and treatment
- Research Articles
- Half-body irradiation with dose escalation in the era of advanced systemic therapies: unveiling new therapeutic opportunities
- Characteristics of MMR protein expression in colorectal cancer and MMR gene variations in Vietnamese patients with Lynch syndrome
- BMPR1A promotes the proliferation of colorectal cancer cells through the activation of Smad1
- Construction and validation of a diagnostic model for cholangiocarcinoma based on tumor-educated platelet RNA expression profiles
- Prognostic implications of PCSK9 expression in HER2-positive breast cancer
- Rapid Communication
- Potential impact of hormone replacement therapy on the risk of hepatocellular carcinoma in women of the PLCO cohort
- Article Commentary
- “Plant-based and ketogenic diets as diverging paths to address cancer”: a commentary concerning the supposed superiority of a plant-based diet
- Novel insights into molecular landscape of advanced renal cell carcinoma
- Corrigendum
- Corrigendum to “Y27632 induces tongue squamous cell carcinoma cell apoptosis through MAPK-ERK/JNK signal”
Articles in the same Issue
- Frontmatter
- Review Articles
- Targeting HER2 in metastatic urothelial carcinoma: a contemporary review
- Advances in research on the impact of exosomal miRNAs on liver metastasis of colorectal cancer
- Sebaceous carcinoma of the intraoral origin: a literature review
- Role of emerging theranostic technologies in precision oncology: revolutionizing cancer diagnosis and treatment
- Research Articles
- Half-body irradiation with dose escalation in the era of advanced systemic therapies: unveiling new therapeutic opportunities
- Characteristics of MMR protein expression in colorectal cancer and MMR gene variations in Vietnamese patients with Lynch syndrome
- BMPR1A promotes the proliferation of colorectal cancer cells through the activation of Smad1
- Construction and validation of a diagnostic model for cholangiocarcinoma based on tumor-educated platelet RNA expression profiles
- Prognostic implications of PCSK9 expression in HER2-positive breast cancer
- Rapid Communication
- Potential impact of hormone replacement therapy on the risk of hepatocellular carcinoma in women of the PLCO cohort
- Article Commentary
- “Plant-based and ketogenic diets as diverging paths to address cancer”: a commentary concerning the supposed superiority of a plant-based diet
- Novel insights into molecular landscape of advanced renal cell carcinoma
- Corrigendum
- Corrigendum to “Y27632 induces tongue squamous cell carcinoma cell apoptosis through MAPK-ERK/JNK signal”

