The role of synthetic peptides derived from bovine lactoferricin against breast cancer cell lines: a mini-review
-
Manuela de la Rosa Arbeláez
und Guilherme Diniz Tavares
Abstract
Breast cancer represents the most commonly diagnosed cancer worldwide, accounting for approximately one in eight cancers diagnosed. Despite significant advances in the diagnosis and detection of this disease, there is still a great need for more effective therapies to combat the invasive forms, especially those with a high incidence of metastasis. For that reason, bioactive molecules as peptides, including bovine lactoferricin (LfcinB), have been investigated. In this sense, there are reports that 20RRWQWR25 motif derivate from the LfcinB has shown activity against different cancer cell lines. Thus, current studies are being carried out with synthetic derivatives (linear, palindromic, dimer and tetrameric structures) that contain the 20RRWQWR25 motif in order to increase its activity against cancer cell lines by altering its hydrophobicity and net positive charge. In this regard, studies have focused on the use of LfcinB derivatives to combat breast cancer cell lines, with encouraging results. Therefore, in this mini-review, we present the state of the art regarding the activity of LfcinB and its analogs against breast cancer cell lines.
Introduction
Breast cancer has emerged as a burgeoning global health concern, being categorized as the prevalent form of cancer among women and the leading cause of cancer-related fatalities. Epidemiological statistics reveal a significant escalation, with approximately 2.26 million new cases recorded in 2020, accounting for 11.7 % of all cancer diagnoses across genders [1].
Systemic treatments such as chemotherapy, hormone therapy, and radiotherapy still face challenges, including drug resistance, adverse effects, toxicity, high costs, and the disease’s heterogeneity, which is categorized into different subtypes known as Luminal A, Luminal B, HER2 positive, and Triple-negative [1], [2], [3], [4].
Several strategies are being explored to discover more selective and less invasive therapeutic agents, including the use of anticancer peptides. One such peptide is bovine lactoferricin (LfcinB: 17FKCRRWQWRMKKLGAPSITCVRRAF41), which comprises 25 amino acids derived from the N-terminal region of a protein known as bovine lactoferrin (BLF) [5]. LfcinB exhibits both antimicrobial and anticancer activities against various cancer cell lines including colon, lung, liver, leukemia, and breast cancer [6, 7].
It is hypothesized that LfcinB is attracted to the cell membrane through electrostatic interactions involving cationic amino acids. This interaction leads to membrane disruption and cellular lysis, as illustrated in Figure 1 [6], [7], [8]. In addition, studies in immunodeficient NOD-SCID-gamma (NSG) mice have shown that intratumoral injections of the LfcinB peptide result in reduced tumor size and density, as observed by live imaging with IVIS. This treatment induces apoptosis and inhibits invasion without affecting normal cells [9].
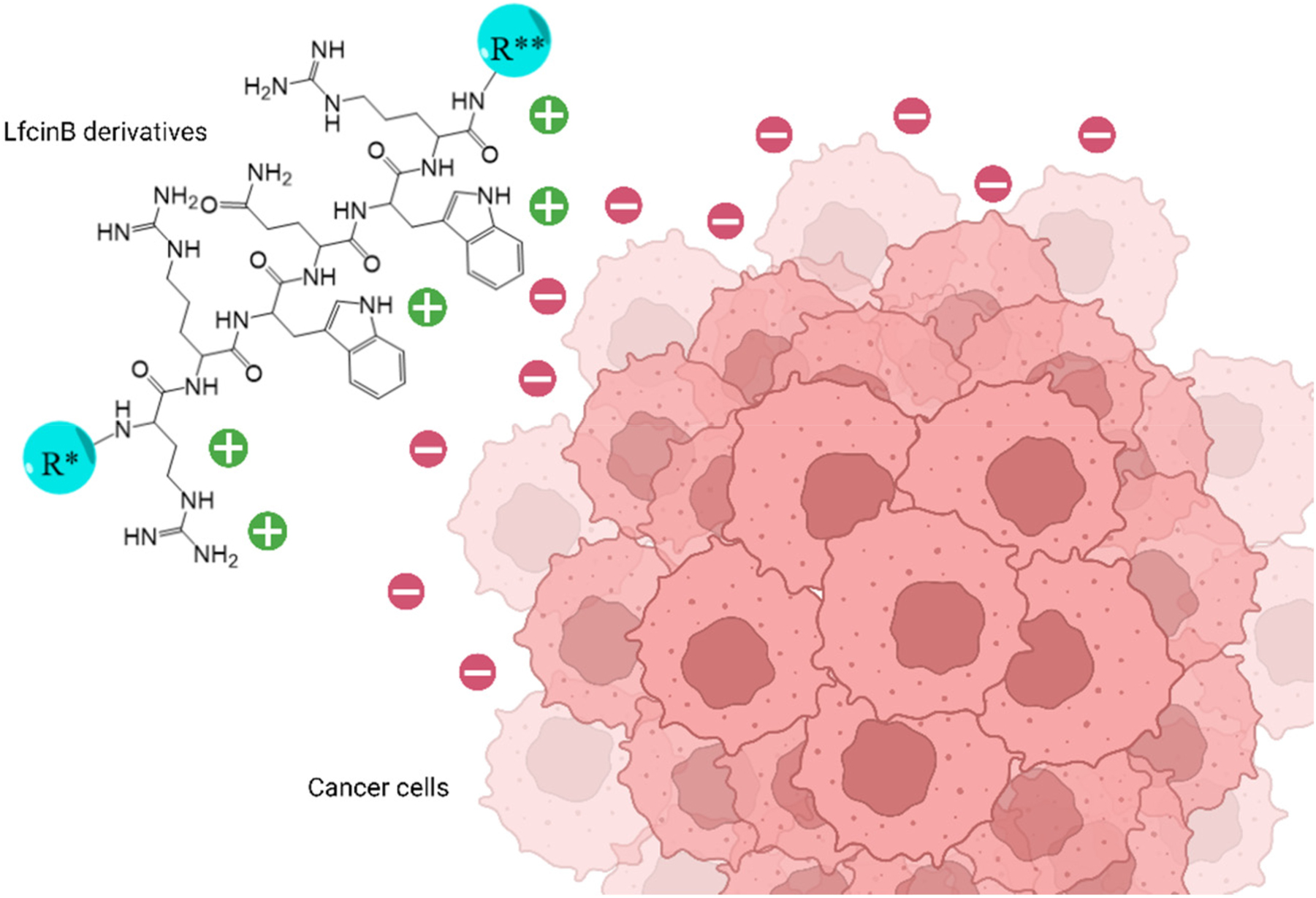
Electrostatic interactions within cancer cell membranes and the minimal motif and LfcinB derivatives. R* and R**=hydrogen atom or different amino acid sequences.
Previous studies have suggested that the anticancer activity of LfcinB is due to the 20RRWQWR25 fragment. Therefore, current in vitro studies are underway to evaluate synthetic variants (linear, dimeric, tetrameric, cyclic, and palindromic peptide structures) containing this sequence against cancer cell lines such as MCF-7, MDA-MB-231, and MDA-MB-468. In Table 1, we present a summary of information gathered over the last 10 years regarding these LfcinB derivatives.
Bovine lactoferricin derivates, its amino acid sequence, and breast cancer cell lines used as experimental models.
| Peptide | Amino acid sequence | BCCL | Observations | Ref. |
|---|---|---|---|---|
| 26[D]-LfcinB (20–30)2 | (RRWQWRDKKLG)2-K*-Ahx | MDA-MB-468 MCF-7 |
The cytotoxic effect increases when hydrophobicity increases: 26[F]>26[L]>26[A]>26[M]. Dimeric peptides show low cytotoxic effect against non-tumorigenic cell line MCF-12 and it is also suggested by cytometry that they generate cell death through the apoptosis pathway. | [4] |
| 26[K]-LfcinB (20–30)2 | (RRWQWRKKKLG)2-K*-Ahx | |||
| 26[M]-LfcinB (20–30)2 | (RRWQWRMKKLG)2-K*-Ahx | |||
| 26[A]-LfcinB (20–30)2 | (RRWQWRAKKLG)2-K*-Ahx | |||
| 26[L]-LfcinB (20–30)2 | (RRWQWRLKKLG)2-K*-Ahx | |||
| 26[F]-LfcinB (20–30)2 | (RRWQWRFKKLG)2-K*-Ahx | |||
| LfcinB (20–25) | 20RRWQWR25 | MDA-MB-231 MDA-MB-468 |
Dimeric and tetrameric peptides show higher cytotoxic effect against triple-negative cancer cell lines when compared with linear peptides derived of LfcinB. Also, it was observed low cytotoxic effect against in fibroblast (PCS-201-012). Cyclic peptides show low cytotoxic effect against breast cancer cell lines. |
[5] |
| LfcinB (20–25)2 | (RRWQWR)2-K*-Ahx | |||
| LfcinB (20–25)4 | (RRWQWR)4-K*2-Ahx2-C2 | |||
| LfcinB (20–25)cyc | Ca-RRWQWR-Ahx2-Ca | |||
| LfcinB (20–30) | 20RRWQWRMKKLG30 | |||
| LfcinB (20–30)2 | (RRWQWRMKKLG)2-K*-Ahx | |||
| LfcinB (20–30)4 | (RRWQWRMKKLG)4-K*2-Ahx2-C2 | |||
| LfcinB (20–30)cyc | Ca-RRWQWRMKKLG-Ahx-Ca | |||
| 19[A]-LfcinB (17–31) | 17FKARRWQWRMKKLGA31 | |||
| 19[A]-LfcinB (17–31)2 | (FKARRWQWRMKKLGA)2-K*-Ahx | |||
| 19[A]-LfcinB (17–31)4 | (FKARRWQWRMKKLGA)4-K*2-Ahx2-C2 | |||
| 19[A]-LfcinB (17–31)cyc | Ca-FKARRWQWRMKKLGA-Ahx-Ca | |||
| LfcinB (21–25)Pal | RWQWRWQWR | MDA-MB-231 MCF-7 |
It was observed that the palindromic derivate has great potential in vitro against Luminal A and triple-negative cancer cell lines which had a better result with MCF-7. It was also tested with non-cancerogenic cells showing low toxicity. It was suggested that trough flow cytometry assays LfcinB (21–25)Pal generates late apoptosis in MCF-7. | [6] |
| 1 | _WQWRWQW_NH2 | MDA-MB-231 MCF-7 |
It was suggested that it is not only the positive net charge of the peptide that increase the cytotoxic effect against cancer cell lines but also the position in which is add. In that order RR-1-R peptide increased its activity while maintaining selectivity. It was shown that RR-1-R peptide activates intrinsic apoptosis pathways and reduced the migration of MCF-7 cells. | [7] |
| R-1-R | R-WQWRWQW-R | |||
| RR-1-RR | RR-WQWRWQW-RR | |||
| R-1-RR | R-WQWRWQW-RR | |||
| RR-1-R | RR-RWQWRWQW-R | |||
| R-1 | R-WQWRWQW_NH2 | |||
| 1-R | _WQWRWQWR-NH2 | |||
| LfcinB (21–25)Pal | RWQWRWQWR | MDA-MB-468 | It is suggested that substituting each amino acid in the palindromic peptide for alanine shows a decrease in its activity against MDA-MB-468 compared with the original LfcinB (21–25)Pal peptide indicating that there is a correlation between net charge and hydrophobicity with the cytotoxic effect. | [8] |
| A1 | AWQWRWQWR | |||
| A2 | RAQWRWQWR | |||
| A3 | RWAWRWQWR | |||
| A4 | RWQARWQWR | |||
| A5 | RWQWAWQWR | |||
| A6 | RWQWRAQWR | |||
| A7 | RWQWRWAWR | |||
| A8 | RWQWRWQAR | |||
| A9 | RWQWRWQWA | |||
| LfcinB (20–25)4 | (RRWQWR)4–K*2–Ahx2–C2 | MCF-7 | It was shown that the tetrameric structure of 20RRWQWR25 sequence has great activity against MCF-7 and also is selective when comparing with the low activity against non-tumorogenic trophoblastic cell line. Furthermore, this peptide induces mitochondrial membrane depolarization and increase of cytoplasmic calcium concentration indicating an apoptotic pathway. | [10] |
-
BCCL, breast cancer cell lines; R, arginine (arg); W, tryptophan (trp); Q, glutamine (glu); A, alanine (ala); L, leucine (leu); G, glycine (gly); F, phenylalanine (phe); M, methionine (met); K, lysine (lys); P, proline (pro); S, serine (ser); I, isoleucine; T, threonine (thr); V, valine (val); K*, precursor of lysine for dimeric and tetrameric peptide, Ahx, 6-aminohexanoic residue; C, precursor of tetrameric peptide. aPrecursor of cyclic peptide.
The role of synthetic LfcinB derivatives against breast cancer
In vitro studies have shown that synthetic analogs containing the 20RRWQWR25 motif in their structure increase the activity against Luminal A (MCF-7) and show low cytotoxicity against the human trophoblast cell line (CRL-3271) and the non-tumorigenic cell line MCF-12 [4, 6, 7, 10]. Among these synthetic derivatives, we would like to highlight the study by Insuasty-Cepeda et al., in which the dimeric peptide LfcinB (20–30)2 was synthesized six times with changes in the 26th position amino acid [4]. This study suggested that not only electrostatic interactions of the peptide but also increased hydrophobicity contributed to the cytotoxic effect. They observed that 26[F]-LfcinB (20–30)2 (IC50=6 µM) and 26[L]-LfcinB (20–30)2 (IC50=20 µM) induced cell damage via the apoptosis pathway [4]. Comparable outcomes were achieved using a tetrameric structure, LfcinB (20–25)4, which at a concentration of 12.2 µM (30 μg/mL), selectively damaged MCF-7 cells and led mitochondrial membrane depolarization associated with an apoptotic pathway [10]. However, it is imperative to acknowledge that in vitro experiments cannot fully replicate the intricacies of organ systems. Consequently, ongoing research should prioritize in vivo approaches.
It has been suggested that increasing the net positive charge and peptide length also increases the cytotoxic effect. To investigate this, a study utilizing a linear palindromic peptide analog, Lfcin (21–25)Pal, six peptides were synthesized by adding and deleting arginine (Arg) at the N-terminal, C-terminal, or both ends of the structure. The results showed that adding arginine at the N-terminus increased the cytotoxic effects while maintaining some level of selectivity [7].
Cytometry experiments showed that this palindromic peptide induced apoptotic events in MCF-7 cells, making it a promising molecule with a therapeutic window of 70–140 μM. In addition, it shows a broad spectrum of activity against various cancer cell lines [6]. Notably, Lfcin (21–25)Pal is the only peptide derivative that has been tested in vivo using a Galleria mellonella larvae model, showing a 90 % survival rate after 10 days when administered at a dosage of 800 μg/mg of peptide [7].
Moreover, the 20RRWQWR25 motif and cyclic derivatives containing that sequence showed low cytotoxicity against cancer cell lines that are more aggressive subtypes such as MDA-MB-231 and MDA-MB-468. For this reason, the synthesis of dimeric and tetrameric structures containing the 20RRWQWR25 motif was tested against triple-negative subtype cancer cell lines, as in the case of LfcinB (20–25)4, LfcinB (20–30)2 and LfcinB (20–30)4 in which IC50 is less than 40 μM [5]. Again, it is suggested that increasing hydrophobicity such as 26[F]-LfcinB (20–30)2 and 26[L]-LfcinB (20–30)2 increases the cytotoxic effect against MDA-MB-231 and maintains low cytotoxicity against MCF-12 [4]. Similar results have been shown with the palindrome analog LfcinB (21–25)Pal, which showed promising results against both cancer cell lines [6], [7], [8]. However, there is a lack of preclinical studies and a great need for more effective treatments for triple-negative subtypes.
Finally, in an attempt to investigate the hypothesis that Trp residues within the peptide’s structure play a pivotal role in its internalization into cancer cells, Barragán-Cárdenas et al. synthetized 9 Lfcin (21–25)Pal peptides substituting Ala in each position (Table 1) and evaluated the cytotoxicity effect against MDA-MB-468 [8]. They found that substitution of Trp for Ala decreased its cytotoxic effect suggesting an association between hydrophobicity and Trp position with the mode of its internalization.
In conclusion, there is a strong relationship between the position, hydrophobicity and net charge of LfcinB derivatives with their cytotoxic effect against cancer cells. Furthermore, it would be very interesting to study how each family of derivatives (linear, dimeric, tetrameric, palindromic or cyclic) affects preclinic models in vivo.
Future perspectives
Overall, the potential of LfcinB and its derivatives against specific breast cancer subtypes, such as triple-negative and Luminal A, is remarkable. Here, we particularly highlight the palindrome peptide Lfcin (20–25)Pal. However, further studies are needed to gain a better understanding of their mechanisms of action. Additionally, preclinical studies should be conducted to establish their safety and efficacy in vivo. Furthermore, it is important to encourage and emphasize future research focused on the green synthesis of peptides and the development of drug delivery systems that ensure their stability in biological systems, such as nanostructured systems.
Acknowledgments
Authors would like to thank Fundação de Amparo à Pesquisa do Estado de Minas Gerais – FAPEMIG, Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq, as well as Coordenação de Aperfeiçoamento de Pessoal de Nível Superior CAPES for fellowships.
-
Research ethics: Not applicable.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Author contributions: All authors confirm contribution to the paper as follows: study conception and design: F.P., and G.D.T; data collection: M.R.A and D.T.A.; draft manuscript preparation: M.R.A, D.T.A, and A.C.B.D.; manuscript revision: F.P. and G.D.T. Authors reviewed the results and approved the final version of the manuscript.
-
Competing interests: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Chhikara, BS, Parang, K. Global Cancer Statistics 2022: the trends projection analysis. Chem Biol Lett 2023;10:451.Suche in Google Scholar
2. Smolarz, B, Nowak, AZ, Romanowicz, H. Breast cancer—epidemiology, classification, pathogenesis and treatment (review of literature). Cancers 2022;14:2569. https://doi.org/10.3390/cancers14102569.Suche in Google Scholar PubMed PubMed Central
3. Dai, X, Cheng, H, Bai, Z, Li, J. Breast cancer cell line classification and its relevance with breast tumor subtyping. J Cancer 2017;8:3131–41. https://doi.org/10.7150/jca.18457.Suche in Google Scholar PubMed PubMed Central
4. Insuasty-Cepeda, DS, Barragán-Cárdenas, AC, Ochoa-Zarzosa, A, López-Meza, JE, Fierro-Medina, R, García-Castañeda, JE, et al.. Peptides derived from (RRWQWRMKKLG)2-K-Ahx induce selective cellular death in breast cancer cell lines through apoptotic pathway. Int J Mol Sci 2020;21:4550. https://doi.org/10.3390/ijms21124550.Suche in Google Scholar PubMed PubMed Central
5. Vargas Casanova, Y, Rodríguez Guerra, JA, Umaña Pérez, YA, Leal Castro, AL, Almanzar Reina, G, García Castañeda, JE, et al.. Antibacterial synthetic peptides derived from bovine lactoferricin exhibit cytotoxic effect against MDA-MB-468 and MDA-MB-231 breast cancer cell lines. Molecules 2017;22:1641. https://doi.org/10.3390/molecules22101641.Suche in Google Scholar PubMed PubMed Central
6. Barragán-Cárdenas, A, Insuasty-Cepeda, DS, Niño-Ramírez, VA, Umaña-Pérez, A, Ochoa-Zarzosa, A, López-Meza, JE, et al.. The nonapeptide RWQWRWQWR: a promising molecule for breast cancer therapy. Chem Select 2020;5:9691–700. https://doi.org/10.1002/slct.202002101.Suche in Google Scholar
7. Barragán-Cárdenas, AC, Insuasty-Cepeda, DS, Vargas-Casanova, Y, López-Meza, JE, Parra-Giraldo, CM, Fierro-Medina, R, et al.. Changes in length and positive charge of palindromic sequence RWQWRWQWR enhance cytotoxic activity against breast cancer cell lines. ACS Omega 2023;8:2712–22. https://doi.org/10.1021/acsomega.2c07336.Suche in Google Scholar PubMed PubMed Central
8. Barragán-Cárdenas, A, Urrea-Pelayo, M, Alfonso Niño-Ramírez, V, Umaña-Pérez, A, Paul Vernot, J, Marcela Parra-Giraldo, C, et al.. Selective cytotoxic effect against the MDA-MB-468 breast cancer cell line of the antibacterial palindromic peptide derived from bovine lactoferricin. RSC Adv 2020;10:17593–601. https://doi.org/10.1039/d0ra02688c.Suche in Google Scholar PubMed PubMed Central
9. Rahman, R, Fonseka, AD, Sua, SC, Ahmad, M, Rajendran, R, Ambu, S, et al.. Inhibition of breast cancer xenografts in a mouse model and the induction of apoptosis in multiple breast cancer cell lines by lactoferricin B peptide. J Cell Mol Med 2021;25:7181–9. https://doi.org/10.1111/jcmm.16748.Suche in Google Scholar PubMed PubMed Central
10. Rodríguez Guerra, J, Barragán Cárdenas, A, Ochoa-Zarzosa, A, López Meza, J, Pérez, AU, Fierro-Medina, R, et al.. The tetrameric peptide LfcinB (20–25) 4 derived from bovine lactoferricin induces apoptosis in the MCF-7 breast cancer cell line. RSC Adv 2019;9:20497–504. https://doi.org/10.1039/c9ra04145a.Suche in Google Scholar PubMed PubMed Central
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Review Articles
- Mitochondrial thermogenesis in cancer cells
- Application of indocyanine green in the management of oral cancer: a literature review
- Long non-coding RNA, FOXP4-AS1, acts as a novel biomarker of cancers
- The role of synthetic peptides derived from bovine lactoferricin against breast cancer cell lines: a mini-review
- Single cell RNA sequencing – a valuable tool for cancer immunotherapy: a mini review
- Research Articles
- Global patterns and temporal trends in ovarian cancer morbidity, mortality, and burden from 1990 to 2019
- The association between NRF2 transcriptional gene dysregulation and IDH mutation in Grade 4 astrocytoma
- More than just a KRAS inhibitor: DCAI abrogates the self-renewal of pancreatic cancer stem cells in vitro
- DUSP1 promotes pancreatic cancer cell proliferation and invasion by upregulating nephronectin expression
- IMMT promotes hepatocellular carcinoma formation via PI3K/AKT/mTOR pathway
- MiR-100-5p transfected MSCs-derived exosomes can suppress NSCLC progression via PI3K-AKT-mTOR
- Inhibitory function of CDK12i combined with WEE1i on castration-resistant prostate cancer cells in vitro and in vivo
- Prognostic potential of m7G-associated lncRNA signature in predicting bladder cancer response to immunotherapy and chemotherapy
- Case Reports
- A rare FBXO25–SEPT14 fusion in a patient with chronic myeloid leukemia treatment to tyrosine kinase inhibitors: a case report
- Stage I duodenal adenocarcinoma cured by a short treatment cycle of pembrolizumab: a case report
- Rapid Communication
- ROMO1 – a potential immunohistochemical prognostic marker for cancer development
- Article Commentary
- A commentary: Role of MTA1: a novel modulator reprogramming mitochondrial glucose metabolism
Artikel in diesem Heft
- Frontmatter
- Review Articles
- Mitochondrial thermogenesis in cancer cells
- Application of indocyanine green in the management of oral cancer: a literature review
- Long non-coding RNA, FOXP4-AS1, acts as a novel biomarker of cancers
- The role of synthetic peptides derived from bovine lactoferricin against breast cancer cell lines: a mini-review
- Single cell RNA sequencing – a valuable tool for cancer immunotherapy: a mini review
- Research Articles
- Global patterns and temporal trends in ovarian cancer morbidity, mortality, and burden from 1990 to 2019
- The association between NRF2 transcriptional gene dysregulation and IDH mutation in Grade 4 astrocytoma
- More than just a KRAS inhibitor: DCAI abrogates the self-renewal of pancreatic cancer stem cells in vitro
- DUSP1 promotes pancreatic cancer cell proliferation and invasion by upregulating nephronectin expression
- IMMT promotes hepatocellular carcinoma formation via PI3K/AKT/mTOR pathway
- MiR-100-5p transfected MSCs-derived exosomes can suppress NSCLC progression via PI3K-AKT-mTOR
- Inhibitory function of CDK12i combined with WEE1i on castration-resistant prostate cancer cells in vitro and in vivo
- Prognostic potential of m7G-associated lncRNA signature in predicting bladder cancer response to immunotherapy and chemotherapy
- Case Reports
- A rare FBXO25–SEPT14 fusion in a patient with chronic myeloid leukemia treatment to tyrosine kinase inhibitors: a case report
- Stage I duodenal adenocarcinoma cured by a short treatment cycle of pembrolizumab: a case report
- Rapid Communication
- ROMO1 – a potential immunohistochemical prognostic marker for cancer development
- Article Commentary
- A commentary: Role of MTA1: a novel modulator reprogramming mitochondrial glucose metabolism

