Abstract
Reaction of dimethyl N-cyanodithioiminocarbonate, NCNC(SCH3)2 (L), and SnPh3Cl led to the formation of [ClPh3SnNCNC(SCH3)2] (1) which crystallizes in the monoclinic space group C2/c with Z=8, a=19.152(2) Å, b=12.8659 (16) Å, c=19.063(3) Å, β=108.608(3) and V=4451.6(10) Å3. The structure of 1 consists of a SnPh3 moiety trans-coordinated in apical positions by one L ligand, N-coordinated, and one chlorine atom involving a trigonal bipyramid geometry around the Sn(IV) atom. To our knowledge, this is the first isolation of a discrete triorganotin(IV) complex with a N-cyanodithioiminocarbonate adduct. The structural characterization of 1 was completed by infrared and nuclear magnetic resonance spectroscopy, and elemental analysis which confirm the X-ray elucidation.
In the past, there were relatively few investigations in the literature on the main group metal complexes with cyanamide adducts (N≡C-N-). Early studies on this domain date back to the 1970s (Seltzer, 1968; Jain and Rivest, 1970). More recently, organoheterobimetallic cyanodithioimidocarbonates involving tin-based complexes have been also reported for electrical conducting properties (Singh and Kumar, 2008). To the best of our knowledge and up to now, only three examples, especially of tin(IV) derivatives, have been crystallographically resolved, exhibiting essentially polymeric structures: (CH3)2Sn[N(CN)2]2 and (CH3)2Sn[N(CN)2] (Chow, 1971), and (CH3)2Sn[NCNNO2] (Jäger et al., 1997).
In our ongoing studies on N-ligands coordinated to organotin(IV) moieties (Sow et al., 2012) we focused on the reactivity of dimethyl N-cyanodithioiminocarbonate, NCNC(SCH3)2 (L), a precursor for the synthesis of quinazolinone derivatives (Al-Salahi et al., 2014), with Ph3SnCl. The resulting complex 1 has been characterized by infrared and multinuclear nuclear magnetic resonance (NMR) spectroscopy (1H, 13C{1H}, and 119Sn{1H}), and its structure was finally determined by an X-ray crystallographic analysis. While the cyanodithioimidocarbonate, [NCNCS2]2-, dianion commonly adopts the S,S-bidentate bridging coordination mode (Burchell et al., 2004), L in which both sulfur atoms are methylated was found in 1 to be a monodentate N-ligand (Scheme 1). To date, such a structure is rather uncommon. The only examples are described for transition metal complexes. The first one was the copper complex, CuCl2[NCNC(SCH3)2]2 (Kojic-Prodic et al., 1992). Recently, some of us have isolated two new specimens, reporting the crystal structure of dichloridobis(dimethyl N-cyanodithioiminocarbonate)cobalt(II) (Diop et al., 2016a) and then dichloridobis(dimethyl N-cyanodithioiminocarbonate)zinc (Diop et al., 2016b).
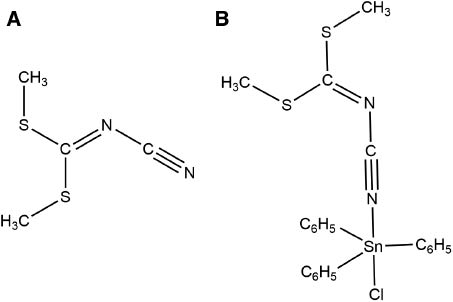
Molecular representations of (A) L and (B) 1.
Compound 1 was first studied by middle and far infrared spectroscopy [attenuated total reflectance (ATR) mode], and its spectrum was compared to those of the free L ligand and Ph3SnCl (Figures S1 and S2, respectively). The shift of the ν(C≡N) band to a higher frequency by 20 cm-1 constitutes the most notable observation which can be explained by the coordination of L to Sn atom through the nitrogen atom of the cyano group. Additional vibration bands, characteristic of phenyl ligands (Ph, C6H5), are also observed at 732 and 696 cm-1 corresponding to δ(Csp2-H) and δ(C=C) elongations, respectively. The far infrared spectrum of 1 combines the fingerprints of Ph3SnCl and L and evidences in the region of 330 cm-1 the absence of the ν(Sn-Cl) band (Tudela and Calleja, 1993), as already observed before for such Ph3SnCl adducts (Sall et al., 1995). The CHNS elemental analysis performed on crystals also supports the proposed formula. Furthermore, compound 1 is well soluble in chlorinated and aromatic solvents. 1H and 13C{1H} NMR spectra in CDCl3 and toluene-d8 highlight the characteristic signals of L and phenyl groups and confirm the 1:3 ratio (Figures S2 and S3). The 119Sn{1H} NMR spectrum in CDCl3 exhibits only one resonance at -47.8 ppm (SnMe4 as external reference, Figure S4) showing a very slight shift to higher field with respect to SnPh3Cl used as organotin precursor (δ=44.7 ppm in CDCl3, Carcelli et al., 1992). A comparable observation was made in toluene-d8 with δ=50.9 ppm. A concentration dependence study was also investigated in CD2Cl2 solution at 300 K involving a limited effect on the values of δ(119Sn{1H}) [0.5 m, δ=-49.9 ppm; 1 m, δ=-51.2 ppm; 2 m, δ=-52.1 ppm] (Figure S6). However, the spectra recorded for the 0.5 and 1 m solutions exhibit broad signals. Such 119Sn{1H} NMR chemical shift values are in line with the presence of four-coordinate species (Holeček et al., 1983). On the basis of the five-coordination of 1 (Scheme 1b), values in the range of -180 to -260 ppm would rather be expected (Holeček et al., 1983). Thus, the probable dissociation of L occurs in solution. Indeed, triphenyltin(IV) adducts are known to be unstable in solution releasing Ph3SnCl. To date, several examples have been already reported in the literature (Momeni et al., 2010; Gholivand et al., 2014). Similar conclusions are also supported by middle infrared measurements (transmission mode) in solution (CH2Cl2). The frequency of the ν(CN) band recorded for 1 fits nearly with that of the free ligand L measured under the same conditions (Figure S7).
The coordination of L to an organotin(IV) moiety was definitively verified in the solid state by an X-ray crystallographic analysis performed on suitable crystals. Crystallographic data and refinement details are summarized in Table 1. An Ortep view with selected bond lengths and angles (Å, °) is shown in Figure 1. The dimethyl N-cyanodithioiminocarbonate ligand was found disordered over two positions with occupation factors equal to 0.5, due to the steric hindrance between the two closer molecules. The structure of 1 corresponds to a SnPh3 moiety trans-coordinated by one L ligand, N-coordinated, and one chlorine atom, terminally bonded. The environment around the tin(IV) atom can be viewed as trigonal bipyramidal (tbp). Thus, the three phenyl groups occupy the equatorial planes [Sn-C1 2.1198(17), Sn-C7 2.1345(17), and Sn-C13 2.1345(17) Å]. The sum of the angles at tin [C1-Sn-C7 121.00(6), C1-Sn-C13 114.49(6), and C13-Sn-C7 121.86(6)] is equal to 357.35°. The Sn-C distances are in the range of those found in tbp SnPh3 moieties (Pelizzi and Pelizzi, 1983). The apical positions are occupied by chlorine and nitrogen atoms [Sn-Cl 2.4717(5) and Sn-N1 2.5360(14) Å]. The Cl-Sn-N angle [177.13(4)°] reveals a slight deviation from linearity. Compared to the two existing examples of chlorotriphenyltin complexes with a cyano adduct, already described in the literature (Carini et al., 1990; Carcelli et al., 1992), some structural disparities are observed with 1 and are reported in Table 2. Thus, the Sn-N distance of 1, longer than in the two examples given, reflects the weak coordination of L and can explain the facile dissociation observed in solution, leading to the release of the starting tin(IV) complex.
Crystal data and structure refinement of 1.
| Formula | C22H21ClN2S2Sn | |
| Formula weight, g/mol | 531.67 | |
| Temperature | 100(2) K | |
| Crystal system | Monoclinic | |
| Space group | C2/C | |
| Unit cell dimensions | a=19.152(2) Å | α=90° |
| b=12.8659(16) Å | β=108.608(3)° | |
| c=19.063(3) Å | γ=90° | |
| Volume | 4451.6(10) Å3 | |
| Z | 8 | |
| Density (calculated) | 1.587 mg/m3 | |
| Absorption coefficient | 1.466 mm-1 | |
| F(000) | 2128 | |
| Crystal size, mm | 0.50×0.20×0.12 | |
| θ range for data collection | 3.235 to 57.584° | |
| Index ranges | -24≤h≤24, -16≤k≤16, -24≤l≤24 | |
| Reflections collected | 88554 | |
| Independent reflections | 5141 [R(int)=0.0213] | |
| Completeness to θmax | 99.7% | |
| Refinement method | Full-matrix least-squares on F2 | |
| Data/restraints/parameters | 5141/255/298 | |
| Goodness-of-fitc on F2 | 1.186 | |
| Final R indices [I>2σ(I)] | R1=0.0205a, wR2=0.0411b | |
| R indices (all data) | R1=0.0236, wR2=0.0436 | |
| Largest difference peak/hole, e/Å3 | 0.791/-0.371 | |
| CCDC number | 1472446 |
aR1=Σ(||Fo|-|Fc||)/Σ|Fo|; bwR2=[Σw(Fo2-Fc2)2/Σ[w(Fo2)2]1/2 where w=1/[σ2(Fo2)+11.0969P+(0.0071P)2] where P=(max(Fo2,0)+2*Fc2)/3; cgoodness of fit=[Σw(Fo2-Fc2)2/(No-Nv)]1/2.
![Figure 1: Molecular structure of [ClPh3SnNCNC(SCH3)2] (1) showing 30% probability ellipsoids and the crystallographic numbering scheme [Ortep views. A) Showing the two positions of the dimethyl N-cyanodithioiminocarbonate ligand, B) showing the positioning between two molecules].Selected bond lengths and angles (Å, °): Sn-Cl 2.4717(5), Sn-N1 2.5360(14), Sn-C1 2.1198(17), C1-Sn-Nl 83.30(5), Sn-C7 2.1345(17), Sn-C13 2.1345(17), N1-C19 1.148(2), C19-N2 1.342(14), N2-C20 1.290(13), C20-S1 1.740(3), C20-S2 1.724(3), S1-C21 1.799(10), S2-C22 1.748(2); Cl-Sn-N1 177.13(4), C1-Sn-Cl 95.03(4), C1-Sn-N1 83.30(5), C1-Sn-C7 121.00(6), C1-Sn-C13 114.49(6), C7-Sn-Cl 94.97(5), C7-Sn-N1 83.93(5), C13-Sn-Cl 96.24(5), C13-Sn-N1 86.58(6), C13-Sn-C7 121.86(6), C19-N1-Sn 175.75(14), C20-N2-C19 125.0(11), N1-C19-N2 172.0(6), N2-C20-S1 121.6(7), N2-C20-S2 120.3(7), S1-C20-S2 118.1(2). Symmetry transformation: (i) 1-x, y, 0.5-z.](/document/doi/10.1515/mgmc-2016-0015/asset/graphic/j_mgmc-2016-0015_fig_001.jpg)
Molecular structure of [ClPh3SnNCNC(SCH3)2] (1) showing 30% probability ellipsoids and the crystallographic numbering scheme [Ortep views. A) Showing the two positions of the dimethyl N-cyanodithioiminocarbonate ligand, B) showing the positioning between two molecules].
Selected bond lengths and angles (Å, °): Sn-Cl 2.4717(5), Sn-N1 2.5360(14), Sn-C1 2.1198(17), C1-Sn-Nl 83.30(5), Sn-C7 2.1345(17), Sn-C13 2.1345(17), N1-C19 1.148(2), C19-N2 1.342(14), N2-C20 1.290(13), C20-S1 1.740(3), C20-S2 1.724(3), S1-C21 1.799(10), S2-C22 1.748(2); Cl-Sn-N1 177.13(4), C1-Sn-Cl 95.03(4), C1-Sn-N1 83.30(5), C1-Sn-C7 121.00(6), C1-Sn-C13 114.49(6), C7-Sn-Cl 94.97(5), C7-Sn-N1 83.93(5), C13-Sn-Cl 96.24(5), C13-Sn-N1 86.58(6), C13-Sn-C7 121.86(6), C19-N1-Sn 175.75(14), C20-N2-C19 125.0(11), N1-C19-N2 172.0(6), N2-C20-S1 121.6(7), N2-C20-S2 120.3(7), S1-C20-S2 118.1(2). Symmetry transformation: (i) 1-x, y, 0.5-z.
Comparison of Sn-Cl, Sn-N, and (Sn)N≡C bond lengths (Å), and Cl-Sn-N angles (°) in cyano derivatives of chlorotriphenyltin(IV) complexes.
| Compounds | Sn-Cl (Å) | Sn-N (Å) | (Sn)N≡C (Å) | Cl-Sn-N (deg) | CSD Identifier |
|---|---|---|---|---|---|
| [(ClPh3Sn)2(μ-NC)2Fe(CN)2(dmso)2]- (Carini et al., 1990) | 2.535(3) | 2.340(7) | 1.16(1) | 175.5(2) | JEFTET |
| [ClPh3Sn(m-NC)Ag(CN)]- (Carcelli et al., 1992) | 2.518(23) | 2.436(3) | 1.138(8) | 175.5(1) | JOYVEY |
| [ClPh3SnNCNC(SCH3)2] (1) (this work) | 2.4717(5) | 2.5360(14) | 1.148(2) | 177.13(4) |
As mentioned before, X-ray structures of triorganotin(IV) complexes with cyanamide adducts were rather rare and until now exclusively describe polymeric networks. Thus and to the best of our knowledge, the isolation of 1 in the solid state is the first example of a monomeric organotin complex bearing a terminal dimethyl N-cyanodithioiminocarbonate ligand. Further work is in progress with the aim to isolate such new derivatives.
Experimental
General
Dimethyl N-cyanodithioiminocarbonate [NCNC(SCH3)2, 90% purity] and triphenyltin chloride (SnPh3Cl, 95% purity) were purchased from Sigma-Aldrich (Steinheim am Albuch, Germany) and were used without any further purification. Middle infrared spectra (4000–500 cm-1) of solid samples were recorded on a Bruker Vector 22 spectrometer (Wissembourg, France) equipped with a Specac Golden GateTM ATR device. The far FT-IR measurements (700–70 cm-1) were performed by using a Bruker Vertex 70v spectrometer under vacuum (Wissembourg, France), with an ATR module. Middle infrared spectra of liquid solutions were recorded in transmission mode on a Bruker Alpha spectrometer (Wissembourg, France). The NMR spectra were recorded on a Bruker Avance III 500 MHz spectrometer with a wide-band sensor broad band fluorine observation (BBFO). 1H and 13C{1H} chemical shifts (δ, ppm) were determined from the residual solvent signal (CHCl3δ=7.26 and CHCl3δ=77.16). 119Sn{1H} chemical shifts (δ, ppm) were reported downfield from (CH3)4Sn used as external standard. Elemental analyses were performed at the Institut de Chimie Moléculaire (Université de Bourgogne Franche-Comté, Dijon-France) using a Thermo Electron CHNS/O Flash EA 112 Series analyser.
Synthesis and isolation of [ClPh3SnNCNC(SCH3)2] (1)
Ethanolic solutions (25 mL) of dimethyl N-cyanodithioiminocarbonate (0.235 g, 1.607 mmol) and triphenyltin chloride (0.619 g, 1.607 mmol) were mixed together at room temperature. A white precipitate is quickly obtained, which is then filtered off after 2 h stirring. After 4 days of slow evaporation at room temperature, colorless crystals, suitable for an X-ray crystallographic analysis, were grown from the filtrate and were finally characterized as 1 (0.503 g, 59% yield).
1H-NMR (CDCl3, 300 K): δ=0.96 (m, 6H, S-CH3), 7.42 (m, 9H, CH, Ph), 7.64 (m, 6H, CH, Ph). 13C{1H} (CDCl3, 300 K): δ=193.6 ((CH3S)2C=N-), 137.3 (Ph-C), 136.1 (Ph-C), 130.4 (Ph-C), 129.1 (Ph-C), 112.2 (N≡C-N=), 16.0 (SCH3). 119Sn{1H} NMR (CDCl3, 300 K): δ=-47.8. δ=119Sn{1H} NMR (toluene-d8, 300 K): δ=-50.9. IR (ATR, cm-1): 3055 (w), 2991 (w), 2917 (w), 2202 (s), 1476 (s), 1426 (s), 1307 (m), 950 (m), 1070 (m), 1023 (m), 998 (m), 732 (s), 696 (s), 563 (m). Anal. Calcd. for C22H21ClN2S2Sn (531.71): C 49.70, H 3.98, N 5.27; S 12.06. Found: C 49.69; H 4.15; N 5.33; S 10.89.
X-ray crystallography
A single colorless prism-shaped crystal with dimensions 0.50×0.20×0.12 mm3 of 1 was selected and used for data collection using a Bruker D8 Venture triumph Mo diffractometer equipped with an Oxford Cryosystems low-temperature apparatus operating at T=100 K. Data were measured using MoKα radiation (λ=0.71073 Å). The total number of runs and images was based on the strategy calculation from the program Apex2 (Bruker, 2014). Cell parameters were retrieved and refined using the saint software (Bruker, 2014). Data reduction was performed using the Saint software which corrects for Lorentz polarization. The structure was solved by direct methods using the ShelXT structure solution program (Sheldrick, 2015a) and refined by full matrix least squares on F2 using ShelXL (Sheldrick, 2015b) with the aid of the Olex2 program (Dolomanov et al., 2009). All non-hydrogen atoms were refined anisotropically. Hydrogen atom positions were calculated geometrically and refined using the riding model. The dimethyl N-cyanodithioiminocarbonate ligand was found disordered over two positions with occupation factors equal to 0.5.
CCDC 1472466 (1) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Acknowledgments
The authors gratefully acknowledge the Cheikh Anta Diop University of Dakar (Senegal), the Centre National de la Recherche Scientifique (CNRS, France), and the University of Bourgogne Franche-Comté (Dijon, France). The authors wish also to thank the anonymous reviewers for their helpful and relevant comments.
References
Al-Salahi, R. A.; Marzouk, M. S.; Ghabbour, H. A.; Kun, F. H. Synthesis of novel 2-(methylthio)benzo[g][1,2,4]triazolo[1,5-a]quinazolin- 5-(4H)-one and its derivatives. Lett. Org. Chem. 2014, 11, 759–767.10.2174/1570178611666140813205111Suche in Google Scholar
Bruker V8.34A. Apex2 and Saint, Bruker AXS Inc., Madison, WI, USA, 2014.Suche in Google Scholar
Burchell, C. J.; Aucott, S. M.; Milton, H. L.; Slawin, A. M. Z.; Woollins, J. D. Synthesis and characterization of cyanodithioimidocarbonate [C2N2S2]2- complexes. Dalton Trans. 2004, 3, 369–374.10.1039/b314949hSuche in Google Scholar
Carcelli, M.; Ferrari, C.; Pelizzi, C.; Pelizzi, G.; Predieri, G.; Solinas, C. Easy access to a new class of anionic cyano-bridged di- and tri-nuclear organotin adducts. Crystal structure of [N(PPh3)2)][ClPh3Sn(μ-NC)Ag(CN)]. J. Chem. Soc. Dalton Trans. 1992, 2127–2128.10.1039/DT9920002127Suche in Google Scholar
Carini, C.; Pelizzi, C.; Pelizzi, G.; Predieri, G.; Tarasconi, P.; Vitali, F. Cyano-bridged organotin compounds. Crystal structure of a trinuclear dianion containing a nearly linear array of nine atoms. J. Chem. Soc., Chem. Commun. 1990, 613–614.10.1039/c39900000613Suche in Google Scholar
Chow, Y. M. The crystal structures of dimethyltin(IV) bisdicyanamide and trimethyltin(IV) dicyanamide. Inorg. Chem. 1971, 10, 1938–1942.10.1021/ic50103a022Suche in Google Scholar
Diop, M. B.; Diop, L.; Oliver, A. G. Crystal structure of dichloridobis(dimethyl N-cyanodithioiminocarbonate)cobalt(II). Acta Cryst. 2016a, E72, 417–419.Suche in Google Scholar
Diop, M. B.; Diop, L.; Oliver, A. G. Crystal structure of dichloridobis(dimethyl N-cyanodithioiminocarbonate)zinc. Acta Cryst. 2016b, E72, 417–419.10.1107/S2056989016002607Suche in Google Scholar
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. Olex2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341.10.1107/S0021889808042726Suche in Google Scholar
Gholivand, K.; Gholami, A.; Tizhoush, S. K.; Schenk, K. J.; Fadaei, F.; Bahrami, A. Steric and electronic control over the structural diversity of N-(n-pyridinyl) diphenylphosphonic amides (n=2 and 4) as difunctiona ligands in triphenyltin(IV) adducts. RSC Adv. 2014, 4, 44509–44516.10.1039/C4RA06212DSuche in Google Scholar
Holeček, J.; Nadvornik, M.; Handliř, K.; Lyčka, A. 13C and 119Sn NMR study of some four- and five-coordinate triphenyltin(IV) compounds. J. Organomet. Chem. 1983, 241, 177–184.10.1016/S0022-328X(00)98505-XSuche in Google Scholar
Jäger, L.; Tretner, C.; Biedermann, M.; Hartung, H. Pseudoelementverbindungen IX. Organostannylierung von Cyanamidonitrat – Molekül- und Kristallstruktur von N-Trimethylstannyl-N′-nitro-carbodiimid. J. Organomet. Chem. 1997, 530, 13–17.10.1016/S0022-328X(96)06554-0Suche in Google Scholar
Jain, S. C.; Rivest, R. Coordination complexes of some group (IV) halides. Preparation and infrared spectra of the complexes of group (IV) halides with cyanamide and diethylcyanamide as ligands. J. Inorg. Nucl. Chem. 1970, 32, 1117–1123.10.1016/0022-1902(70)80105-1Suche in Google Scholar
Kojic-Prodic, B.; Kiralj, R.; Zlata, R.; Sunjic, V. Promoting effect of copper ions in vinyl-like nucleophilic substitution in N-cyanoazomethines, analysis based on crystal structure data. Bull. Slovenian Chem. Soc. 1992, 39, 367–381.Suche in Google Scholar
Momeni, B. Z.; Shahbazi, S.; Khavasi, H. R. Synthesis and characterization of some organotin(IV) adducts containing a related series of pyridines: Crystals structure of [SnMe2Cl2(bu2bpy)]. Polyhedron2010, 29, 1393–1398.10.1016/j.poly.2010.01.025Suche in Google Scholar
Pelizzi, C.; Pelizzi, G. Structural characterization of a binuclear tin adduct: μ-[1,2-Bis(diphenylarsoryl)ethane-O:O′]-bis(chlorotriphenyltin). J. Chem. Soc. Dalton Trans. 1983, 847–849.10.1039/DT9830000847Suche in Google Scholar
Sall, A. S.; Diop, L.; Russo, U. Et4NNO3·3SnPh3Cl: Synthesis, infrared, Mössbauer and NMR studies. Main Group Met. Chem. 1995, 18, 243–244.10.1515/MGMC.1995.18.5.243Suche in Google Scholar
Seltzer, R. The reaction of organotin chlorides with the cyanodithioimidocarbonate anion. J. Org. Chem. 1968, 33, 3896–3900.10.1021/jo01274a044Suche in Google Scholar
Sheldrick, G. M. ShelXT. Acta Cryst. 2015a, A71, 3–8.10.1107/S2053273314026370Suche in Google Scholar
Sheldrick, G. M. ShelXL, Acta Cryst. 2015b, C71, 3–8.10.1107/S2053229614024218Suche in Google Scholar
Singh, N.; Kumar, A. Organoheterobimetallic cyanodithioimidocarbonates and their I2-doped products: synthesis, characterization and conducting properties. Synt. Met. 2008, 158, 442–446.10.1016/j.synthmet.2008.03.005Suche in Google Scholar
Sow, Y.; Diop, L.; Molloy, K. C.; Kociok-Köhn, G. Di-μ-hydroxido-bis[dimethyl(thiocyanato-κN)tin(IV)]. Acta Cryst.2012, E68, m1436.10.1107/S1600536812043462Suche in Google Scholar
Tudela, D.; Calleja, J. M. Tin-carbon and tin-chlorine stretching frequencies in triphenyltin chloride. Spectrochim. Acta1993, 46A, 1023.10.1016/0584-8539(93)80224-XSuche in Google Scholar
Supplemental Material:
The online version of this article (DOI: 10.1515/mgmc-2016-0015) offers supplementary material, available to authorized users.
©2016 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- Research Articles
- Synthesis, spectroscopic, and theoretical studies of tin(II) complexes with biologically active Schiff bases derived from amino acids
- A density functional theory insight into the structure and reactivity of diphenyltin(IV) derivative of glycylphenylalanine
- Synthesis, crystal structure, and antibacterial activity of tricyclohexyltin salicylates
- Fungal strain Aspergillus flavus F3 as a potential candidate for the removal of lead (II) and chromium (VI) from contaminated soil
- Two SrII coordination compounds based on tetrazole-carboxylate ligands
- Short Communications
- Crystal structure of the triphenyltin(IV) chloride dimethyl N-cyanodithioiminocarbonate adduct
- Triorganotin carboxylates – synthesis and crystal structure of 2-methyl-1H-imidazol-3-ium catena-O,O′-oxalatotriphenylstannate
- Organotin(IV) scorpionates – X-ray structure and crystal packing of TpSn(Cl)2(n-Bu) [Tp=hydrotris(pyrazol-1-yl)borate]
Artikel in diesem Heft
- Frontmatter
- Research Articles
- Synthesis, spectroscopic, and theoretical studies of tin(II) complexes with biologically active Schiff bases derived from amino acids
- A density functional theory insight into the structure and reactivity of diphenyltin(IV) derivative of glycylphenylalanine
- Synthesis, crystal structure, and antibacterial activity of tricyclohexyltin salicylates
- Fungal strain Aspergillus flavus F3 as a potential candidate for the removal of lead (II) and chromium (VI) from contaminated soil
- Two SrII coordination compounds based on tetrazole-carboxylate ligands
- Short Communications
- Crystal structure of the triphenyltin(IV) chloride dimethyl N-cyanodithioiminocarbonate adduct
- Triorganotin carboxylates – synthesis and crystal structure of 2-methyl-1H-imidazol-3-ium catena-O,O′-oxalatotriphenylstannate
- Organotin(IV) scorpionates – X-ray structure and crystal packing of TpSn(Cl)2(n-Bu) [Tp=hydrotris(pyrazol-1-yl)borate]

