Abstract
New tin(II) complexes of general stoichiometry [Sn(L)2] with bidentate ligand, derived from amino acids with isatin, 5-chloroisatin have been synthesized. It is characterized by elemental analyses, molar conductance measurements, and molecular weight determinations. Bonding of these complexes is discussed in terms of their UV-visible, infrared, and nuclear magnetic resonance (1H, 13C, and 119Sn NMR) spectroscopic studies. The ligands act as bidentate towards metal ions, via the azomethine nitrogen and deprotonated oxygen of the respective ligands. The bond length, bond angles, highest occupied molecular orbital, lowest unoccupied molecular orbital, chemical hardness, global softness, and the lowest energy model structure of the complexes have been determined by density functional theory (DFT) calculations at B3LYP/LanL2DZ level. A few representative ligands and their tin complexes have been screened for their antibacterial activities and found to be active in this respect.
Introduction
Schiff bases are an important class of compounds in both the medicinal (Kelley et al., 1995; Singh et al., 2007) and pharmaceutical (Pandeya et al., 1999; Turan-Zitouni et al., 2007; Amanullah et al., 2011) activities. In recent years, there has been a rapidly growing interest in the synthesis of Schiff base and their metal complexes, due to their potential applications in the field of coordination and organometallic chemistry (Ferrari et al., 1999; Tarafder et al., 2002; Cozzi, 2004; Raman et al., 2007; Arulmurugan et al., 2010). Amino acids, which constitute a very important class of biomolecules, can act as potential oxygen and nitrogen donor ligands. It has been found that they utilize their functional groups as fully as possible in metal coordination (Arish and Nair, 2012). Therefore, several metal derivatives of different amino acids have been reported (Singh et al., 2014) and some of these have been found to have significant biological activity. For example, tricyclohexyltin alaninate has been found to be active as a fungicide and bactericide for seeds and plants (Liu et al., 2013). It has also been reported that metal complexes of amino acid Schiff bases with transition metals have anticarcinogenic (Zuo et al., 2013), antimicrobial (Nath and Goyal, 1995), and antitumor (Wang et al., 1999) activities.
In the present studies, ligands are obtained by the condensation reaction between amino acids (alanine, isoleucine, valine, methonine, tryptophan, and histidine) and isatin/chloroisatin with the hope that it may provide us valuable theoretical information for exploring metal-based bacteriostatic and carcinostatic pharmaceuticals with high efficacy and low toxicity. In this effort, we have also introduced an azomethine (>C=N–) linkage with the concern that it may permit a notable variety in the remarkable chemistry and behavior of such compounds. The synthesized amino acid derived compounds [Sn(L1–8)2] have been exposed to act as bidentate towards divalent metal atom solely through the azomethine nitrogen and carboxylate oxygen forming a stable five-membered chelate ring. Unlike tin(IV) compounds which usually prefer regular tetrahedral or bipyramidal geometries depending on the coordination numbers, tin(II) compounds are mostly bent, pyramidal, or distorted. In order to confirm the geometries and the bonding featured by these tin(II) compounds, density functional theory (DFT) calculations are performed.
Results and discussion
The reactions of tin(II) acetate with these ligands have been carried out in 1:2 molar ratios using anhydrous benzene and absolute methanol in 3:1 ratio as solvent. These reactions proceed with the liberation of acetic acid, which was azeotropically removed, are indicated below (Scheme 1).
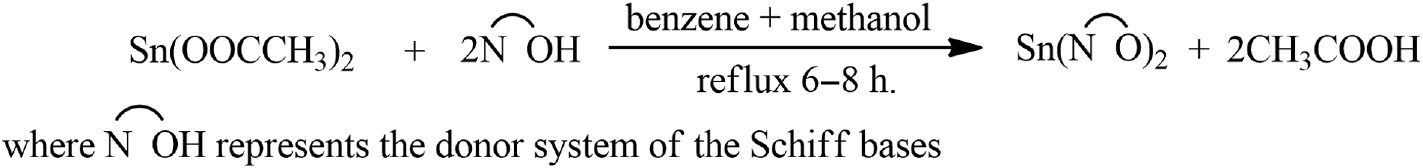
Representative equation illustrating the formation of Sn(II) complexes.
The reactions in Scheme 1 were found to be facile and could be completed in 5–8 h of heating the reaction mixtures at reflux. All these complexes are intensively colored and are solids. They are insoluble in common organic solvents and only soluble in dimethyl formamide (DMF) and dimethyl sulfoxide (DMSO). Molar conductance values of the complexes in DMF (10–3m solution at 25°C) were 14–21 mho cm2 mol–1, indicating their nonelectrolytic nature. Their physical properties and analytical data are shown in Table 1.
Analytical and physical data of tin(II) complexes.
| C. no | Products and color | M.P. °C (d) | Yield (%) | Elemental analysis | Mol. Wt found (Calcd) | Λma | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| %Sn | %C | %H | %N | %S | ||||||
| Sn(L1)2 | C22H18N4O6Sn | 150 | 78 | 21.54 | 47.69 | 3.25 | 10.01 | – | 549.57 | 14.7 |
| red | (21.46) | (47.77) | (3.28) | (10.13) | (553.11) | |||||
| Sn(L2)2 | C28H30N4O6Sn | 260 | 83 | 18.57 | 52.63 | 4.76 | 8.72 | – | 642.46 | 18.3 |
| light red | (18.63) | (52.77) | (4.74) | (8.79) | (637.27) | |||||
| Sn(L3)2 | C22H16Cl2N4O6Sn | 166 | 74 | 19.01 | 42.40 | 2.56 | 9.08 | – | 624.86 | 19.1 |
| red | (19.09) | (42.48) | (2.59) | (9.01) | (622.00) | |||||
| Sn(L4)2 | C26H26N4O6S2Sn | 154 | 86 | 17.65 | 46.33 | 3.85 | 8.35 | 9.48 | 668.55 | 15.7 |
| brown | (17.63) | (46.38) | (3.89) | (8.32) | (9.52) | (673.35) | ||||
| Sn(L5)2 | C26H24Cl2N4O6S2Sn | 164 | 85 | 15.89 | 41.96 | 3.21 | 7.62 | 8.59 | 744.63 | 16.7 |
| light red | (15.99) | (42.07) | (3.26) | (7.55) | (8.64) | (742.24) | ||||
| Sn(L6)2 | C26H24Cl2N4O6Sn | 144 | 68 | 17.43 | 46.14 | 3.55 | 8.22 | – | 674.57 | 18.9 |
| brown | (17.51) | (46.05) | (3.57) | (8.26) | (678.11) | |||||
| Sn(L7)2 | C38H28N6O6Sn | 142 | 88 | 15.23 | 58.17 | 3.56 | 10.78 | – | 780.15 | 15.7 |
| dark brown | (15.15) | (58.26) | (3.60) | (10.73) | (783.38) | |||||
| Sn(L8)2 | C28H22N8O6Sn | 172 | 69 | 17.25 | 49.17 | 3.19 | 16.30 | – | 688.94 | 20.5 |
| brown | (17.32) | (49.08) | (3.24) | (16.35) | (685.23) | |||||
aMolar conductance (Ω–1 mol–1cm2).
Electronic spectra
The spectra of the ligands and their complexes were recorded in dry DMSO. The various bands observed were assigned to inter-ligand and charge transfer of n–π* transitions according to their energies and intensities. Two intense maxima are observed in the complexes at 212–218 and 380–385 nm which may be assigned to n–π* transition of the carboxylate (Ahmad et al., 2002) and of the C=N chromophore. The appreciable shifting observed in the n–π* transition (~374 nm) is due to the polarization in the >C=N– bond caused by tin-ligand electron interaction. This clearly indicates the coordination of the azomethine nitrogen to the tin atom. A band in the region 350–340 nm in the spectra of the Schiff bases and complexes is likely to be the secondary band of the benzene ring coupled with the intramolecular charge transfer transition taking place within the ligand moiety. Furthermore, sharp bands were observed in the region 310–330 nm in the spectra of the complexes which could be assigned to the charge transfer transition from ligand to tin.
Infrared spectra
The infrared (IR) spectra of the complexes were compared with those of the free ligands (Table 2) in order to determine the coordination sites that may be involved in chelation. The position and the intensities of these peaks are expected to change on chelation. The IR spectra of all the ligands show (Kobakhidze et al., 2010) the absence of bands at 3250 and 1742 cm–1 due to ν(NH2) group of amino acids and ν(C=O) group of isatin. Instead, a new prominent band at 1628±5 cm–1 due to azomethine ν(C=N) linkage appeared in all the ligands indicating (Aman and Matela, 2013) that condensation between ketone moiety of isatin and that of amino group of amino acid has taken place resulting in the formation of the desired ligands (L1H)–(L8H). Moreover, on comparison of the IR spectra of the ligands with their tin(II) complexes showed (Singh, 2010) a major shift to lower wave numbers by 10–20 cm–1 in azomethine ν(C=N) at 1609–1618 cm–1 suggesting the involvement of the azomethine nitrogen with the tin(II) ion (Hingorani and Agarwala, 1993; Dey et al., 2004). The new bands, which appeared in the region of 420–432 cm–1 in the spectra of the complexes, are assigned to stretching frequencies of ν(Sn←N) bond formations (Wiecek et al., 2010).
Important IR spectral data (cm–1) of Schiff bases and their corresponding tin(II) complexes.
| Compounds | ν(OH) | ν(C=N−) | ν(C=O) | ν(COO)asym | ν(COO)sym | Δν | ν(Sn-O) | ν(Sn←N) |
|---|---|---|---|---|---|---|---|---|
| L1H | 3105–2790 br | 1635 s | 1730 s | – | – | – | – | – |
| Sn(L1)2 | – | 1618 s | 1728 s | 1592 vs | 1320 s | 272 | 540 w | 430 m |
| L2H | 3100–2740 br | 1625 s | 1730 s | – | – | – | – | – |
| Sn(L2)2 | – | 1610 s | 1732 s | 1604 s | 1328 m | 276 | 547 m | 424 s |
| L3H | 3095–2790 br | 1630 s | 1720 s | – | – | – | – | – |
| Sn(L3)2 | – | 1615 s | 1721 s | 1594 s | 1325 m | 269 | 552 m | 425 m |
| L4H | 3090–2750 br | 1625 s | 1720 s | – | – | – | – | – |
| Sn(L4)2 | – | 1609 s | 1719 s | 1595 s | 1322 s | 273 | 545 s | 420 w |
| L5H | 3110–2750 br | 1622 s | 1735 s | – | – | – | – | - |
| Sn(L5)2 | – | 1610 s | 1730 s | 1587 vs | 1320 s | 267 | 540 w | 425 w |
| L6H | 3105–2810 br | 1632 s | 1725 s | – | – | – | – | – |
| Sn(L6)2 | – | 1615 s | 1726 s | 1600 vs | 1334 vs | 270 | 544 w | 432 m |
| L7H | 3108–2795 br | 1630 s | 1725 s | – | – | – | – | – |
| Sn(L7)2 | – | 1610 s | 1722 s | 1585 s | 1320 s | 265 | 538 m | 429 s |
| L8H | 3090–2740 br | 1620 s | 1728 s | – | – | – | – | – |
| Sn(L8)2 | – | 1611 s | 1730 s | 1598 s | 1324 m | 274 | 548 m | 427 w |
br, Broad; vs, very sharp; v, sharp; m, medium; w, weak.
The spectra of the ligands contain a broad absorption band appeared in the region 3110–2740 cm–1 which is assigned to hydrogen-bonded ν(OH). This band disappears on complexation, suggesting chelation of the oxygen to the tin atom (Beltrán et al., 2003). The infrared spectra of complexes revealed that the νasym(COO–) was shifted to a lower wave number compared to the parent ligands which signify that the coordination took place via the oxygen atom of the carboxylate anion. Complexes showed that νasym(COO–) and νsym(COO–) are in the range of 1604–1585 and 1332–1320 cm–1, respectively (Baul et al., 2007; Win et al., 2010). Upon complexation, the structures of the ligands are altered; all alterations can be observed through the shift of the characteristic bands. The remarkable change is the disappearance of ν(OH) (3110–2740 cm–1). This is attributable to νasym(COO–) and νsym(COO–) existing in the spectra of all the complexes, thus supporting the deprotonation of carboxylic group and coordination of its carboxylic oxygen to the tin(II) ion. The magnitude of Δν=[νasym(COO–)–νsym(COO–)] for the complexes falls in the range of 276–265 cm–1, indicating that the carboxyl group in all the complexes is bound in a monodentate manner (Ho and Zuckerman, 1973). Moreover, for complexes Δν below 200 cm–1 would be expected for bridging or chelating carboxylate, but >200 cm–1 for the monodentate bonding carboxylate anions. Further evidence for the coordination to tin via oxygen atom was revealed by the presence of the ν(Sn-O) (Singh et al., 2014) stretching bands in the spectra of the complexes in the region of 538–552 cm–1.
1H NMR spectra
All the protons were found as to be in their expected region (Table 3). The conclusions drawn from these studies lend further support to the mode of bonding discussed in their IR spectra. In the spectra of tin(II) complexes, coordination of the ligands via azomethine nitrogen and carboxylate oxygen was established by downfield shifting of these signals in the tin(II) complexes due to the increased conjugation and coordination. The number of protons calculated from the integration curves and those obtained from the values of the expected CHN analyses agree with each other. It was observed that DMSO did not have any coordinating effect, neither on the spectra of the ligands nor on its metal complexes. The ligands also exhibit the OH proton signal at δ 11.25–12.55 ppm and is absent in the spectra of the corresponding tin complexes, showing thereby chelation of the ligand moiety through the deprotonated carboxylic oxygen. The ligands show a complex multiplet signal in the region δ 6.95–8.05 ppm (m) for the aromatic protons and it remains almost at the same position in the spectra of the metal complexes. The appearance of signals due to NH protons at the same positions in the ligands and its complexes show the noninvolvement of this group in coordination.
1H NMR spectral dataa of the ligands and their corresponding tin(II) complexes.
| Compounds | Chemical shift (δ, ppm) |
|---|---|
| L1H | 11.25 (s, 1H, COOH), 4.72 (q, 1H, CH), 8.34 (s, 1H, sec. amide), 1.31 (d, 3H, CH3), 7.21–7.82 (m, 4H, aromatic) |
| Sn(L1)2 | 3.72 (q, 1H, CH), 8.36 (s, 1H, sec. amide), 1.22 (d, 3H, CH3), 7.18–7.98 (m, 4H, aromatic) |
| L2H | 11.39 (s, 1H, COOH), 3.96 (d, 1H, CH), 2.10 (m, 1H, CH), 8.18 (s, 1H, sec. amide), 1.66 (m, 2H, CH2), 0.96 (d, 3H, CH3), 7.20–7.80 (m, 4H, aromatic) |
| Sn(L2)2 | 2.88 (d, 1H, CH), 2.02 (m, 1H, CH), 8.12 (s, 1H, sec. amide), 1.60 (m, 2H, CH2), 0.95 (d, 3H, CH3), 7.19–8.05 (m, 4H, aromatic) |
| L3H | 11.32 (s, 1H, COOH), 7.98 (s, 1H, sec. amide), 1.40 (d, 3H, CH3), 7.25–7.90 (m, 4H, aromatic) |
| Sn(L3)2 | 2.85 (d, 1H, CH), 8.00 (s, 1H, sec. amide), 1.26 (d, 3H, CH3), 7.20–7.96 (m, 4H, aromatic) |
| L4H | 11.74 (s, 1H, COOH), 4.72 (d, 1H, CH), 8.05 (s, 1H, sec. amide), 2.30 (m, 2H, CH2), 2.04 (d, 3H, CH3), 7.18–7.72 (m, 4H, aromatic) |
| Sn(L4)2 | 2.98 (d, 1H, CH), 8.00 (s, 1H, sec. amide), 2.10 (m, 2H, CH2), 2.06 (d, 3H, CH3), 7.16–7.80 (m, 4H, aromatic) |
| L5H | 11.72 (s, 1H, COOH), 4.80 (d, 1H, CH), 8.15 (s, 1H, sec. amide), 2.30 (m, 2H, CH2), 2.10 (d, 3H, CH3), 6.95–7.76 (m, 4H, aromatic) |
| Sn(L5)2 | 2.86 (d, 1H, CH), 3.12 (m, 1H, CH), 8.10 (s, 1H, sec. amide), 2.20 (m, 2H, CH2), 1.99 (d, 3H, CH3), 7.10–7.95 (m, 4H, aromatic) |
| L6H | 11.41 (s, 1H, COOH), 4.08 (d, 1H, CH), 8.54 (s, 1H, sec. amide), 2.30 (m, 2H, CH), 0.94(d, 3H, CH3), 7.38–7.80 (m, 4H, aromatic) |
| Sn(L6)2 | 3.16 (d, 1H, CH), 8.50 (s, 1H, sec. amide), 2.48 (m, 2H, CH), 0.92 (d, 3H, CH3), 7.30–7.80 (m, 4H, aromatic) |
| L7H | 11.46 (s, 1H, COOH), 4.39 (d, 1H, CH), 8.12 (s, 1H, sec. amide), 10.15 (s, 1H, indole), 3.02 (m, 2H, CH2), 7.15–7.85 (m, 4H, aromatic) |
| Sn(L7)2 | 3.15 (d, 1H, CH), 8.08 (s, 1H, sec. amide), 10.16 (s, 1H, indole), 2.89 (m, 2H, CH2), 7.10–8.01 (m, 4H, aromatic) |
| L8H | 12.55 (s, 1H, COOH), 4.61 (d, 1H, CH), 8.10 (s, 1H, sec. amide), 12.92 (s, 1H, imidazole), 3.15 (m, 2H, CH2), 7.08–7.80 (m, 4H, aromatic) |
| Sn(L8)2 | 3.68 (d, 1H, CH), 8.00 (s, 1H, sec. amide), 12.79 (s, 1H, imidazole), 3.05 (m, 2H, CH2), 7.10–7.90 (m, 4H, aromatic) |
aChemical shift (δ) in ppm – multiplicity is given as: s, Singlet; d, doublet; t, triplet; q, quartet; m, complex pattern.
13C NMR spectra
The 13C NMR spectroscopic data for L1H, L2H, L4H, L7H and its corresponding tin complexes have been recorded in dry DMSO (Table 4). The shifting in the position of resonance of carbon attached to OH group suggests the bonding of oxygen to the tin atom. Further, the shifting of the azomethine (>C=N–) carbon signal in the spectra of the complexes as compared to the ligands, clearly indicates that the azomethine moiety has been involved in coordination. Though, it is also possible that the shifting of azomethine carbon is due to the change in hybridization of nitrogen attached to carboxylate group but in the light of IR, UV, and 1HNMR spectroscopic studies it seems more reasonable that the shifting in these carbons is due to the involvement of carboxylate oxygen and azomethine nitrogen in bonding.
13C NMR spectral data of the ligands and their corresponding tin(II) complexes.
| Compounds | Chemical shift in (δ ppm) | ||||
|---|---|---|---|---|---|
| COOH | CH | C=N | CH3 | Aromatic carbons | |
| L1H | 172.6 | 63.5 | 155.8 | 20.2 | 159.9, 130.4, 129.5, 133.8, 110.9, 148.5, 121.3 |
| Sn(L1)2 | 183.3 | 64.8 | 160.2 | 20.7 | 153.4, 130.8, 129.4, 134.1, 111.3, 148.3, 120.6 |
| L2H | 178.5 | 65.5 | 162.4 | 21.3 | 167.3, 145.8, 133.2, 130.5, 126.5, 124,0, 119.3 |
| Sn(L2)2 | 185.5 | 66.4 | 154.5 | 20.6 | 167.5, 145.5, 133.0, 130.8, 126.2, 124.1, 119.6 |
| L4H | 178.2 | 60.4 | 162.9 | 17.6 | 157.3, 144.6, 136.1, 132.3, 128.4, 122.5, 116.8 |
| Sn(L3)2 | 184.1 | 59.6 | 153.7 | 16.4 | 156.9, 145.0, 135.8, 132.2, 129.5, 122.8, 117.0 |
| L7H | 177.4 | 72.6 | 162.4 | – | 160.2, 150.7, 138.1,131.5, 130.4, 128.2, 124.5, 124.1,123.8, 122.6, 121.2, 120.6, 117.4, 112.2, 109.4 |
| Sn(L7)2 | 185.4 | 72.1 | 153.7 | – | 160.1, 150.6, 138.3, 131.5, 130.0, 128.4, 124.6,124.5, 123.2, 122.5, 121.4, 120.3, 118.9, 112.6, 111.3 |
119Sn NMR spectra
The range of 119Sn chemical shift is as: for tetra-, penta-, or hexa-coordinated organotin complexes, δ=200 to –60, δ=–90 to –190, and δ=–210 to –400 ppm, respectively (Singh and Singh 2014). But 119Sn chemical shift is strongly dependent upon other factors, such as electronegativity of the ligands, temperature, and concentration employed in the experiments. 119Sn NMR of all the complexes shows only one resonance signal in the range of –48.85 to –57.5 ppm. 119Sn NMR values are characteristic for the four coordinated tin atoms observed in the tin complexes (Jain et al., 1995; Zöller et al., 2011a; Singh et al., 2016).
Thus based on the earlier discussion, it is clear that the ligands by coordinating to tin atom through the azomethine nitrogen and carboxylate oxygen, behaves as bidentate ligands and all the resulting tin(II) complexes are monomeric. Figure 1 is the proposed structure of the tin(II) complexes.
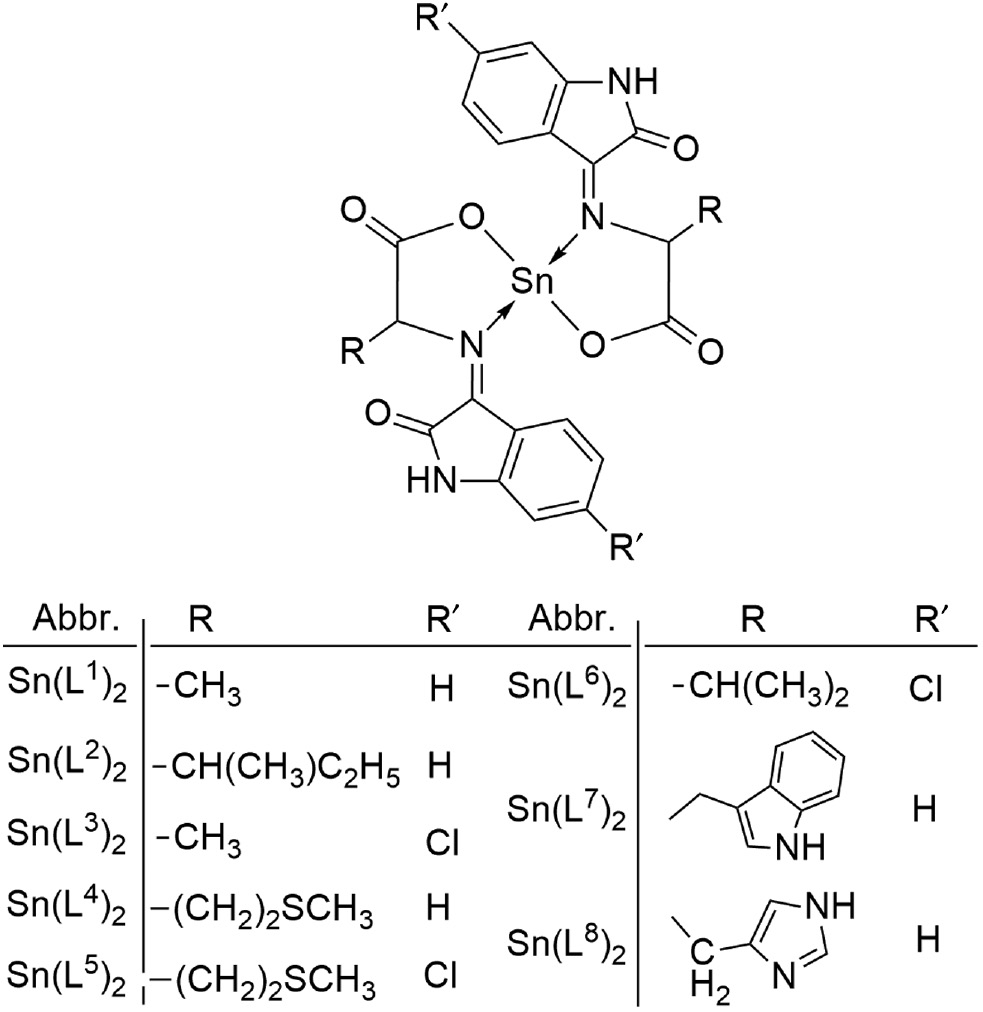
Structure of Sn(II) complexes.
Theoretical calculations
Several attempts to grow appropriate crystal for X-ray crystallography were unsuccessful. Due to this problem the geometries of Sn(L1)2 and Sn(L3)2 complexes were optimized by Gaussian 03 with DFT/B3LYP/ LanL2DZ method to support the spectroscopic data. The utility of DFT calculations has been previously demonstrated for calculating, with good confidence, geometries of molecules for which X-ray data are not available. Therefore, DFT calculations were carried out to understand the structures of the compounds in detail. As the synthesized compounds are related and differ only in substituted R group, two compounds Sn(L1)2 and Sn(L3)2 were theoretically studied. Geometry optimization of the compounds Sn(L1)2 and Sn(L3)2 at the B3LYP/LanL2DZ level also suggests the distorted tetrahedral geometry for Sn(L1)2 and Sn(L3)2. The optimized structure for the compound Sn(L1)2 is shown in Figure 2 and pertinent bond parameters are given in Table 5. The bond angles around tin atom, for example O5-Sn1-O9 angle of 119.53, N2-Sn1-N6 angle of 117.38 ° in Sn(L1)2 are the representatives of the distorted tetrahedral. The two Sn-O bond distances are close to be identical values whereas the two intramolecular Sn-N bond distances differ considerably. The calculated Sn-O bond distances of 2.0447/2.0444, 2.0438/2.0448 in Sn(L1)2, and Sn(L3)2 , respectively, are also close to the already reported Sn-O distances in {CH2N(Me)CH(Me)CH(Ph)O}2Sn, 2.048(2)/2.078(2) (Zöller et al., 2011a,b), in SaleanH2Sn(where Salean=N,N′-(1,2-ethylene)bis-(salicylaldaminato), 2.095(7)/2.202(6) (Atwood et al., 1995). The two different, intramolecular Sn-N distances of Sn1-N2/Sn1-N6 in compounds Sn(L1)2 and Sn(L3)2 are 2.0874/2.0877, 2.0868/2.0876, which are similar to the already reported structures, SaleanH2Sn, (Berends et al., 2009) {CH2N(Me)CH(Me)CH(Ph)O}2Sn, (Atwood et al., 1995) {Me(NCH2CH2O)2Sn}2, (Zöller et al., 2011a,b), and [MeN(CH2CMe2O){(S)-CH(Me)-(R)-CH(Ph)O}Sn]2 (Iovkova-Berends et al., 2011). The two different Sn-N bond lengths are already observed (2.14–2.54 Å) for the compound bis(ethylcysteinato)tin(II) (Anderson et al., 1992) and the difference was attributed to, at least in part from a hydrogen bonding interaction between N atom with long distance, and O atom in a neighboring molecule.
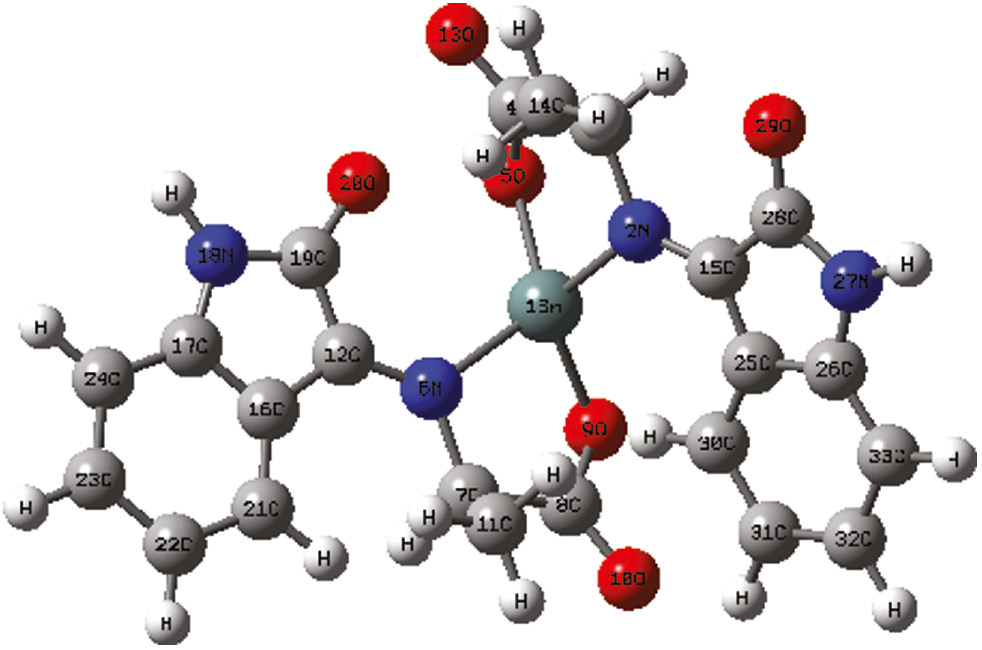
B3YLP/LanL2DL optimized geometry for the compound Sn(L1)2.
Selected lengths (Å) and bond angles of (°) of complex Sn(L1)2 and Sn(L3)2.
| Atoms | Compounds | |
|---|---|---|
| Sn(L1)2 | Sn(L3)2 | |
| N(2)-Sn(1) | 2.087 | 2.087 |
| N(6)-Sn(1) | 2.088 | 2.088 |
| O(5)-Sn(1) | 2.045 | 2.044 |
| O(9)-Sn(1) | 2.044 | 2.045 |
| N(2)-Sn(1)-N(6) | 117.38 | 116.85 |
| N(2)-Sn(1)-O(9) | 118.15 | 117.61 |
| N(2)-Sn(1)-O(5) | 92.81 | 93.29 |
| N(6)-Sn(1)-O(9) | 93.14 | 93.04 |
| N(6)-Sn(1)-O(5) | 118.11 | 119.36 |
| O(9)-Sn(1)-O(5) | 119.53 | 118.91 |
Electronic structure and ionization potentials
The HOMO and LUMO are called frontier orbitals and are very important parameters in quantum chemistry. The energy of the HOMO is directly related to the ionization potential and LUMO energy is directly related to the electron affinity. The frontier orbital gap, the difference between their energy levels, helps us to characterize the chemical reactivity and kinetic stability of the molecule. A molecule with a small frontier orbital gap is more polarizable and is generally associated with a high chemical reactivity and low kinetic stability and is also termed as the soft molecule. A large frontier orbital gap implies high stability for the molecule in the sense of its lower reactivity in chemical reactions.
The highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) of the compound Sn(L1)2, is shown in Figure 3. It is interesting that both orbitals are substantially distributed over the conjugation plane. In addition, it can be observed in Figure 3 that the HOMO orbitals are located on the substituted molecule, while the LUMO orbitals resembled those obtained for the unsubstituted molecule. Therefore, the substitution influenced the electron donation ability while imposing only a small impact on the electron acceptance ability. The orbital energy levels of the HOMO and LUMO of ligands and their tin complexes are listed in Table 6. An electronic system with a larger HOMO-LUMO gap should be less reactive than one having a smaller gap. In the present study, the LUMO-HOMO energy gap values of L1H, L3H, Sn(L1)2 and Sn(L3)2 are 4.414, 3.144, 0.901, and 0.766 eV, respectively. The lower value in the HOMO and LUMO energy gap would explain the eventual charge-transfer interaction taking place within the molecules. The low HOMO values for ligands, indicated that this molecule had low ionization energies, suggesting that it could lose electrons easily. The band gap values of the complexes are less than that of the free ligands and tin acetate. This means that in any excitation process, the tin complexes need less energy than that for free ligands and tin acetate (Hameed, 2006).

HOMO and LUMO orbitals of the compound Sn(L1)2.
Computed molecular descriptions of ligands L1H, L3H and their tin(II) complexes.
| Atoms | Compounds | ||||
|---|---|---|---|---|---|
| L1H | Sn(L1)2 | L3H | Sn(L3)2 | Sn(OAc)2 | |
| Total energy (a.u.) | −760.351 | −1522.373 | −1219.929 | −1551.187 | −460.291 |
| HOMO (eV) | −9.810 | −6.462 | −8.888 | −5.950 | −7.697 |
| LUMO (eV) | −5.396 | −5.561 | −5.744 | −5.184 | −4.557 |
| Energy Gap (eV) | 4.414 | 0.901 | 3.144 | 0.766 | 3.140 |
| Chemical potential (μ, eV)=ELUMO-EHOMO/2 | −2.207 | −0.4505 | −1.572 | −0.383 | −1.57 |
| Electronegativity (χ=–μ) | 2.207 | 0.4505 | 1.572 | 0.383 | 1.57 |
| Chemical hardness (η, eV) | 4.414 | 0.901 | 3.144 | 0.766 | 3.140 |
| Global softness (S, eV–1) | 0.226 | 1.109 | 0.318 | 1.305 | 0.318 |
| Electrophilicity index (ω=μ2/2η) | 0.552 | 0.113 | 0.393 | 0.096 | 5.977 |
| Dipole moment (Debye) | 6.535 | 7.301 | 4.573 | 7.128 | 2.684 |
Furthermore, the chemical hardness of a system implies resistance to charge transfer, whereas global softness is proportional to the polarizability of the system (Table 6). The observed values of chemical hardness and global softness for the studied complexes imply that the complexes resist the charge transfer and hence possess low polarizability.
The natural bond orbital (NBO) analysis is performed on the present molecule using DFT/ B3LYP method with LanL2DZ basis set and the results are presented in Table 7. One may be expect that electron density (charge) of atoms in ligands is larger than in their neutral complexes. However, NBO treatments indicate the reverse trend for coordinated heteroatoms (azomethine nitrogen and carboxylate oxygen). It seems that this is due to high positive charge of tin atom in these complexes. In NBO analysis, the most negative atomic charges (Table 7) are attributed to oxygen and nitrogen atoms in ligands and complexes systems. In all ligands and complexes, the negative charges of two oxygen atoms in the NBO theory are higher than the nitrogen ones. In five-membered chelate rings in complexes, the carbons connected to azomethine nitrogen and caboxylate oxygen have more positive charge than other carbon atom. This high positive charge can be due to oxygen and nitrogen neighbors coordinated to tin. The presence of the 5s2 one pair on the tin atom results in the distortion of the geometry in these compounds. The NBO analysis was performed to get more insight on the bonding nature in these compounds. The computed Wiberg bond indices for the Sn1-O5 and Sn1-O9 are 0.39, and for the Sn1-N2 and Sn1-N6, 0.26 and 0.24, respectively, indicating significant bonding interactions between Sn, O, and Sn, N atoms. This is a similar trend observed in all other compounds computed in this study. The presence of the lone pair electron in the 5s2 orbital of the tin atom is also revealed by the NBO analysis.
Atom characteristics charge, in terms of NBO analysis, a comparison between ligands and their complexes.
| Natural (NBO) charge | Atoms | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sn1 | O2 | N5 | C7 | C8 | O3 | N4 | C6 | C13 | |
| Sn(L1)2 | 2.49125 | −0.86690 | −0.86206 | 0.76925 | −0.08570 | −0.86687 | −0.86119 | 0.76925 | −0.08572 |
| Sn(L3)2 | 2.27871 | −0.87716 | −0.87372 | 0.78979 | −0.13772 | −0.87188 | −0.89543 | 0.78883 | −0.13862 |
| L1H | – | −0.72378 | −0.42165 | 0.84122 | −0.11332 | – | – | – | – |
| L3H | – | −0.71510 | −0.38861 | 0.81848 | −0.13444 | – | – | – | – |
Antibacterial results
In vitro antibacterial activity of the few representative ligands and their corresponding tin(II) complexes were carried out. The antibacterial activity was tested against four bacterial strains; two Gram-positive (MTCC 0430, Bacillus cereus; MTCC 1534, Nocardia sp.) and one Gram-negative (MTCC 2824, Enterobacter aerogenes) strains. The agar well-diffusion method was used in these assays and each experiment was performed in triplicate. The zone of inhibition value represents the mean value of three readings, which are shown in Table 8. The results show that all compounds exhibit antibacterial activity and in many case, the tin complexes are more potent in their inhibition properties than the free ligands. This can be explained in terms of the greater lipid solubility and cellular penetration of the complexes. The increase in the activity of tin(lI) complexes as compared to the parent ligand may be due to the complex formation in which the ligand is coordinated to the central tin atom through the oxygen and azomethine nitrogen leading to an increased biocidal action. Almost all the compounds were found to be more active against all the microorganisms used than the ligands themselves. The preliminary results achieved have led us to conclude that these types of complexes should be studied in detail for their applications in diverse areas.
Antibacterial activity of ligands and their tin(II) complexes.
| Compounds | Zone of inhibition (mm)a | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| MTCC 0430b | MTCC 2824b | MTCC 1534b | |||||||
| 50 μg | 125 μg | 250 μg | 50 μg | 125 μg | 250 μg | 50 μg | 125 μg | 250 μg | |
| L1 | Inact. | 2±0.18 | 7±0.14 | Inact. | Inact. | Inact. | Inact. | 4±0.14 | 7±0.14 |
| Sn(L1)2 | 4±0.12 | 6±0.26 | 8±0.19 | Inact. | Inact. | Inact. | 4±0.14 | 5±0.17 | 10±0.17 |
| L2 | Inact. | 10±0.17 | 13±0.17 | Inact. | 4±0.13 | 5±0.10 | 2±0.23 | 9±0.19 | 13±0.14 |
| Sn(L2)2 | Inact. | 12±0.21 | 14±0.18 | 3±0.22 | 6±0.22 | 8±0.16 | 4±0.16 | 11±0.15 | 15±0.19 |
| L4 | 9±0.14 | 10±0.14 | 13±0.32 | 6±0.12 | 10±0.15 | 13±0.10 | 7±0.10 | 8±0.12 | 10±0.16 |
| Sn(L4)2 | 12±0.20 | 15±0.08 | 20±0.29 | 9±0.16 | 12±0.10 | 16±0.14 | 10±0.16 | 12±0.23 | 16±0.14 |
| L5 | 11±0.09 | 14±0.15 | 17±0.12 | 10±0.24 | 12±0.12 | 15±0.21 | 16±0.22 | 17±0.16 | 20±0.15 |
| Sn(L5)2 | 14±0.22 | 20±0.10 | 20±0.14 | 14±0.18 | 16±0.18 | 17±0.16 | 17±0.12 | 19±0.15 | 25±0.10 |
aThe zone of inhibition was measured with respect to control. DMSO (dimethyl sulfoxide) was taken as control as well as solvent).
bMTCC 0430: Bacillus cereus, MTCC 2824: Enterobacter aerogenes; MTCC 1534: Nocardia sp.
Conclusions
A new series of tin(II) complexes with amino acid Schiff bases were successfully synthesized and characterized. Based on various physiochemical and structural investigations, it was concluded that the ligands act as bidentate and coordinated through azomethine nitrogen and carboxylate oxygen to the tin atom. The HOMO-LUMO energy gap values of the tin complexes are less than that of the free ligands. This means that in any excitation process, the tin complexes need less energy than that for free ligands and high chemical reactivity. Biological studies revealed that generally tin complexes have higher activities than the free ligands.
Experimental section
Chemicals (Aldrich and Merch) and solvents used were dried and purified by standard methods and moisture was excluded from the glass apparatus using CaCl2 drying tubes. Melting points were determined in open capillaries and are uncorrected. The ligands were prepared by the condensation of isatin and 5-chloroisatin with amino acids (alanine, isoleucine, valine, tryptophan, methonine, and histidine) as described earlier (Singh and Singh, 2013).
Syntheses of tin(II) complexes
A weighed amount of tin(II) acetate (1.35 mmol) was added to the calculated amount of the ligands (2.7 mmol) in 1:2 molar ratio in dry benzene (60 mL), methanol (20 mL) mixture as solvent in an oxygen-free nitrogen atmosphere. The contents were refluxed on a fractionating column for 5–8 h and the acetic acid liberated in the reaction was removed azeotropically with solvent. Excess solvent was removed under reduced pressure and the compound was dried in vacuum at 45°C±5°C after repeated washing with dry cyclohexane. The compounds were purified by recrystallization from methanol (Table 1). The purity of the compounds was checked by thin layer chromatography (TLC) using silica gel-G as an adsorbent.
Analytical methods and spectroscopic measurements
Tin was determined gravimetrically as SnO2. Nitrogen and sulfur were determined by Kjeldahl’s and Messenger’s methods, respectively. Molar conductance measurements were made in anhydrous dimethylformamide at 40°C±5°C using a Systronics conductivity bride model 305. Molecular weight determinations were carried out by the Rast camphor method. The electronic spectra were recorded on a Thermo, double-beam spectrophotometer UV 1, in the range of 800–200 nm. The IR spectra of the ligands and metal complexes were recorded in KBr pellets using a Perkin-Elmer RX1 FTIR spectrophotometer in the range of 4000–400 cm–1. 1H and 13C NMR spectra were recorded on a Bruker AVANCE II (400 MHz) FTNMR spectrometer at SAIF, Punjab University, Chandigarh, using DMSO-d6 as solvent at 400 and 100 MHz, respectively. Tetramethylsilane was used as internal reference for 1H NMR and 13C NMR. The 119SnNMRspectra with proton noise decoupling were recorded on a BRUKER AVANCE II spectrometer using dry DMSO as the solvent at 149.21 MHz and tetramethyltin as an external standard.
Theoretical investigations
Density functional theory calculations were carried out using the Gaussian 03 software package, and Gauss view visualization program (Hay and Wadt, 1985; Becke, 1986, 1993; Frisch et al., 2004). The geometry is optimized at B3LYP/6-31G/LanL2DZ basic sets to predict the molecular structures. Calculations were carried out at department of Applied Sciences, MUST, Rajasthan with Becke’s three parameter hybrid model using the Lee-Yang-Parr correlation functional (B3LYP) method.
Antibacterial assay
Synthesized compounds were screened for their antibacterial activity against Bacillus cereus (MTCC 0430), Nocardia spp.(MTCC 1534), and Enterobacter aerogenes (MTCC 2824) at various concentrations of 50, 125, and 250 μg by the agar well-diffusion method (Singh et al., 2001; Singh and Singh, 2012). An aliquot (5 mL) of nutrient broth was inoculated with the test organisms and incubated at 37°C for 24 h sterile nutrient agar plates were also prepared and holes of 5 mm diameter were cut using a sterile cork borer ensuring proper distribution. The test organisms after 24 h of incubation were spread onto separate agar plates. The chemical compounds dissolved in DMSO were poured into appropriately labeled holes using a pipette in aseptic conditions. A hole containing DMSO served as a control. Triplicate plate of each bacterial strain was prepared. The plates were incubated aerobically at 37°C for 24 h. The antimicrobial activity was determined by measuring the diameter of the zone (mm) showing complete inhibition with respect to control (DMSO).
Acknowledgments
The author is thankful to the Dean CASH and Dean, CET, Mody University of Science and Technology, Lakshmangarh, Sikar, for providing necessary laboratory facilities to carry out this research work. The author is also thankful to Dr. K. P. Sharma, Department of Microbiology, MUST, for providing antimicrobial screening facilities.
References
Ahmad, F.; Pervez, M.; Ali, S.; Mazhar, M.; Munir, A. Synthesis and spectral studies of tri- and diorganotin(iv) complexes with 5-benzoyl-α-methyl-2-thiopheneacetic acid: crystal structure of [(CH3)3Sn(C14H11O3S)]. Synth. React. Inorg. Met. Org. Chem. 2002, 32, 665–687.10.1081/SIM-120004439Search in Google Scholar
Aman, R.; Matela, G. Tin(IV) Complexes of Schiff base derived from amino acid: synthesis and characteristic spectral studies. J. Chem.2013, 2013, 1–4.10.1155/2013/637290Search in Google Scholar
Amanullah, M.; Sadozai, S. K.; Rehman, W.; Hassan, Z.; Rauf, A.; Iqbal, M. Cytotoxic, antibacterial activity and physico-chemical properties of some acid catalyzed Schiff bases. Afr. J. Biotechnol. 2011, 10, 209–213.Search in Google Scholar
Anderson, J. E.; Sawtelle, S. M.; Thompson, J. S.; Kretchmar, S. A.; Calabrese, J. Ligand addition reactions and the electron-transfer properties of tin dichloride dihydrate and tin tetrachloride pentahydrate. Molecular structure of bis(ethyl cysteinato)tin(II). Inorg. Chem.1992, 31, 2778–2785.10.1021/ic00039a022Search in Google Scholar
Arish, D.; Nair, M. S. Synthesis, characterization and biological studies of Co(II), Ni(II), Cu(II) and Zn(II) complexes with pyrral-l-histidinate. Arabian J. Chem.2012, 5, 179–186.10.1016/j.arabjc.2010.08.011Search in Google Scholar
Arulmurugan, S.; Kavitha, H. P.; Venkatraman, B. R. Biological activities of Schiff base and its complexes: a review. Rasayan J. Chem. 2010, 3, 385–410.Search in Google Scholar
Atwood, D. A.; Jegier, J. A.; Martin, K. J.; Rutherford, D. Tetradentate–N2O2 ligand complexes of tin(II): X-ray crystal structure of [N,N′-(1,2-ethylene) bis (salicylaldamine)]tin(II), (SaleanH2Sn). J. Organomet. Chem. 1995, 503, C4–C7.10.1016/0022-328X(95)05666-DSearch in Google Scholar
Baul, T. S. B.; Masharing, C.; Ruisi, G.; Jirásko, R.; Holčapek, M.; Vos, D.; Wolstenholme, D.; Linden, A. Self-assembly of extended Schiff base amino acetate skeletons, 2-{[(2Z)-(3-hydroxy-1-methyl-2-butenylidene)]amino}phenylpropionate and 2-{[(E)-1-(2-hydroxyaryl)alkylidene]amino}phenylpropionate skeletons incorporating organotin(IV) moieties: synthesis, spectroscopic characterization, crystal structures, and in vitro cytotoxic activity. J. Organomet. Chem. 2007, 692, 4849–4862.10.1016/j.jorganchem.2007.06.061Search in Google Scholar
Becke, A. D. Density functional calculations of molecular bond energies. J. Chem. Phys.1986, 84, 4524.10.1007/978-94-009-3855-7_22Search in Google Scholar
Becke, A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys.1993, 98, 5648–5652.10.1063/1.464913Search in Google Scholar
Beltrán, H. I.; Zamudio-Rivera, L. S.; Mancilla, T.; Santillan, R.; Farfán, N. One-step preparation, structural assignment, and x-ray study of 2,2-di-n-butyl- and 2,2-diphenyl-6-aza-1,3-dioxa-2-stannabenzocyclononen-4-ones derived from amino acids. Chem. A Eur. J.2003, 9, 2291–2306.10.1002/chem.200204260Search in Google Scholar PubMed
Berends, T.; Iovkova, L.; Bradtmoller, G.; Oppel, I.; Schurmann, M.; Jurkschat, K. LSn(OCH2CH2)2NR (L = lone pair, W(CO)5; R = Me, t-Bu). The molecular structures of 5-Aza-2,8-dioxa-1-stannabicyclo[3.3.0]1.5octanes and their tungstenpentacarbonyl complexes. Z. Anorg. Allg. Chem. 2009, 635, 369–374.10.1002/zaac.200800434Search in Google Scholar
Cozzi, P. G. Metal-Salen Schiff base complexes in catalysis: practical aspects (review). Chem. Soc. Rev. 2004, 33, 410–421.10.1039/B307853CSearch in Google Scholar
Dey, D. K.; Lycka, A.; Mitra, S.; Rosair, G. M. Simplified synthesis, 1H, 13C, 15N, 119Sn NMR spectra and X-ray structures of diorganotin(IV) complexes containing the 4-phenyl-2,4-butanedionebenzoylhydrazone(2–) ligand. J. Organomet. Chem. 2004, 689, 88–95.10.1016/j.jorganchem.2003.09.035Search in Google Scholar
Ferrari, M. B.; Capacchi, S.; Pelosi, G.; Reffo, G.; Tarasconi, P.; Albertini, R.; Pinelli, S.; Lunghi, P. Synthesis, structural characterization and biological activity of helicin thiosemicarbazone monohydrate and a copper(II) complex of salicylaldehyde thiosemicarbazone. Inorg. Chim. Acta1999, 286, 134–141.10.1016/S0020-1693(98)00383-1Search in Google Scholar
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Montgomery, J. A.; Vreven, Jr.T.; Kudin, K. N.; Burant, J. C.; et al. GAUSSIAN 03, Revision C.01,Gaussian Inc., Wallingford CT. 2004.Search in Google Scholar
Hameed, A. J. Computational note on substitution effects on the structural and electronic properties of 1-(para-substituted phenyl diazenyl)pyrrolidinofullerenes. J. Mol. Struct. Theochem. 2006, 766, 73–75.10.1016/j.theochem.2006.04.012Search in Google Scholar
Hay, P. J.; Wadt, W. R. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys.1985, 82, 284–298.10.1063/1.448800Search in Google Scholar
Hingorani, S.; Agarwala, B. V. Structural elucidation of o-vanillin isonicotinoyl hydrazone and its metal complexes. Trans. Met. Chem. 1993, 18, 576–578.10.1007/BF00191126Search in Google Scholar
Ho, B. Y. K.; Zuckerman, J. J. Trialkyltin derivatives of amino acids and dipeptides. Inorg. Chem.1973, 12, 1552–1556.10.1021/ic50125a016Search in Google Scholar
Iovkova-Berends, L.; Berends, T.; Dietz, C.; Bradtmoller, G.; Schollmeyer, D.; Jurkschat, K. Syntheses, structures and reactivity of new intramolecularly coordinated tin alkoxides based on an enantiopure ephedrine derivative. Eur. J. Inorg. Chem. 2011, 2011, 3632–3643.10.1002/ejic.201100352Search in Google Scholar
Jain, A.; Saxena, S.; Bohra R.; Rai, A. K. Crystal structure and stereochemical implications of the tin(II) lone pair in a novel sterically demanding heterocyclic ß-diketone derivative, C25H26N4O5Sn. Main Group Met. Chem. 1995, 18, 661–668.10.1515/MGMC.1995.18.12.660Search in Google Scholar
Kelley, J. L.; Linn, J. A.; Bankston, D. D.; Burchall, C. J.; Soroko, F. E.; Cooper, B. R. 8-Amino-3-benzyl-1,2,4-triazolo[4,3-a]pyrazines. Synthesis and anticonvulsant activity. J. Med. Chem. 1995, 38, 3676–3679.10.1021/jm00018a029Search in Google Scholar PubMed
Kobakhidze, N.; Farfán, N.; Romero, M.; Méndez-Stivalet, J. M.; Ballinas-López, M. G.; García-Ortega, H.; Domínguez, O.; Santillan, R.; Sánchez-Bartéz, F.; Gracia-Mora, I. New pentacoordinated Schiff-base diorganotin(IV) complexes derived from nonpolar side chain α-amino acids. J. Organomet. Chem.2010, 695, 1189–1199.10.1016/j.jorganchem.2010.01.024Search in Google Scholar
Liu, Y. T.; Lian, G. D.; Yin, D. W.; Su, B. J. Synthesis, characterization and biological activity of ferrocene-based Schiff base ligands and their metal(II) complexes. Spectrochim. Acta Part A2013, 100, 131–137.10.1016/j.saa.2012.03.049Search in Google Scholar
Nath, M.; Goyal, S. Spectral studies and bactericidal, fungicidal, insecticidal and parasitological activities of organotin(IV) complexes of thio Schiff bases having NO donor atoms. Metal-Based Drugs1995, 2, 297–309.10.1155/MBD.1995.297Search in Google Scholar
Pandeya, S. N.; Sriram, D.; Nath, G.; De Clercq, E. Synthesis, antibacterial, antifungal and anti-HIV evaluation of Schiff and Mannich bases of isatin derivatives with 3-amino-2-methylmercapto quinazolin-4(3H)-one. Pharm. Acta Helv. 1999, 74, 11–17.10.1016/S0031-6865(99)00010-2Search in Google Scholar
Raman, N.; Raja, J. D.; Sakthivel, A. Synthesis, spectral characterization of Schiff base transition metal complexes: DNA cleavage and antimicrobial activity studies. J. Chem. Sci. 2007, 119, 303–310.10.1007/s12039-007-0041-5Search in Google Scholar
Singh, H. L. Synthesis and characterization of tin(II) complexes of fluorinated Schiff bases derived from amino acids. Spectrochim. Acta A2010, 76, 253–258.10.1016/j.saa.2010.03.029Search in Google Scholar PubMed
Singh, H. L.; Singh, J. B. Synthesis, spectral, 3D molecular modeling and antibacterial studies of dibutyltin(IV) Schiff base complexes derived from substituted isatin and amino acids. Nat. Sci.2012, 4, 170–178.10.4236/ns.2012.43025Search in Google Scholar
Singh, H. L.; Singh, J. B. Synthesis and characterization of new lead(II) complexes of Schiff bases derived from amino acids. Res. Chem. Intermed. 2013, 39, 1997–2009.10.1007/s11164-012-0732-5Search in Google Scholar
Singh, H. L.; Singh J. Synthesis, spectroscopic, molecular structure, and antibacterial studies of dibutyltin(IV) Schiff base complexes derived from phenylalanine, isoleucine, and glycine. Bioinorg. Chem. Appl. 2014, 2014, 1–12.10.1155/2014/716578Search in Google Scholar PubMed PubMed Central
Singh, H.L.; Khungar, B.; Tripaati, U.D.; Varshney, A.K. Spectral and antimicrobial studies of organotin(IV) complexes with bidentate Schiff bases having nitrogen and sulphur donor ligands. Main Group Met. Chem. 2001, 24, 5–12.10.1515/MGMC.2001.24.1.5Search in Google Scholar
Singh, K.; Barwa, M. S.; Tyagi, P. Synthesis and characterization of cobalt(II), nickel(II), copper(II) and zinc(II) complexes with Schiff base derived from 4-amino-3-mercapto-6-methyl-5-oxo-1,2,4-triazine. Eur. J. Med. Chem. 2007, 42, 394–402.10.1016/j.ejmech.2006.10.016Search in Google Scholar PubMed
Singh, H. L.; Singh, J.; Chauhan, S. S.; Mukherjee, A.; Dewa, T. Synthetic, structural, theoretical and biological study of triorganotin(IV) Schiff base complexes derived from amino acids. J. Chem. Pharm. Res.2014,6:248–257.Search in Google Scholar
Singh, H. L.; Singh, J. B.; Bhanuka, S. Synthesis, spectral, DFT and antimicrobial studies of tin(II) and lead(II) complexes with semicarbazone and thiosemicarbazones derived from (2-hydroxyphenyl)(pyrrolidin-1-yl)methanone. J. Coord. Chem.2016, 69, 343–353.10.1080/00958972.2015.1115485Search in Google Scholar
Tarafder, M. T. H.; Kasbollah, A.; Saravanan, N.; Crouse, K. A.; Ali, A. M.; Tin Oo, K. S-methyldithiocarbazate and its Schiff bases: evaluation of bondings and biological properties. J. Biochem. Mol. Biol. Biophys. 2002, 6, 85–91.10.1080/10258140290027207Search in Google Scholar
Turan-Zitouni, G.; Kaplancikli, Z. A.; Özdemir, A.; Chevallet, P. Studies on 1,2,4-triazole derivatives as potential anti-inflammatory agents. Arch. Pharm. Chem. Life Sci. 2007, 340, 586–590.10.1002/ardp.200700134Search in Google Scholar
Wang, R. M.; Hao, C. J.; Wang, Y. P.; Li, S. B. Amino acid Schiff base complex catalyst for effective oxidation of olefins with molecular oxygen. J. Mol. Catal. A: Chem.1999, 147, 173–178.10.1016/S1381-1169(99)00143-0Search in Google Scholar
Wiecek, J.; Kovala-Demertzi, D.; Ciunik, Z.; Zervou, M.; Demertzis, M. A. Diorganotin complexes of a thiosemicarbazone, synthesis: properties, X-ray crystal structure, and antiproliferative activity of diorganotin complexes. Bioinorg. Chem. Appl. 2010, 2010, 1–9.10.1155/2010/867195Search in Google Scholar PubMed PubMed Central
Win, Y. F.; Teoh, S. G.; Vikneswaran, M. R.; Ha, S. T.; Ibrahim, P. Synthesis and characterization of organotin(IV) complexes derived of 4-(diethylamino) benzoic acid: in vitro antibacterial screening activity. Int. J. Phys. Sci. 2010, 5, 1263–1269.Search in Google Scholar
Zöller, T.; Iovkova-Berends, L.; Berends, T.; Dietz, C.; Bradtmoller, G.; Jurkschat, K. Intramolecular N→Sn coordination in tin(II) and tin(IV) compounds based on enantiopure ephedrine derivatives. Inorg. Chem. 2011a, 50, 8645–8653.10.1021/ic201203uSearch in Google Scholar PubMed
Zöller, T.; Iovkova-Berends, L.; Dietz, C.; Berends, T.; Jurkschat, K. On the reaction of elemental tin with alcohols: a straight forward approach to tin(II) and tin(IV) alkoxides and related tinoxo clusters. Chem. A Eur. J.2011b, 17, 2361–2364.10.1002/chem.201003338Search in Google Scholar PubMed
Zuo, J.; Bi, C.; Fan, Y.; Buac, D.; Nardon, C.; Daniel, K. G.; Dou, Q. P. Cellular and computational studies of proteasome inhibition and apoptosis induction in human cancer cells by amino acid Schiff base–copper complexes. J. Inorg. Biochem.2013, 118, 83–93.10.1016/j.jinorgbio.2012.10.006Search in Google Scholar PubMed PubMed Central
©2016 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Research Articles
- Synthesis, spectroscopic, and theoretical studies of tin(II) complexes with biologically active Schiff bases derived from amino acids
- A density functional theory insight into the structure and reactivity of diphenyltin(IV) derivative of glycylphenylalanine
- Synthesis, crystal structure, and antibacterial activity of tricyclohexyltin salicylates
- Fungal strain Aspergillus flavus F3 as a potential candidate for the removal of lead (II) and chromium (VI) from contaminated soil
- Two SrII coordination compounds based on tetrazole-carboxylate ligands
- Short Communications
- Crystal structure of the triphenyltin(IV) chloride dimethyl N-cyanodithioiminocarbonate adduct
- Triorganotin carboxylates – synthesis and crystal structure of 2-methyl-1H-imidazol-3-ium catena-O,O′-oxalatotriphenylstannate
- Organotin(IV) scorpionates – X-ray structure and crystal packing of TpSn(Cl)2(n-Bu) [Tp=hydrotris(pyrazol-1-yl)borate]
Articles in the same Issue
- Frontmatter
- Research Articles
- Synthesis, spectroscopic, and theoretical studies of tin(II) complexes with biologically active Schiff bases derived from amino acids
- A density functional theory insight into the structure and reactivity of diphenyltin(IV) derivative of glycylphenylalanine
- Synthesis, crystal structure, and antibacterial activity of tricyclohexyltin salicylates
- Fungal strain Aspergillus flavus F3 as a potential candidate for the removal of lead (II) and chromium (VI) from contaminated soil
- Two SrII coordination compounds based on tetrazole-carboxylate ligands
- Short Communications
- Crystal structure of the triphenyltin(IV) chloride dimethyl N-cyanodithioiminocarbonate adduct
- Triorganotin carboxylates – synthesis and crystal structure of 2-methyl-1H-imidazol-3-ium catena-O,O′-oxalatotriphenylstannate
- Organotin(IV) scorpionates – X-ray structure and crystal packing of TpSn(Cl)2(n-Bu) [Tp=hydrotris(pyrazol-1-yl)borate]

