Abstract
Objectives
Kawasaki disease (KD) is a systemic vasculitis that results in abnormalities in the coronary arteries. Podoplanin (PDPN) and its receptor C-type lectin-like receptor 2 (CLEC-2) are involved in inflammatory hemostasis and have also been proved to be effective predictive markers for early evaluation of sepsis and coagulation. This study aimed to investigate the expression of PDPN and its receptor CLEC-2 in the patients with KD, and evaluate the relationship between PDPN and clinical laboratory parameters.
Methods
Plasma samples were obtained from a cohort of 63 patients diagnosed with KD and 31 healthy children. Patients with KD were further categorized into two groups: KD with coronary artery lesions (KD-CALs) and KD non-coronary artery lesions (KD-NCALs). The plasma levels of PDPN, CLEC-2, and GPVI were quantified using ELISA.
Results
Our findings indicated that the plasma concentrations of PDPN, CLEC-2, and GPVI were significantly elevated in KD patients compared to the healthy controls. Moreover, positive correlations between plasma PDPN levels and erythrocyte sedimentation rate (ESR), interleukin-6, and D-dimer were observed in the KD-CALs group. Receiver operating characteristic curve analysis showed that the area under the curve of PDPN was 0.8377, with PDPN providing 45.45 % sensitivity and 90.48 % specificity, which proved the value of PDPN as a predictor of CALs in KD.
Conclusions
The findings suggest that PDPN may play a role in the pathogenesis of KD, and upregulation of PDPN may contribute to the hypercoagulable state observed in KD. Therefore, PDPN could potentially be a diagnostic biomarker for KD.
Introduction
Kawasaki disease (KD), an acute febrile and systemic vasculitis syndrome, also referred to as mucocutaneous lymph node syndrome, mainly occurs in infants and children under 5 years old. At present, the etiology of KD remains unidentified. In the pathological process of KD, endothelial damage stimulates the release of pro-inflammatory cytokines, thus promoting the leukocyte activation and fostering a hypercoagulable state [1]. Consequently, this cascade culminates in the development of coronary artery lesions (CALs). For the prevention of CALs, the standard of treatment for KD patients is formulated that a single dose of intravenous immunoglobulin (IVIG) combined with aspirin was delivered to the patients within the first 10 days of onset, and the dose of aspirin is gradually adjusted from the high dose of 30∼50 mg/(kg·d) to the middle dose of 3∼5 mg/(kg·d) [2]. This treatment can reduce systemic inflammation and platelet aggregation, resulting in a 3–5% reduction in CA lesions [3], 4]. Nevertheless, endothelial damage might be pro-longed in IVIG-treated patients [5], and the use of high-dose aspirin can increase the risk of liver injury, bleeding tendency and gastrointestinal discomfort [4].
C-type lectin-like receptor 2 (CLEC-2) is expressed specifically at high levels on platelets and megakaryocytes, and podoplanin (PDPN) is the only known endogenous ligand of CLEC-2. CLEC-2-PDPN interaction leads to the degranulation and activation of blood platelets due to the oligomerization of CLEC-2, thereby facilitating the formation of additional platelet aggregates [6]. CLEC-2 has been proved to be an effective predictive marker for early evaluation of sepsis [7], coagulation and disseminated intravascular coagulation (DIC) diagnosis [8]. The occurrence of venous thrombosis in patients with primary brain tumor has been reported closely related to the overexpression of PDPN [9]. Now recognized as an integral player in inflammation-driven thrombosis, PDPN-positive macrophages have capacity for platelets binding and activation via CLEC-2 [10]. Activated platelet particles can release many pro-coagulants and other circulating inflammatory markers, promoting blood hypercoagulability and thrombosis [11]. The definition of hypercoagulability is the propensity of thrombosis development, which means that patients with hypercoagulability have higher potential to develop thrombosis than normal people [12]. Blood coagulation dysfunction is easy to appear in KD due to the abnormalities of platelet activation [13]. Nevertheless, the investigation into the involvement of PDPN/CLEC-2 in KD remains unexplored, while the correlation between the plasma levels of PDPN/CLEC-2 and the coagulation state of blood is uncertain.
Glycoprotein VI (GPVI) serves as a primary signaling receptor for collagen, being activated by collagen present in the subendothelial matrix, as well as by fibrin and fibrinogen within the thrombus. This activation process contributes significantly to platelet activation and coronary artery thrombosis [14], 15]. However, there is currently a lack of evidence regarding the presence of circulating GPVI in patients with KD.
Here, the plasma levels of PDPN/CLEC-2 and GPVI were measured in 63 cases of KD patients and 31 healthy children to examine the platelet activation, and their correlation was discussed. Furthermore, the association between PDPN level and blood coagulation and inflammation parameters in KD patients was analyzed.
Materials and methods
Study population
Sixty-three KD patients aged from 2 to 6 years admitted to the Department of Cardiology, Children’s Hospital of Zhejiang University School of Medicine, China, from January to October, 2023, were enrolled in this study. The rigorous diagnostic criteria utilized in this study were derived from 2017 American Heart association (AHA) scientific statement for diagnosis of KD [3]. All the enrolled children were excluded free of hematological disorders, experiencing the acute stage of infection, cancer, or recent surgical procedures, as well as various cardiovascular conditions. In addition, 31 healthy children, matched by age and sex, who were hospitalized in the general surgery department, were recruited to the control group. This study (2021-IRB-320) was approved by the Research Ethics Committee of the Children’s Hospital of Zhejiang University School of Medicine and informed consent was provided by all the patients’ parents or legal guardians.
Clinical data collection
Clinical parameters of the samples were collected using an electronic medical record database, including WBC white blood cell counts (WBC), mean platelet volume (MPV), erythrocyte sedimentation rate (ESR), c-reactive protein (CRP), procalcitonin (PCT), complement3(C3), complement4 (C4), Immunoglobulin E (IgE), interleukin (IL), tumor necrosis factor-α (TNF-α), Brain Natriuretic Peptide (BNP), prothrombin time (PT), activated partial thromboplastin time (APTT), D-dimer (D-D), and fibrinogen (FIB).
Plasma collection and CALs were determined by echocardiography
During the acute phase of KD (The first 2 weeks of disease onset) [16], venous blood samples were collected from patients, and it was ensured that they had not received IVIG or anticoagulants therapy. Plasma samples were then obtained through centrifugation for a duration of 10 min, and promptly stored at −80 °C until further analysis. The collection of plasma from healthy children adhered to the identical procedure.
Echocardiography was performed before initial IVIG treatment in children with KD. CALs were defined by either (1) Z score≥2, or (2) internal diameter of a segment≥1.5-times that of an adjacent segment, or (3) demonstration of clearly irregular lumen [3].
Determination of plasma PDPN, CLEC-2, and GPVI by ELISA
The plasma levels of PDPN, CLEC-2, and GPVI were assessed using ELISA kits in accordance with the manufacturer’s provided instructions (Lengton Bioscience, Shanghai, China). All samples were analyzed in duplicate.
Statistical analysis
SPSS 22.0 (SPSS Inc., Chicago, IL), R Studio (version 4.1.0) and GraphPad Prism 10 (GraphPad, La Jolla, CA) were applied for statistical analysis and figure construction. The quantitative variables were described as mean ± SD, while the qualitative variables were expressed as frequency (%). Comparisons between two groups of KD and HC were conducted by Student’s t-test. Chi-squared tests were performed to compare differences between qualitative results. Pearson correlation was adopted to analyze the relationship between measured indicators and clinical parameters. The predictive value of PDPN was assessed by calculating the area under the receiver-operating curve (AUC). The optimal sensitivity/specificity cutoff point was determined using Youden’s index. The Pearson correlation coefficient was employed to examine the correlation. All statistical tests were two-sided, and a significance level of p<0.05 was used.
Results
The clinical characteristics in Kawasaki disease patients and healthy controls
A total of 63 patients diagnosed with Kawasaki disease (KD) and 31 healthy controls (HC) were included in this study. The baseline information, including CRP (c-reactive protein), D-D (D-dimer) and FIB (fibrinogen) levels, leukocyte and PLT counts, were presented in Table 1. The KD patients exhibited significantly elevated levels of CRP, D-D and FIB, as well as leukocyte and PLT counts, compared to the HC group. Simultaneously, we noticed no notable distinction in age, sex, APTT, PT, and MPV.
Demographics of the patients with KD and HC.
| HC (n=31) | KD (n=63) | p-Value | |
|---|---|---|---|
| Age, years | 3.10 ± 1.41 | 4.02 ± 1.23 | 0.788 |
| Gender, M (%) | 20 (64.5) | 22 (51.2) | 0.879 |
| Leukocyte, × 109/L | 7.17 ± 1.99 | 12.24 ± 4.06 | <0.001 |
| CRP, mg/L | 1.23 ± 0.58 | 50.43 ± 38.94 | <0.001 |
| PLT, × 109/L | 324.90 ± 80.04 | 420.53 ± 186.64 | =0.009 |
| MPV, fL | 9.56 ± 0.85 | 9.37 ± 1.11 | 0.897 |
| FIB, g/L | 2.14 ± 0.31 | 4.94 ± 2.04 | <0.001 |
| D-D, mg/L FEU | 0.16 ± 0.06 | 1.11 ± 0.96 | <0.001 |
| PT, s | 11.13 ± 0.59 | 12.04 ± 0.89 | 0.789 |
| APTT, s | 29.05 ± 2.11 | 30.05 ± 4.05 | 0.842 |
-
KD, Kawasaki disease; HC, healthy controls; CRP, c-reactive protein; MPV, mean platelet volume; PT, prothrombin time; APTT, activated partial thromboplastin time; D-D, D-dimer; FIB, fibrinogen.
Plasma concentrations of PDPN, CLEC-2, and GPVI
Obviously, patients with KD exhibited significantly higher plasma levels of PDPN and CLEC-2 compared to healthy controls (HC) (p<0.001, Figure 1A and B). Furthermore, the plasma concentrations of GPVI in KD patients (59.59 ± 19.48 ng/mL) were markedly elevated when compared to those observed in HC individuals (29.74 ± 11.19 ng/mL) (p<0.001, Figure 1C).
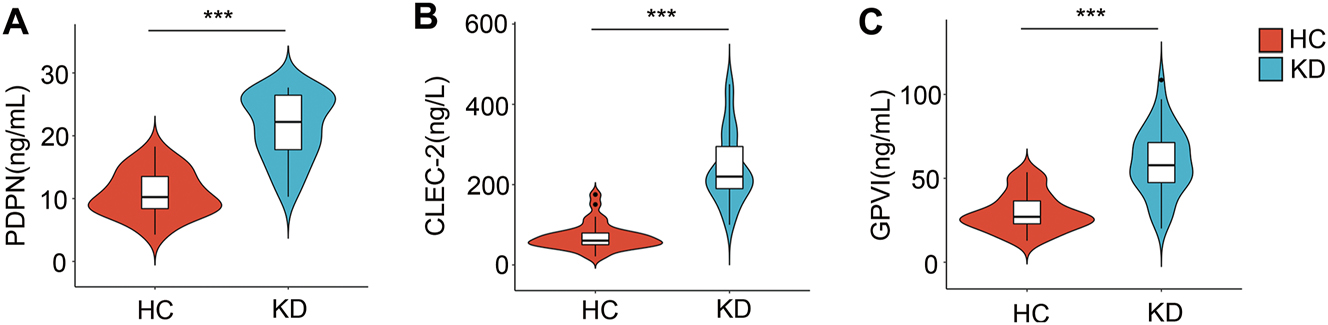
The levels of plasma PDPN, CLEC-2 and GPVI in KD and HC. Comparison of PDPN (A), CLEC-2 (B), and GPVI(C) levels in patients with KD and HC. *, p<0.05; **, p<0.01; ***, p<0.001. KD, Kawasaki disease; HC, healthy controls.
Correlation analysis between plasma PDPN, CLEC-2 and GPVI expression
In order to investigate the potential association between the upregulation of PDPN and CLEC-2 and platelet activation in KD, the correlation analysis was performed to evaluate the levels of PDPN and CLEC-2 in relation to GPVI. According to the results depicted in Figure 2, a significant positive correlation was observed between plasma PDPN and GPVI levels (r=0.716, p<0.001). Conversely, there was a weak correlation between plasma CLEC-2 and GPVI levels in patients with KD (r=0.249, p=0.108).
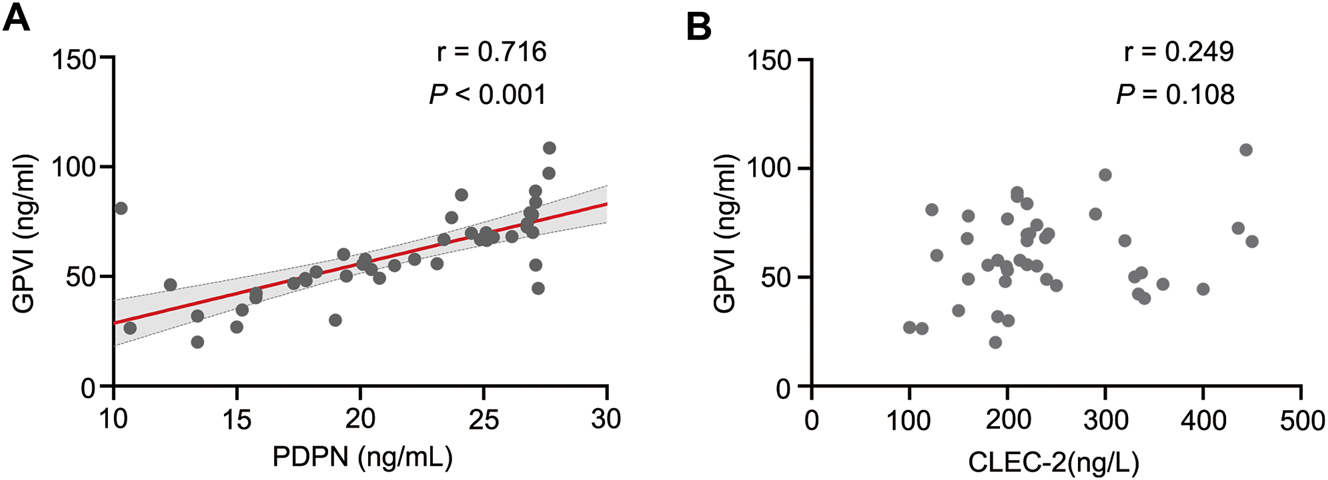
Correlation between plasma PDPN, CLEC-2 and GPVI levels assessed using the pearson correlation coefficient. (A) Correlation analysis of PDPN with GPVI plasma levels in 63 KD patients. (B) Correlation analysis of CLEC-2 with GPVI plasma levels in 63 KD patients. *, p<0.05; **, p<0.01; ***, p<0.001. KD, Kawasaki disease.
Clinical correlates of increased PDPN levels in KD
To ascertain the association between PDPN and molecular markers linked to coagulation and inflammation, we performed Pearson correlation analysis (Table 2). Our findings revealed a positive correlation between plasma PDPN levels and ESR, IL-6, FIB, and D-D (p<0.001) among patients with KD. In the KD-CALs group, plasma PDPN levels were positively correlated with ESR, D-D, and IL-6 (p<0.001).
Correlations between plasma PDPN levels and clinical parameters in the KD-CAL and KD-NCAL groups.
| KD (n=63) | KD-CAL (n=25) | KD-NCAL (n=38) | ||||
|---|---|---|---|---|---|---|
| r | p-Value | r | p-Value | r | p-Value | |
| WBC, 109/L | 0.17 | 0.731 | 0.15 | 0.772 | −0.02 | 0.683 |
| PCT, ng/mL | 0.20 | 0.392 | 0.24 | 0.672 | −0.12 | 0.782 |
| ESR, mm/h | 0.62 | <0.001 | 0.58 | 0.013 | 0.63 | <0.001 |
| CRP, mg/L | 0.31 | 0.041 | 0.32 | 0.673 | 0.28 | 0.032 |
| FIB, g/L | 0.78 | <0.001 | 0.46 | 0.698 | 0.79 | 0.010 |
| D-D, mg/L | 0.52 | <0.001 | 0.78 | <0.001 | 0.22 | 0.782 |
| PLT, 109/L | 0.18 | 0.032 | 0.11 | 0.851 | −0.24 | 0.765 |
| IL-8, pg/mL | −0.10 | 0.702 | −0.12 | 0.728 | 0.03 | 0.743 |
| TNF-α, pg/mL | 0.05 | 0.450 | 0.09 | 0.560 | −0.12 | 0.657 |
| IL-1β, pg/mL | −0.12 | 0.652 | −0.13 | 0.782 | 0.04 | 0.873 |
| IL-6, pg/mL | 0.84 | <0.001 | 0.77 | <0.001 | 0.65 | 0.032 |
| IL-10, pg/mL | 0.21 | 0.793 | 0.23 | 0.783 | 0.24 | 0.872 |
| C3, mg/L | 0.25 | 0.784 | 0.13 | 0.748 | 0.288 | 0.763 |
| C4, mg/L | 0.11 | 0.672 | −0.03 | 0.660 | 0.13 | 0.847 |
| IgE, IU/mL | 0.20 | 0.690 | −0.10 | 0.672 | 0.30 | 0.783 |
| BNP, ng/L | −0.03 | 0.667 | −0.02 | 0.579 | 0.13 | 0.789 |
-
KD, Kawasaki disease; CALs, coronary artery lesions; NCALs, non-CALs; WBC, white blood cell counts; ESR, erythrocyte sedimentation rate; D-D, D-dimer; FIB, fibrinogen; CRP, c-reactive protein; PCT, procalcitonin; C3, complement3; C4, complement4; IgE, immunoglobulin E; IL, interleukin; TNF-α, tumor necrosis factor-α; BNP, brain natriuretic peptide.
Predictive utility of plasma PDPN for CALs in patients with KD
To access the predictive utility of plasma PDPN for KD, the ROC curve of KD was performed (Figure 3A). Accordingly, the area under curve (AUC) was 0.8095. The Youden index yielded a value of 0.626, while the optimal cutoff value was determined to be 20.61 ng/mL, corresponding to a sensitivity and specificity of 80.1 % and 76.2 %, respectively (OR=9.667; 95 % CI, 0.6705–0.9486, p<0.001). Next, the diagnostic value of PDPN concentration for KD-CALs was further estimated (Figure 3B). The AUC was 0.8377 (95 % CI, 0.7121–0.9632, p<0.001), with a sensitivity of 45.45 % and specificity of 90.48 %. The critical value of PDPN in KD was determined to be 25.67 ng/mL. We also employed PDPN in conjunction with GPVI to construct the ROC curve (Supplementary Figure S1). The results indicated that the AUC was 0.838. While the area under the curve increased when compared to using PDPN alone, the combined diagnostic approach for KD did not demonstrate statistical significance (p>0.05).
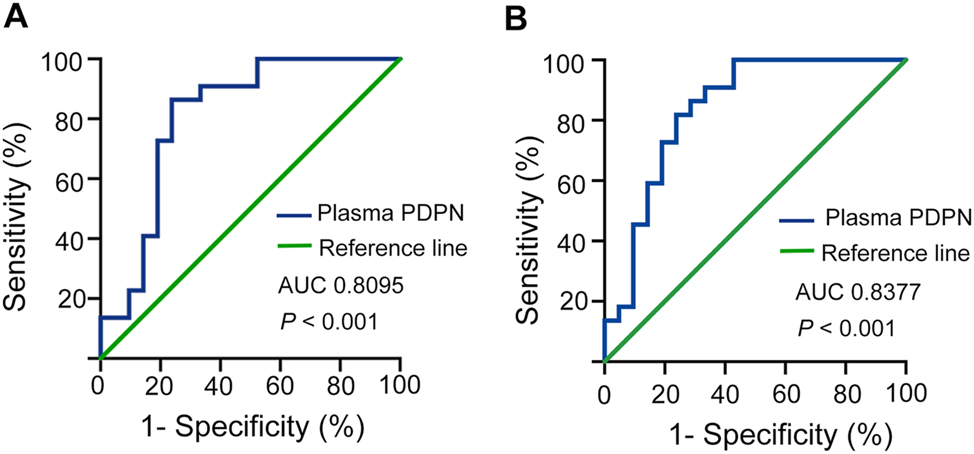
ROC curves for evaluating the predictive utility of PDPN in patients with KD and distinguishing between patients with CALs and NCALs. *, p<0.05; **, p<0.01; ***, p<0.001. AUC, area under the curve; ROC, receiver operator characteristic; KD, Kawasaki disease; CALs, coronary artery lesions; NCALs, non-CALs.
Discussion
Kawasaki disease has now become one of the common children heart diseases in some countries and regions [3]. The prevalence and incidence of KD in East Asia are increasing [1]. The hypercoagulability of blood in acute stage is considered to be an important factor in the occurrence of CA damage [17]. In the present study, we confirmed that the plasma D-D levels were increased in KD patients with CALs compared to NCALs, which indicating the hypercoagulable state in the KD patients with CALs (Supplementary Table S1). Despite the notable reduction in the occurrence of CA aneurysm when IVIG is administered in conjunction with aspirin, it is critical to acknowledge that CA damage remains present. Moreover, it is crucial to consider that the administration of high doses of aspirin to children may pose potential risks [1], 18].
CLEC-2 is an essential platelet receptor, is clustered by ligand binding on the platelet surface, which is mediated by tyrosine kinase Syk in the activation process, ultimately triggering platelet activation and aggregation [19]. PDPN, the only endogenous ligand of CLEC-2, is absent in normal vascular endothelial cells, while up-regulated in the damaged endothelial wall, contributing to platelet activation and thrombus formation [20]. Recently, in the study of deep vein thrombosis (DVT) model of mice [21], it was found that both anti-PDPN antibody therapy and induced deletion of CLEC-2 would lead to the decrease of thrombosis, thus preventing DVT. Therefore, we speculated that there was some relationship between PDPN/CLEC-2 and hypercoagulability of KD. GPVI is also a key platelet receptor, the center of activating collagen receptor, and an ITAM receptor with a similar signal pathway and pathophysiological function as CLEC-2, which leads to platelet activation and thrombosis [20]. GPVI has emerged as a highly promising target for antithrombotic therapy due to its ability to provide robust protection against experimental arterial thrombosis when blocked or depleted by antibodies, while not interfering with platelet hemostatic functions [22].
To the best of our knowledge, this is the first investigation into the function of PDPN/CLEC-2 in patients with KD. The ELISA analysis confirmed significantly elevated levels of PDPN/CLEC-2 and GPVI in KD compared to HC, thereby establishing a foundation for our subsequent investigation into the correlation between PDPN/CLEC-2 and platelet activation. It has been proved that antibody-mediated blocking of mouse GPVI or CLEC-2 can suppress their expression and biological activity on platelet surface, while thrombus growth can be inhibited completely with both inactivated. The mutual compensation of receptors showed that their targeted blocking played a powerful antithrombotic role [23]. Not unexpectedly, we observed a robust positive correlation between plasma PDPN and GPVI. Regarding plasma CLEC-2, The proposal by Yoshiki [24] suggested that there was no correlation between CLEC-2 levels and plasma GPVI levels in patients with thrombotic microangiopathy, thereby supporting the hypothesis of a negative feedback mechanism between CLEC-2 and GPVI that regulates the release of platelet granules [25], aligning with our own results. Compared with GPVI, the release of platelet receptor CLEC-2 is cleaved into soluble form by proteases and can also be affected by damaged platelet fragments [26]. Given the robust association between PDPN and the platelet-activated receptor GPVI (Figure 2A), PDPN was recognized as the pivotal role in the subsequent investigation.
D-dimer is produced when factor XIIIa acts on fibrin monomers and polymers during the degradation of cross-linked fibrin by the endogenous fibrinolytic system in the organism [27]. In clinical settings, FIB and D-D possess crucial guiding implications for the assessment of hypercoagulability and thrombosis in vivo. The analysis of coagulation parameters revealed that KD exhibited significantly elevated levels of D-D, FIB, and PLT counts compared to HC. The KD-CALs group exhibited a higher level of D-D compared to the KD-NCALs group, which indicating the apprarent hypercoagulable state in KD-NCALs. Here, we analyzed the correlation between PDPN and coagulation parameters in patients with KD. The results indicated that the plasma level of PDPN was positively correlated with D-D and FIB in the KD group, as well as a significant positive correlation with D-D in the KD-CALs group. In other words, patients with KD are more likely to suffer from hypercoagulability due to the increased PDPN.
PDPN on interstitial fibroblasts has been observed to elicit inflammatory alterations and assume a pivotal function in triggering plaque rupture [28]. Bacterial-induced thrombosis is mediated by CLEC-2 in a manner reliant on inflammation-driven mechanisms, which are concurrently associated with the up-regulation of PDPN [29]. Leukocytes are recruited to the vascular wall at the early stages of DVT, with platelets playing a role in enhancing this recruitment. The release of various inflammatory cytokines and metalloproteinases by neutrophils further stabilizes and facilitates the process of thrombosis [30], 31]. It can be said that the mutual promotion of inflammation and thrombosis gives rise to the outcome of DVT. DVT may be regarded as a thromboinflammatory process, rather than a solely thrombotic event. Similarly, KD is recognized as a condition characterized by thrombotic progression accompanied by systemic inflammation. Our previous study found that the regulation of CLEC-2 can impact the secretion of inflammatory cytokines in gastric cancer [32]. Therefore, in present study, we also explored the correlation between PDPN and cytokines, along with systemic inflammation indicators. Prior studies have substantiated the involvement of IL-6 in the pathogenesis of CALs in KD [33]. Additionally, ESR is considered a prognostic indicator for the development of CALs in KD [34]. In the KD-CALs group, the results of the correlation analysis indicated a significant positive association between PDPN and IL-6, ESR, suggesting that PDPN may collaborate with IL-6 to induce inflammatory thrombosis in the development of CALs in KD. It was consistent with the result that PDPN score was related to enhanced IL-6 and systemic inflammation [35]. Furthermore, evaluation of the diagnostic efficacy of PDPN by ROC curve analysis demonstrated that PDPN had excellent potential to distinguish KD patients from healthy children, with an AUC of 0.8095, 80.1 % sensitivity and 76.2 % specificity. The AUC of plasma PDPN for predicting CALs was 0.8377, with a sensitivity of 45.45 % and a specificity of 90.48 %. Therefore, we speculate that using PDPN as a predictive marker enables the prediction of CALs in KD with a high probability, thereby presenting a promising avenue for future therapeutic interventions in KD.
However, our selection of plasma samples was restricted to a specific time, warranting the need for prospective cohort study. The age of enrollment was limited due to discrepancies in the reference intervals of clinical indicators across various age groups, leading to a relatively small sample size. In the future, we will broaden the inclusion criteria for the subjects and further validate the accuracy of the research. Additionally, further explorations are also required to investigate on the mechanism of PDPN/CLEC-2 regulating coagulation-related molecules in KD and the formation of CALs.
Funding source: China Postdoctoral Science Foundation
Award Identifier / Grant number: 2023M733076
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 82200551
-
Research ethics: This study was approved by the Research Ethics Committee of the Children’s Hospital of Zhejiang University School of Medicine (Code: 2021-IRB-320).
-
Informed consent: Not applicable.
-
Author contributions: Ling Xu and Li Wei: plasma collection, implement of experiments and original draft editing. Zhimin Geng and Zhaoyang Peng: plasma collection and manuscript editing. Anna Sun, Wenjun Qin, Chunhong Xie: data analysis and graphic production. Fangqi Gong and Hongqiang Shen: concept organization, project establishment and manuscript revision. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: This research was funded by the National Natural Science Foundation of China [grant numbers: 82200551] and China Postdoctoral Science Foundation [certificate Number: 2023M733076].
-
Data availability: Not applicable.
References
1. Shulman, ST, Rowley, AH. Kawasaki disease: insights into pathogenesis and approaches to treatment. Nat Rev Rheumatol 2015;11:475–82. https://doi.org/10.1038/nrrheum.2015.54.Search in Google Scholar PubMed
2. Li, D, Chen, X, Li, X, Yuan, Y, Jin, H, Liu, G, et al.. Effectiveness and safety of dual antiplatelet therapy in coronary aneurysms caused by Kawasaki disease in children: study protocol for a multicenter randomized clinical trial. Transl Pediatr 2021;10:1914–23. https://doi.org/10.21037/tp-21-74.Search in Google Scholar PubMed PubMed Central
3. McCrindle, BW, Rowley, AH, Newburger, JW, Burns, JC, Bolger, AF, Gewitz, M, et al.. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American heart association. Circulation 2017;135:e927–99. https://doi.org/10.1161/cir.0000000000000484.Search in Google Scholar PubMed
4. Fukazawa, R, Kobayashi, J, Ayusawa, M, Hamada, H, Miura, M, Mitani, Y, et al.. JCS/JSCS 2020 guideline on diagnosis and management of cardiovascular sequelae in Kawasaki disease. Circ J 2020;84:1348–407. https://doi.org/10.1253/circj.cj-19-1094.Search in Google Scholar
5. Sakurai, Y, Takatsuka, H, Onaka, M, Takada, M, Nishino, M. Persistent endothelial damage after intravenous immunoglobulin therapy in Kawasaki disease. Int Arch Allergy Immunol 2014;165:111–8. https://doi.org/10.1159/000368402.Search in Google Scholar PubMed
6. Suzuki-Inoue, K. Platelets and cancer-associated thrombosis: focusing on the platelet activation receptor CLEC-2 and podoplanin. Blood 2019;134:1912–8. https://doi.org/10.1182/blood.2019001388.Search in Google Scholar PubMed
7. Ishikura, H, Irie, Y, Kawamura, M, Hoshino, K, Nakamura, Y, Mizunuma, M, et al.. Early recognition of sepsis-induced coagulopathy using the C2PAC index: a ratio of soluble type C lectin-like receptor 2 (sCLEC-2) level and platelet count. Platelets 2022;33:935–44. https://doi.org/10.1080/09537104.2021.2019694.Search in Google Scholar PubMed
8. Kazama, F, Nakamura, J, Osada, M, Inoue, O, Oosawa, M, Tamura, S, et al.. Measurement of soluble C-type lectin-like receptor 2 in human plasma. Platelets 2015;26:711–9. https://doi.org/10.3109/09537104.2015.1021319.Search in Google Scholar PubMed
9. Wang, X, Liu, B, Xu, M, Jiang, Y, Zhou, J, Yang, J, et al.. Blocking podoplanin inhibits platelet activation and decreases cancer-associated venous thrombosis. Thromb Res 2021;200:72–80. https://doi.org/10.1016/j.thromres.2021.01.008.Search in Google Scholar PubMed
10. Meng, D, Luo, M, Liu, B. The role of CLEC-2 and its ligands in thromboinflammation. Front Immunol 2021;12:688643. https://doi.org/10.3389/fimmu.2021.688643.Search in Google Scholar PubMed PubMed Central
11. Sang, Y, Roest, M, de Laat, B, de Groot, PG, Huskens, D. Interplay between platelets and coagulation. Blood Rev 2021;46:100733. https://doi.org/10.1016/j.blre.2020.100733.Search in Google Scholar PubMed PubMed Central
12. Fainchtein, K, Tera, Y, Kearn, N, Noureldin, A, Othman, M. Hypercoagulability and thrombosis risk in prostate cancer: the role of thromboelastography. Semin Thromb Hemost 2023;49:111–8. https://doi.org/10.1055/s-0042-1758116.Search in Google Scholar PubMed
13. Lee, JK. Hygiene hypothesis as the etiology of Kawasaki disease: dysregulation of early B cell development. Int J Mol Sci 2021;22. https://doi.org/10.3390/ijms222212334.Search in Google Scholar PubMed PubMed Central
14. Perrella, G, Nagy, M, Watson, SP, Heemskerk, JWM. Platelet GPVI (glycoprotein VI) and thrombotic complications in the venous system. Arterioscler Thromb Vasc Biol 2021;41:2681–92. https://doi.org/10.1161/atvbaha.121.316108.Search in Google Scholar PubMed PubMed Central
15. Watanabe, N, Shinozaki, Y, Ogiwara, S, Miyagasako, R, Sasaki, A, Suzuki, Y, et al.. Diphenyl-tetrazol-propanamide derivatives act as dual-specific antagonists of platelet CLEC-2 and GPVI. Thromb Haemost 2024;124:203–22. https://doi.org/10.1055/a-2211-5202.Search in Google Scholar PubMed
16. Egami, K, Muta, H, Ishii, M, Suda, K, Sugahara, Y, Iemura, M, et al.. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr 2006;149:237–40. https://doi.org/10.1016/j.jpeds.2006.03.050.Search in Google Scholar PubMed
17. Kong, WX, Ma, FY, Fu, SL, Wang, W, Xie, CH, Zhang, YY, et al.. Biomarkers of intravenous immunoglobulin resistance and coronary artery lesions in Kawasaki disease. World J Pediatr 2019;15:168–75. https://doi.org/10.1007/s12519-019-00234-6.Search in Google Scholar PubMed
18. Soni, PR, Noval Rivas, M, Arditi, M. A comprehensive update on Kawasaki disease vasculitis and myocarditis. Curr Rheumatol Rep 2020;22:6. https://doi.org/10.1007/s11926-020-0882-1.Search in Google Scholar PubMed
19. Nicolson, PLR, Nock, SH, Hinds, J, Garcia-Quintanilla, L, Smith, CW, Campos, J, et al.. Low-dose Btk inhibitors selectively block platelet activation by CLEC-2. Haematologica 2021;106:208–19. https://doi.org/10.3324/haematol.2019.218545.Search in Google Scholar PubMed PubMed Central
20. Furukoji, E, Yamashita, A, Nakamura, K, Hirai, T, Asada, Y. Podoplanin expression on endothelial cells promotes superficial erosive injury and thrombus formation in rat carotid artery: implications for plaque erosion. Thromb Res 2019;183:76–9. https://doi.org/10.1016/j.thromres.2019.10.015.Search in Google Scholar PubMed
21. Brill, A, Fuchs, TA, Chauhan, AK, Yang, JJ, De Meyer, SF, Köllnberger, M, et al.. von Willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood 2011;117:1400–7. https://doi.org/10.1182/blood-2010-05-287623.Search in Google Scholar PubMed PubMed Central
22. Cherpokova, D, Bender, M, Morowski, M, Kraft, P, Schuhmann, MK, Akbar, SM, et al.. SLAP/SLAP2 prevent excessive platelet (hem)ITAM signaling in thrombosis and ischemic stroke in mice. Blood 2015;125:185–94. https://doi.org/10.1182/blood-2014-06-580597.Search in Google Scholar PubMed PubMed Central
23. Bender, M, May, F, Lorenz, V, Thielmann, I, Hagedorn, I, Finney, BA, et al.. Combined in vivo depletion of glycoprotein VI and C-type lectin-like receptor 2 severely compromises hemostasis and abrogates arterial thrombosis in mice. Arterioscler Thromb Vasc Biol 2013;33:926–34. https://doi.org/10.1161/atvbaha.112.300672.Search in Google Scholar PubMed PubMed Central
24. Yamashita, Y, Suzuki, K, Mastumoto, T, Ikejiri, M, Ohishi, K, Katayama, N, et al.. Elevated plasma levels of soluble C-type lectin-like receptor 2 (CLEC2) in patients with thrombotic microangiopathy. Thromb Res 2019;178:54–8. https://doi.org/10.1016/j.thromres.2019.03.018.Search in Google Scholar PubMed
25. Alshahrani, MM, Yang, E, Yip, J, Ghanem, SS, Abdallah, SL, deAngelis, AM, et al.. CEACAM2 negatively regulates hemi (ITAM-bearing) GPVI and CLEC-2 pathways and thrombus growth in vitro and in vivo. Blood 2014;124:2431–41. https://doi.org/10.1182/blood-2014-04-569707.Search in Google Scholar PubMed PubMed Central
26. Suzuki-Inoue, K, Fuller, GL, García, A, Eble, JA, Pöhlmann, S, Inoue, O, et al.. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood 2006;107:542–9. https://doi.org/10.1182/blood-2005-05-1994.Search in Google Scholar PubMed
27. Righini, M, Perrier, A, De Moerloose, P, Bounameaux, H. D-Dimer for venous thromboembolism diagnosis: 20 years later. J Thromb Haemostasis 2008;6:1059–71. https://doi.org/10.1111/j.1538-7836.2008.02981.x.Search in Google Scholar PubMed
28. Tang, C, Wang, L, Sheng, Y, Zheng, Z, Xie, Z, Wu, F, et al.. CLEC-2-dependent platelet subendothelial accumulation by flow disturbance contributes to atherogenesis in mice. Theranostics 2021;11:9791–804. https://doi.org/10.7150/thno.64601.Search in Google Scholar PubMed PubMed Central
29. Payne, H, Ponomaryov, T, Watson, SP, Brill, A. Mice with a deficiency in CLEC-2 are protected against deep vein thrombosis. Blood 2017;129:2013–20. https://doi.org/10.1182/blood-2016-09-742999.Search in Google Scholar PubMed PubMed Central
30. Fuchs, TA, Brill, A, Wagner, DD. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler Thromb Vasc Biol 2012;32:1777–83. https://doi.org/10.1161/atvbaha.111.242859.Search in Google Scholar PubMed PubMed Central
31. Brinkmann, V, Reichard, U, Goosmann, C, Fauler, B, Uhlemann, Y, Weiss, DS, et al.. Neutrophil extracellular traps kill bacteria. Science 2004;303:1532–5. https://doi.org/10.1126/science.1092385.Search in Google Scholar PubMed
32. Xu, L, Liu, F, Li, C, Li, S, Wu, H, Guo, B, et al.. Fucoidan suppresses the gastric cancer cell malignant phenotype and production of TGF-beta1 via CLEC-2. Glycobiology 2020;30:301–11. https://doi.org/10.1093/glycob/cwz097.Search in Google Scholar PubMed
33. Liu, R, He, B, Gao, F, Liu, Q, Yi, Q. Relationship between adipokines and coronary artery aneurysm in children with Kawasaki disease. Transl Res 2012;160:131–6. https://doi.org/10.1016/j.trsl.2012.01.013.Search in Google Scholar PubMed
34. Qiu, H, He, Y, Rong, X, Ren, Y, Pan, L, Chu, M, et al.. Delayed intravenous immunoglobulin treatment increased the risk of coronary artery lesions in children with Kawasaki disease at different status. Postgrad Med J 2018;130:442–7. https://doi.org/10.1080/00325481.2018.1468712.Search in Google Scholar PubMed
35. Juneja, P, Sharma, A, Shasthry, SM, Kumar, G, Tripathi, DM, Rajan, V, et al.. Podoplanin-positive dilated lymphatic vessels in duodenum associates with three-month mortality in patients with cirrhosis. Front Physiol 2023;14:1045983. https://doi.org/10.3389/fphys.2023.1045983.Search in Google Scholar PubMed PubMed Central
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/labmed-2024-0214).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Original Articles
- A comparative study between the Chrono-log 700 and the Sysmex CS-2100i analyzers for assessing ristocetin cofactor activity in patients with von Willebrand disease
- Evaluation of hemolysis index thresholds for 18 biochemistry assays: implications for laboratory-developed tests in the era of the IVDR
- Elevation of plasma podoplanin in Kawasaki disease
- Epidemiology characteristics of Epstein-Barr virus among children in Hangzhou from 2019 to 2023
- Mean platelet volume vs. optical platelet volume measurement: which is a better indicator for neonatal sepsis?
Articles in the same Issue
- Frontmatter
- Original Articles
- A comparative study between the Chrono-log 700 and the Sysmex CS-2100i analyzers for assessing ristocetin cofactor activity in patients with von Willebrand disease
- Evaluation of hemolysis index thresholds for 18 biochemistry assays: implications for laboratory-developed tests in the era of the IVDR
- Elevation of plasma podoplanin in Kawasaki disease
- Epidemiology characteristics of Epstein-Barr virus among children in Hangzhou from 2019 to 2023
- Mean platelet volume vs. optical platelet volume measurement: which is a better indicator for neonatal sepsis?


