A comparative study between the Chrono-log 700 and the Sysmex CS-2100i analyzers for assessing ristocetin cofactor activity in patients with von Willebrand disease
-
Mohamed-Rachid Boulassel
, Hussein Abdellatif
Abstract
Objectives
A variety of methods are currently used to measure von Willebrand factor (VWF) activity, but still the VWF ristocetin cofactor (VWF:RCo) assay using the manual aggregometry technique is the reference method, even having high inter-laboratory variability. The automated coagulation analyzers offer several advantages for routine testing. Herein the performance of the automated Sysmex CS2000/2100i analyzer was compared to the manual aggregometer Chrono-log 700 for assessing VWF:Co activity in patients suspected of having von Willebrand disease (VWD).
Methods
Plasma samples from 136 patients were prospectively collected, and blindly analyzed on both instruments, simultaneously. Linear regression analysis, Bland-Altman test, intra-class correlation coefficient (ICC), and area under receiver-operator characteristic (ROC) curve were used to evaluate the performance of the automated VWF:RCo assay.
Results
There was a strong positive correlation between the two assays (r=0.86, p<0.0001) with an excellent reliability ICC value of 0.81 (95 % CI: 0.74–0.86). A very good degree of agreement between the two assays was also evidenced with an estimated bias of −0.055 (−0.58 to 0.46). The ROC curve for the automated VWF:RCo assay was 0.86 (95 % CI: 0.78–0.92; p<0.0001). Using a cut-off value of 0.44 UI/mL for VWF:RCo activity, the sensitivity and specificity values were 91.2 %, and 88.2 % for the automated assay. The positive and negative positive values for VWD detection were 72.9 %, and 96.7 %, respectively.
Conclusions
Collectively, these findings indicate that the automated VWF:RCo assay yields comparable results to the manual aggregometry assay, with very good accuracy and precision to help diagnose patients suspected with VWD.
Introduction
In current clinical practice, the diagnosis of von Willebrand disease (VWD) is based on personal and family history of mucocutaneous bleeding, and supported by specific laboratory investigations, which are central in the classification and management of this secondary hemostasis disorder [1], 2]. Virtually, all clinical laboratories performed a variety of assays to measure von Willebrand factor (VWF) activity and its plasmatic protein concentration based on available resources and geographic locations [3]. The VWF ristocetin cofactor (VWF:RCo), which is based on the ability of circulating VWF to aggregate normal platelets in the presence of ristocetin, an antibiotic no longer clinically used, is still employed in many laboratories [4]. While being the reference test, the VWF:RCo aggregometry assay still requires thorough optimization and validation steps, and the interpretation of the test results are sometimes challenging for both laboratory specialists and treating physicians [5]. Therefore, new assays have been developed to improve on consistent results and diagnosis of VWD either in addition to, or instead of VWF:RCo test [6].
Regardless of limited comparative trials, a number of clinical laboratories have shifted focus from the VWF:RCo test to new assays that can be easily implemented on automated coagulation analyzers, thereby generating faster and more reproducible results [6], [7], [8], [9]. The fully automated analyzer, Sysmex CS2000/2100i (Sysmex, Kobe, Japan) was introduced with improved analytical algorithms, and equipped with multiple detection techniques ranging from clotting to platelet aggregometry. This coagulation analyzer can perform routine and specialized tests on a single platform that greatly simplifies the hemostasis testing in clinical laboratories processing a large volume of specimens. So far, several VWF:Co assay protocols have been reported using automated coagulation instruments yielding reliable measures even in samples with low circulating levels of VWF [9], [10], [11], [12]. However, more research data is needed to determine which instrument provides better results than others to help obtain assay protocol generalizability. As the Sysmex CS2000/2100i is becoming widely used, it is of importance to verify its performance status in assessing VWF:Co activity under the local laboratory environment. Therefore, this study aimed to assess the agreement between the manual light transmission aggregometer Chrono-log 700 (Chrono-log Corporation USA) and the Sysmex CS-2100i automated coagulation analyzer for VWF:Co activity in patients suspected of having VWD in daily routine practice.
Materials and methods
Study design and patients
This was a prospective study in which patients suspected of having VWD were recruited during a routine clinical visit at the Sultan Qaboos University (SQU) Hospital, Sultanate of Oman, from January 2022 to December 2023. The main eligibility criteria included patients of any age, both gender and having a bleeding phenotype in a favor of VWD. The key exclusion criteria included presence of data suggesting any other concomitant bleeding disorders, taking medicines, and suspicion of any neoplastic or non-neoplastic condition. For each participant, the socio-demographic characteristics, clinical and laboratory data and outcomes were collected using a special case report form. All completed data collection forms were examined for consistency by two independent investigators, and patients were categorized as having VWD or not. This study was performed according to the Declaration of Helsinki, and approved by the Institutional Research Ethic Committee of the SQU, Sultanate of Oman (Ref # 1485).
Blood samples
For each enrolled patient, a fresh blood sample was collected on sodium citrate Vacutainer tube (BD Biosciences, USA), and submitted to the coagulation laboratory within half an hour of collection. The tubes were centrifuged at 1,500 g for 15 min at 25 °C and then plasma was separated. The plasma was re-centrifuged again to produce platelet poor plasma, and stored at −80 °C until used, as previously reported [13].
Reagents
For both analyzers the same commercial reagents and lots were used. Ristocetin (7.5 mg/vial, Siemens, Germany) was reconstituted with 750 μL sterile distilled water to get a final concentration of 10 mg/mL. Lyophilized human platelets (Siemens, Germany) was prepared with 6 mL of Tris buffered saline, while von Willebrand reference normal and deficient plasma samples (Siemens, Germany) were reconstituted with 1 and 0.5 mL of sterile distilled water, respectively, as provided by the manufacturer. All reagents were stored between 2 and 8 °C when reconstituted, and kept at room temperature for 15–20 min before used. After plasma thawing, the assays were performed simultaneously within 2 h and run blindly on both instruments.
Chrono-log 700 assay
The manual reference method for VWF:Co activity was performed on the Chrono-log 700 aggregometer. This technique is based on the absorbance at 450 nm using spectrophotometry principal. For each set of assays, a three-point standard curve was prepared using the von Willebrand reference normal plasma diluted from 1:2 to 1:8. In brief, diluted plasma samples were added to a standardized suspension of platelets, and ristocetin, and incubated in a cuvette at 37 °C under agitation. Platelet agglutination was monitored over 3 min, and the VWF:Co activity was calculated from the standard curve using the integrated computation program. Within each assay, calibration and controls were run, as per the manufacturer recommendation, and a test result of less than 0.44 IU/mL was considered abnormal and indicative of VWD.
Sysmex CS-2100i assay
The automated assay for VWF:Co activity was performed on the Sysmex2000i analyzer. The preparation of reagents was similar to the Chrono-log 700 assay, as stated above. The analysis was automatically conducted using dilutions to establish the standard curve and analyzed by photo-optical system at 405 nm. The agglutination of platelets in the presence of ristocetin and VWF results in an increase of light transmission. The VWF activity was directly proportional to the rate of platelet aggregation reaction. Similar to the Chrono-log 700 assay, the test result on the Sysmex2001i analyzer was expressed as IU/mL.
Other laboratory tests
The complete blood count was measured on the CELL-DYN Sapphire (Abbott Diagnostics, USA) and coagulation factor VIII (FVIII:C) by a chromogenic assay (Precision BioLogic, USA), as previously reported [14]. The VWF antigen (VWF:Ag) and VWF functional activity (VWF:Ac) were measured by immunoassays (Siemens, Germany), while the collagen binding assay (VWF:CB) was assessed by an enzyme-linked immunosorbent assay (Diapharma, USA).
Statistical analysis
Analyses were performed using GraphPad Prism, Version 10 (GraphPad Software, Inc., San Diego, California, USA). Descriptive statistics for VWF:RCo levels were produced for each method. The paired Student’s t-test and Pearson correlation coefficient were used to evaluate the difference in mean VWF:RCo activity and to measure the linear regression between two assays, respectively. The Bland-Altman method was used to assess agreement between VWF:RCo measurements. The reliability of the VWF:RCo measurements was evaluated by the intra-class correlation coefficient (ICC). Area under receiver-operator characteristic (ROC) curve, and positive predictive value (PPV) and negative predictive value (NPV) were used for evaluating the accuracy of the Sysmex CS-2100i assay. All tests were two-tailed with an alpha level of 0.05.
Results
Study population characteristics
Over the study period, 136 patients who met the inclusion criteria were enrolled. The mean age of the study population was 18 ± 2.9 years with a range of 1–52 years. As expected, the proportion of females was higher (61 %) than males (39 %). The most common clinical indications were mild bleeding (66 %) followed by hemorrhage (17 %) and epistaxis (17 %). The mean hemoglobin level was 11.8 ± 1.8 g/dL (8.2–17.2), whereas the while blood cell count ranged from 2.4 to 20 × 109/L. In almost all patients (95 %) the platelet count was in the normal range from 150 to 400 × 109/L, except in six patients (5 %), the count ranged from 950 to 1,561 × 109/L. The prothrombin time, activated partial thromboplastin time and thrombin time mean values ranged from 9.2 to 17.4, 26 to 81, and 12–23 s, respectively. The mean fibrinogen level was 2.9 ± 0.8 g/L (1.5–5.4). The most common blood ABO type was O with a frequency of 85 %, followed by blood type A (11 %), and then type B (2 %) and type AB (2 %).
Diagnosis criteria for van Willebrand disease
In our clinical setting, VWD is established on a documented clinical evidence of bleeding, and an abnormal VWF:RCo activity result, along with other VWF workup findings (Table 1). Based on these findings, the patients were categorized as having VWD (25 %), or normal VWD workup (75 %). Of note, the VWD group was further categorized as type 1 (n=21), type 2 (n=12) and type 3 (n=1). Of note, 25 % of VWD patients were already diagnosed with this condition, and their VWD laboratory work-up was carried out for clinical follow-up. The distribution of the subtypes of type 2 VWD included type 2A (n=7) and type 2M (n=5). In this group of patients the most common clinical presentation was recurrent unexplained bruising, followed by epistaxis and then menorrhagia. It is also worth mentioning that in patients with type 2 VWD, the VWF:RCo activity to VWF antigen ratio is always less than 0.6 UI/mL and consistent with the genetic testing.
Von Willebrand disease workup in study population.
| Parameters | Patients with VWD (n=34) | Patients without VWD (n=102) | p-Value |
|---|---|---|---|
| Age, years | 13.5 ± 2.3 | 19.8 ± 1.2 | 0.02 |
| Gender, n, % Female |
10 (29.5) |
74 (72.5) |
0.001 |
| Blood type O, n, % | 28 (82.4) | 88 (86.2) | 0.58 |
| FVIII:C, IU/mL | 0.72 ± 0.06 | 1.13 ± 0.04 | <0.0001 |
| VWF:Ag, IU/mL | 0.57 ± 0.04 | 1.04 ± 0.04 | <0.0001 |
| VWF:CB, IU/mL | 0.41 ± 0.03 | 0.88 ± 0.03 | <0.0001 |
| VWF:Ac, IU/mL | 0.52 ± 0.04 | 0.97 ± 0.02 | <0.0001 |
-
Data are shown as mean ± standard deviation. FVIII:C, factor VIII procoagulant activity; VWF, von Willebrand factor; VWF:Ag, VWF antigen; VWF:CB, VWF collagen binding assay; VWF:Ac, VWF activity.
Levels of VWF ristocetin cofactor activity
As shown in Figure 1A, the mean ± standard deviation (SD) and range values of VWF:RCo activity were 0.80 ± 0.03 (0.02–2.24) and 0.75 ± 0.03 (0.1–2.42) for the Chrono-log 700 and the Sysmex CS-2100i instruments, respectively. Of note, no significant difference in VWF:RCo activity was evidenced between the two assays (p=0.91). Linear regression analysis revealed a very strong correlation between the two assays (r=0.86, p<0.0001) indicating that the two methods are yielding closely the same VWF:RCo result (Figure 1B). The estimated ICC value was 0.81 (95 % confidence interval: 0.74–0.86) suggesting an excellent reliability between the two assays (Table 2). In line with this finding is the good degree of agreement between the two methods with an estimated bias of −0.055 ranging from −0.58 to 0.46 (Figure 2). The area under the curve for the Sysmex CS-2100i VWF:RCo assay was 0.86, which is reasonably very good (Figure 3). Using a cut-off value of 0.44 UI/mL for VWF:RCo activity, which works better in our testing platform to distinguish VWD, the automated assay demonstrated a sensitivity of 91.2 %, with a specificity of 88.2 %. The PPV, NPV and accuracy values were 72.9 %, 96.7% and 88.9 %, respectively.
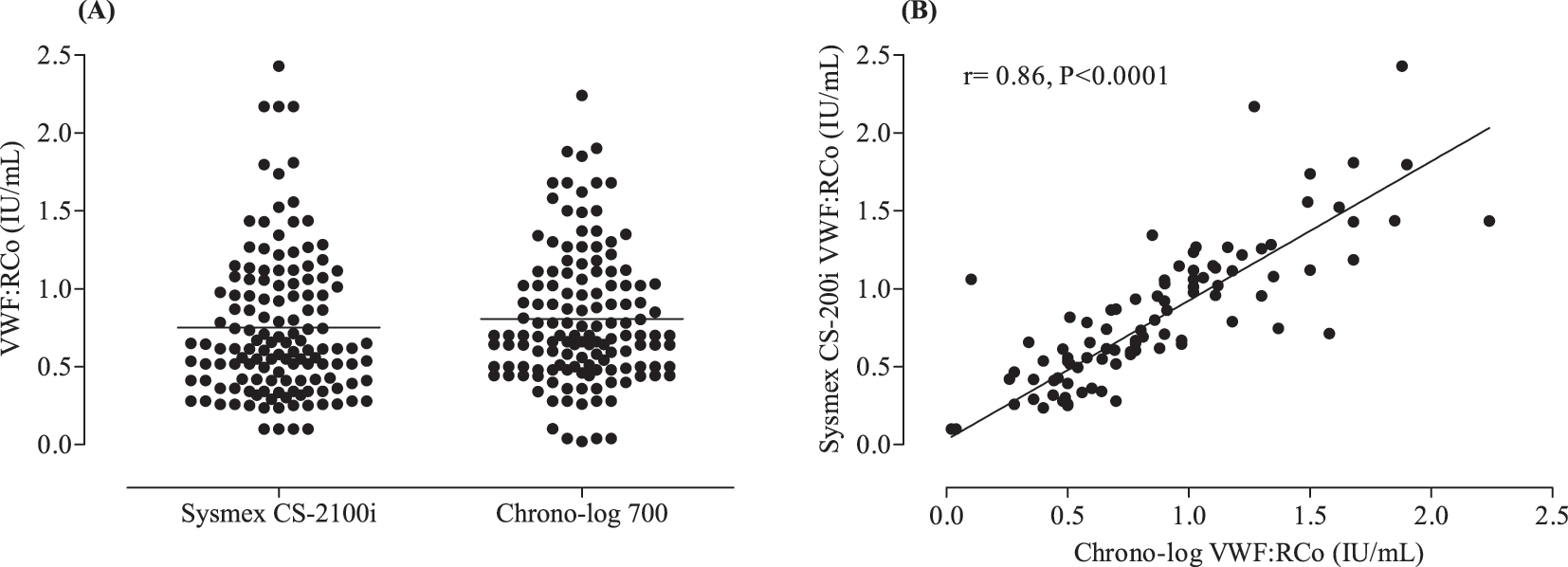
Levels of VWF ristocetin cofactor activity in studied population. (A) Comparison of VWF:Co activity and (B) linear regression analysis between the two assays.
Reliability analysis of VWF:Co activity between the Chrono-log 700 and the Sysmex CS-2100i analyzers.
| Measures | Intra-class correlation coefficient | 95 % confidence interval | p-Value | |
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| Single | 0.83 | 0.74 | 0.86 | <0.0001 |
| Average | 0.89 | 0.85 | 0.92 | <0.0001 |
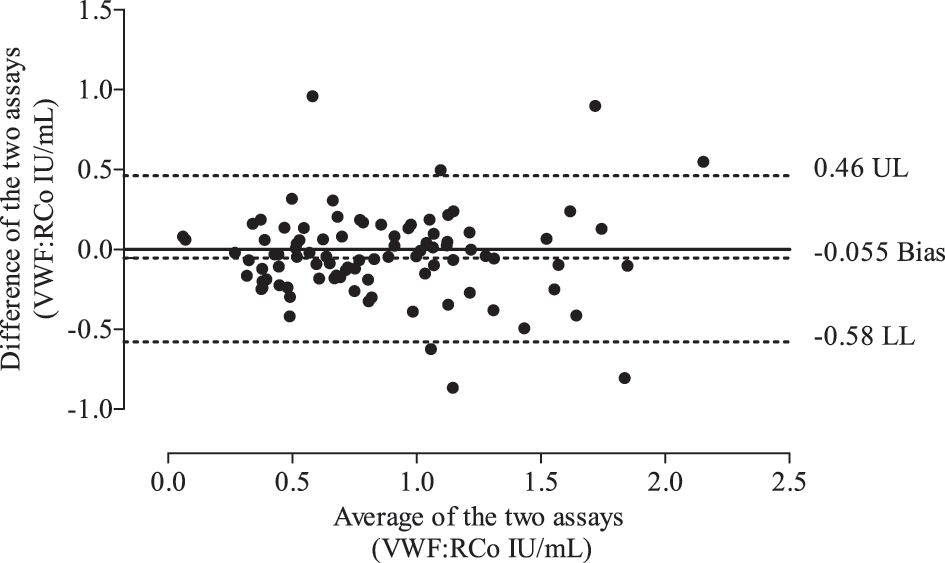
Bland-Altman plot depicting the degree of agreement between the Chrono-log 700 and the Sysmex CS-2100i analyzers in assessing levels of VWF ristocetin cofactor activity. The solid line represents zero difference between the two assays. The dotted lines represent the bias, upper limits (UL), and lower limits (LL) of agreement between the two assays.
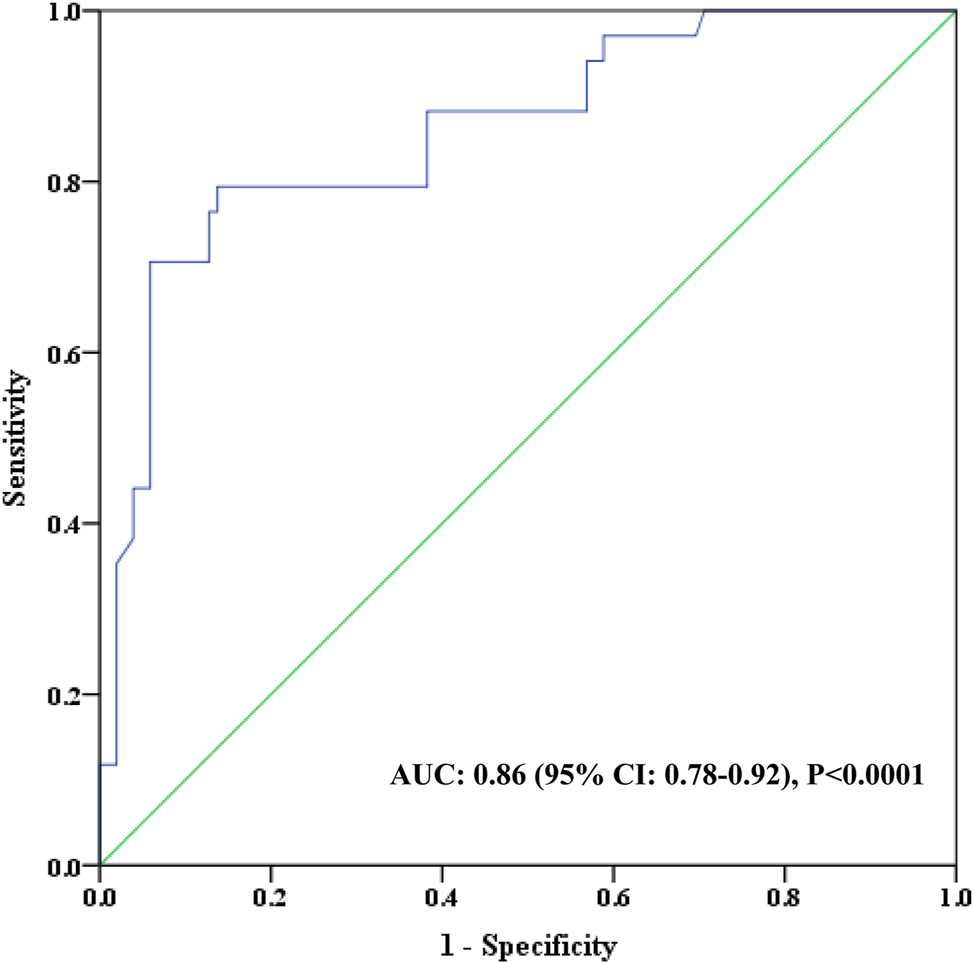
Receiving-operator curve (ROC) analysis of VWF ristocetin cofactor activity for the Sysmex CS-2100i analyzer. AUC, area under the curve; CI, confidence interval.
Discussion
There remains some debate regarding the measurement of VWF:RCo activity using the manual aggregometry technique. White some studies report that automated VWF:RCo assays have a great precision and accuracy in the diagnosis and monitoring of VWD, other have shown that the manual aggregometry tests still yield reliable results to diagnose all types of VWD, even if they are associated with high inter-laboratory variability [2], 15]. An accurate and rapid VWF:RCo assay is essential to categorize VWD subtypes and to monitor the effects of therapy prior to surgical interventions or post traumatic events. Taking advantages of automated performance testing, the VWF:RCo assay was implemented on the Sysmex CS-2100i coagulation instrument to allow its broader routine application.
The current study found that the automated VWF:RCo assay yielded similar results to the reference manual aggregometry method. The comparison of the two methods demonstrated a very strong positive correlation indicating that the two techniques are interchangeable. The same association was also observed with the ICC value, which determines the reliability of the automated VWF:RCo assay to yield the same or comparable VWF:RCo activity to the manual aggregometry method. The ICC is calculated using variance estimates, which are obtained from the analysis of VWF:RCo measurement variance, and is measured on a scale from 0 to 1, where the closer the value is to 1, the higher the reliability. Excellent reliability is usually determined by an ICC value of ≥0.75, which was obtained in the current study [16]. Additionally, the Bland-Atman limits of agreement, which assume that differences are constant throughout the range of VWF:RCo measurements, further indicate that the automated VWF:RCo assay produced similar VWF:RCo activity with a low level of bias in comparison to the manual aggregometry method. Collectively, the results of this study confirm and extend those findings showing that the automated VWF:RCo assay is highly correlated with the reference manual aggregometry method providing the same diagnostic ability in all types of VWD [7], 8], 10]. Using the Sysmex CS-2100i, Bowyer et al., reported a good correlation between the manual and automated VWF:RCo assays, and a very good agreement between the two methods in VWD type 1 patients who received replacement therapy with Desmopressin, a drug used to induce secretion of residual VWF from endothelial cells [10]. Of note, Bowyer et al., study differs in many aspects from ours in terms of demographic characteristics and sample size of the study population, although both studies were implemented on the automated Sysmex CS-2100i to assess the VWF:RCo activity [10].
So far, there is no consensus regarding the best cut-off value for evaluating VWF:RCo activity but is generally accepted to be between 0.5 and 20 UI/mL [17]. Levels less than 50 UI/mL are considered abnormal, although the degree to which these levels are reduced on a population level is still controversial in the diagnosis of VWD. Early studies have shown that blood O type individuals exhibit almost 25 % reduction in VWF levels compared to blood type A individuals [18]. In our laboratory settings, the accepted cut-off value was 0.44 IU/mL for VWF:RCo activity to indicate for VWD, regardless of the blood type. It is worth mentioning that three thirds of current study population has blood type O. The automated VWF:RCo assay showed good sensitivity and specificity, along with excellent diagnostic performance characterized by higher ROC curve, PPV, NPV, specificity and accuracy. These findings are in agreement with those reported by other groups attempting to implement the VWF:RCo assay on various coagulation instruments such as the ACL analyzers [7], 8].
The current study is subjected to some limitations. Given its study design, very few patients with VWD type 3 were enrolled in this study. This may generate a bias toward the selection of participants. However, patients were enrolled over a period of 12 months, which gave opportunity for those patients to participate in the study. In fact, patients with VWD attending the SQUH are coming from all over the country, with a disease burden equitably similar across all regions, and thereby the sample used in this study is at least moderately representative of patients with VWD type 3. Additionally, although the sensitivity limit of the two methods was quite similar, the number of samples tested for low levels of VWF:RCo was relatively small. This may challenge the validity of the automated method to detect even very low levels of VWF:RCo.
In conclusion, the findings of the current study provide evidence that the automated VWF:RCo assay yields comparable results to the manual aggregometry assay, with very good accuracy and precision to help diagnose patients with VWD. As a result, embedding the automated VWF:RCo assay into the laboratory tests should greatly improve the reliability of routine techniques for detecting VWD.
Acknowledgments
The authors wish to thank all the participants and staff from Department of Hematology for providing technical assistance.
-
Research ethics: Research involving human subjects complied with all relevant national regulations, institutional policies and is in accordance with the tenets of the Helsinki Declaration (as revised in 2013), and has been approved by the authors’ Institutional Review Board Ethics Committee (1485).
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: Raw data were generated at the Sultan Qaboos University (SQU), and are not publicly available. Derived data supporting the findings of this study are available from the corresponding author on special request.
References
1. Weyand, AC, Flood, VH. Von Willebrand disease: current status of diagnosis and management. Hematol Oncol Clin N Am 2021;35:1085–101. https://doi.org/10.1016/j.hoc.2021.07.004.Search in Google Scholar PubMed PubMed Central
2. Connell, NT, Flood, VH, Brignardello-Petersen, R, Abdul-Kadir, R, Arapshian, A, Couper, S, et al.. ASH ISTH NHF WFH 2021 guidelines on the management of von Willebrand disease. Blood Adv 2021;5:301–25. https://doi.org/10.1182/bloodadvances.2020003264.Search in Google Scholar PubMed PubMed Central
3. Favaloro, EJ, Pasalic, L. Laboratory diagnosis of von Willebrand disease in the age of the new guidelines: considerations based on geography and resources. Res Pract Thromb Haemost 2023;7:102143. https://doi.org/10.1016/j.rpth.2023.102143.Search in Google Scholar PubMed PubMed Central
4. Harris, NS, Pelletier, JP, Marin, MJ, Winter, WE. Von Willebrand factor and disease: a review for laboratory professionals. Crit Rev Clin Lab Sci 2022;59:241–56. https://doi.org/10.1080/10408363.2021.2014781.Search in Google Scholar PubMed
5. Higgins, RA, Goodwin, AJ. Automated assays for von Willebrand factor activity. Am J Hematol 2019;94:496–503. https://doi.org/10.1002/ajh.25393.Search in Google Scholar PubMed
6. Vangenechten, I, Mayger, K, Smejkal, P, Zapletal, O, Michiels, J, Moore, G, et al.. A comparative analysis of different automated von Willebrand factor glycoprotein Ib-binding activity assays in well typed von Willebrand disease patients. J Thromb Haemost 2018;16:1268–77. https://doi.org/10.1111/jth.14145.Search in Google Scholar PubMed
7. Redaelli, R, Corno, AR, Borroni, L, Mostarda, G, Nichelatti, M, Morra, E, et al.. von Willebrand factor ristocetin cofactor (VWF:RCo) assay: implementation on an automated coagulometer (ACL). J Thromb Haemost 2005;3:2684–8. https://doi.org/10.1111/j.1538-7836.2005.01628.x.Search in Google Scholar PubMed
8. Lai, SW, Chang, CY, Cheng, SN, Hu, SH, Lai, CY, Chen, YC. A comparative evaluation of an automated functional assay for von Willebrand factor activity in type 1 von Willebrand disease. Int J Gen Med 2021;14:5167–74. https://doi.org/10.2147/ijgm.s321605.Search in Google Scholar PubMed PubMed Central
9. Kalot, MA, Husainat, N, Abughanimeh, O, Diab, O, El Alayli, A, Tayiem, S, et al.. Laboratory assays of VWF activity and use of desmopressin trials in the diagnosis of VWD: a systematic review and meta-analysis. Blood Adv 2022;6:3735–45. https://doi.org/10.1182/bloodadvances.2021005431.Search in Google Scholar PubMed PubMed Central
10. Bowyer, AE, Shepherd, F, Kitchen, S, Makris, M. A rapid, automated VWF ristocetin cofactor activity assay improves reliability in the diagnosis of Von Willebrand disease. Thromb Res 2011;127:341–4. https://doi.org/10.1016/j.thromres.2010.11.029.Search in Google Scholar PubMed
11. Patzke, J, Budde, U, Huber, A, Méndez, A, Muth, H, Obser, T, et al.. Performance evaluation and multicentre study of a von Willebrand factor activity assay based on GPIb binding in the absence of ristocetin. Blood Coagul Fibrinolysis 2014;25:860–70. https://doi.org/10.1097/mbc.0000000000000169.Search in Google Scholar PubMed
12. Szederjesi, A, Baronciani, L, Budde, U, Castaman, G, Lawrie, A, Liu, Y, et al.. An international collaborative study to compare different von Willebrand factor glycoprotein Ib binding activity assays: the COMPASS-VWF study. J Thromb Haemost 2018;16:1604–13. https://doi.org/10.1111/jth.14206.Search in Google Scholar PubMed PubMed Central
13. Boulassel, MR, Al-Ghonimi, M, Al-Balushi, B, Al-Naamani, A, Al-Qarni, Z, Wali, Y, et al.. Regulatory B cells are functionally impaired in patients having hemophilia A with inhibitors. Clin Appl Thromb Hemost 2018;24:618–24. https://doi.org/10.1177/1076029617702244.Search in Google Scholar PubMed PubMed Central
14. Boulassel, MR, Al-Naamani, A, Al-Zubaidi, A, Al-Qarni, Z, Khan, H, Oukil, A, et al.. Coexistence of sickle cell disease and systemic lupus erythematosus is associated with quantitative and qualitative impairments in circulating regulatory B cells. Hum Immunol 2022;83:818–25. https://doi.org/10.1016/j.humimm.2022.09.005.Search in Google Scholar PubMed
15. Kitchen, S, Jennings, I, Woods, TA, Kitchen, DP, Walker, ID, Preston, FE. Laboratory tests for measurement of von Willebrand factor show poor agreement among different centers: results from the United Kingdom National External Quality Assessment Scheme for Blood Coagulation. Semin Thromb Hemost 2006;32:492–8. https://doi.org/10.1055/s-2006-947863.Search in Google Scholar PubMed
16. Rafdzah, Z, Bulgiba, A, Ismail, NA. Method comparison studies in medicine. J Health Transl Med 2013;16:1–7.10.22452/jummec.vol16no1.4Search in Google Scholar
17. Ng, C, Motto, DG, Di Paola, J. Diagnostic approach to von Willebrand disease. Blood 2015;125:2029–37. https://doi.org/10.1182/blood-2014-08-528398.Search in Google Scholar PubMed PubMed Central
18. Gill, JC, Endres-Brooks, J, Bauer, PJ, Marks, WJJr, Montgomery, RR. The effect of ABO blood group on the diagnosis of von Willebrand disease. Blood 1987;69:1691–5. https://doi.org/10.1182/blood.v69.6.1691.bloodjournal6961691.Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Original Articles
- A comparative study between the Chrono-log 700 and the Sysmex CS-2100i analyzers for assessing ristocetin cofactor activity in patients with von Willebrand disease
- Evaluation of hemolysis index thresholds for 18 biochemistry assays: implications for laboratory-developed tests in the era of the IVDR
- Elevation of plasma podoplanin in Kawasaki disease
- Epidemiology characteristics of Epstein-Barr virus among children in Hangzhou from 2019 to 2023
- Mean platelet volume vs. optical platelet volume measurement: which is a better indicator for neonatal sepsis?
Articles in the same Issue
- Frontmatter
- Original Articles
- A comparative study between the Chrono-log 700 and the Sysmex CS-2100i analyzers for assessing ristocetin cofactor activity in patients with von Willebrand disease
- Evaluation of hemolysis index thresholds for 18 biochemistry assays: implications for laboratory-developed tests in the era of the IVDR
- Elevation of plasma podoplanin in Kawasaki disease
- Epidemiology characteristics of Epstein-Barr virus among children in Hangzhou from 2019 to 2023
- Mean platelet volume vs. optical platelet volume measurement: which is a better indicator for neonatal sepsis?

