Abstract
Objectives
To investigate the diagnostic value of serum soluble endorphin (sENG) combined with BISAP score for severe acute pancreatitis (SAP) complicated with septic shock.
Methods
A total of 150 cases of SAP complicated with sepsis were selected and categorized into the group with shock (n=88) and the group without shock (n=42). The general clinical data and laboratory indexes of the two groups were compared. The factors affecting the occurrence of septic shock were explored, and the correlation between serum sENG, BISAP, APACHEII, and SOFA scores was analyzed. The value of sENG and BISAP scores for diagnosis of SAP complicated with sepsis was assessed.
Results
APACHEII score, SOFA score, BISAP score, and serum sENG levels were higher in the group that developed septic shock. Increased BISAP score and elevated serum sENG level were independent risk factors for septic shock in patients with SAP. sENG level was positively correlated with BISAP score, APACHEII, and SOFA score in patients with SAP-complicated sepsis, and BISAP score was also positively correlated with APACHEII and SOFA score. sENG level and BISAP score had a predictive value for patients with SAP complicated with septic shock (AUC=0.723, 0.703), and the combination of the two had the highest value for the diagnosis of SAP complicated with septic shock (AUC=0.838). In addition, the AUC values of the two in predicting poor prognostic outcomes in patients with SAP complicated with sepsis were 0.757 and 0.706, respectively, and the AUC of the combination was 0.796.
Conclusions
Serum sENG and BISAP scores are predictors of septic shock in patients with SAP, and the combination of the two has a more powerful predictive effect and better evaluation significance.
Introduction
Acute pancreatitis is an inflammatory injury caused by a variety of causes that lead to the digestion of pancreatic tissue and cause pancreatic edema, hemorrhage and necrosis. Acute pancreatitis often has upper abdominal pain and other symptoms as the first manifestation. It has the characteristics of acute onset, rapid progress and high mortality [1]. Systemic inflammatory response syndrome (SIRS) and multiple organ failure (MOF) are one of the mortality factors in severe acute pancreatitis (SAP) [2]. Two weeks after the onset of SAP, sepsis-associated complications caused by SIRS or pancreatic infections or bacterial translocation frequently occur [3]. Although most patients are mild and the course of disease is self-limiting, 20 % of patients develop into severe or necrotizing pancreatitis. These patients are often accompanied by local or systemic complications and persistent organ failure [4]. However, early identification of high-risk patients who may progress to septic shock remains a challenging task. The high cost of treatment, the prevalence of the disease, and the critical nature of the condition urgently require us to find indicators that can provide a rapid assessment and guide prognosis. Currently, clinicians rely on symptoms, signs, and clinical examination for initial assessment, but also incorporate existing scoring systems for further evaluation of patients, including acute physiology and chronic health assessment (APACHE) II [5], Sequential Organ Failure Assessment (SOFA) [6], and qSOFA) [7]. BISAP, a commonly used scoring system in recent years, has the advantages of simplicity, accuracy, sensitivity and specificity [8]. However, no study has applied BISAP to the diagnosis of SAP combined with septic shock.
Endoglin (ENG) is an adhesion molecule located on the surface of endothelial cells and is a subunit of the TGF-β receptor system. Oxidative stress activates the production of sENG, which is considered a marker of endothelial activation/dysfunction [9]. sENG has been shown to be potentially used as a prognostic biomarker in patients with infectious shock [10], 11]. The present study attempted to investigate the diagnostic and prognostic value of sENG and BISAP score in SAP complicated with septic shock.
Materials and methods
General information
The sample size required for the study was calculated by G-power, and α=0.05 and β=0.05 were selected to formulate the test level α and test efficacy 1-β. The effect size was selected as 0.5, the sample size of a single group was calculated to be 105, and the total sample size was predicted to be 111 cases considering that 5 % of the samples might be lost to visit and so on. Finally, 130 patients with SPA complicated with sepsis were included in the present study. A total of 130 cases of SAP complicated with sepsis admitted to Anhua Peoples Hospital from January 2019 to June 2023 were selected, all of which met the diagnostic criteria for SAP and sepsis [2], 12].
Exclusion criteria: Patients with a recent history of anticoagulation or thrombolytic therapy; patients with organic lesions such as heart, liver and kidney; patients with glucocorticosteroids or other immune function-affecting drugs in the 14 days prior to admission; patients with multiple traumas and massive blood loss; patients with psychiatric disorders.
Among the 130 cases of SAP complicated with sepsis, there were 86 males and 44 females; their ages were from 23 to 64 years old (40.86 ± 10.53); body mass index was from 20.0 to 28.8 (23.94 ± 2.06) kg/m2. Patients were divided into two groups according to whether septic shock occurred or not, namely the group without shock (88 cases) and the group with shock (42 cases). All patients were followed up for a period of 30 days. The patients were categorized into survival group (102 patients) and death group (28 patients) according to the outcome of follow-up. The study was executed with the approval and consent of the Medical Ethics Committee of Anhua Peoples Hospital, and the patients and/or their families signed the relevant informed consent forms.
Diagnostic criteria for septic shock
Diagnostic criteria: 1) Clinical manifestations of infection, laboratory evidence (e.g., increased PCT) or imaging evidence. 2) SOFA score ≥2; 3) persistent hypotension despite adequate fluid resuscitation, requirement of antihypertensive drugs to maintain mean arterial pressure above 8.65 kPa, and blood lactate above 2 mmol/L [7].
Research methods
General data such as gender, age, underlying diseases, and triggers of SAP were collected from all included patients. The patients were scored using the APACHEII and SOFA scale to assess the severity of the disease. An APACHEII score of >17 was classified as a severe disease, and the score was positively correlated with the severity of the disease. The SOFA score ranges from 0 to 24, with the higher score suggesting the more severe the condition and the worse the prognosis [13]. BISAP was calculated.
Fasting peripheral venous blood (6 mL) was collected from patients early in the morning of the day after admission, and the serum was separated after centrifugation. Blood amylase and ALT were measured by enzyme-linked immunosorbent assay kits (Sangon, Shanghai, China). CRP was measured by immunoturbidimetric assay kits (BioLab, Beijing, China). TNF-α and IL-6 were determined by radioimmunoassay kits (Multi Science, Hangzhou, China).
Statistical methods
All data were processed and analyzed by SPSS 22.0 statistical software. Enumeration data were expressed as percentages, and differences between groups were compared by the χ2 test. Measurement data were expressed as mean ± standard deviation after normal test, and differences between groups were compared by the t-test. Skewed-distributed measurement data were expressed as medians (25th–75th percentiles) and compared by the Mann-Whitney U test. Post hoc tests (Bonferroni correction) were performed. Multifactorial logistic regression analysis was applied to explore the factors influencing the occurrence of septic shock in patients with SAP. Spearman’s correlation analysis was applied to explore the correlation between sENG levels, BISAP scores, APACHEII, and SOFA scores. ROC curves and the areas under the curve (AUC) were used to explore the value of sENG and BISAP score in the diagnosis of SAP complicated with septic shock. MedCalc software was utilized to compare whether there was a statistical difference in AUC between the different indexes. A p-value <0.05 was considered statistically significant.
Results
General information
The APACHEII score, SOFA score, BISAP score, and sENG level of the group that developed shock were higher than that of the group that did not develop shock (p<0.01). There was no statistically significant difference (p>0.05) in the comparison of biochemical indexes such as gender, age, BMI, underlying diseases, triggers of disease, ALT, and blood amylase between the two groups (Table 1).
Comparison of general information between the two groups with/without shock in SPA.
| Shock group (n=42) | No shock group (n=88) | p-Value | |
|---|---|---|---|
| Age | 4 0.40 ± 11.17 | 4 1.08 ± 1 0.22 | 0.719 |
| Male | 29 (69.05 %) | 57 (64.77 %) | 0.164 |
| BMI | 23.86 ± 2.12 | 24.13 ± 1.93 | 0.471 |
| Fatty liver disease | 15 (40.48 %) | 28 (31.82 %) | 0.311 |
| Coronary heart disease | 8 (19.05 %) | 23 (26.14 %) | 0.45 |
| Diabetes | 7 (16.67 %) | 18 (20.45 %) | 0.518 |
| High blood pressure | 13 (30.95 %) | 26 (29.55 %) | 0.363 |
| Causes of acute pancreatitis | 0.955 | ||
| Biliary origin | 17 (40.17 %) | 36 (40.90 %) | |
| Hyperlipidemic | 8 (19.05 %) | 20 (22.72 %) | |
| Ethanol | 13 (30.95 %) | 25 (28.40 %) | |
| Others | 4 (9.83 %) | 7 (7.98 %) | |
| APACHEII scoring (IQR) | 23 (17–33) | 18 (13–24) | < 0.001 |
| SOFA score (IQR) | 11 (6–18) | 8 (5–11) | < 0.001 |
| BISAP scoring (IQR) | 3.00 (2.00–4.00) | 2.00 (2.00–3.00) | < 0.001 |
| ALT, U/L | 187.21 ± 21.32 | 194.01 ± 17.53 | 0.0563 |
| Blood amylase, U/L | 1,014.47 ± 595.03 | 1,059.38 ± 452.66 | 0.635 |
| CRP, mg/L | 174.56 ± 11.52 | 173.16 ± 14.13 | 0.577 |
| TNF-α, ng/L | 62.14 ± 28.67 | 58.74 ± 32.83 | 0.567 |
| IL-6, ng/L | 114.6 ± 54.32 | 125.64 ± 55.73 | 0.289 |
| sENG, ng/mL | 12.94 ± 1.46 | 11.74 ± 1.09 | < 0.001 |
-
Data are presented as mean ± standard deviation, n (percentage), or IQR. Comparison of data between the two groups was done by Mann Whitney test, t-test or Fisher exact test.
Analysis of factors affecting the occurrence of septic shock in SAP
Multifactorial logistic regression analysis was performed using whether sepsis occurred in SAP patients as the dependent variable (not occurring was assigned a value of 0, and occurring was assigned a value of 1), age as the correction variable, and indicators with statistically significant differences in Table 1 (original values were entered) as the independent variables. The results showed that APACHEII, SOFA score, BISAP score, and sENG were independent risk factors for septic shock in patients with SAP (p<0.05) (Table 2).
Multifactorial logistic regression analysis of factors influencing the occurrence of septic shock in patients with SAP.
| Indicators | OR | 95 % CI | p-Value |
|---|---|---|---|
| APACHEII score | 1.15 | 1.06–1.25 | < 0.001 |
| SOFA score | 1.30 | 1.13–1.49 | < 0.001 |
| BISAP score | 2.79 | 1.20–6.49 | 0.017 |
| sENG | 1.41 | 1.04–2.06 | 0.042 |
Correlation between sENG levels, BISAP scores, APACHEII scores, and SOFA scores in patients with SAP complicated with sepsis
Spearman correlation analysis showed a positive correlation between the sENG levels and BISAP scores, APACHEII and SOFA scores in patients with SAP complicated with sepsis (p<0.001), and BISAP score was also positively correlated with APACHEII and SOFA scores (p<0.001) (Figure 1).
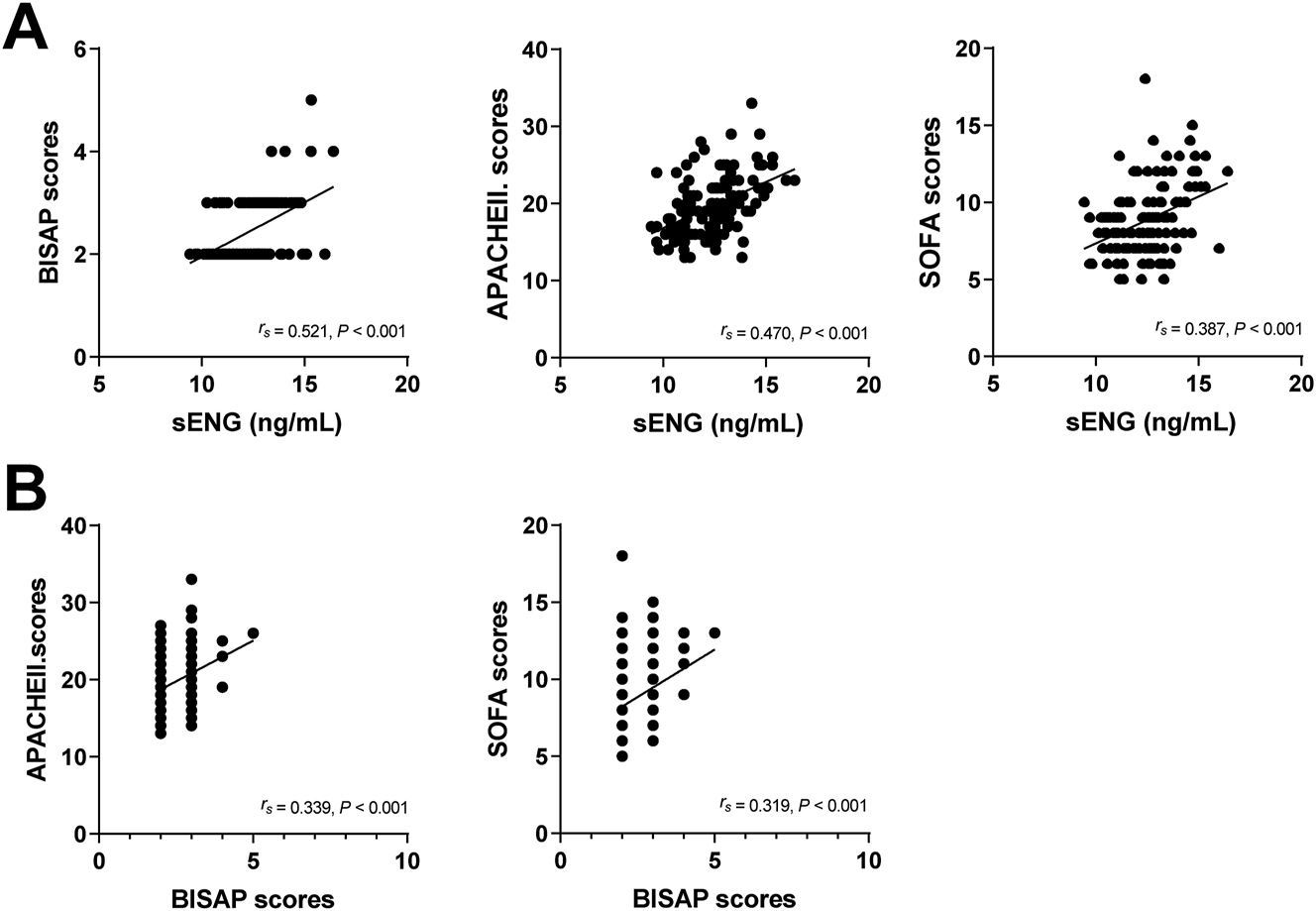
Correlation analysis between sENG levels and BISAP scores, APACHEII scores, and SOFA scores. Correlation analysis between BISAP scores and APACHEII scores and SOFA scores.
Value of sENG level, BISAP score alone and the combination of the two in diagnosing SAP complicated with septic shock
The results of ROC analysis showed that the cut-off values of sENG level and BISAP score for the diagnosis of SAP complicated with septic shock were sENG=13.32 ng/mL and BISAP score=2.5, with the AUC of 0.723 and 0.703 in that order. In addition, the combination of the two had the highest value for the diagnosis of SAP complicated with septic shock, with an AUC of =0.838, as shown in Table 3, Figure 2.
Value of sENG level, BISAP score single and in combination for diagnosis of SAP complicated by septic shock.
| Items | Cut-off value | AUC | 95 % CI | p-Value | Sensitivity | Specificity | False-negative rate | False-positive rate |
|---|---|---|---|---|---|---|---|---|
| sENG | >13.32 ng/mL | 0.723 | 0.622–0.824 | <0.001 | 52.38 % | 88.77 % | 47.62 % | 11.23 % |
| BISAP rating | >2.5 | 0.703 | 0.549–0.758 | 0.0048 | 62.67 % | 70.45 % | 37.33 % | 29.55 % |
| Combined | 0.838 | 0.722–0.895 | <0.001 | 82.52 % | 91.18 % | 17.48 % | 8.82 % |
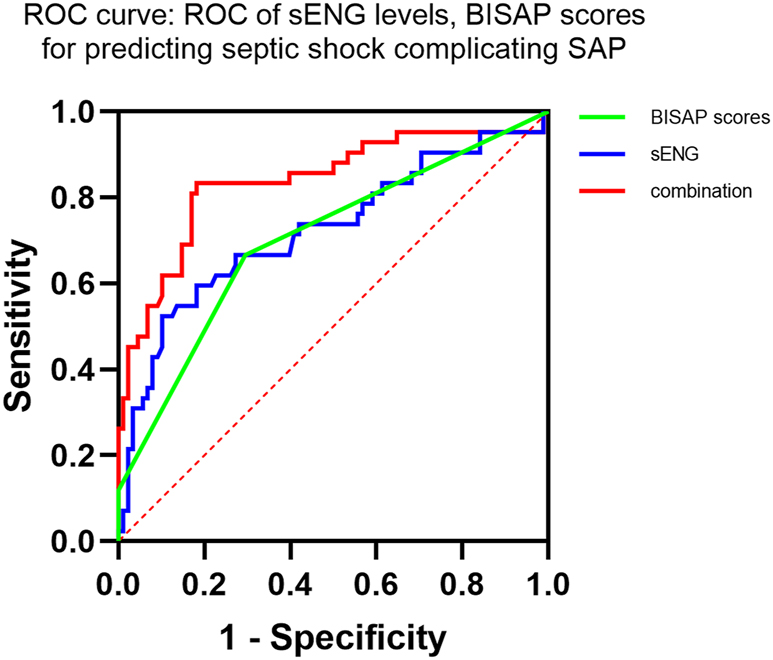
ROC curves of sENG levels and BISAP scores for predicting septic shock in SAP patients.
Prognostic value of sENG level, BISAP score alone and both combined in assessing SAP complicated with septic shock after 30 days
The sENG level and BISAP score of patients in the poor prognosis group were higher than those in the good prognosis group (p<0.05, Table 4). The ROC curve analysis showed that the cut-off values of the sENG level and the BISAP score for predicting the prognosis of the patients with SAP complicated with septic shock were sENG=13.13 ng/mL and BISAP score=2.5, respectively. The AUC values were 0.757 and 0.706, in that order, with the highest value (AUC=0.796) of the combination of the two for diagnosing SAP complicated with septic shock, as shown in Table 5 and Figure 3.
sENG levels, BISAP scores in patients with different prognoses.
| Good prognosis (n=102) | Poor prognosis (n=28) | p-Value | |
|---|---|---|---|
| sENG level | 12.29 (11.06–12.96) | 13.45 (12.41–14.71) | <0.001 |
| BISAP score | 2.0 (2.0–3.0) | 3.0 (2.5–3.0) | <0.001 |
sENG levels, BISAP score alone and the combination of both predict prognosis in patients with SAP complicated by sepsis.
| Indicators | Cut-off value | AUC | 95 % CI | p-Value | Sensitivity | Specificity | False-negative rate | False-positive rate |
|---|---|---|---|---|---|---|---|---|
| sENG | >13.13 ng/mL | 0.757 | 0.648–0.866 | <0.001 | 67.86 % | 80.39 % | 32.14 % | 19.61 % |
| BISAP score | >2.5 | 0.706 | 0.592–0.820 | <0.001 | 75.00 % | 57.84 % | 25.00 % | 42.16 % |
| Combination | 0.796 | 0.697–0.896 | <0.001 | 71.43 % | 79.41 % | 28.57 % | 20.59 % |
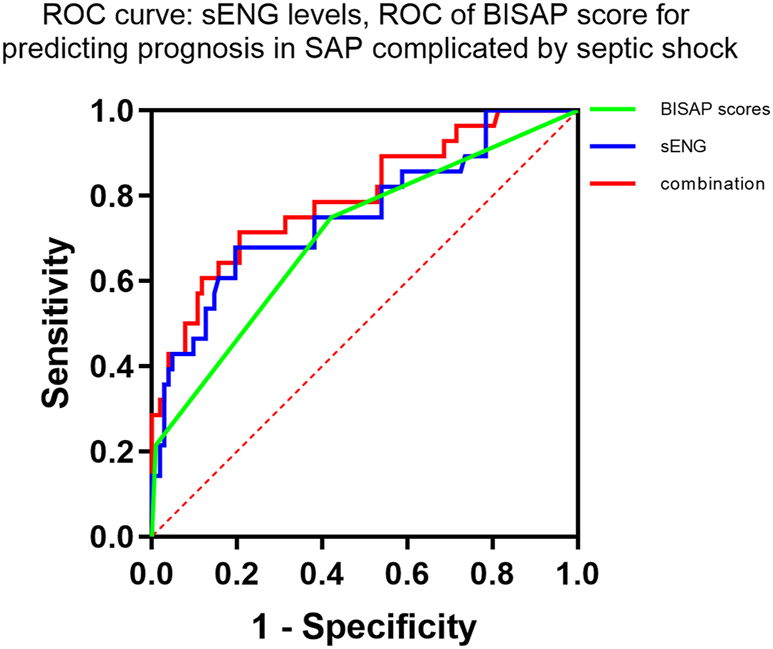
ROC curves of sENG levels and BISAP scores predicting prognosis in SAP complicated by septic shock.
Discussion
Moderately severe or SAP is characterized by rapid progression and high clinical mortality rates [14]. Sepsis is a major complication of SAP [15]. Sepsis may further progress to septic shock, which is characterized by elevated blood lactate levels and persistent hypotension and requires adequate volume resuscitation. The incidence of sepsis/septic shock is increasing and the mortality rate continues to be high [16]. Therefore, predicting the risk of early complications of SAP and carrying out comprehensive treatment are the core means to improve the prognosis of patients.
Multiple scoring systems have been applied to stratify severity and predict early prognosis in patients with SAP, but these scores have limitations [17]. Exploration of blood markers to predict disease and risk of complications in patients with SAP has become an important area of research in recent years. Although many studies have used early infection markers to predict and diagnose abdominal infections and related complications in patients with SAP, there is no single marker that meets the clinical needs [18]. Therefore combining blood markers with scoring systems for diagnosis may help to improve the diagnostic results.
BISAP is a comprehensive assessment of the patient’s condition in five main areas: blood urea nitrogen, Glasgow score, SIRS, age, and pleural exudate [19]. BISAP has the advantages of high sensitivity and specificity, and has the characteristics of easy operation and high accuracy compared with APACHEII. In Hagjer’s study, BISAP scoring system has a better predictive performance than the other scoring systems [20]. sENG has been reported as a blood marker in sepsis patients and has a good predictive value [10], 11], 21]. The validity of both in recognizing SAP complicated with sepsis and predicting the occurrence of septic shock needs to be improved. In this study, the combination of the two was used to predict septic shock in SAP. The results showed that the APACHEII score, SOFA score, BISAP, and sENG levels were higher in patients with SAP who developed shock. BISAP score and serum sENG were independent risk factors for septic shock in patients with SAP.
APACHEII and SOFA scores are now commonly used scoring systems to clinically evaluate the severity of illness in critically ill patients [22]. They are useful for dynamically monitoring the course of organ dysfunction and predicting the prognosis of patients with sepsis [23]. In this study, sENG level was positively correlated with BISAP score, APACHEII and SOFA score in patients with SAP complicated with sepsis, and BISAP score was also positively correlated with APACHEII and SOFA score. It indicates that sENG level and BISAP score also have some value in assessing whether shock occurs in patients with SAP. However, due to the complexity of the condition of patients with SAP complicated with septic shock, the combination of multiple indicators helps to improve the diagnostic efficacy compared with single-indicator diagnosis. The AUC values of sENG level, BISAP score alone and the combination of the two in diagnosing SAP complicated septic shock were 0.723, 0.703 and 0.838 in that order. The AUC values of sENG level and BISAP score for predicting poor prognosis in patients with SAP complicated with septic shock were 0.757 and 0.706, respectively, and the AUC of the two combined was greater, being 0.796.
This study, however, faces limitations like overestimating the effect size, which resulted in a small sample size that failed to achieve sufficient test efficacy, thereby decreasing the study’s reliability and accuracy. In addition, this study was a single-center study and lacked external validation. As an acute and severe condition, SAP has timely treatment methods. This research did not exclude the influence of prompt treatment on sENG levels, did not track the dynamic changes in sENG levels, and failed to explore the role of serum sENG in the progression of the disease. In addition, sepsis is a very heterogeneous syndrome, and we could not validate its individual effects. Therefore, the results of this study need to be further justified by expanding the sample size and conducting multicenter studies.
Conclusions
sENG level and BISAP have certain guiding significance for the condition and prognosis of SAP patients, but when the two are used in combination, the AUC value increases, the prediction effect is better.
-
Research ethics: All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All subjects were approved by Anhua Peoples Hospital (No.201712HN13).
-
Informed consent: Informed consent was obtained from all individuals included in this study, or their legal guardians or wards.
-
Author contributions: Conceptualization, Jinbei Yuan and Qiubao Yang; methodology, Jinbei Yuan; formal analysis, Qiubao Yang; investigation, Chao Chen; data curation, Chao Chen; writing – original draft preparation, Jinbei Yuan and Qiubao Yang; writing – review and editing, Chao Chen. All authors have read and agreed to the published version of the manuscript.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: The raw data can be obtained on request from the corresponding author.
References
1. Garg, PK, Singh, VP. Organ failure due to systemic injury in acute pancreatitis. Gastroenterology 2019;156:2008–23. https://doi.org/10.1053/j.gastro.2018.12.041.Suche in Google Scholar PubMed PubMed Central
2. Italian Association for the Study of the Pancreas, Pezzilli, R, Zerbi, A, Campra, D, Capurso, G, Golfieri, R, et al.. Consensus guidelines on severe acute pancreatitis. Dig Liver Dis 2015;47:532–43. https://doi.org/10.1016/j.dld.2015.03.022.Suche in Google Scholar PubMed
3. Zhang, DL, Zheng, HM, Yu, BJ, Jiang, ZW, Li, JS. Association of polymorphisms of IL and CD14 genes with acute severe pancreatitis and septic shock. World J Gastroenterol 2005;11:4409–13. https://doi.org/10.3748/wjg.v11.i28.4409.Suche in Google Scholar PubMed PubMed Central
4. Greenberg, JA, Hsu, J, Bawazeer, M, Marshall, J, Friedrich, JO, Nathens, A, et al.. Clinical practice guideline: management of acute pancreatitis. Can J Surg 2016;59:128–40. https://doi.org/10.1503/cjs.015015.Suche in Google Scholar PubMed PubMed Central
5. Knaus, WA, Draper, EA, Wagner, DP, Zimmerman, JE. Apache II: a severity of disease classification system. Crit Care Med 1985;13:818–29. https://doi.org/10.1097/00003246-198510000-00009.Suche in Google Scholar
6. Vincent, JL, Moreno, R, Takala, J, Willatts, S, De Mendonca, A, Bruining, H, et al.. The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Intensive Care Med 1996;22:707–10. https://doi.org/10.1007/bf01709751.Suche in Google Scholar
7. Singer, M, Deutschman, CS, Seymour, CW, Shankar-Hari, M, Annane, D, Bauer, M, et al.. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315:801–10. https://doi.org/10.1001/jama.2016.0287.Suche in Google Scholar PubMed PubMed Central
8. Karabuga, B, Gemcioglu, E, Konca Karabuga, E, Baser, S, Ersoy, O. Comparison of the predictive values of CRP, CRP/albumin, RDW, neutrophil/lymphocyte, and platelet/lymphocyte levels in determining the severity of acute pancreatitis in patients with acute pancreatitis according to the BISAP score. Bratisl Lek Listy 2022;123:129–35. https://doi.org/10.4149/BLL_2022_020.Suche in Google Scholar PubMed
9. Kumar, S, Pan, CC, Bloodworth, JC, Nixon, AB, Theuer, C, Hoyt, DG, et al.. Antibody-directed coupling of endoglin and MMP-14 is a key mechanism for endoglin shedding and deregulation of TGF-beta signaling. Oncogene 2014;33:3970–9. https://doi.org/10.1038/onc.2013.386.Suche in Google Scholar PubMed PubMed Central
10. Helan, M, Malaska, J, Tomandl, J, Jarkovsky, J, Helanova, K, Benesova, K, et al.. Kinetics of biomarkers of oxidative stress in septic shock: a pilot study. Antioxidants 2022;11. https://doi.org/10.3390/antiox11040640.Suche in Google Scholar PubMed PubMed Central
11. Tomaskova, V, Mytnikova, A, Hortova Kohoutkova, M, Mrkva, O, Skotakova, M, Sitina, M, et al.. Prognostic value of soluble endoglin in patients with septic shock and severe COVID-19. Front Med 2022;9:972040. https://doi.org/10.3389/fmed.2022.972040.Suche in Google Scholar PubMed PubMed Central
12. Rhodes, A, Evans, LE, Alhazzani, W, Levy, MM, Antonelli, M, Ferrer, R, et al.. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017;43:304-77, https://doi.org/10.1007/s00134-017-4683-6.Suche in Google Scholar PubMed
13. Dorooshi, G, Samsamshariat, S, Gheshlaghi, F, Zoofaghari, S, Hasanzadeh, A, Abbasi, S, et al.. Comparing sequential organ failure assessment score, acute physiology and chronic health evaluation II, modified acute physiology and chronic health evaluation II, simplified acute physiology score II and poisoning severity score for outcome prediction of pesticide poisoned patients admitted to the intensive care unit. J Res Pharm Pract 2023;12:49–57. https://doi.org/10.4103/jrpp.jrpp_43_23.Suche in Google Scholar PubMed PubMed Central
14. De Waele, E, Malbrain, M, Spapen, HD. How to deal with severe acute pancreatitis in the critically ill. Curr Opin Crit Care 2019;25:150–6. https://doi.org/10.1097/mcc.0000000000000596.Suche in Google Scholar
15. Rau, BM, Kemppainen, EA, Gumbs, AA, Buchler, MW, Wegscheider, K, Bassi, C, et al.. Early assessment of pancreatic infections and overall prognosis in severe acute pancreatitis by procalcitonin (PCT): a prospective international multicenter study. Ann Surg 2007;245:745–54. https://doi.org/10.1097/01.sla.0000252443.22360.46.Suche in Google Scholar PubMed PubMed Central
16. Hotchkiss, RS, Moldawer, LL, Opal, SM, Reinhart, K, Turnbull, IR, Vincent, JL. Sepsis and septic shock. Nat Rev Dis Primers 2016;2:16045. https://doi.org/10.1038/nrdp.2016.45.Suche in Google Scholar PubMed PubMed Central
17. Wan, Z, Shen, B, Cen, D, Yu, H, Cai, X. Minimally invasive treatment for severe acute pancreatitis with superior mesenteric vein and common bile duct stenosis: a case report and review of the literature. Pancreas 2019;48:e61–3. https://doi.org/10.1097/mpa.0000000000001379.Suche in Google Scholar
18. Huang, H, Chen, W, Tang, G, Liang, Z, Qin, M, Qin, M, et al.. Optimal timing of contrast-enhanced computed tomography in an evaluation of severe acute pancreatitis-associated complications. Exp Ther Med 2019;18:1029–38. https://doi.org/10.3892/etm.2019.7700.Suche in Google Scholar PubMed PubMed Central
19. Arif, A, Jaleel, F, Rashid, K. Accuracy of BISAP score in prediction of severe acute pancreatitis. Pak J Med Sci 2019;35:1008–12. https://doi.org/10.12669/pjms.35.4.1286.Suche in Google Scholar PubMed PubMed Central
20. Hagjer, S, Kumar, N. Evaluation of the BISAP scoring system in prognostication of acute pancreatitis - a prospective observational study. Int J Surg 2018;54:76–81. https://doi.org/10.1016/j.ijsu.2018.04.026.Suche in Google Scholar PubMed
21. Atreya, MR, Cvijanovich, NZ, Fitzgerald, JC, Weiss, SL, Bigham, MT, Jain, PN, et al.. Serum soluble endoglin in pediatric septic shock-associated multiple organ dysfunction syndrome. Shock 2023;60:379–84. https://doi.org/10.1097/shk.0000000000002183.Suche in Google Scholar
22. Cuenca Fito, E, Gonzalez-Castro, A, Gomez Acebo, I. Predictive value of the APACHEII and SOFA scales in the mortality of cancer patients in intensive care. Med Clin 2023;161:547–8. https://doi.org/10.1016/j.medcle.2023.11.003.Suche in Google Scholar
23. Siregar, GA, Siregar, GP. Management of severe acute pancreatitis. Open Access Maced J Med Sci 2019;7:3319–23. https://doi.org/10.3889/oamjms.2019.720.Suche in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Review
- Contact phase inhibitors: the future of anticoagulation?
- Original Articles
- Validation and implementation of an intraoperative parathyroid hormone assay and workflow: practical advice for endocrine surgery centres
- Serum soluble endorphin combined with BISAP score predicts severe acute pancreatitis combined with septic shock
- Serum LDH and its isoenzymes (LDH2 and LDH5) associated with predictive value for refractory mycoplasma pneumoniae pneumonia in children
- Upregulation of hsa_circ_0000745/hsa_circRNA_101996 in peripheral blood monocytes is associated with coronary heart disease
- Congress Report
- Congress report: 6th German POCT symposium, September 25–26, 2024, Bremen
- Images from the Medical Laboratory
- Gelatinous urine
Artikel in diesem Heft
- Frontmatter
- Review
- Contact phase inhibitors: the future of anticoagulation?
- Original Articles
- Validation and implementation of an intraoperative parathyroid hormone assay and workflow: practical advice for endocrine surgery centres
- Serum soluble endorphin combined with BISAP score predicts severe acute pancreatitis combined with septic shock
- Serum LDH and its isoenzymes (LDH2 and LDH5) associated with predictive value for refractory mycoplasma pneumoniae pneumonia in children
- Upregulation of hsa_circ_0000745/hsa_circRNA_101996 in peripheral blood monocytes is associated with coronary heart disease
- Congress Report
- Congress report: 6th German POCT symposium, September 25–26, 2024, Bremen
- Images from the Medical Laboratory
- Gelatinous urine


