Validation and implementation of an intraoperative parathyroid hormone assay and workflow: practical advice for endocrine surgery centres
-
Phillip Staibano
, Basma Ahmed
Abstract
Objectives
Intraoperative PTH (IOPTH) can be challenging to offer through central laboratories despite its clinical benefit. We describe the implementation of a central laboratory-based IOPTH assay and workflow in a tertiary care centre.
Methods
The Elecsys® PTH STAT assay was assessed in EDTA plasma on the Cobas® e411 analyzer. Assay validation included precision, linearity, coefficient of variation (CV), accuracy, stability, and dilution. Samples were transported to the central laboratory and resulted via telephone to the operating room. We describe a case series of patients with primary hyperparathyroidism (PHPT) who underwent parathyroid surgery using our described IOPTH workflow.
Results
Within- and between-day CV was ≤3.0 % for quality control material that ranged from 2.2–44.6 pmol/L. Passing–Bablok regression yielded a slight proportional negative bias between the two Cobas e411 instruments [Elecsys® PTH our centre=0.95 (95 % CI: 0.90–1.00) × Elecsys® PTH Toronto − 0.05 (95 % CI: −0.20 to 0.09) (n=22)], but high correlation (r=0.99) as compared to PTH measured on the Vitros® XT 7600 analyzer [Elecsys® PTH=0.91 (95 % CI: 0.73–1.1) × Vitros® PTH + 0.1 (95 % CI: −0.34 to 0.76), r=0.96 (n=40)]. The mean operating time across ten patients surgically cured for PHPT was 47.1 min (±9.1) and no patients required intraoperative frozen tissue analysis.
Conclusions
The Elecsys® PTH STAT assay demonstrated acceptable analytical performance, and the described IOPTH workflow was implemented successfully via a collaborative hospital-wide initiative. We discuss our model to help guide other institutions in implementing and improving IOPTH workflows.
Introduction
In the United States, primary hyperparathyroidism (PHPT) has an age-adjusted prevalence of 233 per 100,000 women and 85 per 100,000 men [1]. It is associated with osteoporosis, nephrolithiasis, fatigue, constipation, and/or neurocognitive changes [2]. Surgical extirpation of hyperfunctioning parathyroid tissue is required to achieve cure in PHPT. Over 30 years ago, Irvin and colleagues developed intraoperative parathyroid hormone (IOPTH) monitoring, which harnesses the short half-life of parathyroid hormone (PTH) to guide surgeons during parathyroid gland resection [3]. These assays have been adopted by a number of endocrine surgery centres internationally, but challenges remain in terms of optimizing IOPTH sample processing and turnaround time (TAT) and promoting institutional adoption [4]. In 2016, the American Association of Endocrine Surgeons recommended IOPTH monitoring to improve surgical success when performing minimally invasive parathyroidectomy for localized PHPT [5]. Commercially available PTH immunoassays that target “intact” PTH (amino acids 1–84) have shown improvements in their TAT with modern assays demonstrating an analytical time of 10–20 min, but this does not account for time delays secondary to sample transport and result reporting [6]. Central laboratory-based IOPTH assays have also been shown to be more cost-effective when compared to point-of-care IOPTH tests, but this remains somewhat controversial [6], [7], [8], [9]. Turnaround time in central laboratory-based IOPTH assays, however, can be prolonged due to the lack of on-site facilities, delays in processing and assay run-length, and/or a lack of dedicated laboratory staff [6]. As central laboratory-based IOPTH assays must account for added personnel, coordination between surgical and laboratory teams, and feasibility challenges, it is imperative that an efficient workflow exist to standardize IOPTH assay performance and therefore, broaden the worldwide adoption of this recommended intraoperative adjunct. The goals of this study were (1) to implement a central laboratory-based IOPTH monitoring assay; (2) to validate the analytical measuring range and introduce a novel dilutional protocol for analyzing IOPTH levels elevated beyond the upper analytical limit of the assay; (3) to describe an IOPTH workflow that can be adopted by other institutions.
Materials and methods
Study sites and ethics
St. Joseph’s Healthcare Hamilton (SJHH) (Hamilton, Ontario, Canada) is a tertiary care endocrine surgery centre that serves an estimated 100–125 patients per year with all forms of hyperparathyroidism and is uniquely positioned for managing secondary and tertiary hyperparathyroidism due to its high-volume nephrology and transplantation programs that serve the Greater Toronto and Hamilton area, which has a population of over seven million people. The Toronto General Hospital (TGH) (Toronto, Ontario, Canada), which helped support this study and is also a tertiary care endocrine surgery centre that further serves the Greater Toronto area, which has a population of over 5.5 million people. This study received ethics approval via the Hamilton Integrated Research Ethics Board (2021-13875-C).
Elecsys® STAT intraoperative parathyroid hormone assay
We used the third-generation Elecsys® PTH STAT assay (Roche Diagnostics, Indianapolis, Indiana, USA) to measure IOPTH levels using the Cobas® e411 analyzer (Roche Diagnostics, Indianapolis, Indiana, USA), which was installed in the central laboratory at SJHH. Third-generation PTH assays have shown similar precision and correlated well with second-generation assays [10]. Elecsys® PTH STAT is an electrochemiluminescence sandwich immunoassay that determines the levels of intact PTH in human serum and EDTA plasma. A sandwich is formed using a capturing biotinylated monoclonal antibody the binds to the N‐terminal fragment (1–37) of PTH and a monoclonal detection antibody labelled with a ruthenium complex that binds to the C‐terminal fragment (38–84) of PTH. The antibodies used in this assay are reactive with epitopes in the amino acid regions 26–32 and 37–42 of PTH per the manufacturer [10].
Calibration verification, linearity, reportable ranges, and precision
We used five levels of Maine standards (Cumberland Foreside, Maine, USA) with assigned values of 0.60, 128.7, 256.7, 384.8 and 512.9 pmol/L. These were tested in triplicate to assess IOPTH assay calibration, linearity, and reportable ranges. The percent difference from the assigned value for each level was calculated for calibration verification. We used allowable non-linearity limits of 0.66 pmol/L or 6.6 % for IOPTH levels <10.0 pmol/L and ≥10.0 pmol/L, respectively, which reflected one third of the total error allowable for this analyte per the Institute for Quality Management in Healthcare (IQMH) (i.e., 2 pmol/L or 20 % limits per IQMH, Toronto, Ontario, Canada). Three Bio-Rad (Hercules, California, USA) quality control (QC) materials were employed: low (lot no. 64961), normal (lot no. 64962), and high (lot no. 64963). Within-day precision was assessed by repeated measurement of three QC levels (i.e., 20 times per level). Between-day precision was evaluated by measuring each level twice daily over 10 days. Imprecision was calculated as the coefficient of variation (CV, expressed as a percentage). We used IQMH precision goals of 0.66 pmol/L and 6.6 % for IOPTH levels <10.0 pmol/L and ≥10.0 pmol/L, respectively.
Limit of detection, functional sensitivity and dilution protocol
To validate the assay’s limit of detection (LOD) of 0.13 pmol/L, we tested IOPTH in water and saline as needed to first determine the limit of the blank. The functional sensitivity of the assay was the lowest analyte concentration that could be reproducibly measured with a precision CV of ≤20 %. To verify the functional sensitivity (i.e., 0.640 pmol/L), we prepared two pools of patient EDTA plasma samples with low PTH levels (i.e., above and below 0.640 pmol/L). Each pool was tested ten times to assess SD and CV. Similar pools of plasma EDTA with measured IOPTH concentrations of 0.384 and 0.629 pmol/L were used to dilute level five of the Maine standard to verify the dilution protocol for samples with IOPTH >500 pmol/L. There is no protocol nor diluent listed for the Elecsys® PTH STAT assay as stated by the instructions for use (IFU): “Dilution not necessary due to the broad measuring range”. As such, we performed manual dilutions with a low pool material as described above with a 1:1 dilution of high patient sample to low patient sample to establish a process for reporting values initially reported as >500 pmol/L.
Relative bias assessment
To verify our accuracy, we tested 22 frozen (i.e., below −20 °C for <3 months) patient EDTA plasma samples for PTH on a Cobas® e411 analyzer used at SJHH. These samples were previously tested using a Cobas® e411 analyzer at TGH and analyzed via Passing–Bablok regression, Pearson correlation, difference plot, and Bland–Altman with 95 % limits of agreement. Additionally, 40 EDTA plasma samples (i.e., stored at 2–8 °C for <1 day) were re-centrifuged, aliquoted, and tested for PTH in duplicate first on the Cobas® e411 analyzer and then immediately on the Vitros® XT 7600 analyzer (Ortho Clinical Diagnostics, Raritan, New Jersey, USA) at SJHH. Agreement was assessed using total allowable error estimates for PTH from IQMH (i.e., ±2 pmol/L or ±20 % for IOPTH levels <10.0 pmol/L and ≥10.0 pmol/L, respectively).
Parathyroid hormone stability
Parathyroid hormone levels were measured in four samples on Cobas® e411 and Vitros® XT 7600 analyzers at baseline and after 3 days of storage at 2–8 °C (i.e., plasma was not separated from blood cells). We then calculated the differences in PTH levels comparing the pre- and post- 3-day values from refrigerated samples to baseline values. The Elecsys® PTH STAT IFU stated that at 2–8 °C, the analyte is stable for 3 days whereas the Ortho Clinical Diagnostics PTH IFU stated that at this temperature the analyte is stable for 2 days. The samples from TGH were frozen for 1 week at −20 °C before testing.
Intraoperative parathyroid hormone processing workflow and case series
All workflows were developed by surgical and laboratory personnel with input provided from corresponding personnel at outside centres. We summarize and present the operational challenges with developing this IOPTH workflow and provide practical steps that can further help optimize its performance and adoption at other centres. We also reported the IOPTH results and outcomes of the first ten patients evaluated using this IOPTH protocol. We included adult patients (≥18 years old) diagnosed with PHPT undergoing any type of parathyroid surgery. All patients underwent localizing imaging prior to surgery using either ultrasonography and parathyroid scintigraphy, or computed tomography. We presented preoperative, intraoperative, and postoperative PTH levels (measured in pmol/L), in addition to final surgical pathology for all patients. We also reported the mean operating time and sample turnaround time (TAT) [and standard deviation (SD)] in minutes. Operating time was measured as the time from skin incision to skin closure. Turnaround time was measured from the time of blood draw to result reporting. We deemed cure as normalized serum PTH and normocalcemia six months following surgery [5]. We performed descriptive analyses to describe biochemical and clinical outcomes and 95 % confidence intervals (95 % CI), where appropriate.
Results
Imprecision and analytical measuring range
The Elecsys® IOPTH assay demonstrated linearity from 505 pmol/L to 0.8 pmol/L (Figure 1). Within-and between-day imprecision CV were ≤3 % for QC material with values from 2.45 pmol/L to 44.56 pmol/L. We were not able to validate the LOD (0.13 pmol/L) as the assay showed non-specific signals with water (1.51 pmol/L) and saline (1.29 pmol/L) when measured and thus we were unable to also calculate the limit of blank, which is needed to calculate the LOD. However, using pooled EDTA samples we obtained an imprecision of 6 and 3 % at concentrations of 0.54 and 0.71 pmol/L. A 1:1 dilution of the Maine standard (i.e., 505 pmol/L) with pooled patient samples with low PTH level (i.e., 0.38 pmol/L) yielded IOPTH of 250.8 pmol/L when corrected for dilution [i.e., (2 × 250.8)–0.38] yielded as result of 501 pmol/L (i.e., >99 % recovery from the established value of 505 pmol/L).
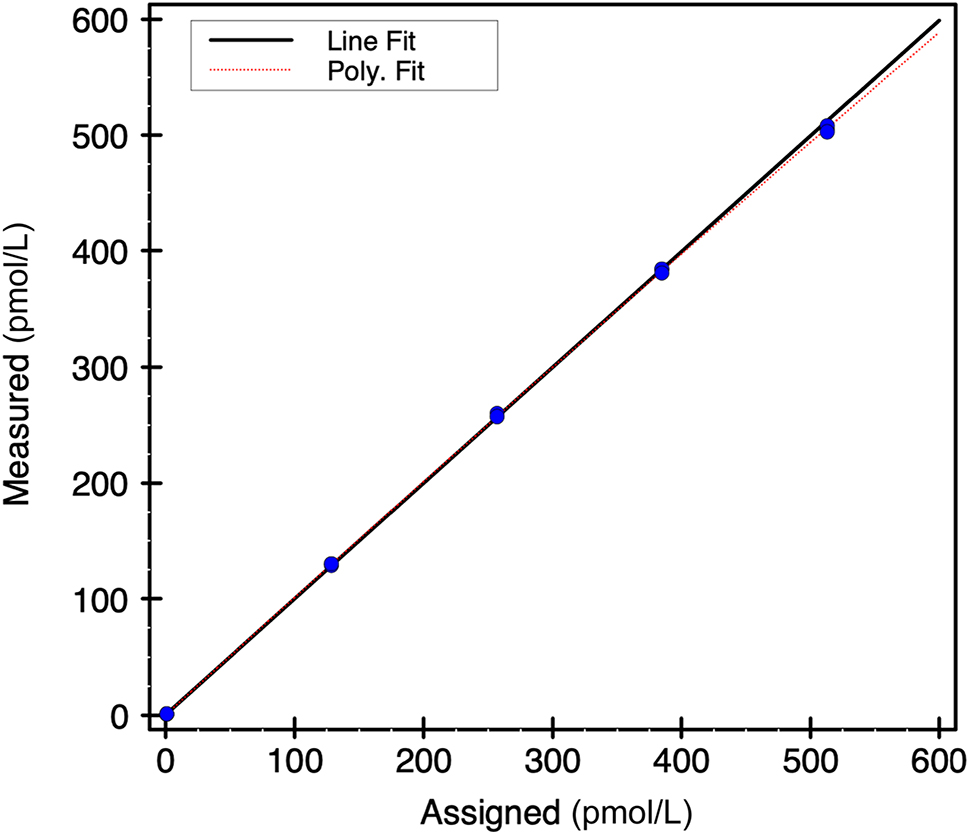
Elecsys® intraoperative parathyroid hormone assay linearity.
Instrument and method comparison
Passing–Bablok regression showed a slight proportional bias of approximately 5 % (i.e., near the observed imprecision of the assay) between the Cobas e411 instruments [SJHH Roche PTH=0.95 (95 % CI: 0.90, 0.99) × TGH Roche PTH − 0.05 (95 % CI: −0.20, 0.09)] (Figure 2). This was confirmed with difference plots (mean % bias=−5.2 %, 95 % CI: −7.8, −2.6) and Bland–Altman analyses demonstrating a mean difference of −5.5 % with 95 % limits of agreement being −17.6–6.5 % and a high correlation between instruments (r=0.998). The method comparison between Roche PTH and Ortho PTH was difficult to assess due to lower correlation (r=0.959) between these methods [SJHH Roche PTH=0.91 (95 % CI: 0.73–1.1) × SJHH Ortho PTH: + 0.1 (95 % CI: −0.34 to 0.76)] (Figure 3).
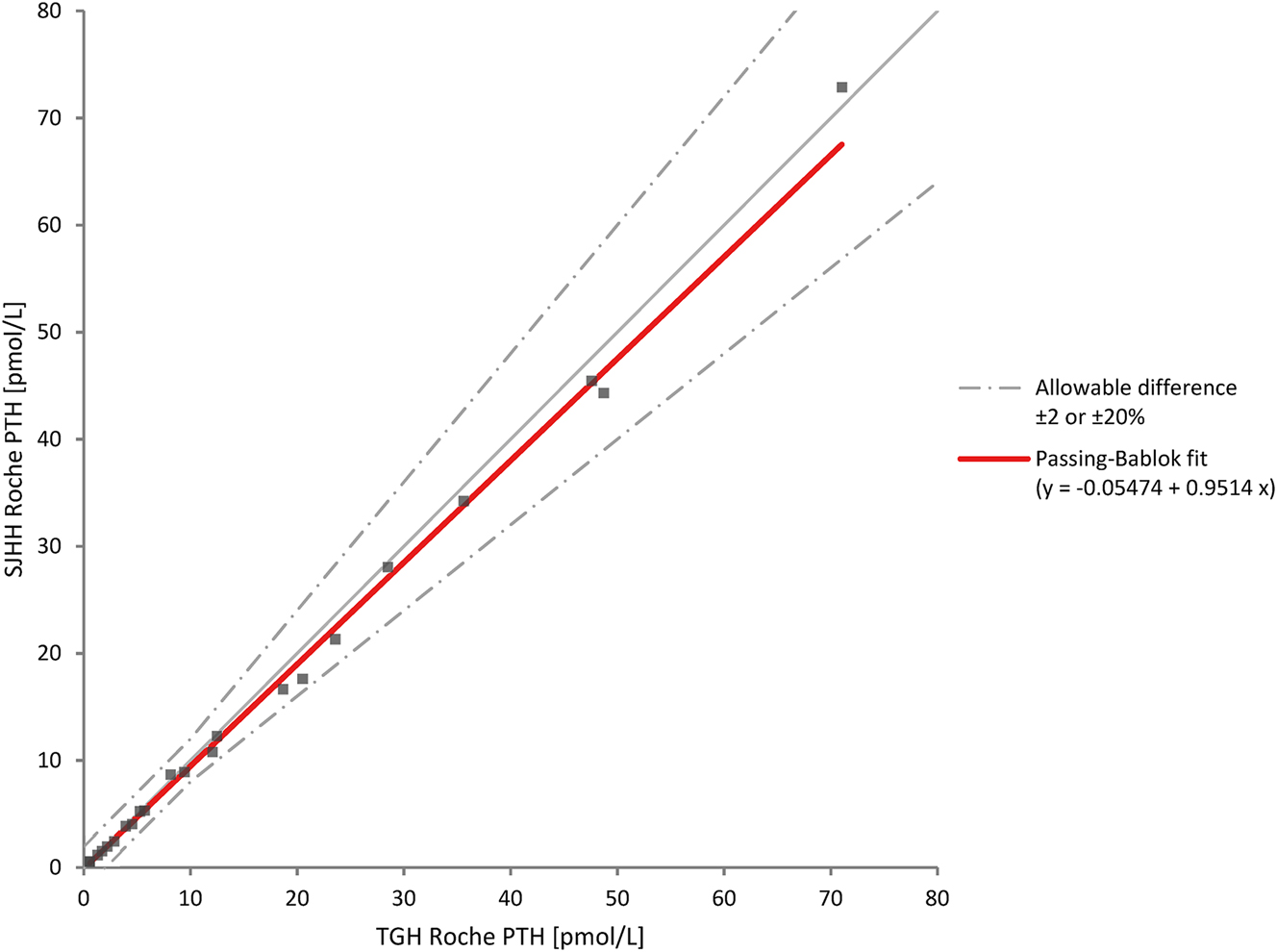
Method comparison of intraoperative parathyroid hormone assays between two Roche Diagnostics Cobas® e411 instruments. PTH, parathyroid hormone; SJHH, St. Joseph’s Hospital Hamilton; TGH, Toronto General Hospital.
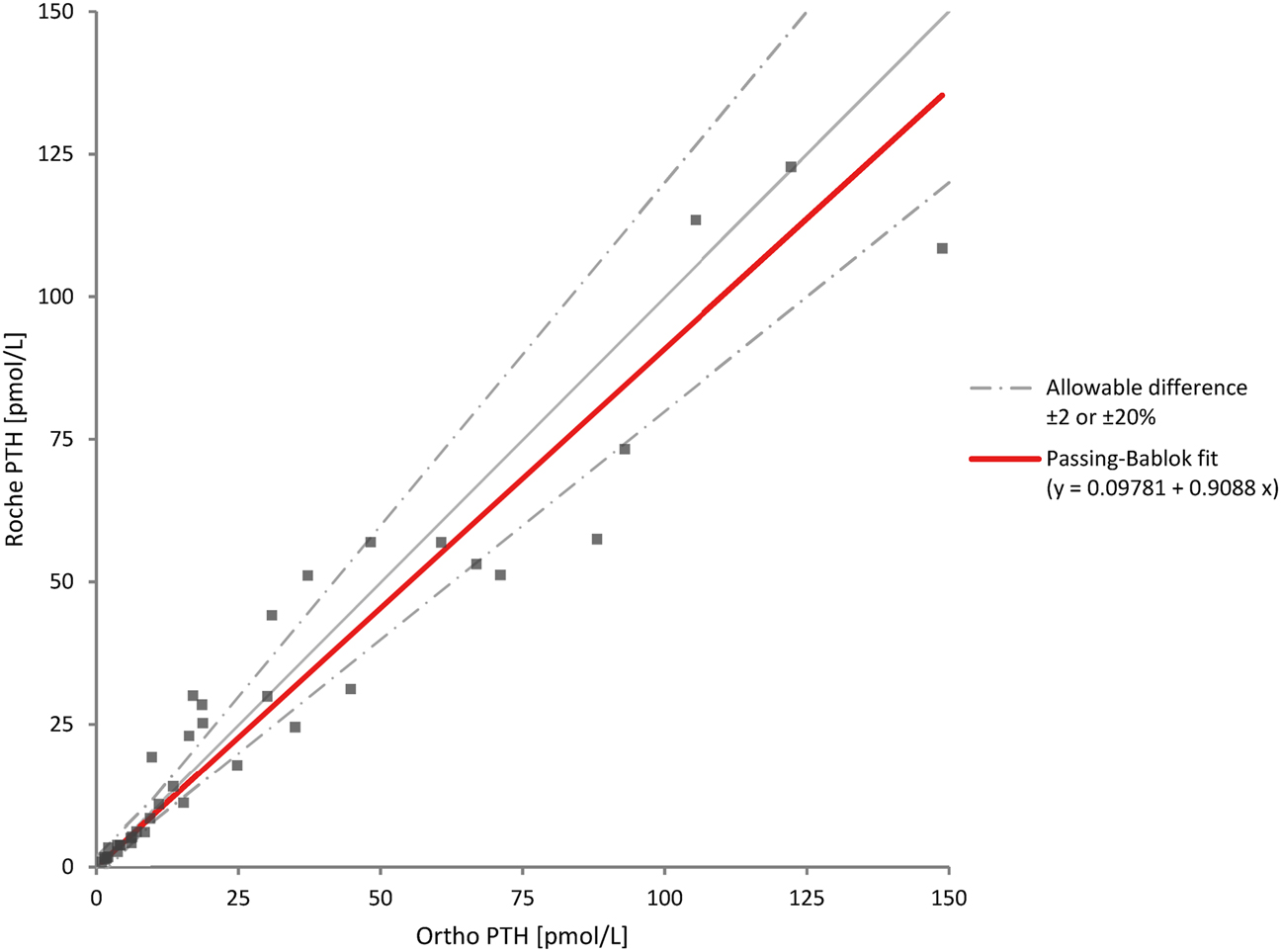
Method comparison of parathyroid hormone measured by Roche Cobas e411 at Ortho Vitros XT 7600 at our centre. PTH, parathyroid hormone.
Parathyroid hormone stability
Four different samples (high to low concentrations) were tested at baseline (Elecsys® PTH: 33.0 pmol/L, 16.3 pmol/L, 7.2 pmol/L, 6.0 pmol/L and Ortho Vitros® 31.5 pmol/L, 14.8 pmol/L, 6.1 pmol/L, 5.5 pmol/L) and then after 3 days of storage at 2–8 °C. For the two high concentrations (>10 pmol/L) the percent difference ranged from −3 % to −11 % for the Elecsys® PTH assay, which fall below the 20 % total error goal; however, for the Ortho Vitros® PTH assay the differences were −23 % to −26 %. For the two lower concentrations the differences ranged from −6 % to −13 % (Table 1).
Stability of parathyroid hormone measured on Roche Cobas e411 and Ortho Vitros XT 7600.
| Roche Cobas e411 | Ortho Vitros XT 7600 | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 1 | Sample 2 | Sample 3 | Sample 4 | |
| Baseline PTH, pmol/L | 33.0 | 16.3 | 7.2 | 6.0 | 31.5 | 14.8 | 6.1 | 5.5 |
| Day 3 (2–8 °C) PTH, pmol/L | 29.3 | 15.8 | 6.8 | 5.2 | 24.2 | 11.0 | 5.7 | 4.8 |
| Difference absolute, pmol/L (%) | −3.7 (−11 %) | −0.5 (−3 %) | −0.4 (−6 %) | −0.8 (−13 %) | −7.3 (−23 %) | −3.8 (−26 %) | −0.4 (−7 %) | −0.7 (−13 %) |
-
PTH, parathyroid hormone.
Intraoperative parathyroid hormone assay workflow and case series
Before we implemented IOPTH at our centre, all parathyroid surgery was aided by use of intraoperative frozen tissue analysis (IFTA). An internal projection identified that transition to using IOPTH would minimize hospital costs and alleviate the pathology sample backlog compounded by the COVID-19 pandemic. We decided to place the Cobas® e411 analyzer in the central laboratory at SJHH, which is located two floors above the operating room. At a casual walking velocity, it takes approximately 140 s to transport samples from the operating suite to the analyzer. We outline in Figure 4 the scheduling, transport, and processing steps required to facilitate open communication between teams and minimize TAT for IOPTH samples. At our centre, the central laboratory immediately reports IOPTH to the operating room via telephone but does not provide calculations of the IOPTH reduction. We report the IOPTH assay results and clinical outcomes for the first 10 consecutive parathyroid surgery patients (Table 2). All patients were diagnosed with PHPT and most underwent minimally invasive parathyroidectomy for a localized single parathyroid adenoma. Our centre uses the Miami IOPTH criteria to declare intraoperative cure and surgical case completion [11]. In this cohort, all patients were diagnosed with localized PHPT and did meet the Miami criteria at 10- or 15-min post-gland excision timepoints. Based on personal preference, surgeons used central or peripheral venous sampling for obtaining IOPTH samples, but this has not been shown to impact relative IOPTH kinetics [12]. The mean time from skin incision to closure (i.e., operating time) was 47.1 min (SD: 9.1 min). The TAT between IOPTH samples ranged from 9–31 min across all included patients with the longest time discrepancy occurring between the baseline and “at excision” IOPTH samples. The mean IOPTH TAT (i.e., from IOPTH sample draw to result reporting) between samples was 9.8 min (SD: 6.7 min). All patients with adequate data entry met criteria for cure at six months and final pathology for all patients was parathyroid adenoma. None of these patients required intraoperative frozen tissue analysis (IFTA) to guide parathyroid exploration. None of the presented patients required revision parathyroid surgery. In contrast, five consecutive parathyroid surgeries performed for localized PHPT prior to implementing our IOPTH program required a mean of 1.6 IFTA samples (range: 0–3 samples) and a mean time operating time of 67.4 min (SD: 19 min).
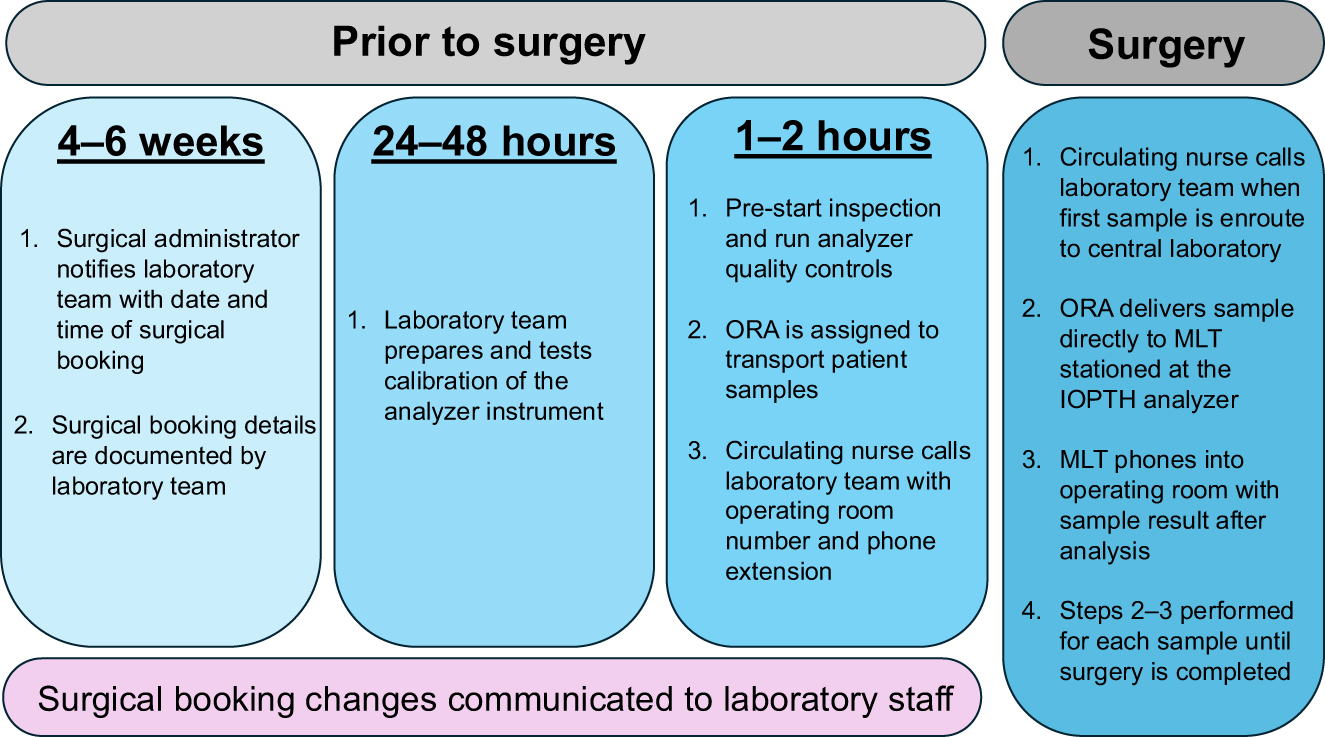
Intraoperative parathyroid assay workflow at our centre. MLT, medical laboratory technologist; ORA, operating room attendant.
Intraoperative parathyroid hormone assay results for first consecutive ten patients.
| Patient number | Sex | Age | Diagnosis | Imaging results | Preoperative corrected calcium, mmol/L | Type of surgery | IOPTH level, pmol/L | Operation time, minutes | Frozen section analysis | PTH at 6 months, pmol/L | Corrected calcium at 6 months, mmol/L | Clinical cure at 6 months | Final pathology | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prior to surgery | Baseline | At gland excision | 10 min after gland excision | 15 min after gland excision | 4 h after surgery | |||||||||||||
| 1 | Male | 67 | PHPT | Single parathyroid adenoma | 2.6 | MIP | 44 | 33 | 16.3 | 7.2 | 6 | 4.3 | 55 | No | 3.8 | 2.4 | Yes | Parathyroid adenoma |
| 2 | Female | 54 | PHPT | Single parathyroid adenoma | 2.7 | MIP | 16.6 | 21.0 | 4.4 | 2.0 | 2.1 | 0.5 | 49 | No | 2.2 | 2.4 | Yes | Parathyroid adenoma |
| 3 | Male | 74 | PHPT | Single parathyroid adenoma | 3.0 | MIP | 34.6 | >>500 (initial); 774 (after dilution) | 31.9 | 11.7 | 8.7 | 3.9 | 52 | No | 3.2 | NA | Yes | Parathyroid adenoma |
| 4 | Female | 75 | PHPT | Single parathyroid adenoma | 2.6 | MIP | 8.8 | 17.1 | 15.1 | 5.4 | 3.7 | 1.5 | 43 | No | NA | NA | NA | Parathyroid adenoma |
| 5 | Female | 65 | PHPT | Single parathyroid adenoma | 2.8 | MIP | 13.8 | 69.4 | 25.9 | 8.8 | 7.2 | 3.7 | 36 | No | 3.6 | 2.4 | Yes | Parathyroid adenoma |
| 6 | Male | 74 | PHPT | Double parathyroid adenoma | 2.9 | BNE | 25.8 | 19.4 | 20.8 | 7.4 | 6.5 | NA | 44 | No | 2.5 | 2.2 | Yes | Parathyroid adenoma |
| 7 | Female | 70 | PHPT | Single parathyroid adenoma | 2.7 | MIP | 8.9 | 19.1 | 9.7 | 3.9 | 2.9 | 1.3 | 63 | No | 3.6 | 2.4 | Yes | Parathyroid adenoma |
| 8 | Female | 71 | PHPT | Single parathyroid adenoma | 2.5 | MIP | 17.5 | 32.6 | 10.4 | 4.9 | 3.8 | NA | 40 | No | 3.9 | 2.5 | Yes | Parathyroid adenoma |
| 9 | Male | 46 | PHPT | Single parathyroid adenoma | 2.9 | MIP | 8.9 | 112.3 | 3.2 | 2.2 | 1.8 | 1.4 | 56 | No | 2.9 | 2.5 | Yes | Parathyroid adenoma |
| 10 | Male | 84 | PHPT | Single parathyroid adenoma | 2.8 | MIP | 11.6 | 22.4 | 20.1 | 5.4 | 3.7 | 3.3 | 33 | No | 4.8 | 2.2 | Yes | Parathyroid adenoma |
-
BNE, bilateral neck exploration; IOPTH, intraoperative parathyroid hormone; PHPT, primary hyperparathyroidism; MIP, minimally invasive parathyroidectomy; NA, not available.
Discussion
Herein, we describe the validation and implementation of an IOPTH assay and workflow protocol at a tertiary care endocrine centre in Canada. In the early 1990s, IOPTH assays were introduced to guide parathyroidectomy, and they have gained popularity amongst surgeons over the past 30 years [4], 13]. Quinn and colleagues demonstrated that IOPTH improves surgical cure and reduces revision surgery rates in patients undergoing focused parathyroidectomy for PHPT, while recent clinical guidelines recommended its use to guide surgery for PHPT [5], 14]. Our group is currently conducting a survey study to describe the adoption of IOPTH across North America [15]. We found via our validation studies that the third-generation Elecsys® IOPTH analyzed via Cobas® e411 performed like other commercially available IOPTH assays, was consistent with this same assay performed at other regional endocrine surgery centres, and we had a mean sample TAT <12 min. These findings are congruent with those of a large retrospective study over 10 years where the Roche Elecsys® PTH assay achieved a median TAT of ≤25 min when evaluated on different analytical platforms and across different hospital testing locations [6], 16]. Our mean TAT is also consistent with studies demonstrating that central laboratory-based IOPTH assays perform similarly or better than point-of-care assays [17], 18]. The Roche Elecsys® PTH assay analytical time of 9 min is important as other assays have significantly longer analytical time (i.e., 10–18 min for other commercial PTH assays) [6], 18], 19]. There are other variables, in addition to the analytical time, that can impact PTH interpretation; these include analyte stability, hemolysis, and/or other assay interferences (e.g., biotin), which can all contribute to discordant findings between assays, as demonstrated in our comparison of Elecsys® and Vitros assays [20]. We also describe a dilutional protocol that may assist other medical laboratories when faced with PTH levels above the upper reference interval since such a protocol is not described in the Elecsys® IOPTH assay IFU. Third-generation IOPTH assays, which detect full-length and post-translationally modified PTH, have higher specificity and can reflect treatment success faster than second-generation assays [21]. Third-generation assays have also been shown to perform better in normocalcaemic PHPT and parathyroid carcinoma, while improving assay performance in secondary and tertiary hyperparathyroidism [21], 22]. Central laboratory-based IOPTH assays have economic advantages and despite concerns for increased TAT, recent studies have shown no impact or possible reduction in operating times when compared to point-of-care assays [6], 7]. As such, we have successfully implemented a third-generation IOPTH assay to guide all parathyroid surgeries at our high-volume centre. Prior to October 2023, all parathyroid surgery was guided by IFTA, but due to the hospital costs and pathology backlog from the COVID-19 pandemic, we discontinued using IFTA and instead transitioned to IOPTH for all parathyroid surgeries. Intraoperative FTA is invasive, misleading when determining parathyroid functionality, pathologist-dependent, and costly when compared to IOPTH – hence, parathyroid surgeons are rethinking the role for IFTA in guiding parathyroid exploration [5], 23], 24]. Consistent with the literature, we also found that IFTA increased operating time by approximately 20 min and that IOPTH was associated with shorter operating time when compared to IFTA for guiding parathyroid exploration [24], [25], [26].
To help ensure that our IOPTH workflow performed optimally, it was developed in collaboration with head and neck surgeons, general surgeons, anesthesiologists, operating room personnel, laboratory medicine personnel, and hospital administrators. We performed modifications to our IOPTH workflow based upon the sample processing efficiencies proposed in a previous study [27]. So far, we have received positive qualitative feedback from hospital stakeholders and maintained operating room case turnover and efficiency as per operating room personnel; thus, we have summarized our IOPTH workflow and provided advice for other institutions planning on developing an IOPTH program (Table 3; Figure 4). We employed our electronic medical record system to facilitate IOPTH order communication, equipment gathering, and sample label generation prior to starting surgery, which is consistent with institutions showing that streamlining IOPTH requisitions and ensuring that all necessary equipment was contained in an assigned tote box improved IOPTH processing time [28]. Our embrace of open and constant communication between surgery and laboratory personnel ensures “closed loop” communication and helps to avoid any delays associated not having analyzer calibrated for surgery [27]. Other critical facets of our IOPTH workflow ensure that dedicated personnel are on standby to transport and analyze IOPTH samples, thus allowing us to avoid unforeseen transport delays associated with pneumatic tube systems. To further improve IOPTH efficiency in the future, we plan on implementing a method that allows laboratory personnel to directly access the surgical schedule; thus, avoiding communication lapses between surgical administrators and laboratory schedulers. As we increase our surgical case volume, we will continue to reflect and improve upon modifiable steps within our IOPTH workflow so that we can further streamline sample processing and resulting.
Keys to streamlining central laboratory-based IOPTH assay turnaround time.
| Workflow design and implementation |
|---|
|
|
|
|
IOPTH sample processing and analysis |
|
|
|
-
IOPTH, intraoperative parathyroid hormone; MLT, medical laboratory technician; OR, operating room; ORA, operating room attendant.
Study limitations
We did not intend to power this study to detect differences in TAT or clinical outcomes between IOPTH and IFTA nor did we perform cost analysis. Our group is also working on a multi-centre prospective evaluation of patient and cost outcomes of IOPTH in secondary and tertiary hyperparathyroidism. Despite the limitations of this study, we believe that the laboratory protocols and IOPTH workflow described in this study can used as a model for other endocrine surgery centres.
Conclusions
In conclusion, we described a validated and efficient central laboratory-based IOPTH workflow that streamlined turnaround time at our endocrine surgery centre. We instituted this collaborative IOPTH program due to the high costs and misleading results associated with IFTA, in addition to our pathology sample backlog compounded by the COVID-19 pandemic. We also provided practical advice and a dilutional protocol for hospitals looking to implement an IOPTH program and thus, advance evidence-based parathyroid surgery around the world.
Acknowledgments
Special thanks to UHN laboratory staff in supplying samples and measurement standards for the Elecsys e411 IOPTH assay.
-
Research ethics: Hamilton Integrated Research Ethics Board (2021-13875-C).
-
Informed consent: Not applicable.
-
Author contributions: P.S. and B.A. contributed equally to this project and manuscript drafting. J.I., J.M., M.A., H.Z., J.D.P., and M.B. all helped with project conception and drafting. All authors approved the final version of the manuscript.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: On request.
References
1. Minisola, S, Arnold, A, Belaya, Z, Brandi, ML, Clarke, BL, Hannan, FM, et al.. Epidemiology, pathophysiology, and genetics of primary hyperparathyroidism. J Bone Miner Res 2022;37:2315–29. https://doi.org/10.1002/jbmr.4665.Suche in Google Scholar PubMed PubMed Central
2. Walker, MD, Silverberg, SJ. Primary hyperparathyroidism. Nat Rev Endocrinol 2018;14:115–25. https://doi.org/10.1038/nrendo.2017.104.Suche in Google Scholar PubMed PubMed Central
3. Irvin, GL3rd, Dembrow, VD, Prudhomme, DL. Operative monitoring of parathyroid gland hyperfunction. Am J Surg 1991;162:299–302. https://doi.org/10.1016/0002-9610(91)90135-z.Suche in Google Scholar PubMed
4. Ahn, D, Kwak, JH. Role and recent trend of intraoperative parathyroid hormone monitoring during parathyroidectomy in patients with primary hyperparathyroidism. Korean J Otorhinolaryngol-Head Neck Surg 2022;65:253–9. https://doi.org/10.3342/kjorl-hns.2022.00332.Suche in Google Scholar
5. Wilhelm, SM, Wang, TS, Ruan, DT, Lee, JA, Asa, SL, Duh, QY, et al.. The American association of endocrine surgeons guidelines for definitive management of primary hyperparathyroidism. JAMA Surg 2016;151:959–68. https://doi.org/10.1001/jamasurg.2016.2310.Suche in Google Scholar PubMed
6. Jacob, D, Lal, G, Voss, DR, Bebber, T, Davis, SR, Kulhavy, J, et al.. Evaluation of switch from satellite laboratory to central laboratory for testing of intraoperative parathyroid hormone. Pract Lab Med 2020;22:e00176. https://doi.org/10.1016/j.plabm.2020.e00176.Suche in Google Scholar PubMed PubMed Central
7. O’Connell, DA, Seikaly, H, Harris, JR. Central laboratory versus point of care testing in intraoperative monitoring of parathyroid hormone levels: cost comparison. J Otolaryngol Head Neck Surg 2008;37:91–7.Suche in Google Scholar
8. Xie, S, Kuriakose, J, Beninato, T, Carayannopoulos, M, Trooskin, SZ, Libutti, SK, et al.. Association of implementation of operating room-based parathyroid hormone testing with operative time and cost. J Am Coll Surg 2022;235:906–12. https://doi.org/10.1097/xcs.0000000000000362.Suche in Google Scholar
9. De Pasquale, L, Gobatti, D, Ravini, ML, Barassi, A, Porreca, W, Melzi d’Eril, GV, et al.. Intra-operative testing for parathyroid hormone: the central Laboratory option. J Endocrinol Investig 2008;31:62–7. https://doi.org/10.1007/bf03345568.Suche in Google Scholar
10. Tan, K, Ong, L, Sethi, SK, Saw, S. Comparison of the Elecsys PTH(1-84) assay with four contemporary second generation intact PTH assays and association with other biomarkers in chronic kidney disease patients. Clin Biochem 2013;46:781–6. https://doi.org/10.1016/j.clinbiochem.2013.01.016.Suche in Google Scholar PubMed
11. Khan, ZF, Lew, JI. Intraoperative parathyroid hormone monitoring in the surgical management of sporadic primary hyperparathyroidism. Endocrinol Metab (Seoul) 2019;34:327–39. https://doi.org/10.3803/enm.2019.34.4.327.Suche in Google Scholar PubMed PubMed Central
12. Woodrum, DT, Saunders, BD, England, BG, Burney, RE, Doherty, GM, Gauger, PG. The influence of sample site on intraoperative PTH monitoring during parathyroidectomy. Surgery 2004;136:1169–75. https://doi.org/10.1016/j.surg.2004.06.043.Suche in Google Scholar PubMed
13. Irvin, GL3rd, Solorzano, CC, Carneiro, DM. Quick intraoperative parathyroid hormone assay: surgical adjunct to allow limited parathyroidectomy, improve success rate, and predict outcome. World J Surg 2004;28:1287–92. https://doi.org/10.1007/s00268-004-7708-6.Suche in Google Scholar PubMed
14. Quinn, AJ, Ryan, EJ, Garry, S, James, DL, Boland, MR, Young, O, et al.. Use of intraoperative parathyroid hormone in minimally invasive parathyroidectomy for primary hyperparathyroidism: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg 2021;147:135–43. https://doi.org/10.1001/jamaoto.2020.4021.Suche in Google Scholar PubMed PubMed Central
15. Staibano, P, McKechnie, T, Thabane, A, Xie, M, Zhang, H, Gupta, MK, et al.. Trends in using intraoperative parathyroid hormone monitoring during parathyroidectomy: protocol and rationale for a cross-sectional survey study of North American surgeons. PLoS One 2024;19:e0301153. https://doi.org/10.1371/journal.pone.0301153.Suche in Google Scholar PubMed PubMed Central
16. Hirsch, BM, Gardner, A, Potter, L, Fish, R, Woeller, E, Atienza, J, et al.. A-110 analytical performance evaluation of Vitros® immunodiagnostic products intact PTH II assay. Clin Chem 2024;70:S1. https://doi.org/10.1093/clinchem/hvae106.109.Suche in Google Scholar
17. Jarrige, V, Nieuwenhuis, JH, van Son, JP, Martens, MF, Vissers, JL. A fast intraoperative PTH point-of-care assay on the Philips handheld magnotech system. Langenbecks Arch Surg 2011;396:337–43. https://doi.org/10.1007/s00423-010-0733-z.Suche in Google Scholar PubMed PubMed Central
18. Leung, EKY, Lee, CC, Angelos, P, Kaplan, EL, Grogan, RH, Sarracino, DA, et al.. Analytical differences in intraoperative parathyroid hormone assays. J Appl Lab Med 2019;3:788–98. https://doi.org/10.1373/jalm.2018.026815.Suche in Google Scholar PubMed
19. Sokoll, LJ, Drew, H, Udelsman, R. Intraoperative parathyroid hormone analysis: a study of 200 consecutive cases. Clin Chem 2000;46:1662–8. https://doi.org/10.1093/clinchem/46.10.1662.Suche in Google Scholar
20. Li, D, Radulescu, A, Shrestha, RT, Root, M, Karger, AB, Killeen, AA, et al.. Association of biotin ingestion with performance of hormone and nonhormone assays in healthy adults. JAMA 2017;318:1150–60. https://doi.org/10.1001/jama.2017.13705.Suche in Google Scholar PubMed PubMed Central
21. Smit, MA, van Kinschot, CMJ, van der Linden, J, van Noord, C, Kos, S. Clinical guidelines and PTH measurement: does assay generation matter? Endocr Rev 2019;40:1468–80. https://doi.org/10.1210/er.2018-00220.Suche in Google Scholar PubMed
22. Lang, BH, Fung, MMH. Intraoperative parathyroid hormone (IOPTH) assay might be better than the second-generation assay in parathyroidectomy for primary hyperparathyroidism. Surgery 2021;169:109–13. https://doi.org/10.1016/j.surg.2020.03.024.Suche in Google Scholar PubMed
23. Dream, S, Kuo, LE, Kuo, JH, Sprague, SM, Nwariaku, FE, Wolf, M, et al.. The American association of endocrine surgeons guidelines for the definitive surgical management of secondary and tertiary renal hyperparathyroidism. Ann Surg 2022;276:e141–76. https://doi.org/10.1097/sla.0000000000005522.Suche in Google Scholar
24. Aksoy, SO, Adiyaman, SC, Cevlik, AD, Guray Durak, M, Secil, M, Sevinc, AI. Intra-operative parathyroid hormone evaluation is superior to frozen section analysis in parathyroid surgery. Am J Otolaryngol 2021;42:102886. https://doi.org/10.1016/j.amjoto.2020.102886.Suche in Google Scholar PubMed
25. Furderer, T, Bouviez, N, Paquette, B, Landecy, G, Heyd, B, Vienney, G, et al.. Frozen tissue examination: is it really no longer of use in parathyroid surgery? Single-center retrospective study on 97 patients treated by minimally invasive approach. World J Endocr Surgery 2017;9:55–60. https://doi.org/10.5005/jp-journals-10002-1211.Suche in Google Scholar
26. Perrier, ND, Ituarte, P, Kikuchi, S, Siperstein, AE, Duh, QY, Clark, OH, et al.. Intraoperative parathyroid aspiration and parathyroid hormone assay as an alternative to frozen section for tissue identification. World J Surg 2000;24:1319–22. https://doi.org/10.1007/s002680010218.Suche in Google Scholar PubMed
27. Pickering, JM, Giles, WH. Improving intraoperative parathyroid hormone lab efficiency. Am Surg 2022;88:915–21. https://doi.org/10.1177/00031348211054556.Suche in Google Scholar PubMed
28. Murgatroyd, J, Wagner, LM, Duh, QY. Improving intraoperative parathyroid hormone specimen handling and processing. AORN J 2024;120:10–8. https://doi.org/10.1002/aorn.14162.Suche in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Review
- Contact phase inhibitors: the future of anticoagulation?
- Original Articles
- Validation and implementation of an intraoperative parathyroid hormone assay and workflow: practical advice for endocrine surgery centres
- Serum soluble endorphin combined with BISAP score predicts severe acute pancreatitis combined with septic shock
- Serum LDH and its isoenzymes (LDH2 and LDH5) associated with predictive value for refractory mycoplasma pneumoniae pneumonia in children
- Upregulation of hsa_circ_0000745/hsa_circRNA_101996 in peripheral blood monocytes is associated with coronary heart disease
- Congress Report
- Congress report: 6th German POCT symposium, September 25–26, 2024, Bremen
- Images from the Medical Laboratory
- Gelatinous urine
Artikel in diesem Heft
- Frontmatter
- Review
- Contact phase inhibitors: the future of anticoagulation?
- Original Articles
- Validation and implementation of an intraoperative parathyroid hormone assay and workflow: practical advice for endocrine surgery centres
- Serum soluble endorphin combined with BISAP score predicts severe acute pancreatitis combined with septic shock
- Serum LDH and its isoenzymes (LDH2 and LDH5) associated with predictive value for refractory mycoplasma pneumoniae pneumonia in children
- Upregulation of hsa_circ_0000745/hsa_circRNA_101996 in peripheral blood monocytes is associated with coronary heart disease
- Congress Report
- Congress report: 6th German POCT symposium, September 25–26, 2024, Bremen
- Images from the Medical Laboratory
- Gelatinous urine

