Abstract
Objectives
To contrast the level of lactate dehydrogenase (LDH) and its isoenzymes between general mycoplasma pneumoniae pneumonia (GMPP) and refractory mycoplasma pneumoniae pneumonia (RMPP) groups and to investigate their predictive value for RMPP in children.
Methods
A total of 160 children with GMPP and 100 children with RMPP were enrolled from August 2022 to April 2023 in our hospital. Serum LDH and its isoenzymes levels were assessed between the two groups. LDH and its isoenzymes were entered into multivariate logistic regression analysis to identify risk factors for RMPP, and variables with significance were used to analyze their diagnostic values for RMPP. ROC curves were drawn, and the AUC was calculated, with sensitivity and specificity obtained.
Results
Children with RMPP displayed more blatant inflammatory responses as well as more alarming imaging findings compared to those with GMPP. The levels of serum LDH and its isoenzymes in children with RMPP were significantly higher than those in children with GMPP. In the multivariate logistic regression analysis, LDH (OR=1.02, p<0.001), LDH2 (OR=1.05, p=0.010) and LDH5 (OR=1.04, p˂0.001) showed statistically significant differences. When the cut-off values were 372.5, 97.46, and 49.29 U/L respectively, the AUCs of LDH (sensitivity=0.80, specificity=0.89), LDH2 (sensitivity=0.83, specificity=0.71), and LDH5 (sensitivity=0.82, specificity=0.72) predicting RMPP were 0.91, 0.81, and 0.82, respectively. The AUC of [LDH + LDH5] (0.92) was the highest.
Conclusions
Serum LDH, LDH2, and LDH5 have good diagnostic values for RMPP and possess the potential to be biological markers in children with RMPP. And the predictive value is higher when used in combination.
Introduction
Mycoplasma pneumoniae (MP) is a common pathogen of respiratory tract infection in children. 40 % of community-acquired pneumonia (CAP) in children is caused by an atypical bacterial pneumonia [1]. All seasons are susceptible to MP, which typically has a subacute start. The symptoms of MP are variable and mainly include fever, cough, sore throat, headache, etc.
It is commonly established that macrolide antibiotics work well to treat MP infections. Recently, there has been an increased incidence of refractory MP pneumonia (RMPP) caused by the abuse of antibiotics and macrolide-resistant bacteria. Patients who have been receiving macrolide antibiotic treatment for seven days or more, meet the diagnostic standards for MPP, have worsened clinical symptoms, a persistent fever, and worsened lung imaging may be candidates for RMPP [2], 3]. Children with MPP have significant airway hyperreactivity and inflammation. One significant immunological mechanism of MPP is the imbalance of Th1/Th2 function following MP infection [4]. Previous studies have shown that in school-age children with RMPP, an excessive host immune response to the pathogen may play a key role, including overexpression of cytokines [5]. The hunt for new molecular diagnostic markers has been a crucial area of research because RMPP cases are rising annually, particularly among youngsters.
Although the exact mechanism of RMPP is not fully understood, reducing the prevalence of RMPP and enhancing its prognosis remains a challenge. Early diagnosis of RMPP in children is crucial, and steroid medication must be started right away.
Lactate dehydrogenase (LDH) is a ubiquitous enzyme, which has been used as an inflammatory marker. It is also considered to replace inflammatory cytokines such as IL-18 as a useful indicator for predicting refractory M. pneumoniae pneumonia [6]. Elevated LDH has been considered as a clinically relevant risk factor for refractory mycoplasma pneumoniae pneumonia [7]. LDH was revealed to be a predictor of RMPP in children in a prior study of ours [8]. LDH is composed of five isoenzymes (LDH1, LDH2, LDH3, LDH4, and LDH5). The role of LDH isoenzymes in severe MPP has not received much attention, though. LDH isoenzyme: a potential RMPP predictor? So, the purpose of our study was to investigate the role of serum LDH and its isoenzymes in RMPP.
Materials and methods
Patients
Children aged 1–13 years who visited the Affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University from August 2022 to April 2023 were enrolled in this research. This study was approved by the Ethics Committee of the Affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University with informed consent ([2022]KY303-01). All of the enrolled children displayed standard pneumonia symptoms and indications. Enrollment of all children was verified by paired serological tests that detected a 4-fold increase in Mycoplasma pneumoniae immunoglobulin M (IgM) and immunoglobulin G (IgG) levels or positive results for Mycoplasma pharyngeal secretion polymerase chain reaction (PCR). All patients were divided into two groups: the RMPP group and the GMPP group. RMPP was defined as a case with persistent fever for more than 7 days and imaging deterioration occurred after the treatment with macrolide antibiotics for more than 7 days [2], 3], despite the exclusion of other pathogenic infections or mixed infections. None of the enrolled children were given macrolide antibiotics before blood collection. All enrolled children were given routine treatment after specimen collection, such as anti-infection, sputum removal, cough suppression, and temperature control. Appropriate treatment with glucocorticoids was given to RMPP children.
Exclusion criteria
The exclusion criteria were the presence of severe concomitant diseases (autoimmune diseases, musculoskeletal disorders, severe liver or kidney disease, cardiovascular disease, neoplasia, and previous chronic lung disease), co-infection with other pathogens (bacteria, adenovirus, syncytial virus, influenza virus, rhinovirus, coxsackievirus, EBV, CMV, or tuberculosis) within 3 months, and missing medical records. A patient who had used glucocorticoids before the enrollment was also excluded.
Specimen collection
Fasting venous blood (5 mL) from all the enrolled children was collected at the time of admission or the next morning, and heparin was used to prevent clotting. Lactate dehydrogenase (LDH) was detected by enzyme immunoassay (AU5800, Beckman Coulter. Inc.). LDH isoenzymes were obtained by agarose gel electrophoresis (HYERASYS2, Sebia Medical Diagnostic Devices [Shanghai] Co. Ltd.). The neutrophil CD64 index was determined by flow cytometry (FCM, BD FACSCanto II). In addition, fasting venous blood was collected and tested by our hospital. The blood routine test, C-reactive protein (CRP), liver function, renal function, complement 3, complement 4, and myocardial enzymes of the two groups were detected.
Data collection
The age, sex, length of hospital stay, imaging changes, and effectiveness of treatment with macrolide were collected upon hospital admission and at the patient discharge.
Statistical analysis
SPSS 19.0 was used to examine all statistical data. When the data had a normal distribution, the continuous variables were expressed as mean with standard deviation (X ± SD), analyzed using the T-test for two independent samples. However, the data were described using a median with an interquartile range if not meeting the normal distribution, with the Mann–Whitney U-test applied. Categorical data were analyzed by the Chi-square test. LDH and its isoenzymes were entered into the multivariate logistic regression model for RMPP risk assessment. Variables with significant differences in the logistic regression were included in the analysis of predictive value for RMPP. The receiver operating characteristic (ROC) curve was drawn to calculate the area under the curve (AUC), with sensitivity and specificity estimated. A p-value of <0.05 was indicated as a statistically significant difference.
Results
Demographic data and clinical profiles of all enrolled children
This study comprised 100 children with RMPP and 160 children with GMPP. Table 1 displays the comparison findings for the clinical traits of the subjects. The RMPP group had a higher mean age (6.77 years) than the GMPP group (4.86 years) (p<0.001). Between the two groups, there was no difference in the gender distribution (p>0.05). In order to minimize the differences in the results from the time point of blood collection, we compared the course of disease before recruitment between the two groups. We found no significant differences between the two groups (p>0.05). The median length of hospital stay (8 days) in the RMPP group was longer than that in the GMPP group (6 days) (p<0.001). Additionally, we discovered that the RMPP group had more severe radiographic results. The levels of WBC, N, PLT, CRP, and neutrophil CD64 index were even higher in the RMPP group compared with the GMPP group with statistical significance, indicating a positive correlation between the levels of these laboratory markers and the severity of MPP. Similarly, the levels of serum C3 and C4 in RMPP children were also higher than in GMPP children. In addition, no differences were found in ALT, AST, CK-MB, and BUN between the two groups. The RMPP group had considerably higher Cr values than the GMPP group in terms of renal function. On the other hand, the RMPP group’s UA levels were dramatically (p=0.007) reduced.
Demographic data and clinical profiles in children with RMPP and GMPP.
| Indicators | GMPP | RMPP | T, Z, or χ2 | p-Value |
|---|---|---|---|---|
| Age, years | 4.86 ± 2.48 | 6.77 ± 2.50 | −6.02 | <0.001 |
| Sex (male, n/%) | 67 (41.88 %) | 42 (42 %) | <0.001 | 0.98 |
| Course of disease before recruitment, days | 5 (4.6) | 5 (4.6–75) | −0.87 | 0.39 |
| Hospital stay, days | 6 (6.7) | 8 (7.9) | −7.85 | <0.001 |
| Imaging findings, n (%) | ||||
| Lobar atelectasis | 6 (3.75 %) | 10 (10 %) | 4.16 | 0.04 |
| Pleural effusion | 1 (6.25 %) | 9 (9 %) | 11.67 | 0.001 |
| Lung consolidation | 27 (16.88 %) | 49 (49 %) | 30.7 | <0.001 |
| WBC, 109/L | 7.44 ± 3.19 | 9.05 ± 4.05 | −3.4 | 0.001 |
| N, 109/L | 3.07 (1.74–4.25) | 4.01 (2.83–6.26) | −4.08 | <0.001 |
| PLT, 109/L | 295.35 ± 98.21 | 351.13 ± 117.95 | −4.12 | <0.001 |
| CRP, mg/L | 5.00 (2.00–14.00) | 20.00 (10.00–32.00) | −7.04 | <0.001 |
| CD64 | 0.15 (0.07–0.30) | 0.20 (0.10–0.76) | −3.16 | 0.02 |
| ALT, U/L | 15.00 (12.00–21.00) | 15.00 (12.00–24.00) | −0.4 | 0.69 |
| AST, U/L | 30.71 ± 9.44 | 33.81 ± 14.01 | −1.95 | 0.053 |
| CK-MB, U/L | 10.00 (7.00–17.00) | 10.00 (7.00–24.00) | −0.66 | 0.51 |
| BUN, mmol/L | 3.60 (2.98–4.14) | 3.28 (2.74–4.09) | −1.53 | 0.13 |
| Cr, umol/L | 28.53 ± 7.57 | 31.13 ± 6.95 | −2.79 | 0.006 |
| UA, umol/L | 258.08 ± 74.57 | 233.39 ± 64.12 | 2.74 | 0.007 |
| C3, g/L | 1.23 ± 0.24 | 1.32 ± 0.28 | −2.89 | 0.004 |
| C4, g/L | 0.32 ± 0.11 | 0.37 ± 0.14 | −3.31 | 0.001 |
-
GMPP, general mycoplasma pneumoniae pneumonia; RMPP, refractory mycoplasma pneumoniae pneumonia; WBC, white blood cell; N, neutrophil; PLT, platelet; CRP, C-reactive protein; ALT, alanine aminotransferase; aspartate aminotransferase; CK-MB, creatine kinase-MB; BUN, blood urea nitrogen; Cr, creatinine; UA, uric acid.
Comparison of serum levels of LDH and its isoenzymes between the GMPP group and the RMPP group
The differences were examined in the levels of LDH and its isoenzymes between the GMPP and RMPP groups (Table 2). The RMPP group presented significantly (p<0.001) increased levels of LDH and its isoenzymes (LDH1, LDH2, LDH3, LDH4, and LDH5) compared with the GMPP group.
Comparison of the LDH and its isoenzymes between the GMPP and RMPP groups.
| Indicators | GMPP | RMPP | T(Z) or χ2 | p-Value |
|---|---|---|---|---|
| LDH, U/L | 306.78 ± 62.58 | 437.18 ± 96.78 | 13.2 | <0.001 |
| LDH1, U/L | 69.42 ± 17.73 | 82.14 ± 23.29 | 4.98 | <0.001 |
| LDH2, U/L | 89.90 ± 20.58 | 119.98 ± 28.40 | 9.88 | <0.001 |
| LDH3, U/L | 68.73 ± 18.84 | 99.71 ± 30.33 | 10.16 | <0.001 |
| LDH4, U/L | 35.93 ± 12.04 | 59.25 ± 22.49 | 10.88 | <0.001 |
| LDH5, U/L | 38.66 (25.94–52.23) | 65.60 (51.97–85.75) | 8.75 | <0.001 |
-
LDH, lactate dehydrogenase; GMPP, general mycoplasma pneumoniae pneumonia; RMPP, refractory mycoplasma pneumoniae pneumonia.
Predictive value of serum LDH and its isoenzymes in children with RMPP
We performed multivariate logistic regression analyses to analyze the independent risk factors for LDH and its isoenzymes for RMPP. The logistic regression analysis demonstrated that LDH (OR=1.02, p<0.001), LDH2 (OR=1.05, p=0.010), and LDH5 (OR=1.04, p<0.001) had significance (p<0.05) for the risk of RMPP (Table 3). As shown in Table 4 and Figure 1, the ROC curve analysis indicated that serum LDH had an optimal AUC of 0.91 with a cut-off value of 372.5 U/L for predicting RMPP, with a sensitivity of 80.0 % and specificity of 89.0 %. LDH2 had an optimal AUC of 0.81 (sensitivity=83.0 %, specificity=71.0 %) with a cut-off value of 97.46 U/L and LDH5 had an optimal AUC of 0.82 (sensitivity=82.0 %, specificity=72.0 %) with a cut-off value of 49.29 U/L for predicting RMPP. In addition, we analyzed the predictive value of the joint indicator. We found that the AUC for [LDH + LDH5] showed the highest value (0.92) for the ROC curve. And the sensitivity of [LDH + LDH5] prediction was also the highest (sensitivity=0.87). Therefore, serum [LDH + LDH5] was a better biomarker than LDH in predicting RMPP.
The multivariate logistic regression analyses of LDH and its isoenzymes for RMPP.
| Variable | β | SE | Wald | p-Value | OR | 95 % CI |
|---|---|---|---|---|---|---|
| LDH | 0.02 | 0 | 63.45 | <0.001 | 1.02 | 1.02–1.03 |
| LDH1 | −0.02 | 0.01 | 1.86 | 0.17 | 0.98 | 0.95–1.01 |
| LDH2 | 0.05 | 0.02 | 6.97 | 0.01 | 1.05 | 1.01–1.1 |
| LDH3 | 0.02 | 0.03 | 0.7 | 0.4 | 1.02 | 0.97–1.08 |
| LDH4 | 0.01 | 0.04 | 0.16 | 0.69 | 1.01 | 0.95–1.09 |
| LDH5 | 0.04 | 0.01 | 13.02 | <0.001 | 1.04 | 1.02–1.07 |
-
LDH, lactate dehydrogenase; OR, odds ratio; CI, confidence interval; SE, standard error; RMPP, refractory mycoplasma pneumoniae pneumonia.
Predictive value of independent correlation factors for RMPP.
| Independent factors | AUC | 95 % CI | Sensitivity | Specificity |
|---|---|---|---|---|
| LDH | 0.91 | 0.87–0.94 | 0.8 | 0.89 |
| LDH2 | 0.81 | 0.76–0.87 | 0.83 | 0.71 |
| LDH5 | 0.82 | 0.77–0.87 | 0.82 | 0.72 |
| LDH2 + LDH5 | 0.91 | 0.88–0.95 | 0.81 | 0.89 |
| LDH + LDH2 | 0.91 | 0.87–0.95 | 0.86 | 0.84 |
| LDH + LDH5 | 0.92 | 0.88–0.95 | 0.87 | 0.84 |
-
LDH, lactate dehydrogenase; AUC, area under the curve; CI, confidence interval; RMPP, refractory mycoplasma pneumoniae pneumonia.
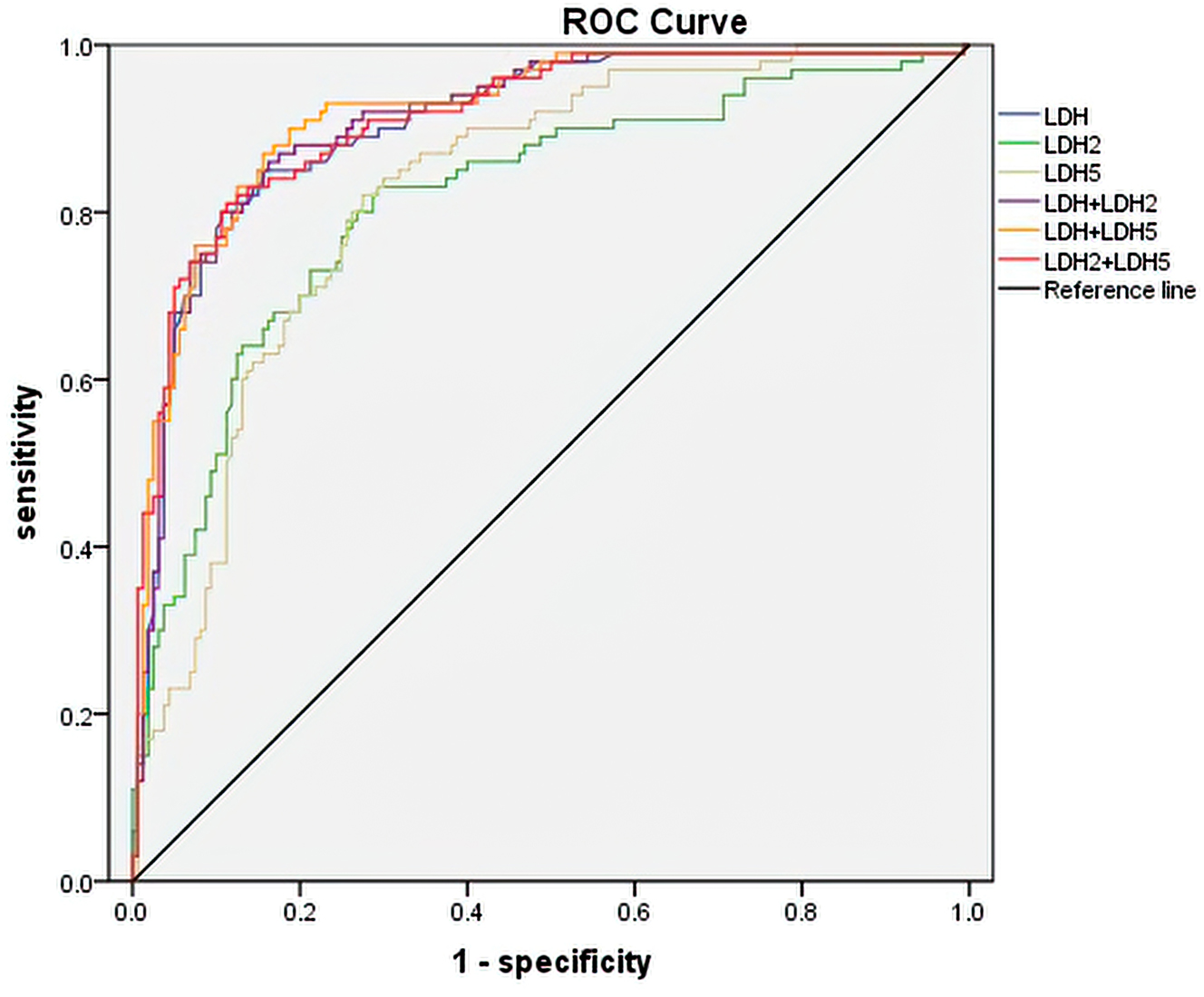
ROC curves of LDH, LDH2, LDH5 and combination indicators for predicting RMPP. ROC: receiver operating characteristic; LDH: lactate dehydrogenase; RMPP: refractory mycoplasma pneumoniae pneumonia.
Discussion
Mycoplasma pneumoniae is one of the most common pathogenic causes of CAP
Although MP pneumonia is a self-limiting disease, some patients often experience recurrent and prolonged disease, and significantly impaired immunity [9]. In recent years, the incidence of RMPP has been relatively high. Additionally, RMPP consequences were generally severe, even causing thrombosis, necrotizing pneumonia, or bronchitis obliterans [10]. As a result, it’s critical to detect the likelihood of RMPP as soon as possible. All children enrolled in this study were treated approximately in the same way after specimen collection. Corticosteroids were added to patients diagnosed with refractory mycoplasma pneumonia during the treatment process. There was no excessive interventional treatment for patients with suspected refractory mycoplasma. In this study, compared with the GMPP group, RMPP children had a longer hospital stay and a higher incidence of lung consolidation, pleural effusion, and atelectasis, as well as significantly higher levels of WBC, neutrophil count, PLT, and CRP in peripheral blood. It further demonstrated that RMPP has a more intense inflammatory response and more severe imaging changes. Therefore, given the serious consequences of RMPP, it is essential to identify RMPP in children as early as possible in our clinical work to reduce the occurrence of RMPP. Furthermore, our study indicated that there was no significant difference between the two groups in terms of liver function damage and myocardial damage. When comparing the renal function of the two groups, it was found that Cr values were higher and UA values were lower in the RMPP group. UA is the end product of purine metabolism in humans. The loss of urease activity has resulted in higher UA levels in humans than in other mammals. In addition, 90 % of UA filtered by the kidneys is reabsorbed rather than excreted from the body. This has led many researchers to think about the possible evolutionary advantages of uricase deficiency and elevated UA levels. A study conducted by Jenn-Haung Lai et al. reported that physiological concentrations of soluble UA showed anti-inflammatory and chondroprotective effects [11]. We can suspect that UA may have a possible protective effect against Mycoplasma pneumoniae pneumonia. This conclusion certainly needs to be further explored subsequently.
Previous studies have found that excessive immune responses to pathogens, including strong expression of cytokines and highly activated cell-mediated immune responses, may play an important role in RMPP [12]. For RMPP, there is a close relationship between the autoinflammatory response and immune function. Therefore, the relevant inflammatory markers are also helpful for the diagnosis and effective treatment intervention of RMPP. When infection occurs, CD64 on the surface of monocytes and lymphocytes does not change. However, changes in CD64 on the surface of neutrophils are thought to reflect the state of infection in a variety of inflammatory conditions. Recent studies have suggested the use of neutrophil CD64 expression as a novel marker for the early diagnosis and prognostic evaluation of bacterial infections [13]. However, there are few clinical studies on the correlation between neutrophil CD64 and children with MPP, especially RMPP. Our data have found that the neutrophil CD64 index was significantly higher in children with RMPP than that in children with GMPP. Therefore, the neutrophil CD64 index correlates with the severity of inflammation, with higher inflammation indicators suggesting a greater likelihood of severe infection.
Some studies have confirmed that the complement system is also involved in lung injury [14]. A previous study demonstrated that the levels of serum C4 and C3 in the acute phase were significantly higher than those in the recovery period for MPP patients [15]. According to our data, serum C3 and C4 levels in RMPP children were higher than those in GMPP children. This observation indicated that the complement system may be involved in the occurrence of RMPP, which can be used as a non-specific indicator for the diagnosis of RMPP.
LDH is an important enzyme in the glycolytic pathway, which is widely found in tissues and cells. When lung tissue is hypoxic and necrotic, cell membrane permeability increases, and enzymes are released from the cells into the blood, raising the level of LDH in the blood [16]. Some studies have acknowledged that LDH may be a predictor for early prediction of refractory MPP [17], 18]. LDH was a noteworthy predictor of RMPP, as demonstrated by our earlier study.8 Therefore, this study evaluated the utility of LDH isoenzymes in childhood RMPP in addition to reiterating the prognostic value of LDH. Our study showed an optimal cut-off level of 372.5 U/L for total LDH, with 80 % sensitivity and 89 % specificity, which was similar to previous reports. Our research also showed that LDH levels can reflect the severity of mycoplasma pneumonia. The types and levels of serum LDH isoenzyme in human tissues are different. The percentage of LDH isoenzymes rose in the healthy state in the following order: LDH2>LDH1>LDH3>LDH4>LDH5. LDH1 and LDH2 are mainly found in the kidney, myocardium, and erythrocytes. LDH3 is present in malignant tumors, lymphoid tissues, and platelets. LDH4 and LDH5 are mainly from lungs, liver, and skeletal muscles [15]. The distribution of LDH isozymes is distinctly tissue-specific. Therefore, it can be used to assist in the diagnosis of a disease based on its distribution characteristics. LDH isoenzymes can improve the diagnostic accuracy of certain diseases, especially lung lesions [19]. At present, the predictive value of LDH isozymes in RMPP is rarely studied. According to a Taiwanese study, the RMPP group exhibited greater levels of LDH2, LDH3, LDH4, and LDH5 [16]. In our present research, the activities of LDH isoenzymes displayed different significant changes as compared with GMPP and RMPP children. The RMPP group showed significantly higher levels of LDH isoenzymes. This may be because RMPP children are more likely to have multiple organ injuries. Besides that, our ROC analysis of LDH isoenzymes suggested that an LDH2 level of 97.46 U/L and an LDH5 level of 49.29 U/L also had high predictive values for RMPP. We considered that the inconsistent results may be due to different sample sizes, different specimen collection time points, etc. Undoubtedly, a larger multicenter investigation is necessary to support our findings. In addition, we analyzed the value of the combined indicators and found that [LDH + LDH5] had the largest AUC area (0.92) and highest sensitivity (0.87). For RMPP [LDH + LDH5] may be a better predictor than LDH.
In addition, our current study has some limitations. Firstly, our study was a single-center study with a relatively small sample size. Although all enrolled children were typical MPP cases, multicenter studies with a larger sample size will be planned in the future. Secondly, although serum specimens from all enrolled children were sampled within 24 h of enrollment, the different disease courses of these children may lead to differences in test results. We were unable to define the same point in the course of the disease as the time point for specimen collection. Finally, we excluded cases with co-infection within 3 months, but some children with co-infection may be not detected. Despite these limitations, our research not only analyzed the differences in inflammatory indicators between RMPP and GMPP children but also preliminarily explored the predictive role of LDH and its isoenzymes on the occurrence of RMPP.
Conclusions
In conclusion, serum LDH and its isoenzymes (LDH2 and LDH5) have good diagnostic performances for RMPP. Therefore, LDH and its isoenzymes (LDH2 and LDH5) possess the potential to be used as valuable prediction markers for children with RMPP. The combined use of these indicators (especially LDH + LDH5) has a higher predictive value. Moreover, more extensive prospective trials are required to replicate this work and fully comprehend the connection between these markers and the emergence of RMPP.
-
Research ethics: This study was approved by the Ethics Committee of The Affiliated Changzhou No.2 People’s Hospital of Nanjing Medical University with informed consent ([2022]KY303-01).
-
Informed consent: Informed consent was obtained from all subjects and/or their legal guardians.
-
Author contributions: LJ, FF provided substantial contribution to the design of the study; LJ, WY, and JF conducted the analysis of data for the work, and drafted the work; LJ, WY, and FF provided substantial contributions to the interpretation of data for the work, and revised it critically for important intellectual content. All authors gave their final approval for the version to be published. All authors have read and agreed to the published version of the manuscript.
-
Competing interests: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
1. Jin, HL, Zhan, L, Mei, SF, Shao, ZY. Serum cytokines and FeNO in school-aged children with mycoplasma pneumoniae pneumonia. Med Sci Mon Int Med J Exp Clin Res 2020;26:e923449. https://doi.org/10.12659/msm.923449.Suche in Google Scholar
2. Subspecialty Group of Respiratory Diseases, The Society of Pediatrics, Chinese Medical Association The Editorial Board, Chinese Journal of Pediatrics. Guidelines for management of community acquired pneumonia in children (the revised edition of 2013) (II). Zhonghua Er Ke Za Zhi 2013;51:856–62.Suche in Google Scholar
3. Tamura, A, Matsubara, K, Tanaka, T, Nigami, H, Yura, K, Fukaya, T. Methylprednisolone pulse therapy for refractory Mycoplasma pneumoniae pneumonia in children. J Infect 2008;57:223–8. https://doi.org/10.1016/j.jinf.2008.06.012.Suche in Google Scholar PubMed PubMed Central
4. Guo, SS, Bao, L, Qu, TG, Mao, X, Gao, YJ, Xu, YL, et al.. Ameliorative effects of infantile Feire Kechuan oral solution on mycoplasma pneumoniae pneumonia in infant mouse and rat models. Evid Based Complement Alternat Med 2018;2018:8139040. https://doi.org/10.1155/2018/8139040.Suche in Google Scholar PubMed PubMed Central
5. Inamura, N, Miyashita, N, Hasegawa, S, Kato, A, Fukuda, Y, Saitoh, A, et al.. Management of refractory Mycoplasma pneumoniae pneumonia: utility of measuring serum lactate dehydrogenase level. J Infect Chemother 2014;20:270–3. https://doi.org/10.1016/j.jiac.2014.01.001.Suche in Google Scholar PubMed
6. Barker, AF, O’Donnell, AT, Flume, P, Thompson, PJ, Ruzi, JD, Gracia, JD, et al.. Aztreonam for inhalation solution in patients with non-cystic fibrosis bronchiectasis (AIR-BX1 and AIR-BX2): two randomised double-blind, placebo-controlled phase 3 trials. Lancet Respir Med 2014;2:738–49. https://doi.org/10.1016/s2213-2600(14)70165-1.Suche in Google Scholar
7. Bi, Y, Zhu, YF, Ma, X, Xu, JJ, Guo, Y, Huang, TY, et al.. Development of a scale for early prediction of refractory Mycoplasma pneumoniae pneumonia in hospitalized children. Sci Rep 2021;11:6595. https://doi.org/10.1038/s41598-021-86086-5.Suche in Google Scholar PubMed PubMed Central
8. Fan, F, Lv, J, Yang, QY, Jiang, F. Clinical characteristics and serum inflammatory markers of community-acquired mycoplasma pneumonia in children. Clin Res J 2023;17:607–17. https://doi.org/10.1111/crj.13620.Suche in Google Scholar PubMed PubMed Central
9. Rogozinski, LE, Alverson, BK, Biondi, EA. Diagnosis and treatment of Mycoplasma pneumoniae in children. Minerva Pediatr 2017;69:156–60. https://doi.org/10.23736/s0026-4946.16.04866-0.Suche in Google Scholar
10. Cheng, Q, Zhang, H, Shang, YX, Zhao, YD, Zhang, Y, Zhuang, DL, et al.. Clinical features and risk factors analysis of bronchitis obliterans due to refractory Mycoplasma pneumoniae pneumonia in children: a nomogram prediction model. BMC Infect Dis 2021;21:1085. https://doi.org/10.1186/s12879-021-06783-4.Suche in Google Scholar PubMed PubMed Central
11. Lai, JH, Luo, SF, Hung, LF, Huang, CY, Lien, SB, Lin, LC, et al.. Physiological concentrations of soluble uric acid are chondroprotective and anti–inflammatory. Sci Rep 2017;7:2359. https://doi.org/10.1038/s41598-017-02640-0.Suche in Google Scholar PubMed PubMed Central
12. Fan, HF, Lu, BT, Yang, DY, Zhang, DW, Shi, TT, Lu, G. Distribution and expression of IL-17 and related cytokines in children with mycoplasma pneumoniae pneumonia. Jpn J Infect Dis 2019;72:387–93. https://doi.org/10.7883/yoken.jjid.2019.113.Suche in Google Scholar PubMed
13. Cui, W, Xu, YY, Fang, H, Tong, WJ, Zhu, LR, Jin, DQ, et al.. Assessment of continuous neutrophil CD64 index measurement for diagnosing sepsis and predicting outcome in a Chinese pediatric intensive care unit: a prospective study. Transl Pediatr 2021;10:1668–76. https://doi.org/10.21037/tp-21-63.Suche in Google Scholar PubMed PubMed Central
14. Ehrnthaller, C, Flierl, M, Perl, M, Denk, S, Unnewehr, H, Ward, PA, et al.. The molecular fingerprint of lung inflammation after blunt chest trauma. Eur J Med Res 2015;20:70. https://doi.org/10.1186/s40001-015-0164-y.Suche in Google Scholar PubMed PubMed Central
15. Mi, YM, Qi, Q, Zhang, L, Wang, XF, Chen, ZM, Hua, CZ. Assessment of serum sialic acid correlated with C3 in children with Mycoplasma pneumoniae pneumonia. J Clin Lab Anal 2020;34:e23078. https://doi.org/10.1002/jcla.23078.Suche in Google Scholar PubMed PubMed Central
16. Liu, TY, Lee, WJ, Tsai, CM, Kuo, KC, Lee, CH, Hsieh, KS, et al.. Serum lactate dehydrogenase isoenzymes 4 plus 5 is a better biomarker than total lactate dehydrogenase for refractory Mycoplasma pneumoniae pneumonia in children. Pediatr Neonatol 2018;59:501–6. https://doi.org/10.1016/j.pedneo.2017.12.008.Suche in Google Scholar PubMed
17. Wang, MJ, Wang, YQ, Yan, YD, Zhu, CH, Huang, L, Shao, XJ, et al.. Clinical and laboratory profiles of refractory Mycoplasma pneumoniae pneumonia in children. Int J Infect Dis 2014;29:18–23. https://doi.org/10.1016/j.ijid.2014.07.020.Suche in Google Scholar PubMed
18. Lu, AZ, Wang, CK, Zhang, XB, Wang, LB, Qian, LL. Lactate dehydrogenase as a biomarker for prediction of refractory mycoplasma pneumoniae pneumonia in children. Respir Care 2015;60:1469–75. https://doi.org/10.4187/respcare.03920.Suche in Google Scholar PubMed
19. Ergenc, I, Capar, E, Erturk, SB, Bahramzade, G, Atalah, F, Kocakaya, D, et al.. Diagnostic performance of lactate dehydrogenase (LDH) isoenzymes levels for the severity of COVID-19. J Med Biochem 2023;42:16–26. https://doi.org/10.5937/jomb0-37234.Suche in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Review
- Contact phase inhibitors: the future of anticoagulation?
- Original Articles
- Validation and implementation of an intraoperative parathyroid hormone assay and workflow: practical advice for endocrine surgery centres
- Serum soluble endorphin combined with BISAP score predicts severe acute pancreatitis combined with septic shock
- Serum LDH and its isoenzymes (LDH2 and LDH5) associated with predictive value for refractory mycoplasma pneumoniae pneumonia in children
- Upregulation of hsa_circ_0000745/hsa_circRNA_101996 in peripheral blood monocytes is associated with coronary heart disease
- Congress Report
- Congress report: 6th German POCT symposium, September 25–26, 2024, Bremen
- Images from the Medical Laboratory
- Gelatinous urine
Artikel in diesem Heft
- Frontmatter
- Review
- Contact phase inhibitors: the future of anticoagulation?
- Original Articles
- Validation and implementation of an intraoperative parathyroid hormone assay and workflow: practical advice for endocrine surgery centres
- Serum soluble endorphin combined with BISAP score predicts severe acute pancreatitis combined with septic shock
- Serum LDH and its isoenzymes (LDH2 and LDH5) associated with predictive value for refractory mycoplasma pneumoniae pneumonia in children
- Upregulation of hsa_circ_0000745/hsa_circRNA_101996 in peripheral blood monocytes is associated with coronary heart disease
- Congress Report
- Congress report: 6th German POCT symposium, September 25–26, 2024, Bremen
- Images from the Medical Laboratory
- Gelatinous urine


