Introduction
Rheumatology, a specialty focused on autoimmune and musculoskeletal disorders, faces persistent diagnostic challenges that hinder timely and effective patient care. In recent years, artificial intelligence (AI), particularly through advancements in machine learning (ML), has emerged as a transformative force across healthcare. By enabling the analysis of complex, multimodal datasets, AI offers unprecedented opportunities to enhance diagnostic precision, personalize treatments, and optimize outcomes for patients with rheumatic diseases. Real-world applications of AI-powered tools are already demonstrating their impact, offering new pathways for earlier detection and more precise treatment strategies. However, their integration into clinical practice also raises important ethical and practical considerations. As AI continues to transform the healthcare landscape, its influence in reshaping rheumatology is becoming increasingly evident—paving the way for more accurate diagnoses, tailored therapies, and enhanced patient care experiences.[1, 2, 3]
Figure 1 illustrates the flowchart for the present article “Artificial intelligence in rheumatology: A transformative perspective”, which systematically examines the integration of AI into rheumatology to address diagnostic challenges and enhance patient care. The flowchart is divided into six key sections: introduction, traditional diagnostic challenges, AI-powered intelligent diagnosis, case studies, challenges and ethical considerations, and future directions. The Introduction section highlights the persistent challenges in diagnosing rheumatic diseases and introduces AI as a groundbreaking tool to address these inefficiencies. The traditional diagnostic challenges section outlines the complexity of clinical presentations, the limitations of biomarkers and imaging techniques, and the procedural constraints that often delay accurate diagnoses. Moving forward, the AI-powered intelligent diagnosis section highlights the capabilities of machine learning algorithms to analyze complex, multimodal datasets and detect early disease patterns, thereby improving diagnostic precision and efficiency. The case studies section showcases real-world applications of AI, including cost-sensitive neural networks that enhance diagnostic accuracy, automated screening tools that expand access to care in underserved areas, and personalized treatment strategies informed by predictive modeling. The challenges and ethical considerations section critically examines data bias, privacy risks, and the necessity of transparent algorithms and clinician education to ensure the ethical and effective deployment of AI. Lastly, the future directions section envisions advancements in predictive modeling, the proliferation of digital health tools, and the importance of interdisciplinary collaboration among healthcare providers, data scientists, and policymakers.
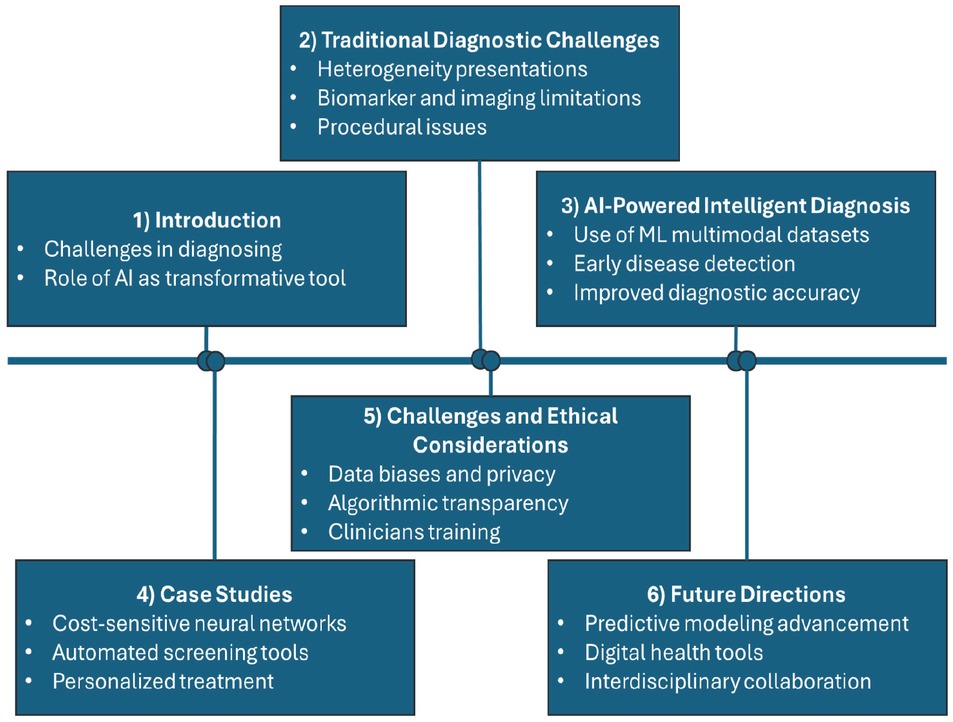
Flowchart of artificial intelligence in rheumatology: A transformative perspective.
Traditional diagnostic challenges in rheumatology
The accurate and timely diagnosis of rheumatological diseases remains a significant challenge due to the inherent complexity of autoimmune and musculoskeletal conditions. Traditional diagnostic methods rely on clinical expertise, laboratory testing, and imaging techniques, each with its critical limitations, which contribute to delays and variability in care. Diagnosis in rheumatology is highly dependent on the expertise of individual clinicians, resulting in substantial interobserver variability. Conditions such as rheumatoid arthritis (RA) often present with subtle and overlapping symptoms, making early recognition particularly difficult. For example, identifying synovitis—a hallmark of RA—requires advanced clinical acumen, and inconsistent recognition can lead to delayed interventions and suboptimal management strategies.[4]
Commonly used biomarkers, such as rheumatoid factor (RF) and anti-cyclic citrullinated peptide (anti-CCP) antibodies, are imperfect tools for diagnosis. RF is present not only in RA but also in other autoimmune diseases and even in healthy individuals, resulting in false positives. Similarly, anti-CCP, while more specific, lacks complete sensitivity, particularly in early-stage RA. These limitations can lead to diagnostic uncertainty, delayed therapy initiation, and missed opportunities for early intervention.[5] Although essential in detecting joint pathology, imaging modalities are limited in their capabilities. X-rays often fail to detect early joint damage or subtle erosions, while ultrasound is operator-dependent, requiring substantial expertise to ensure diagnostic accuracy. Though more precise, magnetic resonance imaging (MRI) is expensive, time-intensive, and impractical for routine use in many healthcare settings. These challenges reduce the utility of imaging as a standalone diagnostic tool in routine practice.[6]
Procedures such as synovial biopsies and arthrocentesis can provide valuable diagnostic insights but are often associated with patient discomfort and risk of complications. For instance, synovial biopsy, although informative, involves risks such as bleeding and infection, leading to patient reluctance. Similarly, arthrocentesis, while helpful in diagnosing synovial inflammation, may be avoided due to pain and the potential for post-procedure joint infections.[7] The cumulative effect of these challenges results in prolonged diagnostic timelines, increased healthcare costs, and a reduced quality of life for patients.
AI-Powered intelligent-assisted diagnosis
ML algorithms have emerged as powerful tools for enhancing the diagnosis of rheumatological diseases. These algorithms leverage computational techniques to analyze complex datasets and identify patterns that may not be readily apparent to human clinicians. In rheumatology, ML algorithms offer several advantages for improving diagnostic accuracy and efficiency. One of the key strengths of ML algorithms is their ability to recognize patterns in multimodal data, including clinical assessments, laboratory results, and imaging studies. By integrating diverse information sources, ML algorithms can generate comprehensive diagnostic models that capture the multifaceted nature of rheumatological diseases. These models can guide further diagnostic evaluations and treatment decisions, leading to more accurate and timely diagnoses. ML algorithms detect subtle patterns in data, making them particularly well-suited for early disease detection. In rheumatology, early diagnosis is critical for initiating appropriate treatment and preventing irreversible joint damage. ML algorithms can analyze imaging studies, such as X-rays and MRI scans, to identify early signs of inflammation and joint damage that may not be apparent to human observers. By detecting these changes at the earliest stages of the disease, ML algorithms can facilitate prompt intervention and improve patient outcomes.[8, 9, 10]
Challenges and ethical considerations
Despite its transformative potential, the implementation of AI in rheumatology faces significant challenges, particularly in terms of data quality, biases, and ethical considerations. AI algorithms rely on diverse, high-quality datasets to ensure accurate predictions, yet many healthcare datasets are incomplete, heterogeneous, or biased. These limitations can skew AI models, exacerbating healthcare disparities, especially for underrepresented populations. Ethical concerns also arise regarding data privacy, security, and transparency. Using sensitive patient data in AI systems poses risks of breaches and misuse, requiring stringent safeguards to protect confidentiality.
Future directions and conclusion
AI is poised to revolutionize rheumatology by enhancing diagnostic accuracy, personalizing treatments, and empowering patients through digital innovations. Predictive modeling using AI can identify novel biomarkers and forecast disease trajectories, enabling earlier and more targeted interventions. Digital health tools, including mobile apps and wearables, promote patient engagement and adherence, fostering a proactive approach to disease management. Importantly, the integration of privacy-preserving technologies, such as federated learning, holds great promise in enabling secure multi-center collaborations without compromising sensitive patient data. These approaches can help build robust, generalizable AI models by aggregating insights across institutions while maintaining strict data confidentiality and privacy.
Interdisciplinary collaboration among rheumatologists, data scientists, and policymakers is crucial to ensure the ethical and practical integration of AI technologies into clinical practice. Equally important is the establishment of structured training frameworks to support clinicians in adopting AI tools. Educational initiatives should focus on developing literacy in data interpretation, algorithmic transparency, and limitations of AI predictions. Moreover, real-world evaluation metrics such as clinical utility, patient outcomes, and system-level performance must be standardized to monitor and continuously improve AI effectiveness in daily clinical settings. Addressing current barriers, such as data quality and algorithmic transparency, AI can transform rheumatology into a more precise, equitable, and patient-centered field, ultimately improving outcomes and satisfaction. As advancements continue, AI-driven rheumatology promises to revolutionize care delivery, achieving unprecedented levels of personalization, prevention, and efficiency.
Funding statement: Zhejiang Provincial Public Welfare Research Program (No. LTGY24H100006); the National Natural Science Foundation of China (No. 82201997). Natural Science Foundation of Zhejiang Province (LQ24F030013), the Fundamental Research Funds for the Provincial Universities of Zhejiang (GK259909299001-305).
Acknowledgements
None.
-
Author Contributions
Conceptualization, L. Ye, D. Chen, H. Ghorbani, G. Zhang and X. Zhou; data curation, H. Ghorbani, X. Zhou, A. Minasyan and L. Ye, D. Chen; formal analysis, B. Zheng, A. Minasyan and H. Ghorbani; funding acquisition, G. Zhang, H. Ghorbani; investigation, H. Ghorbani, G. Zhang, X. Li, X. Pan and X. Zhou; methodology, G. Zhang, A. Minasyan, L. Ye, D. Chen and H. Ghorbani; project administration, G. Zhang and H. Ghorbani; resources, G. Zhang, B. Zheng, X. Li, X. Pan and H. Ghorbani; supervision, A. Minasyan and H. Ghorbani; validation, G. Zhang, L. Ye, D. Chen, X. Pan, X. Zhou, X. Li, B. Zheng and H. Ghorbani; visualization, X. Zhou, G. Zhang and H. Ghorbani; writing—original draft preparation, B. Zheng, L. Ye, D. Chen, A. Minasyan, X. Li, G. Zhang, X. Pan, X. Zhou and H. Ghorbani; writing— review and editing, B. Zheng, X. Zhou, A. Minasyan, L. Ye, D. Chen, X. Li, X. Pan, G. Zhang and H. Ghorbani.
-
Ethical Approval
Not applicable.
-
Informed Consent
Not applicable.
-
Conflict of Interest
The authors declare no conflicts of interest.
-
Use of Large Language Models, AI and Machine Learning Tools
None declared.
-
Data Availability Statement
No additional data is available.
References
1 Shi Y, Zhou M, Chang C, Jiang P, Wei K, Zhao J, et al. Advancing precision rheumatology: applications of machine learning for rheumatoid arthritis management. Front Immunol 2024;15:1409555.10.3389/fimmu.2024.1409555Search in Google Scholar PubMed PubMed Central
2 Hügle T. Advancing Rheumatology Care Through Machine Learning. Pharmaceut Med 2024;38:87-96.10.1007/s40290-024-00515-0Search in Google Scholar PubMed PubMed Central
3 Hügle M, Omoumi P, van Laar JM, Boedecker J, Hügle T. Applied machine learning and artificial intelligence in rheumatology. Rheumatol Adv Pract 2020;4:rkaa005.10.1093/rap/rkaa005Search in Google Scholar PubMed PubMed Central
4 Handa R. Clinical Rheumatology. Springer Singapore; 2021. https://doi.org/10.1007/978-981-33-4885-1.10.1007/978-981-33-4885-1Search in Google Scholar
5 Li H, Li L, Liu C, Cheng L, Yan S, Chen H, et al. Diagnostic value of anti-citrullinated α-enolase peptide 1 antibody in patients with rheumatoid arthritis: A systematic review and meta-analysis. Int J Rheum Dis 2021;24:633-646.10.1111/1756-185X.14093Search in Google Scholar PubMed PubMed Central
6 Silvagni E, Zandonella Callegher S, Mauric E, Chiricolo S, Schreiber N, Tullio A, et al. Musculoskeletal ultrasound for treating rheumatoid arthritis to target-a systematic literature review. Rheumatology (Oxford) 2022;61:4590-4602.10.1093/rheumatology/keac261Search in Google Scholar PubMed PubMed Central
7 Johnsson H, Najm A. Synovial biopsies in clinical practice and research: current developments and perspectives. Clin Rheumatol 2021;40:25932600.10.1007/s10067-020-05512-7Search in Google Scholar PubMed PubMed Central
8 Parashar A, Rishi R, Parashar A, Rida I. Medical imaging in rheumatoid arthritis: A review on deep learning approach. Open Life Sci 2023;18:20220611.10.1515/biol-2022-0611Search in Google Scholar PubMed PubMed Central
9 Lee S, Kang S, Eun Y, Won HH, Kim H, Lee J, et al. Machine learningbased prediction model for responses of bDMARDs in patients with rheumatoid arthritis and ankylosing spondylitis. Arthritis Res Ther 2021;23:254.10.1186/s13075-021-02635-3Search in Google Scholar PubMed PubMed Central
10 Hu X, Liu X, Xu Y, Zhang S, Liu J, Zhou S. Machine learning-based prediction model integrating ultrasound scores and clinical features for the progression to rheumatoid arthritis in patients with undifferentiated arthritis. Clin Rheumatol 2025;44:649-659.10.1007/s10067-025-07304-3Search in Google Scholar PubMed
© 2025 Lusi Ye, Xin Li, Hamzeh Ghorbani, Xiaofei Zhou, Arsen Minasyan, Bolun Zheng, Xiaotian Pan, Guodao Zhang, Dan Chen, published by De Gruyter on behalf of Scholar Media Publishing
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Perspective
- Prehospital neuroprotective intervention: A critical imperative evidenced by the FRONTIER trial outcomes
- Artificial intelligence in rheumatology: A transformative perspective
- Dialysis adequacy revisited: Kt/V's blind spot for phosphorus and iodine
- Review Article
- The plakin family: Potential therapeutic targets for digestive system tumors
- Original Article
- Lnc5q21.2, a novel long intergenic RNA, sensitizes colorectal cancer cells to ATR inhibitor by activating Wnt pathway
- Effect of bone marrow mononuclear cells therapy on long-term survival of patients with liver cirrhosis: A 10-year retrospective cohort study using propensity score matching
- Associations between serum sex steroid hormone metabolites and gastric cancer and precancerous lesions in men: A 11.8-year prospective study
- Comprehensive investigation of cuproptosis-related genes in clinical features, biological characteristics, and immune microenvironment in B-cell Non-Hodgkin lymphoma
- Protocol
- A multicenter, prospective, randomized, open-label, blinded endpoint trial of intravenous thrombolysis with tenecteplase for acute non-large vessel occlusion in extended time window (OPTION): Rationale and design
- Rapid Communication
- Association between anemia and 1-year recurrence of paroxysmal atrial fibrillation after radiofrequency ablation
Articles in the same Issue
- Perspective
- Prehospital neuroprotective intervention: A critical imperative evidenced by the FRONTIER trial outcomes
- Artificial intelligence in rheumatology: A transformative perspective
- Dialysis adequacy revisited: Kt/V's blind spot for phosphorus and iodine
- Review Article
- The plakin family: Potential therapeutic targets for digestive system tumors
- Original Article
- Lnc5q21.2, a novel long intergenic RNA, sensitizes colorectal cancer cells to ATR inhibitor by activating Wnt pathway
- Effect of bone marrow mononuclear cells therapy on long-term survival of patients with liver cirrhosis: A 10-year retrospective cohort study using propensity score matching
- Associations between serum sex steroid hormone metabolites and gastric cancer and precancerous lesions in men: A 11.8-year prospective study
- Comprehensive investigation of cuproptosis-related genes in clinical features, biological characteristics, and immune microenvironment in B-cell Non-Hodgkin lymphoma
- Protocol
- A multicenter, prospective, randomized, open-label, blinded endpoint trial of intravenous thrombolysis with tenecteplase for acute non-large vessel occlusion in extended time window (OPTION): Rationale and design
- Rapid Communication
- Association between anemia and 1-year recurrence of paroxysmal atrial fibrillation after radiofrequency ablation

