Abstract
Objectives
To evaluate ultrasonographic measurements of the fetal adrenal gland in pregnancies complicated by preeclampsia (PE), with and without fetal growth restriction (FGR), and to assess the utility of adrenal gland dimensions and ratios as potential diagnostic radiologic markers for PE.
Methods
This prospective study included pregnant women between 20 and 41 weeks of gestation followed at the Obstetrics and Gynecology Department of Başakşehir Çam and Sakura City Hospital. Participants were divided into three groups: PE (n=63), PE+FGR (n=63), and healthy controls (n=65). Fetal adrenal gland total width and medulla width were measured via ultrasonography. The cortex width = (total width-medulla width) / 2 and the adrenal gland ratio (cortex width/medulla width) were calculated. Statistical analyses were conducted to evaluate differences between groups and to determine the predictive value of the adrenal gland ratio.
Results
Fetal adrenal gland total width (adrenal width), cortex width, and adrenal gland ratio were significantly increased in both the PE and PE+FGR groups compared to healthy controls (p<0.001). An adrenal gland ratio ≥2.8 was significantly associated with PE and PE+FGR (p<0.001), showing high diagnostic accuracy. The adrenal gland ratio was not correlated with gestational age.
Conclusions
Fetal adrenal gland enlargement, particularly increased cortex width, is associated with preeclampsia and may serve as a noninvasive ultrasonographic biomarker. An adrenal glandthe ratio≥2.8 demonstrates strong diagnostic potential for identifying PE and PE+FGR, offering a valuable tool for early diagnosis and management.
Introduction
Hypertensive disorders of pregnancy, particularly preeclampsia (PE), represent a significant cause of maternal and perinatal morbidity and mortality, affecting multiple organ systems and complicating approximately 8 % of pregnancies worldwide [1]. One of the serious fetal complications is fetal growth restriction (FGR), which is defined as an estimated fetal weight below the 10th percentile for gestational age. Although FGR may result from various etiologies, including maternal, fetal, and placental factors, the underlying pathophysiology most commonly involves impaired utero-placental perfusion, leading to suboptimal fetal nutrient and oxygen delivery. These conditions not only pose significant risks in utero life, may also cause preterm birth, neonatal intensive care admission, and long-term neurodevelopmental impairment [2].
PE and FGR share a common pathological basis. The development of PE is a multifactorial process characterized by abnormal trophoblast invasion and failed remodeling of the spiral arterioles. This results in high-resistance, low-flow utero-placental circulation and subsequent placental hypoxia. Hypoxic stress induces the release of pro-inflammatory cytokines and antiangiogenic factors into the maternal circulation, triggering systemic endothelial dysfunction and the clinical manifestations of PE [3]. The hypothalamic-pituitary-adrenal (HPA) axis matures in late gestation, but adverse intrauterine conditions such as hypoxia may induce its precocious activation. This is characterized by increased circulating levels of adrenocorticotropic hormone (ACTH) and adrenal steroids [4].
The fetal adrenal gland plays a critical role in regulating the endocrine response to intrauterine stress. Histologically distinct from the adult adrenal gland, the fetal adrenal gland consists of two distinct zones: the definitive zone and the fetal zone, with the latter being highly steroidogenically active. As early as gestational day 33, the fetal adrenal begins to differentiate, and by the end of the first trimester, the fetal zone becomes prominent. This zone synthesizes large quantities of adrenal androgens, which are subsequently converted into estrogens by the placenta. Sonographically, the fetal adrenal gland appears as an oval structure in the longitudinal plane and lentil-shaped structure in the transverse plane. Beginning from the 20th gestational week, it can be reliably visualized as a hypoechoic rim surrounding a central echogenic line [5], 6].
Moreover, studies have demonstrated that the fetal adrenal gland undergoes dynamic morphological changes in response to stress, including alterations in size and vascular perfusion, particularly in cases of preterm labor and growth restriction. Toward term, the fetal zone comprises approximately 80 % of the adrenal gland volume, but this decreases rapidly postpartum, with a ∼50 % reduction in gland size and complete regression of the fetal zone by the end of the first postnatal year [7]. Autopsy studies further suggest that adrenal gland weights are higher in fetuses delivered preterm due to spontaneous labor compared to those delivered early for other reasons, such as placental abruption or maternal hemorrhage [8].
Given the involvement of the fetal adrenal gland in the stress response and its measurable changes in size and morphology under pathological conditions, ultrasonographic evaluation of this organ may provide valuable insights into fetal well-being. The aim of the present study is to assess fetal adrenal gland dimensions in pregnancies complicated by preeclampsia and preeclampsia with coexisting fetal growth restriction, and to investigate the potential role of these measurements as a non-invasive radiologic marker. To evaluate differences in the fetal adrenal gland, we included three groups: pregnancies complicated by preeclampsia, preeclampsia with intrauterine growth restriction, and healthy pregnancies. Fetal adrenal gland measurements were performed using ultrasonography in all participants.
Materials and methods
This study was designed as a prospective observational study. Pregnant women between 20 and 41weeks of gestation who presented for routine obstetric or perinatology outpatient visits at Başakşehir Çam and Sakura City Hospital, Istanbul, Türkiye, Department of Obstetrics and Gynecology, were informed about the study and provided written informed consent. The research was granted ethical approval by the Ethics Committee of Basaksehir Cam ve Sakura City Hospital, with decision number KAEK/14.02.2024.103, and was conducted in compliance with Declaration of Helsinki.
Demographic and clinical data including maternal age, comorbidities, gravida-parity status, and laboratory parameters (spot urine protein, AST, ALT, urea, creatinine) were recorded. Fetal biometric and Doppler data including estimated fetal weight (EFW), umbilical artery Doppler indices, total fetal adrenal gland width, and adrenal medulla width were obtained via ultrasonography as shown in Figure 1. Cortical width and fetal adrenal gland ratio (FAGR) were calculated from these measurements. Cortical width was computed by subtracting the adrenal medulla width from the total gland width and dividing the result by two. FAGR was defined as the ratio of total gland width to adrenal medulla width.
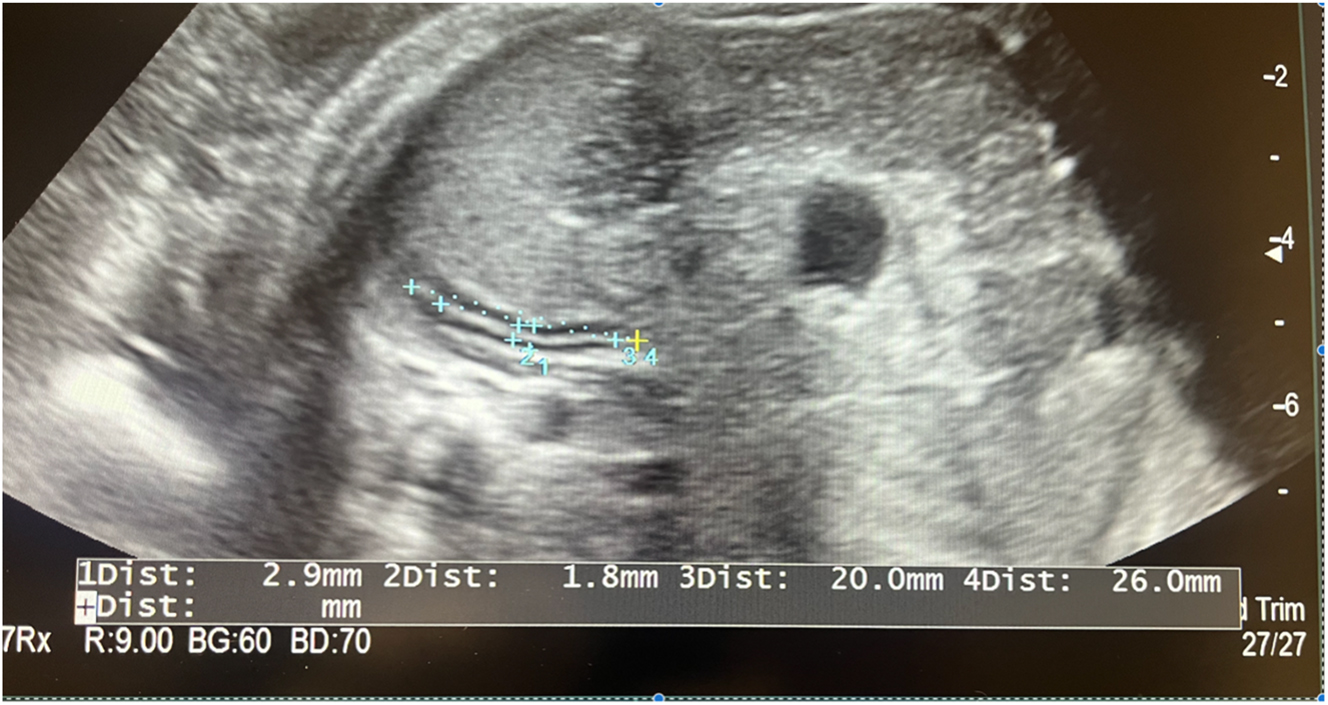
Ultrasound image demonstrating the measurement of the fetal adrenal gland.
All ultrasonographic measurements were performed using the Arietta 65 Diagnostic Ultrasound System (Hitachi, Tokyo, Japan) with a 3.5–5.0 MHz transabdominal convex probe. The adrenal gland was visualized in the transverse plane at its narrowest section. B-mode imaging was magnified to ensure precise measurements. The ultrasound measurements were conducted based on the study by Sharma et al. [6].
Preeclampsia diagnosis was based on the presence of proteinuria detected in spot urine samples. Fetal growth restriction was diagnosed when the EFW was below the 10th percentile for gestational age. The study population consisted of pregnant women between 20 and 41 weeks of gestation who met the inclusion criteria, and voluntarily agreed to participate. Sample size was calculated using the G-Power 3.1.9.7 software. With an alpha error of 0.05, power of 0.80, and effect size (d) of 0.4, the minimum sample size was determined to be 189 patients, with 63 participants in each of the three study groups for analysis via ANOVA.
Inclusion criteria were: pregnant women between 20 and 41 weeks with no pregnancy-related systemic diseases, those with isolated PE, and those diagnosed with PE + FGR followed in the perinatology clinic. Exclusion criteria included multiple pregnancies, gestations below 20 weeks, pregnancies with fetal anomalies, maternal age under 18 years, and pregnancies complicated by conditions such as gestational diabetes mellitus (GDM), pregestational diabetes, and other hypertensive disorders of pregnancy other than PE. PE and PE + FGR patients without proteinuria on spot urine testing were also excluded.
Statistical analysis was performed using SPSS version 27. Quantitative variables were expressed as mean, standard deviation, median, minimum, and maximum values; qualitative variables were summarized as frequencies and percentages. Normality of distribution was assessed using the Shapiro-Wilk test and box plot graphics. For comparisons of quantitative variables among groups, one-way ANOVA test was used. Post-hoc analysis were done by using Tukey test. Relationships between variables were evaluated using Pearson correlation analysis according to data distribution. The Chi-square test was used for comparisons of qualitative variables. Diagnostic screening tests and ROC curve analysis were conducted to assess the predictive value of adrenal gland ratios for PE and PE + FGR. Results were evaluated within a 95 % confidence interval, with statistical significance set at p<0.05.
Results
This study was conducted between February 2024 and December 2024 at , Başakşehir Çam and Sakura City Hospital, Istanbul, Türkiye, including a total of 191 pregnant women. The participants’ ages ranged from 18 to 44 years, with a mean age of 29.40 ± 5.91 years.
The demographic and clinical characteristics of the study population are presented in Table 1. When examining the gravida status of the participants, 38.2 % (n=73) were primigravida, 25.7 % (n=49) had two pregnancies, 14.7 % (n=28) had three pregnancies, and 21.5 % (n=41) had four or more pregnancies. Regarding parity, 42.9 % (n=82) were nulliparous, 28.8 % (n=55) had one previous delivery, 13.6 % (n=26) had two, and 14.7 % (n=28) had three or more previous deliveries. The mode of delivery was distributed: 50.5 % (n=55) underwent vaginal delivery and 49.5 % (n=54) underwent cesarean section. Gestational age at the time of assessment ranged from 21 to 41.4 weeks, with a mean of 33.87 ± 4.27 weeks. Chronic medical conditions were present in 24.1 % (n=46) of the participants.
Demographic and clinical characteristics of entire patients.meth
| n (%) | |
|
|
|
| Age | |
| Avr ± SD | 29.40 ± 5.91 |
| Median (Min-Max) | 29 (18–44) |
| Gravidity | |
| 1 gravida | 73 (38.2) |
| 2 gravida | 49 (25.7) |
| 3 gravida | 28 (14.7) |
| ≥4 gravida | 41 (21.5) |
| Parity | |
| 0 | 82 (42.9) |
| 1 parity | 55 (28.8) |
| 2 parity | 26 (13.6) |
| ≥3 parity | 28 (14.7) |
| Mode of delivery | |
| Vaginal | 55 (50.5) |
| C-section | 54 (49.5) |
| Gestational age | |
| Avr ± SD | 33.87 ± 4.27 |
| Median (Min-Max) | 35.3 (21–41.4) |
| Chronic ilness | |
| No | 145 (75.9) |
| Yes | 46 (24.1) |
| Group | |
| PE | 63 (33.0) |
| PE + FGR | 63 (33.0) |
| Control | 65 (34.0) |
-
Avr, average; SD, standard deviation; Min, minimum; Max, maximum; Gravida, number of pregnancies; parity, number of births after 20 weeks of gestation; vaginal, vaginal delivery; C-section, cesarean section; no, absence of chronic illness; yes, presence of chronic illness; PE, preeclampsia; FGR, fetal growth restriction; control, control group.
Comparisons of demographic and clinical characteristics between the PE, PE+FGR, and control groups are shown in Table 2. Participants were divided into three groups: 33 % (n=63) had preeclampsia, 33 % (n=63) had PE with intrauterine growth restriction, and 34 % (n=65) constituted the control group. There was a statistically significant difference in maternal age among the groups (p=0.001). Post-hoc pairwise comparisons revealed that participants in the PE group were significantly older than those in the PE+FGR and control groups (p=0.043; p=0.001). Gravida count differed significantly among the groups (p=0.002). The rate of two pregnancies was higher in the control group compared to the PE and PE+FGR groups. The rate of three pregnancies was higher in the PE+FGR group than in the PE group. The prevalence of four or more pregnancies was higher in the PE group than in both the PE+FGR and control groups. Parity count also showed significant intergroup differences (p=0.006). Nulliparity was more common in the PE+FGR group than in the control group. The rate of one previous delivery was higher in the control group compared to the PE and PE+FGR groups. The presence of chronic disease differed significantly among the groups (p=0.001). The control group had a lower prevalence of chronic diseases compared to the PE and PE+FGR groups. Among the latter two, the PE group had a higher rate of chronic conditions than the PE+FGR group. No statistically significant differences were found between groups in terms of mode of delivery and gestational age (p=0.207; p=0.108).
Demographic and clinical charecteristics in patients with PE, PE and IUGR, and control population.
| PE (n=63) | PE + FGR (n=63) | Control (n=65) | p-Value | |
|---|---|---|---|---|
| Age | ||||
| Avr ± SD | 31.71 ± 6.45 | 29.22 ± 5.81 | 27.34 ± 4.58 | b0.001c |
| Median (Min-Max) | 32 (18–44) | 28 (19–42) | 27 (19–41) | |
| Gravidity | ||||
| 1 gravida | 24 (38.1) | 29 (46.0) | 20 (30.8) | a0.002c |
| 2 gravida | 13 (20.6) | 12 (19.0) | 24 (36.9) | |
| 3 gravida | 4 (6.3) | 14 (22.2) | 10 (15.4) | |
| ≥4 gravida | 22 (34.9) | 8 (12.7) | 11 (16.9) | |
| Parity | ||||
| 0 | 27 (42.9) | 33 (52.4) | 22 (33.8) | a0.006c |
| 1 parity | 11 (17.5) | 16 (25.4) | 28 (43.1) | |
| 2 parity | 11 (17.5) | 10 (15.9) | 5 (7.7) | |
| ≥3 parity | 14 (22.2) | 4 (6.3) | 10 (15.4) | |
| Mode of delivery | ||||
| Vaginal | 20 (55.6) | 11 (36.7) | 24 (55.8) | a0.207 |
| C-section | 16 (44.4) | 19 (63.3) | 19 (44.2) | |
| Gestational age | ||||
| Avr ± SD | 34.59 ± 4.04 | 33.01 ± 3.95 | 34.01 ± 4.67 | b0.108 |
| Median (Min-Max) | 36 (23.4–41.4) | 33.7 (23.1–39.6) | 35.6 (21–39.9) | |
| Chronic ilness | ||||
| No | 37 (58.7) | 49 (77.8) | 59 (90.8) | a0.001c |
| Yes | 26 (41.3) | 14 (22.2) | 6 (9.2) | |
-
aPearson Chi-square test. bOne-way ANOVA test. cp<0.01. Avr, average; SD, standard deviation; Min, minimum; Max, maximum; gravida, number of pregnancies; parity, number of births after 20 weeks of gestation; vaginal, vaginal delivery; C-section, cesarean section; no, absence of chronic illness; yes, presence of chronic illness. Bold p-values indicate statistical significance (p<0.005).
Medulla width did not differ significantly among the groups (p=0.249). However, a statistically significant difference was found in adrenal gland width (p=0.001). Post-hoc analysis revealed that the adrenal gland width was significantly higher in the PE group compared to both the PE+FGR and control groups (p=0.015; p=0.001). A significant difference in adrenal cortex width was also observed between the groups (p=0.001). Post-hoc pairwise analysis showed that the control group had significantly lower cortex width than the PE and PE+FGR groups (p=0.001; p=0.019), and the PE group showed higher values than the PE+FGR group (p=0.014). Adrenal gland ratio significantly differed among the groups (p=0.001). Post-hoc pairwise analysis showed that the control group had significantly lower ratios compared to the PE and PE+FGR groups (p=0.001; p=0.001).
Table 3 shows the correlation between gestational age and ultrasonographic measurements. A statistically significant negative correlation was found between gestational age and umbilical artery pulsatility index (Umb A PI) values (r = −0.419; p=0.001), indicating a decrease in Umb A PI with advancing gestational age. A significant positive correlation was observed between gestational age and medulla width (r=0.360; p=0.001), adrenal gland width (r=0.385; p=0.001), and cortex width (r=0.322; p=0.001). However, no statistically significant correlation was found between gestational age and adrenal gland ratio (p=0.410).
The relationship between gestational age and ultrasonographic measurement values.
| Umb A PI | |
| r | −0.419 |
| p | 0.001a |
| Medulla width (w) | |
| r | 0.360 |
| p | 0.001a |
| Adrenal gland width (W) | |
| r | 0.385 |
| p | 0.001a |
| Cortex width (W-w/2) | |
| r | 0.322 |
| p | 0.001a |
| Adrenal gland ratio (W/w) | |
| r | 0.060 |
| p | 0.410 |
-
r, Pearson’s correlation test; ap<0.01; Umb A PI, umbilical artery pulsatility index; medulla width (w), width of the adrenal gland medulla; adrenal gland width (W), total width of the adrenal gland; cortex width (W-w/2), calculated cortex width; adrenal gland ratio (W/w), ratio of adrenal gland width to medulla width. Bold p-values indicate statistical significance (p<0.05).
The diagnostic performance of the adrenal gland ratio for predicting PE and PE+FGR is summarized in Table 4. For predicting the presence of preeclampsia, an adrenal gland ratio cutoff value of 2.8 yielded a sensitivity of 68.25 %, specificity of 72.31 %, positive predictive value of 70.5 %, and negative predictive value of 70.1 %. The area under the curve (AUC) on ROC analysis was 73.9 %, with a standard error of 4.4 % as shown in Figure 2. There was a statistically significant association between an adrenal gland ratio≥2.8 and the presence of PE (p=0.001). The risk of detecting PE was 4.5 times higher in cases with an adrenal gland ratio of 2.8 or greater. The odds ratio was 4.500 (95 % CI: 2.994–6.763). In predicting the presence of PE+FGR, a fetal adrenal gland ratio cut-off value of 2.8 yielded a sensitivity of 68.25 %, specificity of 72.31 %, positive predictive value of 70.5 %, and negative predictive value (NPV) of 70.1 %. AUC was calculated as 72.3 %, with a standard error of 4.5 %. A statistically significant association was found between a gland ratio of 2.8 and the presence of PE+FGR (p=0.001). The risk of detecting PE+FGR was found to be 4.5 times higher in cases with a gland ratio equal to or greater than 2.8. The odds ratio for the adrenal gland ratio was 4.500 (95 % CI: 2.994–6.763).
Diagnostic screening tests and ROC curve results for predicting the presence of PE and PE+IUGR based on adrenal gland ratio.
| Diagnostic scan | ROC curve | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| Cut off | Sensitivity | Specificity | Positive predictive value | Negative predictive value | Area | 95 % confidence interval | ||
| PE | ||||||||
|
|
||||||||
| Adrenal gland ratio | ≥2.8 | 68.25 | 72.31 | 70.5 | 70.1 | 0.739 | 0.652–0.826 | 0.001b |
|
|
||||||||
| PE+FGR | ||||||||
|
|
||||||||
| Adrenal gland ratio | ≥2.8 | 68.25 | 72.31 | 70.5 | 70.1 | 0.723 | 0.634–0.812 | 0.001b |
-
≥2.8=threshold value for predicting PE or PE+FGR. Bold AUC values represent the overall diagnostic accuracy of the test. Bold p-values indicate statistical significance (p<0.05).
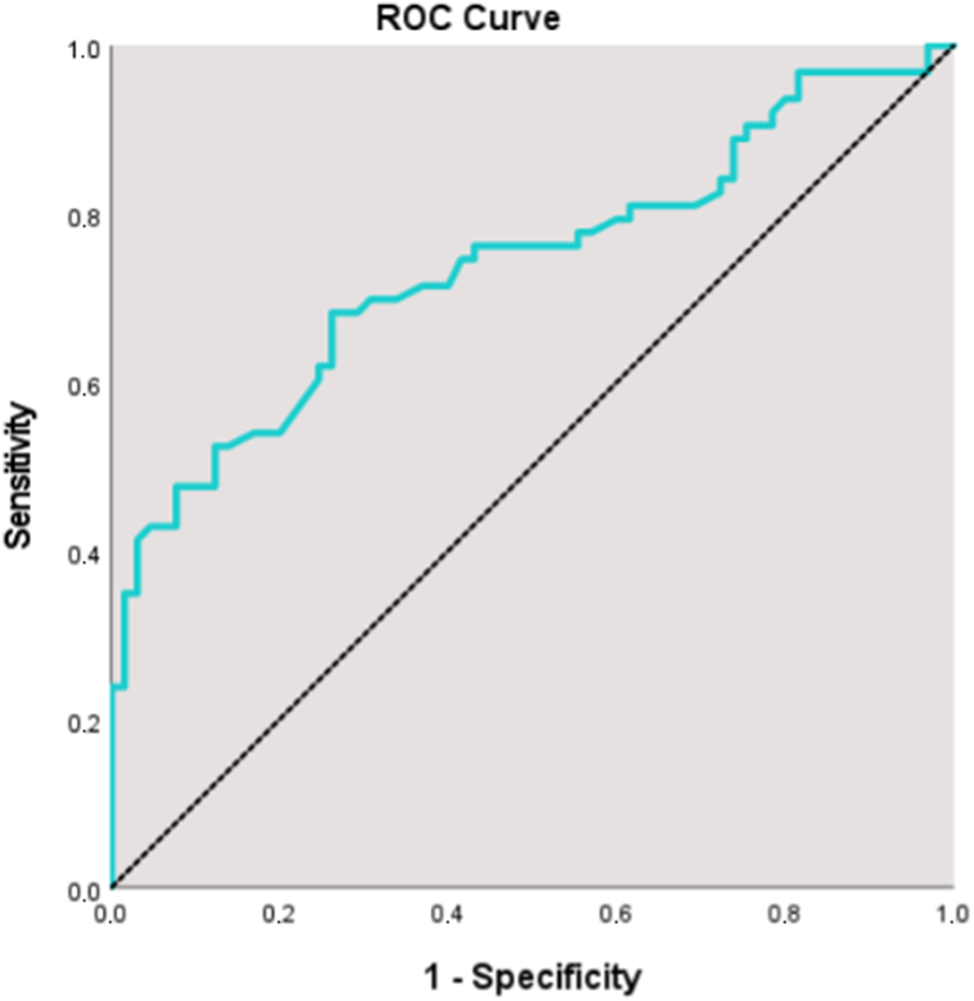
ROC analysis for the fetal adrenal gland ratio in predicting preeclampsia. The curve demonstrates the diagnostic performance of the adrenal gland ratio.
Discussion
Placental insufficiency, characterized by hypoxia, inflammation, and endothelial dysfunction, is one of the fundamental mechanisms in the pathogenesis of preeclampsia [3]. Placental insufficiency may affect fetal circulation and lead to early activation of the HPA axis [9]. All living organisms respond to stress as a survival mechanism. When the HPA axis is stimulated by a stressor, the secretion of corticotropin-releasing hormone (CRH) triggers the anterior pituitary gland to release ACTH, which in turn stimulates the adrenal cortex to produce glucocorticoids [10]. Under stress conditions, fetal adrenal cells release growth factors, resulting in hypertrophy of the fetal adrenal region [11]. Hypoxia-induced transcription factors play a key role in the mechanism of preeclampsia. Adaptive mechanisms of fetal circulation may undergo alterations under the conditions of preeclampsia and fetal growth restriction [12]. It is proposed that this hypoxic environment creates a fetal stress response that activates the HPA axis prematurely, potentially enhancing adrenal steroid production and accelerating fetal maturation [5]. Consistent with our findings, the fetal adrenal gland ratio, total gland width, and cortical width were observed to increase in PE and PE+FGR cases, reflecting a physiological response to this mechanism. This suggests that fetal stress response, as evidenced by adrenal gland measurements, may serve as a significant indicator in pregnancies complicated by preeclampsia.
In our study, a fetal adrenal gland ratio of ≥2.8 was found to be statistically significant in predicting PE and PE+FGR (p<0.001). This suggests that sonographic evaluation of the fetal adrenal gland may serve as a potential radiological marker for preeclampsia and fetal growth restriction. In a study by Heese et al., which included 343 controls and 63 FGR fetuses between 20 and 41 weeks of gestation, both the fetal adrenal gland ratio and cortical width were increased in FGR pregnancies. Moreover, this study demonstrated that the adrenal gland ratio was independent of gestational age [13]. Similarly, our findings revealed increased adrenal cortex width, total gland width, and adrenal gland ratio in both PE and PE+FGR groups. Additionally, while total gland width and cortical width showed a growth trend with gestational age, the fetal adrenal gland ratio appeared to be independent of gestational age. Therefore, assessment of fetal adrenal gland ratio may be an important parameter in the monitoring of high-risk pregnancies regardless of gestational age.
Cortisol synthesized by the fetal adrenal gland contributes to the maturation of several organs, including the lungs. The hormone synthesis and relative hypertrophy of the fetal adrenal gland in response to CRH also play a role in the mechanism of labor. In a study by Sharma et al., the fetal adrenal gland ratio was found to be a more valuable predictor of induction success in pregnancies over 40 weeks when compared with the Bishop score [6]. Furthermore, a study by Turan et al. demonstrated that growth in the fetal adrenal region was a better predictor of preterm labor than transvaginal cervical length [14]. These studies, together with our data, support the notion that the fetal adrenal gland becomes more active under stress conditions, leading to hypertrophy. In parallel with our findings, enlargement of the fetal adrenal gland emerges as a response to intrauterine stress. As evidenced by these studies, fetal adrenal gland hypertrophy is not limited to PE but may be observed in various intrauterine stress conditions. Hence, detecting fetal adrenal gland enlargement may serve as an early indicator of increased risk for FGR, PE, or preterm birth.
Animal studies have demonstrated that hypoxic fetuses redistribute blood flow from other organs to the brain, heart, and adrenal glands, resulting in increased perfusion and decreased vascular resistance in these organs. The reduction in middle cerebral artery resistance observed in chronic hypoxia is an example of the brain-sparing effect. In a study by Dubiel et al., Doppler waveforms of the middle adrenal artery and middle cerebral artery were recorded in 102 hypertensive pregnancies. Among these, 64 cases exhibited adrenal-sparing effects, while 32 showed brain-sparing effects. Notably, all cases with perinatal mortality exhibited adrenal-sparing effects. A positive correlation was also found between fetal adrenal PI and umbilical artery pH [15]. These findings underscore the pivotal role of the fetal adrenal gland in stress responses and its adaptation to hypertensive changes. In line with our study, the increases in adrenal gland ratio, cortical width, and total gland width observed in PE may be related to increased adrenal blood flow. This increased perfusion likely enhances the metabolic activity of the gland, contributing to its hypertrophy.
In animal models, insulin has been shown to inhibit placental aromatase enzyme activity, leading to compensatory hypertrophy and volume increase in the fetal adrenal gland [16]. Therefore, pregnancies complicated by GDM or diabetes mellitus were excluded from our study. A study by Hetkamp et al. revealed increased adrenal cortex thickness, adrenal medulla size, total adrenal gland width, and adrenal gland ratio in pregnancies complicated by GDM. Consistent with our findings, the fetal adrenal gland ratio was also shown to be independent of gestational age [17]. This highlights the potential utility of fetal adrenal gland ratio as a gestational age-independent screening tool.
These findings may contribute to the development of novel approaches in the management of preeclampsia. Further research is needed to explore this relationship in greater detail and to elucidate the clinical relevance of fetal adrenal gland assessment in pregnancies complicated by preeclampsia and fetal growth restriction. Detecting fetuses affected by placental dysfunction remains a clinical challenge. Therefore, measurement of fetal adrenal gland dimensions and adrenal gland ratio may offer a valuable perspective in identifying at-risk fetuses. Since the positive predictive values we observed may vary depending on the population, further studies are needed to strengthen and validate our findings.
Limitations
This study has several important limitations. First, being a single-center study, the generalizability of our findings is inherently limited. Additionally, there may be a lack of standardization among ultrasonographic techniques used for fetal adrenal gland measurements. The use of 2D ultrasound, in particular, introduces potential variability between operators, which may contribute to discrepancies in outcomes across different centers. Future studies with larger sample sizes and multicenter designs are necessary to better elucidate the relationship between fetal adrenal gland size and preeclampsia. Another limitation is the heterogeneity in gestational age at the time of measurement. While this variability may pose a constraint in interpreting the findings, it also offers a potential advantage by providing preliminary insights applicable to a broader range of gestational weeks, thereby laying the groundwork for future investigations. It should be noted that positive predictive value is highly dependent on disease prevalence; in our cohort, the prevalence of PE and PE+FGR was 66.7 %, which may have inflated the observed positive predictive value (PPV). In populations with lower PE and PE+FGR prevalence, the PPV of the adrenal gland ratio would be expected to decrease accordingly.
Interestingly, the ROC analyses for predicting PE and PE+FGR based on adrenal gland ratio yielded nearly identical results. This can be explained by the remarkably similar distributions of adrenal gland ratio values in both groups, as demonstrated in Table 5. Although PE+FGR represents a more severe clinical condition, both groups share common pathophysiological features that may affect fetal adrenal gland development in a similar manner. The overlapping radiologic profiles may have limited discriminative capacity of adrenal gland ratio between these two conditions. Therefore, while adrenal gland ratio shows potential in distinguishing pathological from normal pregnancies, its role in differentiating between PE and PE+FGR requires further investigation in larger and more heterogeneous populations.
Distribution of adrenal gland measurements by groups.
| PE (n=63) | PE + FGR (n=63) | Control (n=65) | ap-Value | |
|---|---|---|---|---|
| Umb A PI | ||||
| Avr ± SD | 0.95 ± 0.17 | 0.92 ± 0.17 | 0.92 ± 0.20 | 0.620 |
| Median (Min-Max) | 0.9 (0.6–1.6) | 0.9 (0.5–1.3) | 0.9 (0.6–1.6) | |
| Medulla width (w) | ||||
| Avr ± SD | 1.38 ± 0.54 | 1.24 ± 0.50 | 1.31 ± 0.37 | 0.249 |
| Median (Min-Max) | 1.3 (0.5–2.9) | 1.1 (0.5–3.1) | 1.3 (0.7–2.2) | |
| Adrenal gland width (W) | ||||
| Avr ± SD | 4.35 ± 1.32 | 3.73 ± 1.37 | 3.34 ± 0.94 | 0.001b |
| Median (Min-Max) | 4.4 (2.2–8.9) | 3.6 (1.6–10.2) | 3.2 (1.7–5.4) | |
| Cortex width (W-w/2) | ||||
| Avr ± SD | 1.48 ± 0.53 | 1.24 ± 0.52 | 1.02 ± 0.34 | 0.001b |
| Median (Min-Max) | 1.4 (0.8–3.3) | 1.2 (0.6–3.6) | 1 (0.4–1.7) | |
| Adrenal gland ratio (W/w) | ||||
| Avr ± SD | 3.37 ± 1.01 | 3.15 ± 0.84 | 2.60 ± 0.48 | 0.001b |
| Median (Min-Max) | 3.2 (1.8–6.4) | 3.1 (1.8–7.3) | 2.6 (1.7–4.1) | |
-
aOne-way ANOVA test. bp<0.01, Avr, average; SD, standard deviation; Min, minimum; Max, maximum; Umb A PI, umblical artery pulsatility index; medulla width (w), width of the adrenal gland medulla; adrenal gland width (W), total width of the adrenal gland; cortex width (W-w/2), calculated cortex width; adrenal gland ratio (W/w), ratio of adrenal gland width to medulla width. Bold p-values indicate statistical significance (p<0.05).
On the other hand, our study possesses several strengths. Notably, no statistically significant differences were found among the gestational age subgroups, which supports the reliability of our data. Furthermore, the exclusion of cases with GDM, pregestational diabetes mellitus, and isolated fetal growth restriction helped to minimize potential confounding factors that could independently influence fetal adrenal gland size.
Conclusions
The total adrenal gland width and cortical width of the fetal adrenal gland are considered important indicators of the fetal stress response in preeclamptic pregnancies. Notably, a fetal adrenal gland ratio (≥2.8) demonstrates high sensitivity and specificity in predicting preeclampsia and PE with fetal growth restriction, suggesting that ultrasonographic evaluation may serve as a valuable tool in the management of preeclampsia. Our findings support the notion that early activation of the fetal hypothalamic-pituitary-adrenal axis during the course of preeclampsia may represent a homeostatic response mechanism to a hypoxic intrauterine environment.
However, larger-scale, prospective studies are required to facilitate the integration of these parameters into clinical practice. Future research should focus on the longitudinal assessment of fetal adrenal gland morphology across different trimesters, in conjunction with other established markers used in the diagnosis of preeclampsia. Furthermore, the relationship between fetal adrenal gland measurements and neonatal as well as long-term outcomes should be investigated.
In conclusion, fetal adrenal gland measurements may represent a significant radiological marker in the diagnosis and monitoring of preeclampsia. Further studies are necessary to enable their incorporation into clinical protocols. Monitoring fetal adrenal gland morphology could contribute to the early detection and effective management of preeclampsia, thereby supporting maternal and fetal health.
-
Research ethics: The research was granted ethical approval by the Ethics Committee of Başakşehir Çam and Sakura City Hospital, Istanbul, Türkiye, with decision number KAEK/14.02.2024.103, and was conducted in compliance with Declaration of Helsinki.
-
Informed consent: Informed consent was obtained from all individuals included in this study, or their legal guardians or wards.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
1. Hypertension in pregnancy. Report of the American college of obstetricians and gynecologists’ task force on hypertension in pregnancy. Obstet Gynecol 2013;122:1122–31.Search in Google Scholar
2. ACOG practice bulletin no. 134: fetal growth restriction. Obstet Gynecol 2013;121:1122–33. https://doi.org/10.1097/01.AOG.0000429658.85846.f9.Search in Google Scholar PubMed
3. Burton, GJ, Redman, CW, Roberts, JM, Moffett, A. Pre-eclampsia: pathophysiology and clinical implications. BMJ 2019;366:l2381. https://doi.org/10.1136/bmj.l2381.Search in Google Scholar PubMed
4. Challis, JR, Sloboda, D, Matthews, SG, Holloway, A, Alfaidy, N, Patel, FA, et al.. The fetal placental hypothalamic-pituitary-adrenal (HPA) axis, parturition and post natal health. Mol Cell Endocrinol 2001;185:135–44. https://doi.org/10.1016/s0303-7207(01)00624-4.Search in Google Scholar PubMed
5. Rainey, WE, Rehman, KS, Carr, BR. Fetal and maternal adrenals in human pregnancy. Obstet Gynecol Clin N Am. 2004;31:817–35, https://doi.org/10.1016/j.ogc.2004.08.006.Search in Google Scholar PubMed
6. Sharma, R, Kumari, A, Tandon, A, Suneja, A, Guleria, K. Ultrasonographic measurement of fetal adrenal gland size for the prediction of success of induction of labor among primigravida beyond 40 weeks gestation. J Obstet Gynaecol India 2023;73:406–13. https://doi.org/10.1007/s13224-023-01774-8.Search in Google Scholar PubMed PubMed Central
7. Helfer, TM, Rolo, LC, Okasaki, NAde BM, de Castro Maldonado, AA, Rabachini Caetano, AC, Perez Zamarian, AC, et al.. Reference ranges of fetal adrenal gland and fetal zone volumes between 24 and 37 + 6 weeks of gestation by three-dimensional ultrasound. J Matern-Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet 2017;30:568–73. https://doi.org/10.1080/14767058.2016.1178226.Search in Google Scholar PubMed
8. Anderson, AB, Laurence, KM, Davies, K, Campbell, H, Turnbull, AC. Fetal adrenal weight and the cause of premature delivery in human pregnancy. J Obstet Gynaecol Br Commonw 1971;78:481–8. https://doi.org/10.1111/j.1471-0528.1971.tb00305.x.Search in Google Scholar PubMed
9. Challis, JR, Lye, SJ, Gibb, W, Whittle, W, Patel, F, Alfaidy, N. Understanding preterm labor. Ann N Y Acad Sci 2001;943:225–34. https://doi.org/10.1111/j.1749-6632.2001.tb03804.x.Search in Google Scholar PubMed
10. Nicolaides, NC, Kyratzi, E, Lamprokostopoulou, A, Chrousos, GP, Charmandari, E. Stress, the stress system and the role of glucocorticoids. Neuroimmunomodulation 2015;22:6–19. https://doi.org/10.1159/000362736.Search in Google Scholar PubMed
11. Beshay, VE, Carr, BR, Rainey, WE. The human fetal adrenal gland, corticotropin-releasing hormone, and parturition. Semin Reprod Med 2007;25:14–20. https://doi.org/10.1055/s-2006-956772.Search in Google Scholar PubMed
12. Rana, S, Lemoine, E, Granger, JP, Karumanchi, SA. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res 2019;124:1094–112. https://doi.org/10.1161/circresaha.118.313276.Search in Google Scholar PubMed
13. Heese, S, Hammer, K, Möllers, M, Köster, HA, Falkenberg, MK, Eveslage, M, et al.. Adrenal gland size in growth restricted fetuses. J Perinat Med 2018;46:900–4. https://doi.org/10.1515/jpm-2017-0339.Search in Google Scholar PubMed
14. Turan, OM, Turan, S, Funai, EF, Buhimschi, IA, Campbell, CH, Bahtiyar, OM, et al.. Ultrasound measurement of fetal adrenal gland enlargement: an accurate predictor of preterm birth. Am J Obstet Gynecol 2011;204:311.e1–10. https://doi.org/10.1016/j.ajog.2010.11.034.Search in Google Scholar PubMed
15. Dubiel, M, Breborowicz, GH, Marsal, K, Gudmundsson, S. Fetal adrenal and middle cerebral artery Doppler velocimetry in high-risk pregnancy. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol 2000;16:414–8. https://doi.org/10.1046/j.1469-0705.2000.00278.x.Search in Google Scholar PubMed
16. Albrecht, ED, Aberdeen, GW, Pepe, GJ. Estrogen elicits cortical zone-specific effects on development of the primate fetal adrenal gland. Endocrinology 2005;146:1737–44. https://doi.org/10.1210/en.2004-1124.Search in Google Scholar PubMed
17. Hetkamp, T, Hammer, K, Möllers, M, Köster, HA, Falkenberg, MK, Kerschke, L, et al.. Fetal adrenal gland size in gestational diabetes mellitus. J Perinat Med 2019;47:941–6. https://doi.org/10.1515/jpm-2019-0146.Search in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Reviews
- Fetal neurobehavior and consciousness: a systematic review of 4D ultrasound evidence and ethical challenges
- Prenatal maternal stress and long-term neurodevelopmental outcomes: a narrative review
- From exencephaly to anencephaly: a catastrophic continuum of neural tube defects from embryogenesis to ultrasonographic diagnosis
- Opinion Papers
- Fetoception: a window into maternal interoception?
- Rationale for the use of fetal ventriculosubgaleal shunts for the treatment of aqueduct stenosis
- Original Articles – Obstetrics
- The fetal occiput-spine angle measurement during first stage of labor as a predictor for vaginal delivery, a systematic review and meta-analysis
- Hemorrhage-related maternal morbidity of secondary compared to primary postpartum hemorrhage
- First-trimester maternal serum PAPP-A levels and hyperemesis gravidarum: unraveling the link – a meta-analysis
- Antepartum cerebroplacental ratio in low risk pregnancy and its relationship with adverse perinatal outcome: a prospective cohort study
- Liver fibrosis markers as predictors of adverse outcomes in pregnancy-related hypertensive disorders
- Endocrine disrupting chemicals: translating mechanisms into perinatal risk assessment
- Low uterine segment thickness in the prediction of cesarean delivery after induction of labor
- Original Articles – Fetus
- Fetal adrenal gland size in preeclamptic pregnancies with and without fetal growth restriction: an ultrasonographic assessment
- Evaluation of safety and performance of CentaFlow™ in the assessment of fetal growth restriction – a randomized trial and prospective cohort study
- Advantages of fully automated AI-enhanced algorithm (5D CNS+™) for generating a fetal neurosonogram in clinical routine
- Reference ranges for fetal tricuspid and mitral annular plane systolic excursions
- FetalDenseNet: multi-scale deep learning for enhanced early detection of fetal anatomical planes in prenatal ultrasound
- Original Articles – Neonates
- Incidence and mortality trends in congenital diaphragmatic hernia in the United States
Articles in the same Issue
- Frontmatter
- Reviews
- Fetal neurobehavior and consciousness: a systematic review of 4D ultrasound evidence and ethical challenges
- Prenatal maternal stress and long-term neurodevelopmental outcomes: a narrative review
- From exencephaly to anencephaly: a catastrophic continuum of neural tube defects from embryogenesis to ultrasonographic diagnosis
- Opinion Papers
- Fetoception: a window into maternal interoception?
- Rationale for the use of fetal ventriculosubgaleal shunts for the treatment of aqueduct stenosis
- Original Articles – Obstetrics
- The fetal occiput-spine angle measurement during first stage of labor as a predictor for vaginal delivery, a systematic review and meta-analysis
- Hemorrhage-related maternal morbidity of secondary compared to primary postpartum hemorrhage
- First-trimester maternal serum PAPP-A levels and hyperemesis gravidarum: unraveling the link – a meta-analysis
- Antepartum cerebroplacental ratio in low risk pregnancy and its relationship with adverse perinatal outcome: a prospective cohort study
- Liver fibrosis markers as predictors of adverse outcomes in pregnancy-related hypertensive disorders
- Endocrine disrupting chemicals: translating mechanisms into perinatal risk assessment
- Low uterine segment thickness in the prediction of cesarean delivery after induction of labor
- Original Articles – Fetus
- Fetal adrenal gland size in preeclamptic pregnancies with and without fetal growth restriction: an ultrasonographic assessment
- Evaluation of safety and performance of CentaFlow™ in the assessment of fetal growth restriction – a randomized trial and prospective cohort study
- Advantages of fully automated AI-enhanced algorithm (5D CNS+™) for generating a fetal neurosonogram in clinical routine
- Reference ranges for fetal tricuspid and mitral annular plane systolic excursions
- FetalDenseNet: multi-scale deep learning for enhanced early detection of fetal anatomical planes in prenatal ultrasound
- Original Articles – Neonates
- Incidence and mortality trends in congenital diaphragmatic hernia in the United States


