Evaluation of safety and performance of CentaFlow™ in the assessment of fetal growth restriction – a randomized trial and prospective cohort study
-
Cathrine Vedel
, Ane Rom
Abstract
Objectives
The aim was to evaluate the sensitivity and specificity of centaflow (CF) in a prospective multicenter study, and secondary to evaluate the safety of the CF device in a randomized multicenter study.
Methods
A sponsor-initiated multicenter randomized controlled clinical trial with termination of the randomization after enough women had been included to evaluate safety. The study proceeded as a prospective multicenter study including high-risk women (estimated fetal weight <−15 %, FGR). The first part randomized women to either standard care (SC) or SC+CF. Participants underwent CF evaluation with subsequent analysis for sensitivity and specificity for FGR at birth. Secondarily, adverse events were evaluated. Clinical assessments of fetal size conducted by midwives served as a reference. The performance of CF and SC was compared by McNemar’s Test. The performance analysis of CF was done per-protocol sample.
Results
A total of 1,601 pregnant women were enrolled, with 886 undergoing CF evaluation. A total of 123 were FGR (<3rd percentile) at birth, of which 88 were evaluated by CF, and 117 had a clinical assessment of the estimated fetal weight. CF demonstrated no evidence of benefit for detecting FGR with a sensitivity of 50 % and specificity of 43 %. Adverse events associated with CF use were limited to minor skin irritation. McNemar’s test showed SC was superior to CF regarding specificity as a screening tool (p=0.014).
Conclusions
While CF was safe to use, we found no evidence that CF can be used as a predictor of FGR. Further refinement of signal analysis is necessary to enhance CFs diagnostic utility.
Key message
Centaflow was safe to use on pregnant women.
There was no evidence that Centaflow can be used to detect fetal growth restriction.
Introduction
Fetal growth restriction (FGR) affects approximately 3 % of all pregnancies [1], 2] and is a condition related to abnormal placental development when no chromosomal aberrations or immunization are responsible [3], 4]. FGR is associated with an increased risk of intrauterine death, preterm birth, and a series of long-term adverse consequences including cardiovascular and metabolic disease in both mother and child and altered neurodevelopmental outcomes in the child [5], [6], [7], [8], [9]. However, FGR is not easily identified prenatally, as in vivo assessment of the placental development and function have not yet been convincing. Therefore, clinical assessment and measurement of the symphysis fundal height as well as the fetal heartbeat using a stethoscope, in locations with no universal 3rd-trimester ultrasound screening, have been the reference standard [10]. Nevertheless, these widely used screening methods have low sensitivities [1], which increases the risk of intrauterine deaths and neonatal complications that could potentially be reduced with correct antenatal identification of FGR [11], 12].
The existence of maternal vascular murmurs (MVM) from the uteroplacental unit and the association with FGR was previously reported by Riknagel et al. based on small cross-sectional studies [13], [14], [15]. They tested a device called Centaflow™ (CF), which is a wireless patch with microphones placed on the lower abdomen of the pregnant women to assess the presence of MVM [13], [14], [15]. Their results showed that the majority of cases of FGR do exhibit MVM, but on the contrary, not all healthy pregnancies or pregnancies with fetuses <10th percentile with normal umbilical artery flow, present MVM [14]. Due to the size and type of previous studies, the possible clinical implications still need to be investigated. Moreover, the overall safety of use of CF on the mother and the child needs to be determined on a larger scale.
Hence, the overall aim was to evaluate the sensitivity and specificity of CF, and secondary to evaluate the safety of the CF device. We hypothesized that the device is safe to use and has a higher sensitivity than standard care.
Materials and methods
This was a sponsor-initiated multicenter randomized controlled clinical trial (RCT) with a termination of the randomization after enough women had been included to evaluate the safety of CF. After the termination of the randomization, the study proceeded as a prospective multicenter study. Performance was evaluated in both the randomized part and the prospective part of the study. The study was conducted between 03/06/2020 and 21/03/2022 at Copenhagen University Hospital Rigshospitalet, Copenhagen and Regional Hospital Central Jutland, Viborg, and Regional Hospital West Jutland, Herning. Women +18 years of age with a singleton pregnancy more than 12 weeks’ gestation up until delivery were invited to participate. Exclusion criteria were fetal abnormality known to affect fetal growth or affect the birth weight (including malformations, genetic disorders, and immunization) diagnosed at any given time prenatally and up to 12 days postpartum. In the prospective study, only high-risk pregnancies, defined as an estimated fetal weight <15 %, were invited to participate to include women with growth restricted babies to evaluate the sensitivity of CF. This part also included women from Copenhagen University Hospital Herlev, Copenhagen and Copenhagen University Hospital North Zealand, Hillerod.
In Denmark, we have tax-paid universal healthcare, hence the prenatal screening program and obstetric care are free of charge for the individual. All pregnant women are offered two prenatal ultrasound screening exams – one in the 1st trimester screening for trisomies and one in the 2nd trimester screening for malformations with an attendance of more than 95 % of the pregnant population [16]. Moreover, women are offered 5–7 antenatal visits with a midwife and three visits with a general practitioner. If any complications are suspected, the patients are referred to the obstetric department for further evaluation. This is nationwide standard. All women were recruited through antenatal visits at the midwife or at the ultrasound clinic.
The Centaflow device
The system is not yet CE-marked under the medical device regulation (MDR) and is investigated as a Class IIa medical device. The indication of use is the detection of murmurs (MVM) as a sign of intra-arterial turbulence in the maternal side of the placenta. The CF System consists of an active device with two sets of bilateral microphones (one set placed on the inner side and another on the outer side) and seven electrocardiogram (ECG) electrodes mounted to an adhesive patch (Figure 1, image). The CF device is placed on the lower abdomen of the pregnant woman. A computer tablet is connected to control the recording where the recorded audio and ECG is stored and sent to a central server. The ECG electrodes measure the maternal pulse to segment the sound recording from the R-wave in the ECG and are not used for other purposes. The body-contact microphones were developed with the Cardio-technology group at Aalborg University (Department of Health Science & Technology) and the Acoustics Group (Department of Electronic Systems) and have previously been tested [15].

Image of the Centaflow device.
Training of investigators and trial personnel included investigational device usage, electronic case report form completion, medical device Good Clinical Practice (GCP) (ISO 14155), and trial personnel responsibilities.
The CF recorded for 3 min. A data complier function provided information of a) sufficient data or b) insufficient data. If b) was displayed, a new option for recording was provided, and by acceptance, an additional three-minute recording proceeded. After this, a new evaluation was performed by the compiler function and provided information of either a) or b). If the data were still insufficient, the examination was registered as incomplete.
All recordings were manually assessed by a minimum of two assessors. The recordings went through several steps of interpretation and analyses: 1) assessment of the quality; 2) evaluation of the recording by two independent, and blinded assessors (blinded for estimated fetal size and outcome); 3) and consensus meeting when disagreement occurred between the assessors. Step 2 was categorized as 1–3) murmur; 4) no murmur; and 5) uncertain. A murmur was classified as persistent systolic high frequency like described in previous studies [13], [14], [15]. A positive signal was defined as a signal classified between 1 and 3, where the signals were grouped 1, 2, or 3 depending on noise.
Sensitivity and specificity of CF
FGR was defined as a birth weight <3rd percentile for the gestational age according to Marsal’s formula (Z score 1.881) [17]. The pilot data reported by Riknagel et al. showed that CF had a sensitivity for detecting FGR of 85 % and a specificity of 88 % [14]. We assumed that the sensitivity of standard care (SC) (symphysis fundal height, clinical assessment, and fetal heart rate) for FGR was 60 % and, for the same screen positive rate as SC, the sensitivity of CF was 80 %. For 90 % power, with a two-sided test at the 5 % level, a conservative estimate of the number of cases of true FGR, to show a significant difference between the sensitivities, was 133.
Randomization for safety evaluation
To evaluate the safety of CF, the first part of the study was randomized and planned to continue for 12 months with a minimum of 500 participants included in the CF group if no adverse events were recorded. Each included woman was randomized to receive either the fetal-growth assessment by [1] SC or [2] SC+CF by simple randomization. Immediately before the patient’s assessments, the randomization sequence was accessed by the healthcare provider from the sponsor’s website. The clinical monitor, the healthcare provider performing the fetal growth screening, and the subject were blinded to the results of the CF assessment, and there was no intervention related to the CF assessment. Each enrolled subject was to remain in the trial until completion of the required follow-up period of 12 days postpartum. However, a subject could be withdrawn from the trial due to death, voluntary withdrawal, withdrawal indicated by the physician, or lost-to-follow-up defined as follows: If the subject missed two consecutive scheduled follow-up time points, and the attempts at contacting the subject were unsuccessful (a minimum of two phone calls on different days followed by a letter). After inclusion, the status of the fetus was evaluated by either SC or SC+CF at each routine visit.
Adverse events
We registered any occurrence of adverse events (AE) and serious adverse events (SAE) during the entire trial period (from inclusion to 12 days postpartum). An AE was defined as any untoward medical occurrence in a subject enrolled in the clinical trial, which did not necessarily have a causal relationship with the trial device. Elective procedures for a pre-existing condition without progression were not considered as AEs. An SAE was defined as an event that included death or were life-threatening and resulted in illness or injury, required inpatient hospitalization or prolongation of existing hospitalization, persistent or significant disability, a congenital malformation, fetal death, fetal distress, or medical or surgical intervention to prevent permanent impairment to body structure or body function. Determination of whether there was a reasonable possibility that the CF device caused or contributed to an AE was determined by the investigator. Determination was based on an assessment of temporal relationships, biological plausibility, association (or lack of association) with underlying disease, and presence (or absence) of a more-likely cause. Definitions for determination of the relationship included: 1) not related: Exposure to the device had not occurred, the occurrence of the AE was not reasonably related in time, there was a definite alternative etiology, or it was biologically implausible for the AE to be related to the use of the device; 2) possible: Exposure to the device had occurred or the AE was reasonably related in time, and the device was more likely than other alternative causes to be responsible for the adverse event; 3) unknown: Exposure to the device and the occurrence of an AE could not be reasonably determined to be unrelated to the device. If the relationship was identified as unknown, it was treated as related to the device. The investigator monitored the occurrence of AEs for each subject during the clinical trial, and all AEs reported by either subject, investigator, or documented in medical records were recorded in the study, whether determined to be related or unrelated to the device. All AEs and SAEs were reported to the National Medicines Agency.
Baseline characteristics and follow-up
Baseline characteristics included age, ethnicity, body mass index (BMI), parity, smoking status, mode of conception, and gestational age at inclusion and were obtained from the patient files after enrollment. After the delivery, data regarding delivery, birth weight, and possible complications were obtained from the mother and child’s patient files with a follow-up time of 12 days postpartum.
Statistical analyses
Baseline characteristics are presented for the randomized/non-randomized groups and for SC/CF groups. The characteristics are described with numbers and proportions and medians with interquartile ranges.
The performance analysis of CF was done per-protocol sample, defined as those women who completed the CF assessment, and had a final diagnosis of FGR or no FGR at birth. The sensitivity and specificity of CF and SC were calculated. SC was defined as true positive if, at any visit, SC screened a fetus FGR, and the child was true FGR at birth. A Chi2 test was used to assess the performances. Moreover, the positive-predictive values, negative predictive values, and false-positive rates were calculated. We calculated Z-scores for the clinical assessment of the estimated fetal weight and for the symphysis-fundal height for all visits and used the lowest Z-scores to establish receiver operating characteristic (ROC) curves [17], 18]. The CF was considered positive, if at any visit, CF resulted in a positive signal (MVM category 1–3).
To compare the performance (sensitivity and specificity) of SC with CF, a cut-off was determined for the CF score that gave the same screen positive rate as SC. McNemar’s test was applied by comparison of discordant counts (discordance between screen positives between CF and SC in FGR fetuses) among the randomized participants. A significance level of 0.05 was chosen.
The safety evaluation including AE and SAE was performed on data where patients were included according to the intention-to-treat protocol, defined as those women who had at least one attempt at an assessment with CF. The assessment did not need to have been completed.
Funding
CentaFlow Holding A/S sponsored the project. The authors of the paper did not receive any funds from CentaFlow Holding A/S. The sponsor designed the study, obtained relevant permissions to conduct the trial, and analyzed the CF signals. Execution of the study was done in collaboration between the sponsor and the study sites. The analyses of the data, interpretation of the results, and writing the paper was done by the authors. The final draft of the paper was approved by the sponsor.
Permissions and consent
Eligible women were given oral and written information about the study, as well as a minimum of 24 h of consideration. If the woman consented to participate, a written consent form was completed. The study was approved by the National Ethical Committee (1-10-72-169-19), the National Medicines Agency (2019-07-3411), and the Danish Data Protection Agency (1-16-02-0323-19). The study was registered on ClinicalTrials.gov (NCT04438668).
The manuscript followed the CONSORT guidelines.
Results
A total of 1,601 pregnant women were included: 1,418 women were randomized to either SC+CF (n=703) or SC (n=715), and 183 women were included in the prospective study after the termination of the randomization (Figure 2, Flowchart). Hence, 886 were evaluated by CF. Baseline characteristics for those randomized are shown in Table 1.
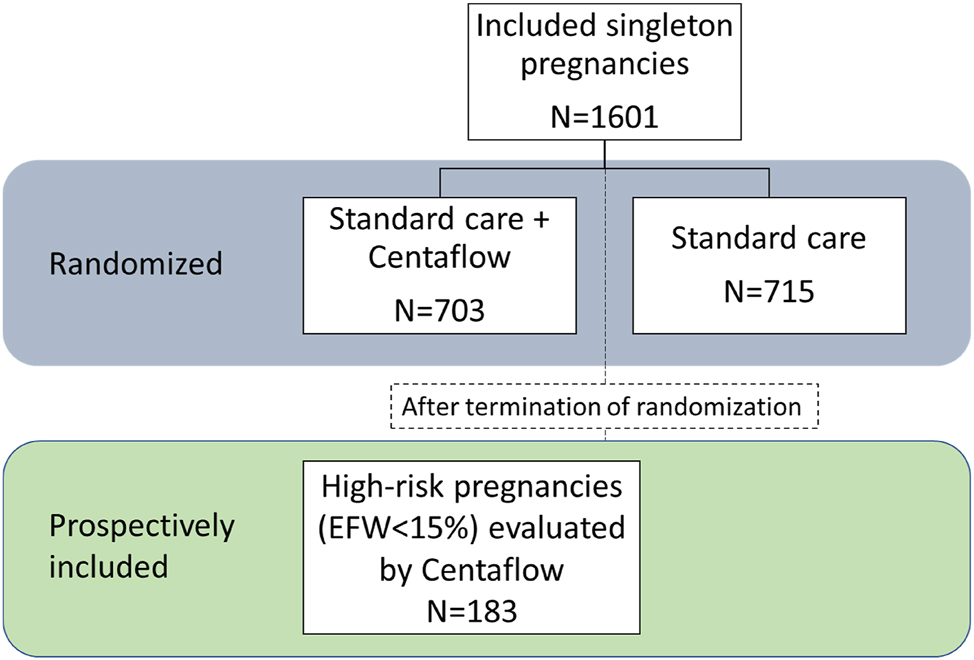
Flowchart of study population.
Baseline characteristics of the randomized study population.
| Randomized to SC n=715 | Randomized to SC+CF n=703 | |
|---|---|---|
| Maternal age in years, median (IQR) | 31 (28–34) | 31 (29–34) |
| Ethnicity, caucasian, n (%) | 679 (95.0) | 660 (93.9) |
| Pregestational BMI, median (IQR) | 22.4 (20.4–25.0) | 22.3 (20.7–25.0) |
| Nulliparous, n (%) | 377 (52.7) | 377 (53.6) |
| Smoker, n (%) Missing |
18 (2.5) 9 (1.3) |
9 (1.3) 3 (0.4) |
| Spontaneous conception, n (%) Missing |
616 (86.2) 13 (1.8) |
627 (89.2) 15 (2.1) |
| Gestational age at inclusion, days (IQR) | 202 (198–207) | 204 (198–214) |
-
SC, standard care; CF, Centaflow.
Of the 886 participants evaluated by CF, 501 were screened positive at least once (56.5 %). A total of 123 children were FGR (<3rd percentile) at birth, of which 88 were evaluated by CF, and 117 had a clinical assessment of the estimated fetal weight. We needed to include 133 pregnancies with true FGR according to our sample size calculation, however, due to an error in the equation in one of the sponsor’s systems used when including patients, we ended up with a population of 123 FGR cases.
In a total of 18 women, no clinical assessment of the fetal weight was registered. Table 2 shows the calculated sensitivity and specificity of CF. There was no evidence of any association between CF and FGR (p=0.192). The CF device gave a sensitivity of FGR of 50 % with a false positive rate of 57 %. Table 3 shows the sensitivity and specificity of clinical assessment of FGR. There was a sensitivity for FGR of 31.6 % with a false positive rate of 4.6 %.
Sensitivity and specificity of Centaflow in all tested participants.
| Outcome | |||||
|---|---|---|---|---|---|
| FGR | AGA | Total | |||
| CF test result | Screen positive | 44 | 457 | 501 | PPV=8.8 % |
| Screen negative | 44 | 341 | 385 | NPV=88.6 % | |
| Total | 88 | 798 | 886 | ||
| Sensitivity=50 % | Specificity=43 % | ||||
-
χ2=1.704, p=0.192. FGR, fetal growth restriction; AGA, appropriate for gestational age; PPV, positive predictive value; NPV, negative predictive value.
Sensitivity and specificity of clinical assessment to assess FGR.
| Outcome | |||||
|---|---|---|---|---|---|
| FGR | AGA | Total | |||
| SC test result | Screen positive | 37 | 68 | 105 | PPV=35.2 % |
| Screen negative | 80 | 1,398 | 1,478 | NPV=94.6 % | |
| Total | 117 | 1,466 | 1,583 | ||
| Sensitivity=31.6 % | Specificity=95.4 % | ||||
-
χ2=127.4, p<0.001. FGR, fetal growth restriction; AGA, appropriate for gestational age; PPV, positive predictive value; NPV, negative predictive value.
Figure 3 shows the ROC-curves for the clinical assessment of the estimated fetal weight and symphysis-fundal height with areas under the curve of 0.82 and 0.73, respectively. For the same false-positive rate as CF, McNemar’s test showed that SC was superior to CF (p=0.014) (Supplementary Table 1).
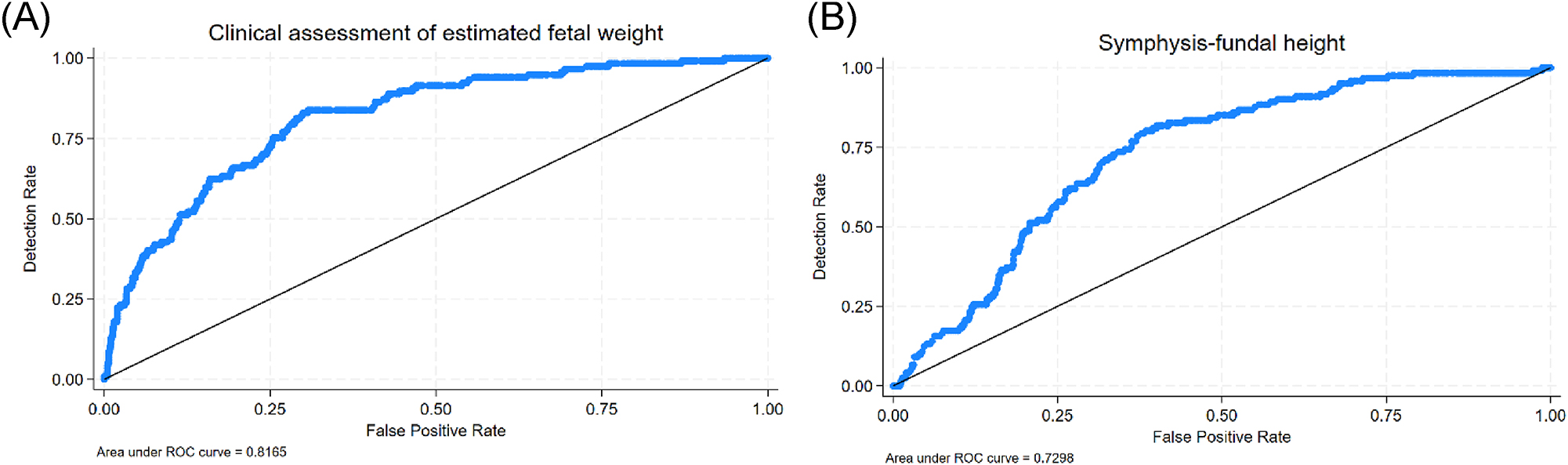
ROC-curves illustrating the performance of the clinical assessment by the midwife (A) and the symphysis-fundal height (B).
In the CF group, two patients experienced AEs as redness after removal of the adhesive patch which resolved spontaneously (0.3 %). The distribution of reported SAEs can be seen in Supplementary Table 2. The SAEs covered abnormal prenatal flow measurements, anhydramnion, polyhydramnion, elevated liver enzymes, hypertension, preeclampsia, HELLP syndrome, maternal infection, pulmonary embolism, shortened cervix, premature rupture of membranes/preterm premature rupture of membranes, intrapartum fever, placental abruptio, instrumental delivery, emergency cesarean section, peripartum hemorrhage, asphyxia, 3rd degree rupture of perineum, preterm birth (<37 weeks’ gestation), endometritis, mastitis, post-partum urinary retention, malformation, neonatal apnea, neonatal hypoglycemia, neonatal icterus, neonatal immunization, neonatal infection, respiratory distress syndrome, neonatal weight loss, foetus mors (n=5), and neonatal death (n=1). There were significantly more SAEs in the SC group compared to the CF group (p<0.001). There were two cases of intrauterine fetal death in the CF group and three cases in the SC group. The case of neonatal death was in the CF group. None of the SAEs were found to be associated with the use of CF.
Discussion
In this study, we evaluated the performance and the safety of CF for the first time in a large randomized controlled study (safety+performance) and a prospective study (performance). Overall, we found that CF is safe with no risk of serious adverse events, and only 0.3 % experienced minor skin problems (redness) from the adhesive patch. We found no evidence that CF can be used as a predictor of FGR, as the CF had a low sensitivity of 50 % and a specificity of 43 %. The positive predictive value was very low (8.8 %), though possibly affected by the low incidence of FGR, while the negative predictive value was acceptable (88.6 %). Thus, we were unable to replicate previous findings regarding the clinical performance of CF.
The performance of CF to detect FGR has previously been evaluated in one study [14]. Riknagel et al. performed a cross-sectional study over three months, including singleton pregnancies between 28- and 34-weeks’ gestation at a single center in Denmark. They investigated the correlation between MVM obtained from the CF device and estimated fetal weight<10th percentile along abnormal Doppler flow. They analyzed 63 pregnant women of which 25 were estimated to be fetal growth restricted based on ultrasound. The sensitivity for the outcome of interest was 36 %, specificity 79 %, and screen positive rate 27 %. The sensitivity and specificity improved markedly when the outcome was combined with abnormal umbilical artery PI (sensitivity 85 % and specificity 88 %). Our current results show a higher sensitivity, possibly due to the definition of FGR in the current study (<3rd-percentile), but a much lower specificity.
There are several possible reasons for the performance results including 1) The interpretation of the CF signals; 2) the design of the study to include patients in the entire third trimester; and 3) the definition of the outcome. This study was planned with manual annotations of the CF signals, i.e., a person listened to the recordings and categorized the sounds/murmurs. Despite efforts to minimize variability, this method implies a relative subjectivity in the evaluation of the sound signals, which introduces bias. It seems possible that it is more difficult for a person to reject abnormality than accept it, which could partly explain the low positive predictive value. Moreover, it is also possible that listening for a murmur is not the correct use of the CF. It is likely that more detailed, systematic, and objective signal analysis and assessment of the sound signals could improve the performance of CF. The study also included women through the entire third trimester, however, it has not been established at which gestational age the performance of CF is best. It may be independent of gestational age, though it appears more likely that the signal changes throughout pregnancy, similar to changes in the uterine artery Doppler flow observed at different gestational ages [19]. Lastly, the outcome was defined as FGR. The CF device can possibly detect adverse blood flow in the placenta, hence possibly detect poor placenta-associated outcomes not limited to FGR – and sometimes not related to FGR. Consequently, the appropriate outcome for CF could be adverse outcome associated with placental insufficiency.
Though the performance of CF was poor in detecting FGR, we found that the clinical assessment of the fetal weight performed by midwives performed well with an AUC of 0.82. The sensitivity was not remarkable, but the specificity of more than 95 % shows how accurately the Danish midwives can identify fetuses with normal growth. The results are better than what has been previously reported on the performance of clinical assessment to identify FGR [20].
The study has limitations. The sample size calculation estimated a need for 133 pregnancies with true FGR. However, due to a systematic error (error in the equation) in one of the systems used when including patients, we ended up with a population of 123 FGR cases. This is unfortunate, but we do not believe it would have changed any results. Also, due to the manual annotations of the signal, we believe that the results from this study should be interpreted with great caution. Moreover, we do not know the implications of the location of the placenta in the annotation of signals. This should be explored further in the future.
Though this study failed to show the possible potential of CF to detect FGR, we, as clinicians, still see the potential in this new biomarker. Despite the limitations, this study has demonstrated that the CF signal analysis and categorizations need to be reevaluated, and time windows of usage should be explored to determine the potential benefit of CF for FGR detection. Hence, future studies need to include 1) a new approach to assess the CF signals, i.e., through machine learning, and 2) more placenta-associated outcomes, i.e., preeclampsia and not only FGR. Moreover, the sounds of the normal pregnancy at low risk needs to be ascertained as well as the changes in the signal over time. By investigating and establishing these parameters, a more solid foundation could be laid to determine the possible usage of this new biomarker – alone or in combination with existing biomarkers.
In conclusion, we found that CF is safe to use, the performance of CF to detect FGR was poor. However, as there is still room for great improvement in both the characterization and analysis of the signal, we have not yet ruled out the possibility of CF as a new biomarker in the detection or prediction of placenta-associated adverse pregnancy outcomes.
-
Research ethics: The study was approved by the National Ethical Committee on the 21st of February, 2020 (1-10-72-169-19), the National Medicines Agency (2019-07-3411), and the Danish Data Protection Agency (1-16-02-0323-19). The study was conducted in accordance with the Declaration of Helsinki.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission. Olav Bjørn Petersen was the primary clinical investigator. Cathrine Vedel was the daily clinical project manager and in charge of the recruitment in the latter part of the inclusion period. Ane Rom, Mie de Wolff, Hanne Hegaard, Richard Farlie, Lise Hald Nielsen, and Anne Hammer Lauridsen were in charge of the recruitment in the first part of the study period at the different study sites. Simone Hansen and Frederikke Huitfeldt Sander recruited participants and performed recordings in the latter part of the study. Dave Wright (DW) was responsible for the statistical analyses. Cathrine Vedel, Ane Rom, Dave Wright, and Olav Bjørn Petersen drafted the paper. All authors critically reviewed the paper and approved the final draft.
-
Use of Large Language Models, AI and Machine Learning Tools: Not applicable.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: The study was fully funded by the company Centaflow. None of the co-authors received any funding in regard to this study.
-
Data availability: The data that support the findings of this study are available from Centaflow. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors with the permission of Centaflow.
References
1. Andreasen, LA, Tabor, A, Nørgaard, LN, Rode, L, Gerds, TA, Tolsgaard, MG. Detection of growth-restricted fetuses during pregnancy is associated with fewer intrauterine deaths but increased adverse childhood outcomes: an observational study. Bjog 2021;128:77–85. https://doi.org/10.1111/1471-0528.16380.Search in Google Scholar PubMed
2. Monier, I, Blondel, B, Ego, A, Kaminiski, M, Goffinet, F, Zeitlin, J. Poor effectiveness of antenatal detection of fetal growth restriction and consequences for obstetric management and neonatal outcomes: a French national study. Bjog 2015;122:518–27. https://doi.org/10.1111/1471-0528.13148.Search in Google Scholar PubMed
3. Mifsud, W, Sebire, NJ. Placental pathology in early-onset and late-onset fetal growth restriction. Fetal Diagn Ther 2014;36:117–28. https://doi.org/10.1159/000359969.Search in Google Scholar PubMed
4. Burton, GJ, Jauniaux, E. Pathophysiology of placental-derived fetal growth restriction. Am J Obstet Gynecol 2018;218:S745–s61. https://doi.org/10.1016/j.ajog.2017.11.577.Search in Google Scholar PubMed
5. Sacchi, C, Marino, C, Nosarti, C, Vieno, A, Visentin, S, Simonelli, A. Association of intrauterine growth restriction and small for gestational age status with childhood cognitive outcomes: a systematic review and meta-analysis. JAMA Pediatr 2020;174:772–81. https://doi.org/10.1001/jamapediatrics.2020.1097.Search in Google Scholar PubMed PubMed Central
6. Flenady, V, Koopmans, L, Middleton, P, Frøen, JF, Smith, GC, Gibbons, K, et al.. Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet 2011;377:1331–40. https://doi.org/10.1016/s0140-6736(10)62233-7.Search in Google Scholar
7. Leon, DA, Lithell, HO, Vâgerö, D, Koupilová, I, Mohsen, R, Berglund, L, et al.. Reduced fetal growth rate and increased risk of death from ischaemic heart disease: cohort study of 15 000 Swedish men and women born 1915-29. Bmj 1998;317:241–5. https://doi.org/10.1136/bmj.317.7153.241.Search in Google Scholar PubMed PubMed Central
8. Crispi, F, Crovetto, F, Rodriguez-López, M, Sepúlveda-Martinez, Á, Miranda, J, Gratacós, E. Postnatal persistence of cardiac remodeling and dysfunction in late fetal growth restriction. Minerva Obstet Gynecol 2021;73:471–81. https://doi.org/10.23736/S2724-606X.21.04823-5.Search in Google Scholar PubMed
9. Colella, M, Frérot, A, Novais, ARB, Baud, O. Neonatal and long-term consequences of fetal growth restriction. Curr Pediatr Rev 2018;14:212–8. https://doi.org/10.2174/1573396314666180712114531.Search in Google Scholar PubMed PubMed Central
10. Wanyonyi, SZ, Orwa, J, Ozelle, H, Martinez, J, Atsali, E, Vinayak, S, et al.. Routine third-trimester ultrasound for the detection of small-for-gestational age in low-risk pregnancies (ROTTUS study): randomized controlled trial. Ultrasound Obstet Gynecol 2021;57:910–6. https://doi.org/10.1002/uog.23618.Search in Google Scholar PubMed
11. Lindqvist, PG, Molin, J. Does antenatal identification of small-for-gestational age fetuses significantly improve their outcome? Ultrasound Obstet Gynecol 2005;25:258–64. https://doi.org/10.1002/uog.1806.Search in Google Scholar PubMed
12. Policiano, C, Fonseca, A, Mendes, JM, Clode, N, Graça, LM. Small-for-gestational-age babies of low-risk term pregnancies: does antenatal detection matter? J Matern Fetal Neonatal Med 2018;31:1426–30. https://doi.org/10.1080/14767058.2017.1317741.Search in Google Scholar PubMed
13. Riknagel, D, Zimmermann, H, Farlie, R, Hammershøi, D, Schmidt, SE, Hedegaard, M, et al.. Separation and characterization of maternal cardiac and vascular sounds in the third trimester of pregnancy. Int J Gynaecol Obstet 2017;137:253–9. https://doi.org/10.1002/ijgo.12151.Search in Google Scholar PubMed
14. Riknagel, D, Farlie, R, Hedegaard, M, Humaidan, P, Struijk, JJ. Association between maternal vascular murmur and the small-for-gestational-age fetus with abnormal umbilical artery Doppler flow. Int J Gynaecol Obstet 2017;139:211–6. https://doi.org/10.1002/ijgo.12268.Search in Google Scholar PubMed
15. Riknagel, D, Dinesen, B, Zimmermann, H, Farlie, R, Schmidt, S, Toft, E, et al.. Digital auscultation of the uterine artery: a measure of uteroplacental perfusion. Physiol Meas 2016;37:1163–71. https://doi.org/10.1088/0967-3334/37/7/1163.Search in Google Scholar PubMed
16. Sundhedsstyrelsen. Retningslinjer for fosterdiagnostik 2020. Available from: https://www.sst.dk/-/media/Udgivelser/2020/Fosterdiagnostik/Retningslinjer-for-fosterdiagnostik.ashx?la=da&hash=406318312FADFC9ED6973ABFDEE02F772DC8DC0E.Search in Google Scholar
17. Marsal, K, Persson, PH, Larsen, T, Lilja, H, Selbing, A, Sultan, B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr 1996;85:843–8. https://doi.org/10.1111/j.1651-2227.1996.tb14164.x.Search in Google Scholar PubMed
18. Papageorghiou, AT, Ohuma, EO, Gravett, MG, Hirst, J, da Silveira, MF, Lambert, A, et al.. International standards for symphysis-fundal height based on serial measurements from the Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project: prospective cohort study in eight countries. Bmj 2016;355:i5662. https://doi.org/10.1136/bmj.i5662.Search in Google Scholar PubMed PubMed Central
19. Cavoretto, PI, Salmeri, N, Candiani, M, Farina, A. Reference ranges of uterine artery pulsatility index from first to third trimester based on serial Doppler measurements: longitudinal cohort study. Ultrasound Obstet Gynecol 2023;61:474–80. https://doi.org/10.1002/uog.26092.Search in Google Scholar PubMed
20. Bais, JM, Eskes, M, Pel, M, Bonsel, GJ, Bleker, OP. Effectiveness of detection of intrauterine growth retardation by abdominal palpation as screening test in a low risk population: an observational study. Eur J Obstet Gynecol Reprod Biol 2004;116:164–9. https://doi.org/10.1016/j.ejogrb.2004.01.037.Search in Google Scholar PubMed
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/jpm-2025-0153).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

