Abstract
Objectives
To investigate the incidence and risk factors of bilobate placenta, as well as to assess its impact on preeclampsia (PE), preterm delivery (PTD) and small-for-gestational age (SGA) neonates.
Methods
A prospective study of singleton pregnancies, undergoing routine anomaly scan at 20+0–23+6 gestational weeks, was conducted, between 2018 and 2022. The impact of prenatally diagnosed bilobate placenta on PE, PTD and SGA was assessed. Multivariate logistic regression models were employed to assess the independent association between bilobate placenta and the main pregnancy outcomes, using specific confounders. Additionally, a risk factor analysis was performed.
Results
The study population included 6,454 pregnancies; the incidence of prenatally diagnosed bilobate placenta was 2.0 % (n=129). Bilobate placenta was associated with PE (aOR: 1.721; 95 % CI: 1.014–2.922), while no statistically significant association was found between this anatomical variation and SGA (aOR: 1.059; 95 % CI: 0.665–1.686) or PTD (aOR: 1.317; 95 % CI: 0.773–2.246). Furthermore, pregnancies with prenatally diagnosed bilobate placenta had an increased prevalence of abnormal cord insertion (marginal or velamentous) (9.8 vs. 27.1 %; p<0.001) and increased mean UtA PI z-score (0.03 vs. 0.23; p=0.039). Conception via ART (aOR: 3.669; 95 % CI: 2.248–5.989), previous history of 1st trimester miscarriage (aOR: 1.814; 95 % CI: 1.218–2.700) and advancing maternal age (aOR: 1.069; 95 % CI: 1.031–1.110) were identified as major risk factors for bilobate placenta.
Conclusions
Bilobate placenta, excluding cases of co-existing vasa previa, is associated with higher incidence of PE, increased mean UtA PI z-score and higher probability of abnormal cord insertion, but not with increased risk for SGA or PTD. It is more common in pregnancies following ART and in women with a previous 1st trimester miscarriage.
Introduction
The bilobate placenta is a structural variant of the typical placenta where two placental lobes are present, with a reported incidence of up to 4 % [1]. In case of a noticeable size discrepancy between the two lobes, the term succenturiate is used to describe the small accessory placental lobe that develops inside the fetal membranes apart from the main placental body and is typically connected with a vessel of fetal origin [2]. Advanced maternal age, previous history of cesarean delivery and conception via assisted reproduction technology (ART) have been reported as possible risk factors for these structural variants of the placenta [3].
The placenta plays a crucial role in regulating fetal development; as a mediator of oxygen, nutrients and metabolic waste exchange and as an immunological and endocrinological regulator, this organ is essential for fetal growth and the maintenance of pregnancy [4]. Consequently, anatomical variants such as the bilobate placenta may be associated with pregnancy complications and adverse outcomes hypothesizing that abnormal placental development is the underlying etiology. Of note, vasa previa is strongly associated with succenturiate placenta, while complications associated with it are non-reassuring fetal heart rate and postpartum hemorrhage, both linked to vasa previa [3, 5]. To date, there is limited research regarding adverse pregnancy outcomes i.e., preeclampsia (PE), small for gestational age neonates (SGA) and preterm delivery (PTD) in pregnancies diagnosed with structural variants of placenta [3]. Due to the gap in the current literature on the bilobate placenta, it is a matter of debate whether such variations should be regarded as pathological or normal. In current practice, in high resource settings, the routine use of sonographic assessment, including color Doppler, enables the differential diagnosis from other placental anomalies and increases the rate of prenatal diagnosis of bilobate placenta, however, the clinical usefulness of this prenatal diagnosis remains largely unknown [6].
Therefore, the objective of this study was to investigate the incidence, associated risk factors and possible adverse pregnancy outcomes of cases prenatally diagnosed with bilobate placenta, in a systematic approach and offer exact and trustworthy effect estimates for hitherto uninvestigated associations.
Materials and methods
Study design, participants
A prospective observational study took place between June 2018 and June 2022, at the Third Department of Obstetrics and Gynecology, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Greece. The study was conducted according to the STROBE statement [7].
All pregnant women with a singleton pregnancy, undergoing both a routine first trimester scan at 11+0–13+6 and an anomaly scan at 20+0–24+0 gestational weeks were eligible to participate in the study. Exclusion criteria were missing data, miscarriage between the two scans, termination of pregnancy and vasa previa. All the scans were performed by maternal-fetal medicine consultants. Fetal scanning, including measurement of uterine artery pulsatility index (UtA PI), was performed transabdominally, according to the ISUOG guidelines [8]. Pregnancies diagnosed with a bilobate placenta (including those with succenturiate) were considered as the study group. Effort was taken for the postnatal confirmation of the finding. No false positive or false negative cases were recognized; however, confirmation was not always feasible, especially in cases of cesarean section. All pregnancies were dated according to the fetal crown-rump length at the first-trimester scan or the date of embryo-transfer in ART cases. A dedicated database (Astraia Software GmbH, Munich, Germany) was used for the storage of the patient data, including demographic and obstetric parameters.
It should be noted that the finding of a bilobate placenta did not affect the obstetric management as there currently is no guideline recommending modification of standard practice in such cases. The finding was mentioned in the ultrasound report with the only intention to increase awareness following a vaginal delivery for the complete removal of the placenta.
All the participants consented that their anonymized files may be used for research. The study protocol was approved by the Bioethics Committee of the School of Medicine, the Aristotle University of Thessaloniki, Greece (3.188/2-5-2018). Of note, no incentives were provided for participation in the study.
Measurements
With regards to the variables of interest, maternal medical and obstetric parameters were collected. Height and weight were measured during the first visit for the body mass index (BMI) calculation. Sociodemographic characteristics (age, parity, smoking, conception via ART) were collected through our database or directly during the recording of the maternal history.
The primary outcome under investigation was PE, defined as gestational hypertension accompanied by end-organ involvement [9]. Secondary outcomes were SGA, defined as birthweight below the 10th percentile [10], PTD, defined as the delivery between 22+0 and 36+6 weeks of gestation [11] and gestational age at delivery. Additionally, we studied all the available risk factors for possible correlation with the presence of bilobate placenta.
Statistical analysis
The continuous variables are represented as means (standard deviation), if normally distributed, otherwise as the medians (interquartile ranges). Categorical variables are expressed as numbers and percentages. Based on the type of statistical distribution, comparisons between groups were made using the parameterized Student’s T-test or nonparametric Mann–Whitney U test (continuous variables) and chi-square test (categorical variables).
Associations with the three main outcomes (PE, SGA and PTD), were assessed using multivariable logistic regression to adjust for the known and available confounders, while multivariable linear regression was conducted for gestational age at delivery. According to the most recent suggestions, a regression model forcing in all the existing factors is needed to calculate the unbiased prognostic effect of the new factor under investigation [12]. Thus, all the available variables that are known or expected to be confounders for PE, SGA and PTD according to published data were selected [13, 14]. Correlation analysis using Spearman’s rank correlation coefficient was performed among the continuous variables to assess multicollinearity.
A risk factor analysis was also conducted by employing a multivariable logistic regression model with the bilobate placenta as the dependent variable to consider possible confounders and offer more exact effect estimates; backward stepwise selection, based on p-value ≤0.1, was employed, in order to differentiate among the risk factors that had a significant prognostic value for bilobate placenta.
A complete-case analysis was employed in our study whereby cases with missing data were excluded from the analysis. This decision was based on the relatively small proportion of affected cases (approximately 2 % of the total population) and the absence of missing values in the study group, thereby not compromising the statistical efficiency by excluding any of our limited study cases [15]. A 5 % critical level of significance was used in every analysis. All analyses were conducted using R 2.15.1 (R foundation, Vienna, Austria).
Results
The study population consisted of 6,454 singleton pregnancies after exclusion from a total population of 6,651 cases undergoing a first trimester scan. Reasons for exclusion were missing data (n=131), miscarriage (n=33), termination of pregnancy (n=28), vasa previa (n=5) (Figure 1). The incidence of the prenatal diagnosis of bilobate in the study population was 2.0 % (129/6,454). The mean maternal age (31.6 vs. 34.5; p<0.001) and UtA PI z-score (0.03 vs. 0.23; p=0.039) were found to be higher in the study group, while the median values of gestational age at delivery (39.0 vs. 38.4; p<0.001) and birthweight (3,250 vs. 3,100; p<0.001) were lower compared to the control group (non-bilobate placenta). Furthermore, ART (4.4 vs. 20.9 %; p<0.001), history of miscarriage (15.4 vs. 30.2 %; p<0.001) and abnormal cord insertion (marginal or velamentous – 9.8 vs. 27.1 %; p<0.001) had a higher incidence in the study group. PE (7.3 vs. 14.7 %, p=0.003) and PTD (8.2 vs. 14 %, p=0.029) had higher incidence in the bilobate placenta group, while no association was found with SGA (Table 1).
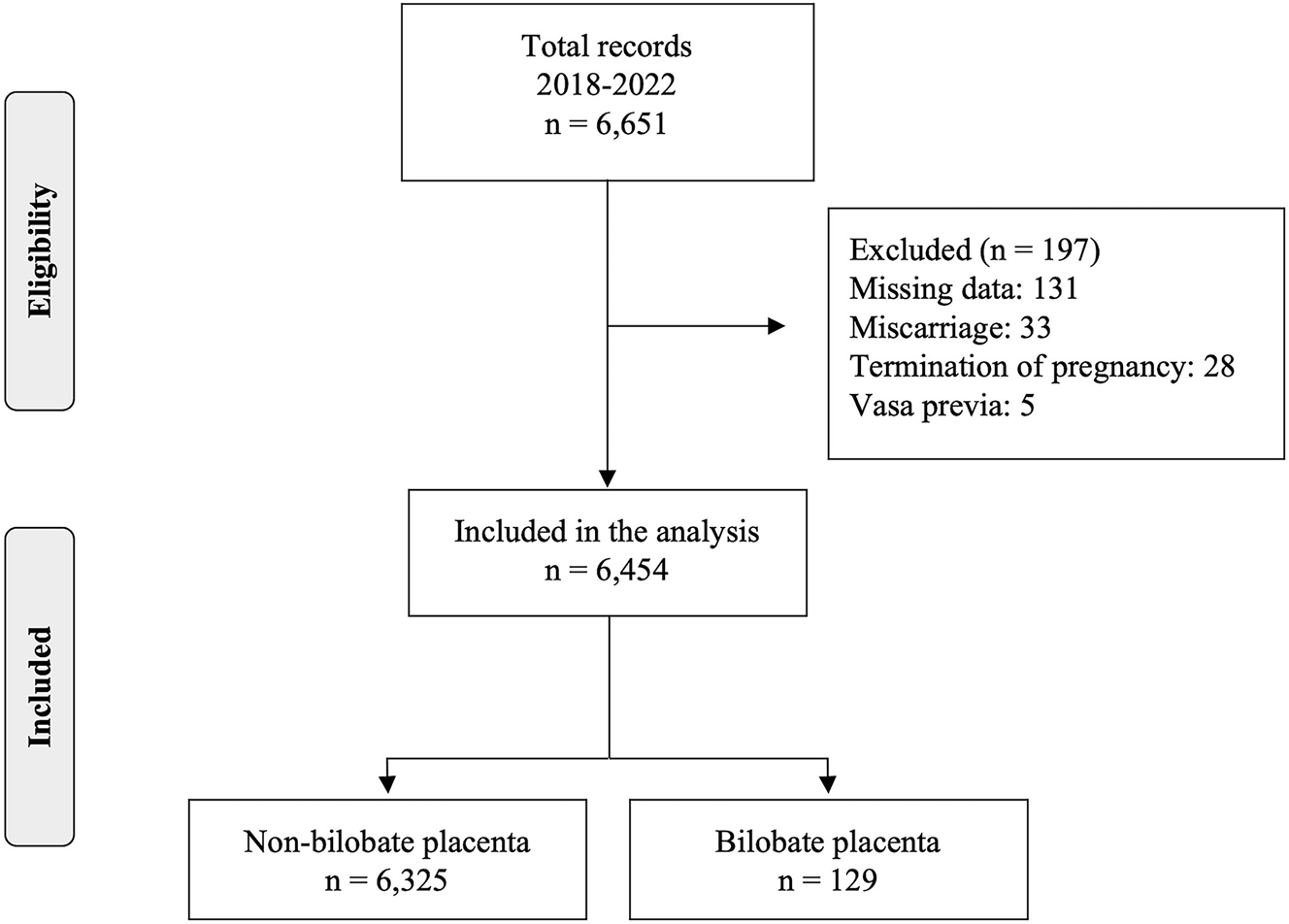
Study population.
Characteristics of the study population
| Variables | Overall (n=6,454) | Non-bilobate placenta (n=6,325) | Bilobate placenta (n=129) | p-Value |
|---|---|---|---|---|
| Maternal age, years, mean (SD) | 31.7 (5.1) | 31.6 (5.1) | 34.5 (5.7) | <0.001 |
| No smoking, n (%) | 4,124 (63.9) | 4,043 (63.9) | 81 (62.8) | 0.405 |
| Quit smoking | 1,648 (25.5) | 1,610 (25.5) | 38 (29.5) | |
| Current smoking | 682 (10.6) | 672 (10.6) | 10 (7.7) | |
| Multiparous, n (%) | 2,593 (40.2) | 2,539 (40.1) | 54 (41.9) | 0.762 |
| ART, n (%) | 307 (4.8) | 280 (4.4) | 27 (20.9) | <0.001 |
| BMI, median [IQR] | 23.0 [21.0, 26.0] | 23.0 [21.0, 26.0] | 23.3 [20.9, 26.3] | 0.595 |
| Bleeding in 1st trimester, n (%) | 323 (5.0) | 315 (5.0) | 8 (6.2) | 0.670 |
| Previous history of 1st trim miscarriage, n (%) | 1,015 (15.7) | 976 (15.4) | 39 (30.2) | <0.001 |
| Previous history of PTD, n (%) | 154 (2.4) | 148 (2.3) | 6 (4.7) | 0.158 |
| Abnormal cord insertion, n (%) | 652 (10.1) | 617 (9.8) | 35 (27.1) | <0.001 |
| UtA PI z-score, mean, SD | 0.04 (1.10) | 0.03 (1.09) | 0.23 (1.29) | 0.039 |
| PE, n (%) | 482 (7.5) | 463 (7.3) | 19 (14.7) | 0.003 |
| PTD, n (%) | 537 (8.3) | 519 (8.2) | 18 (14.0) | 0.029 |
| SGA, n (%) | 1,056 (16.4) | 1,031 (16.3) | 25 (19.4) | 0.415 |
| GA at delivery, median [IQR] | 39.0 [38.1, 39.9] | 39.0 [38.1, 39.9] | 38.4 [37.7, 39.1] | <0.001 |
| BW, median [IQR] | 3,250 [2,970, 3,540] | 3,250 [2,980, 3,550] | 3,100 [2,850, 3,400] | <0.001 |
-
ART, assisted reproductive technology; BW, birthweight; GA US, gestational age during the ultrasound; IQR, interquartile range; PE, preeclampsia; PTD, preterm delivery; SGA, small for gestational age; SD, standard deviation; UtA PI, uterine artery pulsatility index. Bold values stand for statistically significant results.
Bilobate placenta was associated with PE in the univariate analysis; an almost double probability was calculated compared to singleton pregnancies with non-bilobate placenta (OR: 1.922; 95 % CI: 1.144–3.229). The association persisted in the multivariable analysis, which pointed to 1.7 times higher odds of PE among pregnancies with bilobate placenta compared to the control group (aOR: 1.721; 95 % CI: 1.014–2.922). Conversely, bilobate placenta was not associated with SGA, neither in the univariate (OR: 1.234; 95 % CI: 0.794–1.919) nor in the multivariable analyses (aOR: 1.059; 95 % CI: 0.665–1.686). As for PTD, it was associated with bilobate placenta in the univariate analysis (OR: 1.814; 95 % CI: 1.094–3.009), but the association was eliminated when the confounders were included in the model (aOR: 1.317; 95 % CI: 0.773–2.246). However, diagnosis of a bilobate placenta was associated with a reduction in the mean gestational age at delivery of 5 (β=−0.712) and 3 days (β=−0.460), in the univariate and multivariable linear regression models, respectively (Table 2, Supplementary Tables 1–4).
Multivariable logistic regression models regarding the investigated outcomes.
| PE | SGA | PTD | Gestational age at delivery, weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | aOR | 95 % CI | p-Value | aOR | 95 % CI | p-Value | aOR | 95 % CI | p-Value | β | 95 % CI | p-Value |
| Maternal age, years | 1.053 | 1.031, 1.074 | 0.001a | 1.007 | 0.993, 1.022 | 0.310 | 0.998 | 0.979, 1.017 | 0.848 | −0.006 | −0.014, 0.002 | 0.158 |
| ΒΜΙ, kg/m2 | 1.107 | 1.090, 1.125 | 0.001a | 0.956 | 0.941, 0.971 | 0.001a | 0.998 | 1.004, 1.040 | 0.017a | −0.023 | −0.031, −0.015 | 0.001a |
| No smoking | Ref | Ref | Ref | |||||||||
| Quit smoking | 1.280 | 1.030, 1.590 | 0.026a | 0.963 | 0.818, 1.135 | 0.657 | 1.114 | 0.901, 1.378 | 0.320 | −0.100 | −0.192, −0.007 | 0.035a |
| Current smoking | 1.548 | 1.163, 2.061 | 0.003a | 1.853 | 1.513, 2.270 | 0.001a | 1.406 | 1.062, 1.861 | 0.017a | −0.233 | −0.364, −0.102 | 0.001a |
| Multiparity | 0.828 | 0.673, 1.019 | 0.075 | 0.634 | 0.544, 0.739 | 0.001a | 0.902 | 0.737, 1.104 | 0.316 | −0.124 | −0.210, −0.039 | 0.004a |
| ART | 1.476 | 1.014, 2.149 | 0.042a | 1.001 | 0.723, 1.387 | 0.995 | 2.101 | 1.470, 3.003 | 0.001a | −0.762 | −0.956, −0.568 | 0.001a |
| UtA PI z-score | 1.099 | 1.011, 1.195 | 0.027a | 1.558 | 1.468, 1.655 | 0.001a | 1.568 | 1.453, 1.692 | 0.001a | −0.212 | −0.248, −0.176 | 0.001a |
| Bleeding in 1st trimester | 1.723 | 1.210, 2.454 | 0.003a | 0.937 | 0.681, 1.288 | 0.687 | 1.246 | 0.851, 1.824 | 0.258 | −0.323 | −0.503, −0.142 | 0.001a |
| Previous history of 1st trim miscarriage | 1.035 | 0.806, 1.328 | 0.787 | 1.162 | 0.962, 1.403 | 0.119 | 1.276 | 1.006, 1.618 | 0.044a | −0.121 | −0.231, −0.010 | 0.032a |
| Previous history of PTD | 1.170 | 0.665, 2.059 | 0.586 | 1.549 | 1.006, 2.385 | 0.047a | 5.065 | 3.425, 7.490 | 0.001a | −1.160 | −1.421, −0.898 | 0.001a |
| Abnormal cord insertion | 1.014 | 0.747, 1.375 | 0.931 | 1.191 | 0.963, 1.473 | 0.107 | 1.183 | 0.898, 1.557 | 0.232 | −0.102 | −0.234, 0.029 | 0.127 |
| Bilobate placenta | 1.721 | 1.014, 2.922 | 0.044a | 1.059 | 0.665, 1.686 | 0.810 | 1.317 | 0.773, 2.246 | 0.311 | −0.460 | −0.743, −0.177 | 0.001a |
-
β, regression coefficient; aOR, adjusted odds ratio; ART, assisted reproductive technology; CI, confidence intervals; PE, preeclampsia; PTD, preterm delivery; SGA, small for gestational age; UtA PI, uterine artery pulsatility index. aDenotes statistical significance. All the depicted variables were included in each multivariate model.
Following multivariable risk factor analysis, conception via ART appears to be the most important risk factor for bilobate placenta as it increases the probability almost 4 times (aOR: 3.669; 95 % CI: 2.248–5.989), while previous history of 1st trimester miscarriage almost doubles the probability of bilobate placenta (aOR: 1.814; 95 % CI: 1.218–2.700). Advancing maternal age was also associated with a minimal rise of about 7 % in the probability of the bilobate placenta (aOR: 1.069; 95 % CI: 1.031–1.110) (Table 3).
Risk factor analysis on the incidence of bilobate placenta.
| Univariate analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| Variables | OR | 95 % CI | p-Value | aOR | 95 % CI | p-Value |
| Maternal age, years | 1.117 | 1.079, 1.157 | 0.001a | 1.069 | 1.031, 1.110 | 0.001a |
| ΒΜΙ, kg/m2 | 1.002 | 0.966, 1.039 | 0.902 | – | – | – |
| Smoking (reference: no smoking) | ||||||
| Quit smoking | 1.178 | 0.798, 1.739 | 0.410 | – | – | – |
| Current smoking | 0.743 | 0.383, 1.440 | 0.378 | – | – | – |
| Multiparity | 1.074 | 0.754, 1.529 | 0.694 | – | – | – |
| ART | 5.715 | 3.678, 8.880 | 0.001a | 3.669 | 2.248, 5.989 | 0.001a |
| Bleeding in 1st trimester | 1.261 | 0.611, 2.603 | 0.530 | – | – | – |
| Previous history of 1st trim miscarriage | 2.375 | 1.621, 3.479 | 0.001a | 1.814 | 1.218, 2.700 | 0.003a |
| Previous history of PTD | 2.036 | 0.883, 4.695 | 0.095 | – | – | – |
| Male fetus | 1.098 | 0.774, 1.559 | 0.599 | – | – | – |
-
aOR, adjusted odds ratio; ART, assisted reproductive technology; OR, odds ratio; PTD, preterm delivery. aDenotes statistical significance.
Notably, the assumptions of both the logistic and linear regressions were respected and no multicollinearity was detected among the study variables.
Discussion
Principal findings
The main findings of this study were: 1) the incidence of prenatally diagnosed bilobate placenta in singleton pregnancies is 2 %, 2) bilobate placenta is associated with an increased prevalence of abnormal cord insertion and increased mean UtA PI z-score, 3) a statistically significant association between bilobate placenta and PE was identified, while no association was found with SGA and PTD and 4) conception via ART and history of 1st trimester miscarriage are the main risk factors for bilobate placenta.
Interpretation of the findings
Based on the findings of the present study, prenatally diagnosed bilobate placenta involves about 1 in 50 singleton pregnancies and these women have an almost two-fold increased risk for PE, compared to the control group. Given that bilobate placentas are typically regarded as benign anatomical variations, this finding is intriguing and implies that bilobate placenta and PE may share the same pathophysiological procedure of abnormal placentation. Our findings are consistent with a clinicopathological analysis of 130 abnormally shaped placentas, including 48 succenturiate and 12 bilobed, which found an association between the abnormal placental shape and insufficient maternal-fetal perfusion [16]. A systematic review by Schiffer et al. showed that pregnancies complicated by placental syndromes, such as PE and SGA have higher placental thickness and greater presence of placental lakes and calcifications compared to the uncomplicated ones, however, bilobate placenta was not examined individually [17]. This is further supported by the significant association noted between the existence of bilobate placenta and a higher mean UtA PI z-score in our study, indicating a potential indirect mechanism where bilobate placentas may cause PE through abnormally high UtA PI; abnormal UtA PI is a well-studied predictor of PE [18]. Additionally, we found that bilobate placenta was linked to a higher prevalence of abnormal cord insertion (9.8 vs. 27.1 %). Given that the most recently published relevant meta-analyses [19, 20] demonstrated that both marginal and velamentous cord insertions have been linked to an increased risk of PE, this could be another pathophysiological mechanism explaining the association between bilobate placenta and PE. Of note, abnormal cord insertion has been previously found to be associated with higher mean UtA PI as well [21].
With regards to SGA, no association with bilobate placenta was identified; this is in accordance with the findings by Suzuki et al. who analyzed succenturiate placentas and found no statistically significant difference in the incidence of SGA compared to the controls [3]. We additionally investigated PTD, which was also not associated with the bilobate placenta. Of note, bilobate placenta was associated with a 3 days’ decrease in the mean gestational age at delivery; this is of minor clinical importance, since prematurity rates were not affected.
Following a risk factor analysis, conception via ART, history of previous miscarriage in the first trimester and advancing maternal age were identified as independent risk factors for bilobate placenta. These findings are in accordance with those by Suzuki et al., who identified maternal age above 35 years and the use of in vitro fertilization as possible risk factors [3]. The underlying increasing arterial endothelial damage brought on by aging may be the cause of placental anatomical variations like the bilobate placenta in older women [22, 23]. A systematic review assessed the association between ART and bilobate or succenturiate placenta and found an increased rate of succenturiate lobes in ART vs. non-ART pregnancies (OR: 6.97) [24]. The most plausible theory explaining why ART is an important risk factor, is based on the in vitro formation of the chorion that could affect the formation of the placenta and predispose to morphological variations such as bilobate placenta [25].
To our knowledge, this is the first study investigating the risk factors for bilobate placenta, as well as its associations with pregnancy outcomes. We consider the design and methodology of the present study as its major strength; excluding cases of vasa previa eliminated the possible bias of its coexistence with the bilobate placenta and by including only prenatally diagnosed cases, our study is rendered clinically relevant. The main weakness of the study stems from the limited study sample and statistical power. Thus, our results should be interpreted with caution until larger population studies are conducted to enable the extraction of safer and more robust conclusions. Furthermore, the inclusion of succenturiate placenta cases in the study group may have affected the results. Although conducting a complete-case analysis may be perceived as a weakness of our study, it is worth noting that the proportion of cases with missing values was limited (about 2 %) and no study case had missing values. Additionally, the data were missing due to no reporting of some values or due to loss in follow-up, considering both as missing at random; according to the literature complete-case analysis does not introduce any bias [15]. We should also state that statistical significance level was not adjusted for multiple testing, thus p-value is of no confirmatory value. Finally, the substantial inter-observer and intra-observer variability in the sonographic assessment of the placenta should not be disregarded.
Conclusions
Bilobate placenta, excluding vasa previa, was associated with an increased mean UtA PI z-score, higher rates of abnormal cord insertion and higher incidence of PE, suggesting that these women may benefit from closer antenatal care. Significant individual risk factors of bilobate placenta are conception via ART, previous history of miscarriage in the first trimester and advancing maternal age. More high-quality prospective cohorts are needed and future research on the association of bilobate placenta with other adverse pregnancy outcomes may be of clinical importance.
-
Research ethics: The study protocol was approved by the Bioethics Committee of the School of Medicine, the Aristotle University of Thessaloniki, Greece (3.188/2-5-2018).
-
Informed consent: All the participants consented that their anonymized files may be used for research.
-
Author contributions: Themistoklis Dagklis developed the original idea for the study, coordinated and revised the manuscript. Sonia Giouleka and Antonios Siargkas designed, evaluated the results and coordinated the manuscript. Ioannis Tsakiridis designed, coordinated, implemented the project and submitted the article. Apostolos Mamopoulos and Apostolos Athanasiadis cooperated in the analysis and participated in the revision. All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Competing interests: The authors state no conflict of interest.
-
Research funding: None declared.
References
1. Calagna, G, Polito, S, Schiattarella, A, Cucinella, G, Calò, F, Chiantera, V, et al.. Bilobed placenta and management choices. Literature review and two cases studies. Ital J Gynaecol Obstet 2022;35:158–67. https://doi.org/10.36129/jog.2022.59.Suche in Google Scholar
2. Rathbun, KM, Hildebrand, JP. Placenta abnormalities. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC.; 2023.Suche in Google Scholar
3. Suzuki, S, Igarashi, M. Clinical significance of pregnancies with succenturiate lobes of placenta. Arch Gynecol Obstet 2008;277:299–301. https://doi.org/10.1007/s00404-007-0482-6.Suche in Google Scholar PubMed
4. Herrick, EJ, Bordoni, B. Embryology, placenta. Treasure Island (FL): StatPearls; 2022.Suche in Google Scholar
5. Tsakiridis, I, Mamopoulos, A, Athanasiadis, A, Dagklis, T. Diagnosis and management of vasa previa: a comparison of 4 national guidelines. Obstet Gynecol Surv 2019;74:436–42. https://doi.org/10.1097/ogx.0000000000000692.Suche in Google Scholar
6. Cavaliere, AF, Rosati, P, Ciliberti, P, Buongiorno, S, Guariglia, L, Scambia, G, et al.. Succenturiate lobe of placenta with vessel anomaly: a case report of prenatal diagnosis and literature review. Clin Imag 2014;38:747–50. https://doi.org/10.1016/j.clinimag.2014.01.018.Suche in Google Scholar PubMed
7. von Elm, E, Altman, DG, Egger, M, Pocock, SJ, Gotzsche, PC, Vandenbroucke, JP, et al.. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–7. https://doi.org/10.1016/j.ypmed.2007.08.012.Suche in Google Scholar PubMed
8. Salomon, LJ, Alfirevic, Z, Berghella, V, Bilardo, CM, Chalouhi, GE, Da Silva Costa, F, et al.. ISUOG Practice Guidelines (updated): performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obstet Gynecol 2022;59:840–56. https://doi.org/10.1002/uog.24888.Suche in Google Scholar PubMed
9. Tsakiridis, I, Giouleka, S, Arvanitaki, A, Giannakoulas, G, Papazisis, G, Mamopoulos, A, et al.. Gestational hypertension and preeclampsia: an overview of national and international guidelines. Obstet Gynecol Surv 2021;76:613–33. https://doi.org/10.1097/ogx.0000000000000942.Suche in Google Scholar
10. Osuchukwu, OO, Reed, DJ. Small for gestational age. Treasure Island (FL): StatPearls; 2022.Suche in Google Scholar
11. Giouleka, S, Tsakiridis, I, Kostakis, N, Koutsouki, G, Kalogiannidis, I, Mamopoulos, A, et al.. Preterm labor: a comprehensive review of guidelines on diagnosis, management, prediction and prevention. Obstet Gynecol Surv 2022;77:302–17. https://doi.org/10.1097/ogx.0000000000001023.Suche in Google Scholar PubMed
12. Riley, RD, van der Windt, D, Croft, P, Moons, KG. Prognosis research in healthcare: concepts, methods, and impact. Oxford: Oxford University Press; 2019.10.1093/med/9780198796619.001.0001Suche in Google Scholar
13. Bartsch, E, Medcalf, KE, Park, AL, Ray, JG. High Risk of Pre-eclampsia Identification G. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ 2016;353:i1753. https://doi.org/10.1136/bmj.i1753.Suche in Google Scholar PubMed PubMed Central
14. Lang, JM, Lieberman, E, Cohen, A. A comparison of risk factors for preterm labor and term small-for-gestational-age birth. Epidemiology 1996;7:369–76. https://doi.org/10.1097/00001648-199607000-00006.Suche in Google Scholar PubMed
15. Lee, KJ, Tilling, KM, Cornish, RP, Little, RJA, Bell, ML, Goetghebeur, E, et al.. Framework for the treatment and reporting of missing data in observational studies: the Treatment and Reporting of Missing data in Observational Studies framework. J Clin Epidemiol 2021;134:79–88. https://doi.org/10.1016/j.jclinepi.2021.01.008.Suche in Google Scholar PubMed PubMed Central
16. Wang, AC, Xie, JL, Wang, YN, Sun, XF, Lu, LJ, Sun, YF, et al.. Singleton placentas with abnormal shape: a clinicopathological analysis of 130 cases. Zhonghua bing li xue za zhi = Chin J Pathol 2022;51:39–43. https://doi.org/10.3760/cma.j.cn112151-20210508-00346.Suche in Google Scholar PubMed
17. Schiffer, V, van Haren, A, De Cubber, L, Bons, J, Coumans, A, van Kuijk, SM, et al.. Ultrasound evaluation of the placenta in healthy and placental syndrome pregnancies: a systematic review. Eur J Obstet Gynecol Reprod Biol 2021;262:45–56. https://doi.org/10.1016/j.ejogrb.2021.04.042.Suche in Google Scholar PubMed
18. Cnossen, JS, Morris, RK, ter Riet, G, Mol, BW, van der Post, JA, Coomarasamy, A, et al.. Use of uterine artery Doppler ultrasonography to predict pre-eclampsia and intrauterine growth restriction: a systematic review and bivariable meta-analysis. Can Med Assoc J = J de l’Assoc Med Canad 2008;178:701–11. https://doi.org/10.1503/cmaj.070430.Suche in Google Scholar PubMed PubMed Central
19. Siargkas, A, Tsakiridis, I, Pachi, C, Mamopoulos, A, Athanasiadis, A, Dagklis, T. Impact of velamentous cord insertion on perinatal outcomes: a systematic review and meta-analysis. Am J Obstet Gynecol MFM 2023;5:100812. https://doi.org/10.1016/j.ajogmf.2022.100812.Suche in Google Scholar PubMed
20. Siargkas, A, Tsakiridis, I, Pachi, C, Mamopoulos, A, Athanasiadis, A, Dagklis, T. Impact of marginal cord insertion on perinatal outcomes: a systematic review and meta-analysis. Am J Obstet Gynecol MFM 2023;5:100876. https://doi.org/10.1016/j.ajogmf.2023.100876.Suche in Google Scholar PubMed
21. Tsakiridis, I, Dagklis, T, Athanasiadis, A, Dinas, K, Sotiriadis, A. Impact of marginal and velamentous cord insertion on uterine artery Doppler indices, fetal growth, and preeclampsia. J Ultrasound Med 2022;41:2011–8. https://doi.org/10.1002/jum.15883.Suche in Google Scholar PubMed
22. Cleary-Goldman, J, Malone, FD, Vidaver, J, Ball, RH, Nyberg, DA, Comstock, CH, et al.. Impact of maternal age on obstetric outcome. Obstet Gynecol 2005;105:983–90. https://doi.org/10.1097/01.aog.0000158118.75532.51.Suche in Google Scholar
23. Chen, Z, Xiong, L, Jin, H, Yu, J, Li, X, Fu, H, et al.. Advanced maternal age causes premature placental senescence and malformation via dysregulated α-Klotho expression in trophoblasts. Aging Cell 2021;20:e13417. https://doi.org/10.1111/acel.13417.Suche in Google Scholar PubMed PubMed Central
24. Matsuzaki, S, Ueda, Y, Matsuzaki, S, Kakuda, M, Lee, M, Takemoto, Y, et al.. The characteristics and obstetric outcomes of type II vasa previa: systematic review and meta-analysis. Biomedicines 2022;10:3263. https://doi.org/10.3390/biomedicines10123263.Suche in Google Scholar PubMed PubMed Central
25. Shevell, T, Malone, FD, Vidaver, J, Porter, TF, Luthy, DA, Comstock, CH, et al.. Assisted reproductive technology and pregnancy outcome. Obstet Gynecol 2005;106:1039–45. https://doi.org/10.1097/01.aog.0000183593.24583.7c.Suche in Google Scholar
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/jpm-2023-0122).
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Obituary
- A tribute to Professor Moshe Mazor, M.D.
- Mini Review
- Systematic review of the long-term effects of postnatal corticosteroids
- Opinion Paper
- The proposal of the novel fetal shoulder dystocia graduation: a clinical-based opinion
- Corner of Academy
- Prenatal diagnosis of bilobate placenta: incidence, risk factors and impact on pregnancy outcomes
- Original Articles – Obstetrics
- High mobility group box 1 in women with unexplained recurrent pregnancy loss
- Velamentous cord insertion in monochorionic twin pregnancies: a step forward in screening for twin to twin transfusion syndrome and birthweight discordance?
- Advanced maternal age (AMA) and 75 g oGTT glucose levels are pedictors for insulin therapy in women with gestational diabetes (GDM)
- Perinatal, obstetric and parental risk factors for asthma in the offspring throughout childhood: a longitudinal cohort study
- Increased risk of severe COVID-19 in pregnancy in a multicenter propensity score-matched study
- Comparative clinical and placental pathologic characteristics in pregnancies with and without SARS-CoV-2 infection
- An analysis of factors affecting survival in prenatally diagnosed omphalocele
- The impact of abnormal maternal body mass index during pregnancy on perinatal outcomes: a registry-based study from Qatar
- Original Articles – Fetus
- Embryonic and fetal tiny pericardial fluid collections at less than 12 weeks of gestation
- Modeling fetal cortical development by quantile regression for gestational age and head circumference: a prospective cross sectional study
- The effect of 50 GR oral glucose tolerance test on fetal celiac artery and superior mesenteric artery Doppler parameters in healthy pregnancies
- Original Articles – Neonates
- Carboxyhaemoglobin levels in infants with hypoxic ischaemic encephalopathy
- Exploring professionals’ views regarding prenatal counselling in congenital diaphragmatic hernia
- Letter to the Editor
- Cutting of the strangulated double nuchal umbilical cord in a release of the severe shoulder dystocia: forensically justified or controversial procedure
- Retraction
- Retraction of: Pre-operative tranexemic acid vs. etamsylate in reducing blood loss during elective cesarean section: randomized controlled trial
- Retraction of: Lidocaine vs. tramadol vs. placebo wound infiltration for post-cesarean section pain relief: a randomized controlled trial
Artikel in diesem Heft
- Frontmatter
- Obituary
- A tribute to Professor Moshe Mazor, M.D.
- Mini Review
- Systematic review of the long-term effects of postnatal corticosteroids
- Opinion Paper
- The proposal of the novel fetal shoulder dystocia graduation: a clinical-based opinion
- Corner of Academy
- Prenatal diagnosis of bilobate placenta: incidence, risk factors and impact on pregnancy outcomes
- Original Articles – Obstetrics
- High mobility group box 1 in women with unexplained recurrent pregnancy loss
- Velamentous cord insertion in monochorionic twin pregnancies: a step forward in screening for twin to twin transfusion syndrome and birthweight discordance?
- Advanced maternal age (AMA) and 75 g oGTT glucose levels are pedictors for insulin therapy in women with gestational diabetes (GDM)
- Perinatal, obstetric and parental risk factors for asthma in the offspring throughout childhood: a longitudinal cohort study
- Increased risk of severe COVID-19 in pregnancy in a multicenter propensity score-matched study
- Comparative clinical and placental pathologic characteristics in pregnancies with and without SARS-CoV-2 infection
- An analysis of factors affecting survival in prenatally diagnosed omphalocele
- The impact of abnormal maternal body mass index during pregnancy on perinatal outcomes: a registry-based study from Qatar
- Original Articles – Fetus
- Embryonic and fetal tiny pericardial fluid collections at less than 12 weeks of gestation
- Modeling fetal cortical development by quantile regression for gestational age and head circumference: a prospective cross sectional study
- The effect of 50 GR oral glucose tolerance test on fetal celiac artery and superior mesenteric artery Doppler parameters in healthy pregnancies
- Original Articles – Neonates
- Carboxyhaemoglobin levels in infants with hypoxic ischaemic encephalopathy
- Exploring professionals’ views regarding prenatal counselling in congenital diaphragmatic hernia
- Letter to the Editor
- Cutting of the strangulated double nuchal umbilical cord in a release of the severe shoulder dystocia: forensically justified or controversial procedure
- Retraction
- Retraction of: Pre-operative tranexemic acid vs. etamsylate in reducing blood loss during elective cesarean section: randomized controlled trial
- Retraction of: Lidocaine vs. tramadol vs. placebo wound infiltration for post-cesarean section pain relief: a randomized controlled trial

