Abstract
Vaginal progesterone (VP) has been recommended to prevent preterm birth (PTB) in women at high-risk. However, there is controversy as to whether VP is efficacious in some subsets of high-risk women. In this review, we examined the current best evidence on the efficacy of VP to prevent PTB in several subsets of high-risk women and provided recommendations for its clinical use. Compelling evidence indicates that VP reduces the risk of PTB and improves perinatal outcomes in singleton gestations with a short cervix (≤25 mm), both with and without a history of spontaneous PTB. VP appears promising to reduce the risk of PTB in twin gestations with a short cervix (≤25 mm) and in singleton gestations conceived by assisted reproductive technologies, but further research is needed. There is no convincing evidence that supports prescribing VP to prevent PTB in singleton gestations based solely on the history of spontaneous preterm birth. Persuasive evidence shows that VP does not prevent PTB nor does it improve perinatal outcomes in unselected twin gestations and in singleton gestations with a history of spontaneous PTB and a cervical length >25 mm. There is no evidence supporting the use of VP to prevent PTB in triplet or higher-order multifetal gestations, singleton gestations with a positive fetal fibronectin test and clinical risk factors for PTB, and gestations with congenital uterine anomalies or uterine leiomyoma. In conclusion, current evidence indicates that VP should only be recommended in singleton gestations with a short cervix, regardless of the history of spontaneous PTB.
Introduction
In 2021, the rate of preterm birth in the United States, which had declined from 2019 to 2020 (10.23 to 10.09%), increased to 10.49% [1]. This is the highest level reported in at least 14 years. An estimated 14.84 million infants (10.6% of all live births) are born preterm worldwide every year [2]. In 2019, complications relating to preterm birth were the leading cause of mortality in children under 5 years of age worldwide, accounting for 17.7% of all deaths, and for 36.1% of neonatal deaths [3]. Infants born prematurely are at increased risk for neonatal complications such as respiratory distress syndrome (RDS), bronchopulmonary dysplasia, necrotizing enterocolitis, and intraventricular hemorrhage, long-term neurodevelopmental behavioral and cognitive disorders, as well as chronic diseases in adulthood such as diabetes mellitus, hypertension, ischemic heart disease, cerebrovascular disease leading to stroke, and chronic kidney disease [4], [5], [6], [7], [8], [9], [10]. Moreover, preterm birth generates a significant economic burden to society and has a major impact on the quality of life of parents and families [4, 11, 12].
Preterm labor is a complex syndrome associated with multiple etiologic processes such as infection/inflammation, vascular disorders, decidual hemorrhage, uterine overdistention, decline in progesterone action, cervical disease, breakdown of maternal-fetal tolerance, immunologically mediated processes, and maternal stress, among others [13], [14], [15]. This is the reason why a single intervention does not prevent all, or even most, cases of preterm birth.
Several interventions have been proposed to prevent preterm birth: home uterine monitoring to detect preterm labor, risk scoring systems to predict preterm birth, psychosocial interventions, health system interventions (packages of antenatal care, specialized antenatal clinics, incentives for increasing prenatal care, midwifery led care), optimal birth spacing, bed rest, activity restriction, nutritional interventions (nutritional advice, macronutrient and micronutrient supplementation, vitamins, fish oil, zinc, calcium, iron and folic acid), prophylactic antibiotics during the second and third trimesters, screening and prevention/treatment of periodontal disease and infections, low-dose aspirin, prophylactic tocolysis, administration of progestogens, cervical cerclage, and cervical pessary. Unfortunately, most of these interventions have been shown to be ineffective in reducing the risk of preterm birth in singleton and twin gestations [16], [17], [18].
In 2012, both the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) recommended the administration of vaginal progesterone to women with a singleton gestation, no history of spontaneous preterm birth, and a transvaginal cervical length ≤20 mm at ≤24 weeks of gestation [19, 20]. A SMFM statement published in 2017 reaffirmed that “vaginal progesterone should not be considered a substitute for 17OHP-C [17α-hydroxyprogesterone caproate]” in women with a singleton gestation and a history of spontaneous preterm birth [21]. In 2021, the ACOG updated its guidelines, which were endorsed by the SMFM, and recommended the administration of vaginal progesterone to women with a singleton gestation, no history of spontaneous preterm birth, and a transvaginal cervical length <25 mm at 18–22 weeks of gestation [22]. In addition, for the first time, these guidelines recommended offering vaginal progesterone to women with a singleton gestation and a history of spontaneous preterm birth regardless of the cervical length measurement. The evidence base to make this last recommendation was the EPPPIC meta-analysis, an individual patient data (IPD) meta-analysis that included 9 trials comparing vaginal progesterone to placebo/no treatment in women with a singleton gestation considered at high risk for preterm birth due to a history of spontaneous preterm birth, short cervix, congenital uterine anomalies, uterine leiomyomas, pregnancy after assisted reproductive technologies, or a positive fetal fibronectin test combined with other clinical risk factors [23]. This study reported that vaginal progesterone significantly reduced the risk of preterm birth <34 weeks of gestation in high-risk singleton gestations (pooled relative risk [RR], 0.78; 95% confidence interval [CI], 0.68–0.90). There were no significant differences between the vaginal progesterone and placebo/no treatment groups in the risk of the remaining primary outcomes (preterm birth <37 and <28 weeks of gestation, perinatal death, serious neonatal complications, and maternal complications). This IPD meta-analysis has been criticized by the U.S. Food and Drug Administration’s (FDA) Center for Drug Evaluation and Research (CDER) because it grouped together trials of patients with differences in their risk profiles, including combining women with a history of spontaneous preterm birth and those without a history of spontaneous preterm birth, and women with and without a short cervix [24]. Because of this grouping, this IPD meta-analysis does not provide relevant information regarding the efficacy of vaginal progesterone in different subsets of patients at high risk for preterm birth. Hence, an assessment of the efficacy of vaginal progesterone for preventing preterm birth in such subsets of patients is justified.
The objectives of this review were (1) to examine the current best evidence regarding the efficacy of vaginal progesterone to prevent preterm birth and improve perinatal outcomes in several subsets of women at high risk for this entity; and (2) to provide evidence-based recommendations for the clinical use of vaginal progesterone in women at high risk for preterm birth. We prioritized data from randomized controlled trials and systematic reviews and meta-analyses of randomized controlled trials.
Efficacy of vaginal progesterone to prevent preterm birth in high-risk women
Women with a singleton gestation and a midtrimester transvaginal sonographic short cervix
In 2018, a meta-analysis of IPD was published that assessed the efficacy of vaginal progesterone in reducing the risk of preterm birth and adverse perinatal outcomes in asymptomatic women with a singleton gestation and a midtrimester (18–24 weeks of gestation) transvaginal sonographic short cervix (cervical length ≤25 mm) [25]. The primary outcome was preterm birth <33 weeks of gestation. IPD were obtained for 974 women with a cervical length ≤25 mm from 5 double-blind, placebo-controlled, high-quality trials [26], [27], [28], [29], [30]. The daily dose of vaginal progesterone used in the trials was 200 mg in 2 studies [27, 30], 100 mg in 1 study [28], and 90 mg in 2 studies [26, 29], and the treatment was administered from 18+0–24+6 to 34+0–37+0 weeks of gestation. A total of 498 women were assigned to receive vaginal progesterone and 476 to receive placebo. Vaginal progesterone significantly reduced the risk of preterm birth <36 weeks (RR, 0.80; 95% CI, 0.67–0.97), <35 weeks (RR, 0.72; 95% CI, 0.58–0.89), <34 weeks (RR, 0.65; 95% CI, 0.51–0.83), <33 weeks (RR, 0.62; 95% CI, 0.47–0.81), <32 weeks (RR, 0.64; 95% CI, 0.48–0.86), <30 weeks (RR, 0.70; 95% CI (0.49–0.98), and <28 weeks (RR, 0.67; 95% CI, 0.45–0.99). Importantly, vaginal progesterone was also associated with a significant decrease in the risk of RDS (RR, 0.47; 95% CI, 0.27–0.81), composite neonatal morbidity and mortality (RR, 0.59; 95% CI, 0.38–0.91), birthweight <1,500 (RR, 0.62; 95% CI, 0.44–0.86) and <2,500 g (RR, 0.82; 95% CI, 0.68–0.98), and admission to the neonatal intensive care unit (NICU) (RR, 0.68; 95% CI, 0.53–0.88) (Table 1). Moreover, there was a nonsignificant trend toward reduction of neonatal death (RR, 0.44; 95% CI, 0.18–1.07), neonatal sepsis (RR, 0.61; 95% CI, 0.34–1.08), and use of mechanical ventilation (RR, 0.65; 95% CI, 0.41–1.01). Maternal adverse events, congenital anomalies, and adverse neurodevelopmental and health outcomes at 2 years of age did not significantly differ between the vaginal progesterone and placebo groups. According to the GRADE approach [31], evidence was graded as high-quality for all outcomes for which vaginal progesterone significantly reduced their risk. It signifies that we are very confident that the true effect lies close to that of the estimate of the effect and that further research is very unlikely to change our confidence in the estimate of effect. The mechanisms by which vaginal progesterone prevents preterm birth in women with a singleton gestation and a sonographic short cervix are not clear but may involve alterations of molecular pathways involved in premature cervical ripening and/or the anti-inflammatory effects of vaginal progesterone [32], [33], [34].
Statistically significant beneficial effects of vaginal progesterone in women with a singleton gestation and a midtrimester transvaginal sonographic short cervix.
| Outcome | No of trials | Vaginal progesterone | Placebo | Relative risk (95% CI) | p-Value | ||
|---|---|---|---|---|---|---|---|
| Preterm birth <36 weeks | 5 | 139/498 | (28%) | 166/476 | (35%) | 0.80 (0.67–0.97) | 0.02 |
| Preterm birth <35 weeks | 5 | 106/498 | (21%) | 141/476 | (30%) | 0.72 (0.58–0.89) | 0.003 |
| Preterm birth <34 weeks | 5 | 86/498 | (17%) | 126/476 | (26%) | 0.65 (0.51–0.83) | 0.0006 |
| Preterm birth <33 weeks | 5 | 70/498 | (14%) | 107/476 | (22%) | 0.62 (0.47–0.81) | 0.0006 |
| Preterm birth <32 weeks | 5 | 62/498 | (12%) | 92/476 | (19%) | 0.64 (0.48–0.86) | 0.003 |
| Preterm birth <30 weeks | 5 | 49/498 | (10%) | 67/476 | (14%) | 0.70 (0.49–0.98) | 0.04 |
| Preterm birth <28 weeks | 5 | 38/498 | (8%) | 54/476 | (11%) | 0.67 (0.45–0.99) | 0.04 |
| Spontaneous preterm birth <33 weeks | 5 | 60/498 | (12%) | 82/476 | (17%) | 0.70 (0.51–0.95) | 0.02 |
| Spontaneous preterm birth <34 weeks | 5 | 73/498 | (15%) | 97/476 | (20%) | 0.72 (0.55–0.95) | 0.02 |
| Respiratory distress syndrome | 4 | 17/365 | (5%) | 37/358 | (10%) | 0.47 (0.27–0.81) | 0.007 |
| Composite neonatal morbidity and mortality | 4 | 29/365 | (8%) | 49/358 | (14%) | 0.59 (0.38–0.91) | 0.02 |
| Birthweight <1,500 g | 5 | 50/497 | (10%) | 77/473 | (16%) | 0.62 (0.44–0.86) | 0.004 |
| Birthweight <2,500 g | 5 | 144/497 | (29%) | 168/473 | (36%) | 0.82 (0.68–0.98) | 0.03 |
| Admission to NICU | 5 | 83/496 | (17%) | 117/474 | (25%) | 0.68 (0.53–0.88) | 0.003 |
-
Data are presented as number/total number. CI, confidence interval; NICU, neonatal intensive care unit.
Prespecified subgroup analyses showed that the beneficial effect of vaginal progesterone on preterm birth <33 weeks of gestation did not differ significantly between patients with a history of spontaneous preterm birth (RR, 0.59; 95% CI, 0.40–0.88) and those without a history of spontaneous preterm birth (RR, 0.65; 95% CI, 0.45–0.94; p for interaction=0.74), as well as between US women (RR, 0.73; 95% CI, 0.42–1.27) and non-US women (RR, 0.59; 95% CI, 0.43–0.80; p for interaction=0.51). There was no difference in efficacy in the prevention of preterm birth <33 weeks of gestation when either 90–100 mg/d (RR, 0.53; 95% CI, 0.33–0.87) or 200 mg/d (RR, 0.67; 95% CI, 0.49–0.93) of vaginal progesterone was administered. Therefore, either regimen can be used in practice. However, it is recommended to use a daily dose of 90–100 mg of vaginal progesterone because it is the lowest dose that reduced the risk of preterm birth <33 weeks of gestation.
In conclusion, there is compelling evidence that vaginal progesterone decreases the risk of preterm birth and improves perinatal outcomes in women with a singleton gestation and a midtrimester transvaginal sonographic short cervix, both with and without a history of spontaneous preterm birth, without any demonstrable deleterious effects on childhood neurodevelopment.
Women with a singleton gestation, a history of spontaneous preterm birth, and a midtrimester transvaginal sonographic short cervix
In 2011, an IPD meta-analysis assessed the efficacy of cerclage for the prevention of preterm birth and perinatal morbidity and mortality in asymptomatic women with a singleton gestation, a history of spontaneous preterm birth, and a cervical length <25 mm before 24 weeks of gestation [35]. Cerclage was associated with a significant reduction in the risk of preterm birth <37, <35, <32, and <28 weeks of gestation, composite perinatal morbidity and mortality, and birthweight <1,500 g when compared to no cerclage. Given that our 2018 IPD meta-analysis [25] showed that vaginal progesterone significantly reduced the risk of preterm birth <33 weeks among women with a singleton gestation, a history of spontaneous preterm birth, and a midtrimester transvaginal sonographic short cervix (cervical length ≤25 mm), we compared the efficacy of vaginal progesterone and cerclage in preventing preterm birth and adverse perinatal outcomes in this subset of patients by using adjusted indirect comparison meta-analytic techniques [36]. Vaginal progesterone, compared to placebo, significantly reduced the risk of preterm birth <35 weeks (RR, 0.68; 95% CI, 0.50–0.93) and <32 weeks (RR, 0.60; 95% CI, 0.39–0.92), composite perinatal morbidity and mortality (RR, 0.43; 95% CI, 0.20–0.94), neonatal sepsis (RR, 0.38; 95% CI, 0.15–0.96), composite neonatal morbidity (RR, 0.29; 95% CI, 0.11–0.81), and admission to the NICU (RR, 0.46; 95% CI, 0.30–0.70).
Adjusted indirect comparison meta-analyses did not show statistically significant differences between vaginal progesterone and cerclage in the reduction of preterm birth or adverse perinatal outcomes. These results indicate that vaginal progesterone and cerclage are equally efficacious in preventing preterm birth in women with a singleton gestation, a history of spontaneous preterm birth, and a sonographic short cervix.
In conclusion, vaginal progesterone should be offered as an alternative to cerclage in patients with a singleton gestation, a history of spontaneous preterm birth, and a midtrimester transvaginal short cervix (cervical length ≤25 mm).
Women with a twin gestation and a midtrimester transvaginal sonographic short cervix
In February 2022, we published the results of an updated IPD meta-analysis that assessed the efficacy of vaginal progesterone for the prevention of preterm birth and neonatal morbidity and mortality in asymptomatic women with a twin gestation and a midtrimester transvaginal sonographic cervical length ≤25 mm [37]. The primary outcome was preterm birth <33 weeks of gestation. Six double-blind, placebo-controlled, high-quality trials [27, 28, 38], [39], [40], [41], which provided IPD for 95 women and their 190 fetuses/infants, were included in this updated meta-analysis. Vaginal progesterone, as compared to placebo, was associated with a significant decrease in the frequency of preterm birth <33 weeks of gestation (38.5 vs. 55.8%; RR, 0.60; 95% CI, 0.38–0.95). Moreover, the treatment with vaginal progesterone significantly reduced the risk of preterm birth <34 weeks (RR, 0.68; 95% CI, 0.46–0.99), <32 weeks (RR, 0.56; 95% CI, 0.33–0.93), <30 weeks (RR, 0.45; 95% CI, 0.23–0.89) and <28 weeks (RR, 0.41; 95% CI, 0.19–0.91) of gestation, spontaneous preterm birth <33 weeks (RR, 0.53; 95% CI, 0.33–0.87) and <34 weeks (RR, 0.58; 95% CI, 0.38–0.89) of gestation, composite neonatal morbidity and mortality (RR, 0.59; 95% CI, 0.33–0.98), and birthweight<1,500 g (RR, 0.55; 95% CI, 0.33–0.94).
Although this updated IPD meta-analysis showed that vaginal progesterone reduces the risk of preterm birth occurring at <28 to <34 gestational weeks and improves perinatal outcomes, we think further evidence is required before recommending the use of this intervention among women with a twin gestation and a short cervix. The PROSPECT study (NCT02518594) is an ongoing randomized controlled trial assessing the use of vaginal progesterone 200 mg/day or cervical pessary vs. placebo to prevent preterm birth <35 weeks of gestation in 630 women with a twin gestation and a cervical length <30 mm between 16 and 23 weeks of gestation. The estimated completion date of this trial is February 2025. The results of this study will help to establish whether this promising intervention can be recommended to women with a twin gestation and a short cervix.
Women with a singleton gestation and a history of spontaneous preterm birth
In September 2022, we reported the results of a systematic review and meta-analysis that aimed to evaluate the efficacy and safety of vaginal progesterone to prevent preterm birth and adverse perinatal outcomes in women with a singleton gestation and a history of spontaneous preterm birth [42]. The primary outcomes were preterm birth <37 and <34 weeks of gestation. A total of 10 randomized controlled trials, including 2,958 women, that compared vaginal progesterone to placebo/no treatment met the criteria for inclusion [26, 28, 30, 43], [44], [45], [46], [47], [48], [49]. Seven studies had a sample size <150 (small studies) [28, 43], [44], [45], [46], [47, 49] and 3 had a sample size >600 (large studies) [26, 30, 48]. The three large studies had high methodological quality. Among the 7 small studies, only 1 was deemed as high-quality [28]. The remaining 6 small studies were at high risk of bias (4 trials) [43, 45, 46, 49] or some concerns of bias (2 trials) [44, 47]. Overall, meta-analyses with substantial statistical heterogeneity that included data from all 10 trials showed that vaginal progesterone significantly reduced the risk of preterm birth <37 weeks (RR, 0.64; 95% CI, 0.50–0.81) and <34 weeks (RR, 0.62; 95% CI, 0.42–0.92), and the risk of admission to the NICU (RR, 0.53; 95% CI, 0.33–0.85). The quality of evidence according to the GRADE approach [31] for the outcomes of preterm birth <37 and <34 weeks of gestation was deemed as very low, which means that the true effect is probably markedly different from the estimated effect.
A prespecified subgroup analysis according to study sample size indicated that the results were highly conflicting because vaginal progesterone was associated with a large decrease in the risk of preterm birth <37 weeks (RR, 0.43; 95% CI, 0.33–0.55) and <34 weeks (RR, 0.27; 95% CI, 0.15–0.49) and of NICU admission (RR, 0.30; 95% CI, 0.18–0.51) in the small, poor-quality trials, whereas it had no effect in the large, high-quality trials (RR, 0.98; 95% CI 0.88–1.09 for preterm birth <37 weeks; RR, 0.94; 95% CI, 0.78–1.13 for preterm birth <34 weeks; and RR, 0.87; 95% CI, 0.69–1.09 for NICU admission) (Figure 1). Small-study effects, defined as the tendency of small trials to report larger benefits of treatment than large trials do, were clearly demonstrated in these meta-analyses. Sensitivity analyses restricted to the trials at overall low risk of bias showed that vaginal progesterone did not decrease the risk of preterm birth <37 weeks (RR, 0.96; 95% CI, 0.84–1.09) and <34 weeks (RR, 0.90; 95% CI, 0.71–1.15) and the risk of NICU admission (RR, 0.77; 95% CI, 0.53–1.14). In addition, the adjustment for small-study effects resulted in a markedly reduced and nonsignificant effect of vaginal progesterone on preterm birth <37 weeks (RR, 0.86; 95% CI, 0.68–1.10) and <34 weeks (RR, 0.92; 95% CI, 0.60–1.42). There were no substantial differences between the vaginal progesterone and the placebo/no treatment groups in other adverse perinatal and maternal outcomes. Based on these analyses, it was concluded that no convincing evidence supports prescribing vaginal progesterone to prevent preterm birth in singleton gestations with a history of spontaneous preterm birth.
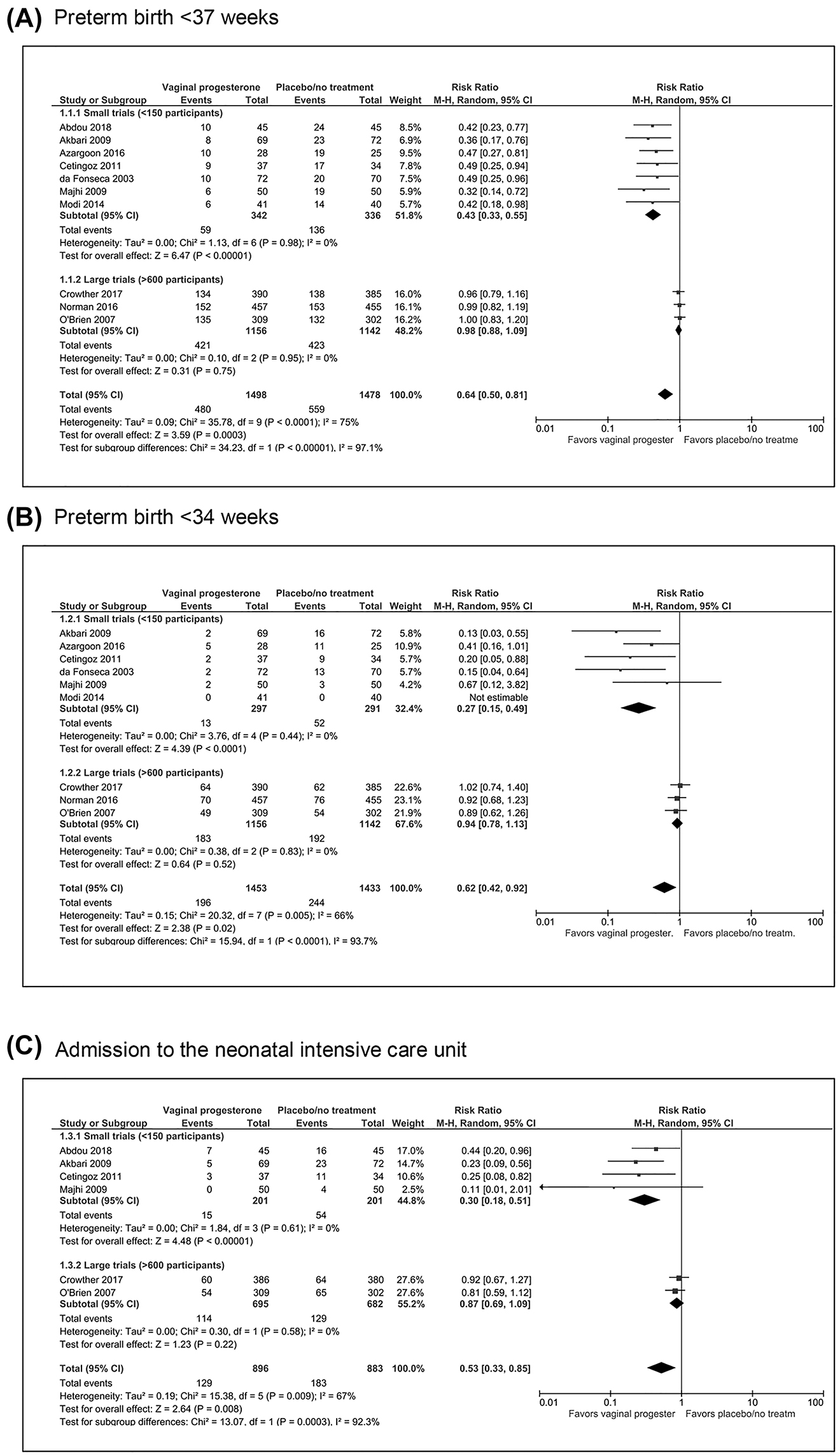
Effect of vaginal progesterone on preterm birth <37 and <34 weeks and on admission to the neonatal intensive care unit in women with a singleton gestation and a history of spontaneous preterm birth according to study sample size.
In summary, vaginal progesterone should not be recommended for preventing preterm birth in women with a singleton gestation based solely on the history of spontaneous preterm birth.
Women with a singleton gestation, a history of spontaneous preterm birth, and a midtrimester transvaginal sonographic cervical length >25 mm
We recently reported the results of a post-hoc subgroup analysis [50] of the recently published meta-analysis [42], which evaluated the efficacy of vaginal progesterone in preventing preterm birth in women with a singleton gestation, a history of spontaneous preterm birth, and a midtrimester transvaginal sonographic cervical length >25 mm. The primary outcomes were preterm birth <37 and <34 weeks of gestation. Four randomized controlled trials [26, 28, 30, 49], comprising 1,308 women with these characteristics, fulfilled the inclusion criteria. The frequency of preterm birth <37 weeks of gestation among women allocated to receive vaginal progesterone was remarkably similar to that observed in women in the placebo/no treatment group (35.4 vs. 35.4%; RR, 0.99; 95% CI, 0.84–1.16; p=0.88). The quality of evidence according to the GRADE approach [31] was considered high for this outcome. There were no significant differences between the vaginal progesterone and placebo/no treatment groups in the risk of preterm birth <34 and <28 weeks of gestation and of adverse perinatal outcomes. Hence, it was concluded that vaginal progesterone does not prevent preterm birth, nor does it improve perinatal outcomes in women with a singleton gestation, a history of spontaneous preterm birth, and a midtrimester transvaginal sonographic cervical length >25 mm.
In summary, findings from this analysis indicate that vaginal progesterone should be offered to patients with a singleton gestation and a history of spontaneous preterm birth only if they have a midtrimester transvaginal sonographic cervical length ≤25 mm.
Women with a singleton gestation conceived by assisted reproductive technologies
We identified a single center, double-blind, placebo-controlled trial that evaluated the efficacy of vaginal progesterone (400 mg/d) from 16–22 to 36 weeks of gestation to prevent preterm birth in 215 singleton gestations conceived by in vitro fertilization or intracytoplasmic sperm injection [51]. Vaginal progesterone administration was associated with a significant reduction in the risk of preterm birth <37 weeks (RR, 0.63; 95% CI, 0.40–0.98). There were no significant differences between the vaginal progesterone and placebo groups in the risk of preterm birth <34 weeks of gestation and adverse perinatal outcomes. Therefore, although vaginal progesterone appears promising to reduce the risk of preterm birth in women with a singleton gestation conceived by assisted reproductive technologies, further trials are needed to confirm the findings of this study before recommending its use in this subset of patients.
Women with an unselected twin gestation
Currently, we are performing a systematic review and meta-analysis on the efficacy of vaginal progesterone in twin gestations (registered with the PROSPERO database of systematic reviews; number CRD42020205184). The primary outcome is preterm birth <34 weeks of gestation. We identified 9 randomized controlled trials that compared vaginal progesterone vs. placebo/no treatment in unselected twin gestations conceived either naturally or by assisted reproductive technologies [28, 38], [39], [40], [41, 51], [52], [53], [54]. All but 1 study [54] were double-blind, placebo-controlled trials. The daily dose of vaginal progesterone used in the trials was 90–100 mg in 3 studies [28, 52, 53], 200 mg in 2 studies [38, 40], 400 mg in 2 studies [51, 54], 600 mg in 1 study [41], and 200 or 400 mg in 1 study [39]. All 9 trials reported that there were no significant differences between the vaginal progesterone and the placebo/no treatment groups in the risk of preterm birth <34 weeks of gestation and adverse perinatal outcomes. Only 1 small trial [28] reported that vaginal progesterone significantly reduced the risk of preterm birth <37 weeks of gestation. The remaining 8 trials reported no significant differences between the vaginal progesterone and the placebo/no treatment groups in preterm birth <37 weeks. A meta-analysis with data from the 9 trials, comprising 3,368 women, showed that there was no significant difference between the vaginal progesterone and the placebo/no treatment groups in the risk of preterm birth<34 weeks of gestation (pooled RR, 1.00; 95% CI, 0.84–1.19). We concluded that vaginal progesterone, regardless of the daily dose used, does not prevent preterm birth nor does it improve perinatal outcomes in unselected twin gestations.
Women with a triplet or higher-order multifetal gestation
Only the study by Wood et al. [53], comparing vaginal progesterone to placebo in multiple gestations, included 3 triplet gestations (two in the vaginal progesterone group and 1 in the placebo group). No results were reported for this subset of patients. Therefore, currently there is no evidence supporting the use of vaginal progesterone to prevent preterm birth in triplet and higher-order multifetal gestations.
Women with a singleton gestation and a positive fetal fibronectin test result combined with other clinical risk factors
The trial by Norman et al. [30] included a subset of patients with a positive fetal fibronectin test at 22–24 weeks of gestation combined with other clinical risk factors for preterm birth such as a history of preterm birth, a second-trimester loss, preterm premature rupture of the membranes, or a history of a cervical procedure to treat abnormal smears (n=343). There were no significant differences between patients allocated to receive vaginal progesterone and those allocated to receive placebo in preterm birth or fetal death before 34 weeks of gestation (odds ratio, 0.91; 95% CI, 0.57–1.46), a composite outcome of neonatal death, bronchopulmonary dysplasia, or brain injury on cerebral ultrasound (odds ratio, 0.69; 95% CI, 0.37–1.30), and a Bayley-III cognitive composite score at 2 years of age (mean difference, −1.09; 95% CI, −5.41 to 3.23). In conclusion, vaginal progesterone does not reduce the risk of preterm birth and adverse neonatal outcomes in women with a singleton gestation and a positive fetal fibronectin test result combined with other clinical risk factors.
Women with congenital uterine anomalies
To date, only 4 randomized controlled trials assessing vaginal progesterone in women at high risk of preterm birth have included patients with congenital uterine anomalies [28, 43, 45, 47]. The studies by Da Fonseca et al. [43] and Akbari et al. [45], comprising a total of 10 women with congenital uterine anomalies, did not report results separately for these patients. We were able to perform meta-analyses including data from the studies by Cetingoz et al. [28] (n=12) and Azargoon et al. [47] (n=15). Overall, no significant differences were observed between the vaginal progesterone and the placebo groups in the risk of preterm birth <37 weeks (pooled RR, 2.10; 95% CI, 0.21–21.27) and <34 weeks (pooled RR, 0.89; 95% CI, 0.40–1.99). Thus, the current evidence suggests that the use of vaginal progesterone in patients with congenital uterine anomalies has no benefit in preventing preterm birth.
Women with uterine leiomyoma
The trial by Azargoon et al. [47], which compared vaginal progesterone to placebo in women at high-risk for preterm birth, was the only one that included patients with uterine leiomyoma (≥7 cm; n=5). No results were reported separately for this subset of patients. Consequently, there is no evidence supporting the administration of vaginal progesterone to women with uterine leiomyoma aiming to prevent preterm birth.
Recommendations for the clinical use of vaginal progesterone in women at high risk for preterm birth
Based on the evidence presented herein, we propose the following recommendations for the clinical use of vaginal progesterone in women at high-risk for preterm birth:
Vaginal progesterone at a dose of 90–100 mg/d should be offered from 18–24 to 36 weeks of gestation to women with a singleton gestation and a transvaginal sonographic cervical length ≤25 mm at 18–24 weeks of gestation, with and without a history of spontaneous preterm birth.
Vaginal progesterone at a dose of 90–100 mg/d should be offered from 18–24 to 36 weeks of gestation to women with a singleton gestation and a history of spontaneous preterm birth only if they have a transvaginal sonographic cervical length ≤25 mm at 18–24 weeks of gestation.
Vaginal progesterone appears promising to reduce the risk of preterm birth in women with a twin gestation and a midtrimester transvaginal sonographic cervical length ≤25 mm and in women with a singleton gestation conceived by assisted reproductive technologies, but further research is needed before recommending its use in these subsets of patients.
Vaginal progesterone should not be offered to women with a singleton gestation and a history of spontaneous preterm birth based solely on such history.
Vaginal progesterone should not be offered to women with an unselected twin gestation and to women with a singleton gestation, a history of spontaneous preterm birth, and a transvaginal sonographic cervical length >25 mm at 18–24 weeks of gestation.
There is no evidence supporting the use of vaginal progesterone in women with a triplet or higher-order multifetal gestation, in women with a singleton gestation and a positive fetal fibronectin test result combined with other clinical risk factors, and in women with congenital uterine anomalies or uterine leiomyoma.
A revised, evidence-based clinical algorithm for the use of vaginal progesterone in preventing preterm birth among women with a singleton gestation is presented in Figure 2.
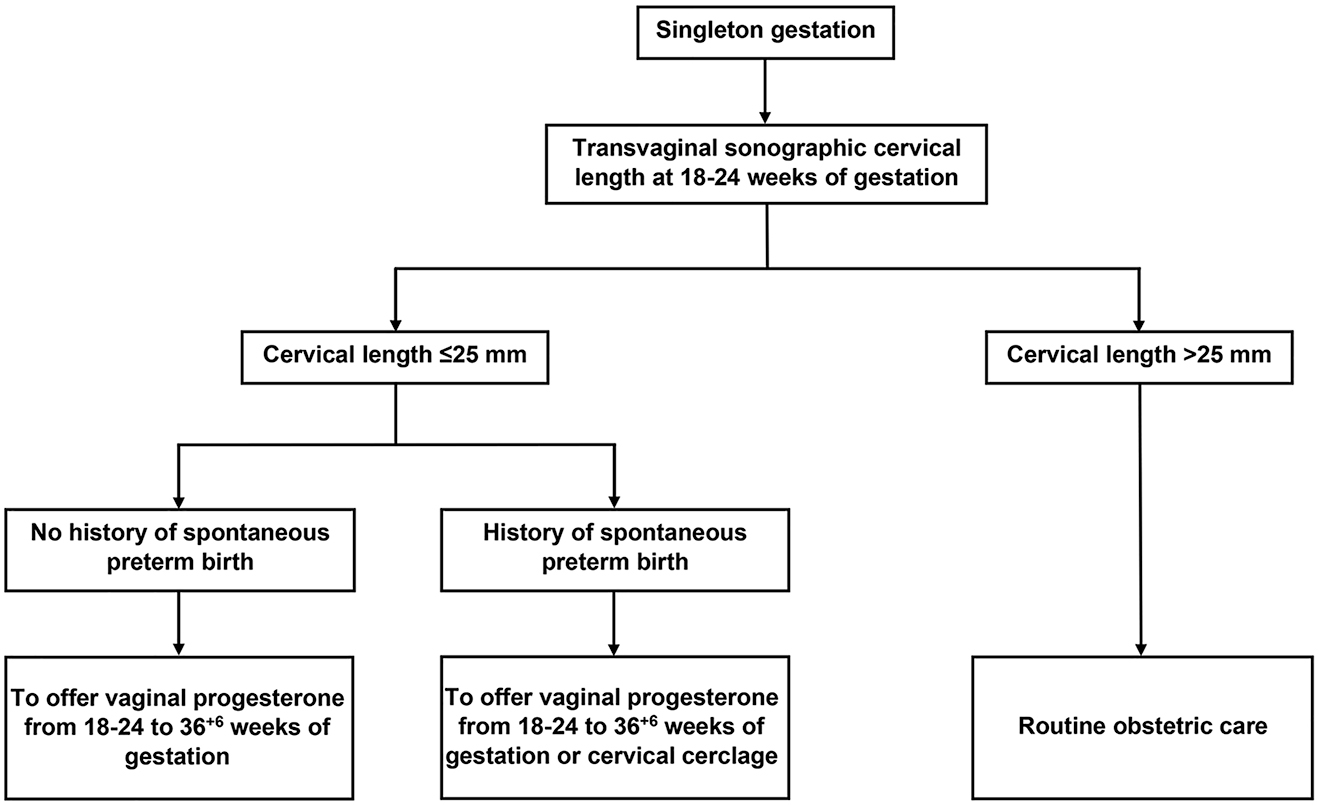
Evidence-based clinical algorithm for the use of vaginal progesterone in preventing preterm birth among singleton gestations.
Award Identifier / Grant number: Contract No. HHSN275201300006C
-
Research funding: This review was supported, in part, by the Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C. Dr. Romero has contributed to this work as part of his official duties as an employee of the United States Federal Government.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: The authors state no conflicts of interest.
-
Informed consent: Not applicable.
-
Ethical approval: Not applicable.
-
Role of the funding source: The funder had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review or approval of the manuscript or the decision to submit the manuscript for publication.
References
1. Martin, JA, Hamilton, BE, Osterman, MJ. Births in the United States, 2021. NCHS Data Brief 2022:1–8.10.15620/cdc:119632Suche in Google Scholar
2. Chawanpaiboon, S, Vogel, JP, Moller, AB, Lumbiganon, P, Petzold, M, Hogan, D, et al.. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Global Health 2019;7:e37–46. https://doi.org/10.1016/s2214-109x(18)30451-0.Suche in Google Scholar
3. Perin, J, Mulick, A, Yeung, D, Villavicencio, F, Lopez, G, Strong, KL, et al.. Global, regional, and national causes of under-5 mortality in 2000-19: an updated systematic analysis with implications for the sustainable development goals. Lancet Child Adolesc Health 2022;6:106–15. https://doi.org/10.1016/s2352-4642(21)00311-4.Suche in Google Scholar PubMed PubMed Central
4. Institute of Medicine Committee on Understanding Premature Birth and Assuring Healthy Outcomes. The national academies collection: reports funded by national institutes of health. In: Behrman, RE, Butler, AS, editors. Preterm birth: causes, consequences, and prevention. Washington (DC): National Academies Press (US), National Academy of Sciences; 2007.Suche in Google Scholar
5. Manuck, TA, Rice, MM, Bailit, JL, Grobman, WA, Reddy, UM, Wapner, RJ, et al.. Preterm neonatal morbidity and mortality by gestational age: a contemporary cohort. Am J Obstet Gynecol 2016;215:103.e1–4.Suche in Google Scholar
6. Catov, JM, Scifres, CM, Caritis, SN, Bertolet, M, Larkin, J, Parks, WT. Neonatal outcomes following preterm birth classified according to placental features. Am J Obstet Gynecol 2017;216:411.e1–4. https://doi.org/10.1016/j.ajog.2016.12.022.Suche in Google Scholar PubMed
7. Saigal, S, Doyle, LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008;371:261–9. https://doi.org/10.1016/s0140-6736(08)60136-1.Suche in Google Scholar
8. Fernández de Gamarra-Oca, L, Ojeda, N, Gómez-Gastiasoro, A, Peña, J, Ibarretxe-Bilbao, N, García-Guerrero, MA, et al.. Long-term neurodevelopmental outcomes after moderate and late preterm birth: a systematic review. J Pediatr 2021;237:168–76.e11. https://doi.org/10.1016/j.jpeds.2021.06.004.Suche in Google Scholar PubMed
9. Robbins, CL, Hutchings, Y, Dietz, PM, Kuklina, EV, Callaghan, WM. History of preterm birth and subsequent cardiovascular disease: a systematic review. Am J Obstet Gynecol 2014;210:285–97. https://doi.org/10.1016/j.ajog.2013.09.020.Suche in Google Scholar PubMed PubMed Central
10. Crump, C. An overview of adult health outcomes after preterm birth. Early Hum Dev 2020;150:105187. https://doi.org/10.1016/j.earlhumdev.2020.105187.Suche in Google Scholar PubMed PubMed Central
11. Petrou, S, Yiu, HH, Kwon, J. Economic consequences of preterm birth: a systematic review of the recent literature (2009–2017). Arch Dis Child 2019;104:456–65. https://doi.org/10.1136/archdischild-2018-315778.Suche in Google Scholar PubMed
12. Amorim, M, Silva, S, Kelly-Irving, M, Alves, E. Quality of life among parents of preterm infants: a scoping review. Qual Life Res 2018;27:1119–31. https://doi.org/10.1007/s11136-017-1771-6.Suche in Google Scholar PubMed
13. Romero, R, Mazor, M, Munoz, H, Gomez, R, Galasso, M, Sherer, DM. The preterm labor syndrome. Ann N Y Acad Sci 1994;734:414–29. https://doi.org/10.1111/j.1749-6632.1994.tb21771.x.Suche in Google Scholar PubMed
14. Romero, R, Espinoza, J, Kusanovic, JP, Gotsch, F, Hassan, S, Erez, O, et al.. The preterm parturition syndrome. BJOG 2006;113:17–42. https://doi.org/10.1111/j.1471-0528.2006.01120.x.Suche in Google Scholar PubMed PubMed Central
15. Romero, R, Dey, SK, Fisher, SJ. Preterm labor: one syndrome, many causes. Science 2014;345:760–5. https://doi.org/10.1126/science.1251816.Suche in Google Scholar PubMed PubMed Central
16. Medley, N, Vogel, JP, Care, A, Alfirevic, Z. Interventions during pregnancy to prevent preterm birth: an overview of cochrane systematic reviews. Cochrane Database Syst Rev 2018;11:CD012505.10.1002/14651858.CD012505.pub2Suche in Google Scholar PubMed PubMed Central
17. Campbell, F, Salam, S, Sutton, A, Jayasooriya, SM, Mitchell, C, Amabebe, E, et al.. Interventions for the prevention of spontaneous preterm birth: a scoping review of systematic reviews. BMJ Open 2022;12:e052576. https://doi.org/10.1136/bmjopen-2021-052576.Suche in Google Scholar PubMed PubMed Central
18. Conde-Agudelo, A, Romero, R, Nicolaides, KH. Cervical pessary to prevent preterm birth in asymptomatic high-risk women: a systematic review and meta-analysis. Am J Obstet Gynecol 2020;223:42–65.e2. https://doi.org/10.1016/j.ajog.2019.12.266.Suche in Google Scholar PubMed PubMed Central
19. Committee on Practice Bulletins—Obstetrics, The American College of Obstetricians and Gynecologists. Practice bulletin no. 130: prediction and prevention of preterm birth. Obstet Gynecol 2012;120:964–73.10.1097/AOG.0b013e3182723b1bSuche in Google Scholar PubMed
20. Society for Maternal-Fetal Medicine Publications Committee, with assistance of Vincenzo Berghella. Progesterone and preterm birth prevention: translating clinical trials data into clinical practice. Am J Obstet Gynecol 2012;206:376–86.10.1016/j.ajog.2012.03.010Suche in Google Scholar PubMed
21. Society for Maternal-Fetal Medicine (SMFM) Publications Committee. The choice of progestogen for the prevention of preterm birth in women with singleton pregnancy and prior preterm birth. Am J Obstet Gynecol 2017;216:B11–3.10.1016/j.ajog.2017.01.022Suche in Google Scholar PubMed
22. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol 2021;138:e65–90.10.1097/AOG.0000000000004479Suche in Google Scholar PubMed
23. EPPPIC Group. Evaluating progestogens for preventing preterm birth international collaborative (EPPPIC): meta-analysis of individual participant data from randomised controlled trials. Lancet 2021;397:1183–94. Erratum in: Lancet 2021;397:1446. https://doi.org/10.1016/s0140-6736(21)00217-8.Suche in Google Scholar
24. US Food and Drug Administration. CDER perspective on recently published results of EPPPIC meta-analysis; 2021. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/cder-perspective-recently-published-results-epppic-meta-analysis [Accessed 14 Sep 2022].Suche in Google Scholar
25. Romero, R, Conde-Agudelo, A, Da Fonseca, E, O’Brien, JM, Cetingoz, E, Creasy, GW, et al.. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: a meta-analysis of individual patient data. Am J Obstet Gynecol 2018;218:161–80. https://doi.org/10.1016/j.ajog.2017.11.576.Suche in Google Scholar PubMed PubMed Central
26. O’Brien, JM, Adair, CD, Lewis, DF, Hall, DR, Defranco, EA, Fusey, S, et al.. Progesterone vaginal gel for the reduction of recurrent preterm birth: primary results from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol 2007;30:687–96. https://doi.org/10.1002/uog.5158.Suche in Google Scholar PubMed
27. Fonseca, EB, Celik, E, Parra, M, Singh, M, Nicolaides, KH. Fetal Medicine Foundation Second Trimester Screening Group. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med 2007;357:462–9. https://doi.org/10.1056/nejmoa067815.Suche in Google Scholar PubMed
28. Cetingoz, E, Cam, C, Sakallı, M, Karateke, A, Celik, C, Sancak, A. Progesterone effects on preterm birth in high-risk pregnancies: a randomized placebo-controlled trial. Arch Gynecol Obstet 2011;283:423–9. https://doi.org/10.1007/s00404-009-1351-2.Suche in Google Scholar PubMed
29. Hassan, SS, Romero, R, Vidyadhari, D, Fusey, S, Baxter, JK, Khandelwal, M, et al.. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol 2011;38:18–31. https://doi.org/10.1002/uog.9017.Suche in Google Scholar PubMed PubMed Central
30. Norman, JE, Marlow, N, Messow, CM, Shennan, A, Bennett, PR, Thornton, S, et al.. Vaginal progesterone prophylaxis for preterm birth (the OPPTIMUM study): a multicentre, randomised, double-blind trial. Lancet 2016;387:2106–16. Erratum in: Lancet 2019;393:228. Erratum in: Lancet 2019;393:1596. https://doi.org/10.1016/s0140-6736(16)00350-0.Suche in Google Scholar PubMed PubMed Central
31. Schünemann, HJ, Higgins, JPT, Vist, GE, Glasziou, P, Akl, EA, Skoetz, N, et al.. Chapter 14. Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins, JPT, Thomas, J, Chandler, J, Cumpston, M, Li, T, Page, MJ, et al.., editors. Cochrane handbook for systematic reviews of interventions version 6.2. (updated Feb 2021). Cochrane; 2021.Suche in Google Scholar
32. Xu, H, Gonzalez, JM, Ofori, E, Elovitz, MA. Preventing cervical ripening: the primary mechanism by which progestational agents prevent preterm birth? Am J Obstet Gynecol 2008;198:314.e1–8. https://doi.org/10.1016/j.ajog.2008.01.029.Suche in Google Scholar PubMed
33. Furcron, AE, Romero, R, Plazyo, O, Unkel, R, Xu, Y, Hassan, SS, et al.. Vaginal progesterone, but not 17α-hydroxyprogesterone caproate, has antiinflammatory effects at the murine maternal-fetal interface. Am J Obstet Gynecol 2015;213:846.e1–9. https://doi.org/10.1016/j.ajog.2015.08.010.Suche in Google Scholar PubMed PubMed Central
34. Mesiano, SA, Peters, GA, Amini, P, Wilson, RA, Tochtrop, GP, van Den Akker, F. Progestin therapy to prevent preterm birth: history and effectiveness of current strategies and development of novel approaches. Placenta 2019;79:46–52. https://doi.org/10.1016/j.placenta.2019.01.018.Suche in Google Scholar PubMed PubMed Central
35. Berghella, V, Rafael, TJ, Szychowski, JM, Rust, OA, Owen, J. Cerclage for short cervix on ultrasonography in women with singleton gestations and previous preterm birth: a meta-analysis. Obstet Gynecol 2011;117:663–71. https://doi.org/10.1097/aog.0b013e31820ca847.Suche in Google Scholar PubMed
36. Conde-Agudelo, A, Romero, R, Fonseca, ED, O’Brien, JM, Cetingoz, E, Creasy, GW, et al.. Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation, previous spontaneous preterm birth, and a short cervix: updated indirect comparison meta-analysis. Am J Obstet Gynecol 2018;219:10–25. https://doi.org/10.1016/j.ajog.2018.03.028.Suche in Google Scholar PubMed PubMed Central
37. Romero, R, Conde-Agudelo, A, Rehal, A, Fonseca, ED, Brizot, ML, Rode, L, et al.. Vaginal progesterone for the prevention of preterm birth and adverse perinatal outcomes in twin gestations with a short cervix: an updated individual patient data meta-analysis. Ultrasound Obstet Gynecol 2022;59:263–6. https://doi.org/10.1002/uog.24839.Suche in Google Scholar PubMed PubMed Central
38. Rode, L, Klein, K, Nicolaides, KH, Krampl-Bettelheim, E, Tabor, A, PREDICT Group. Prevention of preterm delivery in twin gestations (PREDICT): a multicenter, randomized, placebo-controlled trial on the effect of vaginal micronized progesterone. Ultrasound Obstet Gynecol 2011;38:272–80. https://doi.org/10.1002/uog.9093.Suche in Google Scholar PubMed
39. Serra, V, Perales, A, Meseguer, J, Parrilla, JJ, Lara, C, Bellver, J, et al.. Increased doses of vaginal progesterone for the prevention of preterm birth in twin pregnancies: a randomised controlled double-blind multicentre trial. BJOG 2013;120:50–7. https://doi.org/10.1111/j.1471-0528.2012.03448.x.Suche in Google Scholar PubMed
40. Brizot, ML, Hernandez, W, Liao, AW, Bittar, RE, Francisco, RPV, Krebs, VLJ, et al.. Vaginal progesterone for the prevention of preterm birth in twin gestations: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol 2015;213:82.e1–9. https://doi.org/10.1016/j.ajog.2015.02.021.Suche in Google Scholar PubMed
41. Rehal, A, Benkő, Z, Matallana, CDP, Syngelaki, A, Janga, D, Cicero, S, et al.. Early vaginal progesterone versus placebo in twin pregnancies for the prevention of spontaneous preterm birth: a randomized, double-blind trial. Am J Obstet Gynecol 2021;224:86.e1–9. https://doi.org/10.1016/j.ajog.2020.06.050.Suche in Google Scholar PubMed
42. Conde-Agudelo, A, Romero, R. Does vaginal progesterone prevent recurrent preterm birth in women with a singleton gestation and a history of spontaneous preterm birth? evidence from a systematic review and meta-analysis. Am J Obstet Gynecol 2022;227:440–61.e2. https://doi.org/10.1016/j.ajog.2022.04.023.Suche in Google Scholar PubMed PubMed Central
43. Da Fonseca, EB, Bittar, RE, Carvalho, MH, Zugaib, M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol 2003;188:419–24. https://doi.org/10.1067/mob.2003.41.Suche in Google Scholar PubMed
44. Majhi, P, Bagga, R, Kalra, J, Sharma, M. Intravaginal use of natural micronised progesterone to prevent pre-term birth: a randomised trial in India. J Obstet Gynaecol 2009;29:486–91. https://doi.org/10.1080/01443610902980878.Suche in Google Scholar PubMed
45. Akbari, S, Birjandi, M, Mohtasham, N. Evaluation of the effect of progesterone on prevention of preterm delivery and its complications [in Persian]. Sci J Kurdistan Univ Med Sci 2009;14:11–9.Suche in Google Scholar
46. Modi, R, Rathore, AM, Arora, R. Randomized trial of natural progesterone in prevention of preterm birth in high risk women. J Pediatr Obstet Gynecol 2014:101–7.Suche in Google Scholar
47. Azargoon, A, Ghorbani, R, Aslebahar, F. Vaginal progesterone on the prevention of preterm birth and neonatal complications in high risk women: a randomized placebo-controlled double-blind study. Int J Reprod Biomed2016;14:309–16. https://doi.org/10.29252/ijrm.14.5.309.Suche in Google Scholar
48. Crowther, CA, Ashwood, P, McPhee, AJ, Flenady, V, Tran, T, Dodd, JM, et al.. Vaginal progesterone pessaries for pregnant women with a previous preterm birth to prevent neonatal respiratory distress syndrome (the PROGRESS study): a multicentre, randomised, placebo-controlled trial. PLoS Med 2017;14:e1002390. https://doi.org/10.1371/journal.pmed.1002390.Suche in Google Scholar PubMed PubMed Central
49. Abdou, AM. Role of vaginal progesterone in prevention of preterm labor in women with previous history of one or more previous preterm births. Open J Obstet Gynecol 2018;8:329–37. https://doi.org/10.4236/ojog.2018.84036.Suche in Google Scholar
50. Conde-Agudelo, A, Romero, R. Vaginal progesterone does not prevent recurrent preterm birth in women with a singleton gestation, a history of spontaneous preterm birth, and a midtrimester cervical length >25 mm. Am J Obstet Gynecol 2022. Epub ahead of print. PMID: 35926647.10.1016/j.ajogmf.2023.101215Suche in Google Scholar PubMed
51. Aboulghar, MM, Aboulghar, MA, Amin, YM, Al-Inany, HG, Mansour, RT, Serour, GI. The use of vaginal natural progesterone for prevention of preterm birth in IVF/ICSI pregnancies. Reprod Biomed Online 2012;25:133–8. https://doi.org/10.1016/j.rbmo.2012.03.013.Suche in Google Scholar PubMed
52. Norman, JE, Mackenzie, F, Owen, P, Mactier, H, Hanretty, K, Cooper, S, et al.. Progesterone for the prevention of preterm birth in twin pregnancy (STOPPIT): a randomised, double-blind, placebo-controlled study and meta-analysis. Lancet 2009;373:2034–40. https://doi.org/10.1016/s0140-6736(09)60947-8.Suche in Google Scholar
53. Wood, S, Ross, S, Tang, S, Miller, L, Sauve, R, Brant, R. Vaginal progesterone to prevent preterm birth in multiple pregnancy: a randomized controlled trial. J Perinat Med 2012;40:593–9. https://doi.org/10.1515/jpm-2012-0057.Suche in Google Scholar PubMed
54. Shabaan, OM, Hassanin, IM, Makhlouf, AM, Salem, MN, Hussein, M, Mohamed, M, et al.. Vaginal progesterone for prevention of preterm delivery in women with twin pregnancy: a randomized controlled trial. Facts Views Vis Obgyn 2018;10:93–8.Suche in Google Scholar
© 2022 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Editorial
- Journal of Perinatal Medicine: Happy 50th anniversary
- Articles
- Fifty years of the Journal of Perinatal Medicine: an altmetric and bibliometric study
- Early origins of respiratory disease
- Oxygenation of the newborn. The impact of one molecule on newborn lives
- Emergency button cannula vs. umbilical catheter as neonatal emergency umbilical vein access – a randomized cross-over pilot study
- Covid and pregnancy in the United States – an update as of August 2022
- Facts and doubts on the beginning of human life – scientific, legal, philosophical and religious controversies
- Molecular subclasses of preeclampsia characterized by a longitudinal maternal proteomics study: distinct biomarkers, disease pathways and options for prevention
- Maternal telehealth: innovations and Hawaiʻi perspectives
- Prevention of risks of overweight and obesity in pregnant women
- Intraoperative ultrasound during repeat cesarean delivery facilitates sampling of uterine scar tissue
- The effect of abnormal placentation on maternal serum fetal fraction of cell-free DNA
- Prenatal predictors of adverse perinatal outcome in congenital cytomegalovirus infection: a retrospective multicenter study
- Diagnostic approach to fetal ventriculomegaly
- Clinical potential of human amniotic fluid stem cells
- Vaginal progesterone for the prevention of preterm birth: who can benefit and who cannot? Evidence-based recommendations for clinical use
- A second look at intrapartum fetal surveillance and future directions
- Computerized analysis of cardiotocograms in clinical practice and the SisPorto® system thirty-two years after: technological, physiopathological and clinical studies
- Acknowledgment
- Acknowledgment
Artikel in diesem Heft
- Frontmatter
- Editorial
- Journal of Perinatal Medicine: Happy 50th anniversary
- Articles
- Fifty years of the Journal of Perinatal Medicine: an altmetric and bibliometric study
- Early origins of respiratory disease
- Oxygenation of the newborn. The impact of one molecule on newborn lives
- Emergency button cannula vs. umbilical catheter as neonatal emergency umbilical vein access – a randomized cross-over pilot study
- Covid and pregnancy in the United States – an update as of August 2022
- Facts and doubts on the beginning of human life – scientific, legal, philosophical and religious controversies
- Molecular subclasses of preeclampsia characterized by a longitudinal maternal proteomics study: distinct biomarkers, disease pathways and options for prevention
- Maternal telehealth: innovations and Hawaiʻi perspectives
- Prevention of risks of overweight and obesity in pregnant women
- Intraoperative ultrasound during repeat cesarean delivery facilitates sampling of uterine scar tissue
- The effect of abnormal placentation on maternal serum fetal fraction of cell-free DNA
- Prenatal predictors of adverse perinatal outcome in congenital cytomegalovirus infection: a retrospective multicenter study
- Diagnostic approach to fetal ventriculomegaly
- Clinical potential of human amniotic fluid stem cells
- Vaginal progesterone for the prevention of preterm birth: who can benefit and who cannot? Evidence-based recommendations for clinical use
- A second look at intrapartum fetal surveillance and future directions
- Computerized analysis of cardiotocograms in clinical practice and the SisPorto® system thirty-two years after: technological, physiopathological and clinical studies
- Acknowledgment
- Acknowledgment


