Clinical potential of human amniotic fluid stem cells
-
Monique M. Martin
, Eran Bornstein
Abstract
Objectives
To determine whether amniotic fluid derived stem cells maintain their stem cell characteristics (a) after processing by a licensed cell therapy center and (b) after the cells undergo simulated clinical application.
Methods
Amniotic fluid was collected by laparotomy – a small uterine incision was made at proposed site for delivery and a sterile catheter inserted to collect fluid into a sterile bag. After flow stopped the catheter was withdrawn, the cesarean completed and the collected fluid delivered to the cell therapy center for processing and cryostorage. A clinical setting was simulated where amniotic fluid cells received from cell therapy center were thawed at room temperature for a maximum of 3 h and passed through a clinical cell delivery device to monitor cell viability. The cells were examined for viability, stability, growth, differentiation, and markers of stemness.
Results
Amniotic fluid stem cells processed from a clinical cell therapy center behave similarly to amniotic fluid stem cells processed in a research laboratory with respects to viability, stability, growth, differentiation and maintain markers of stemness. There were differences due to heterogeneity of samples which were not methodological. Growth in cell culture and differentiation were satisfactory. Simulation of treating the cells in a clinical environment show a general stability in viability of amniotic fluid cells at room temperature for 3 h minimum and when passed through a clinically approved delivery device.
Conclusions
The data indicate human amniotic fluid processed in a clinical facility could be used therapeutically if proven to be safe.
Introduction
There are various types of stem cells currently being studied; embryonic stem cells, induced pluripotent cells, umbilical cord blood, umbilical cord tissue, placenta and tissue specific stem cells. However, all of these have significant drawbacks for clinical use. We are focused on human term amniotic fluid stem cells because others are less desirable for a variety of reasons such as; need for induction by a viral vector, low yield with prolonged culture, immunogenicity, tumor formation and alterations in the genome of unknown significance. Human term amniotic fluid stem cells are readily available, grow well in culture, do not have these disadvantages and avoid ethical issues associated with embryonic stem cells, since they are sourced from cesarean births and would normally be discarded as a waste product. Human term AFSC, cultured from freshly collected samples show stem cell characteristics [1]. In addition, there is substantial preclinical data suggesting that they will be potentially useful for a variety of clinical purposes in regenerative medicine [2]. Human term amniotic fluid stem cells therefore are a rich source of stem cells for regenerative medicine and have many advantages compared to other sources.
Some clinical trials with other sources have started because the FDA has recognized the potential of regenerative medicine with stem cell therapy. In the words of Scott Gottlieb, FDA Commissioner in 2019; “This is no longer the stuff of science fiction. This is the practical promise of regenerative medicine.” Therefore it seems timely to examine the clinical potential of AFSC.
Using flow cytometry, the presence of stem cell characteristics of term amniotic fluid stem cells have been shown. Term AFSCs have displayed surface markers such as SSEA3, SSEA4, CD90, and TRA 1–60 in addition to transcription factors such as OCT-4, NANOG, REX1, PDX1 and SOX2 [1]. Research using term AFSCs has yielded a range of 100,000–300,000 cells/ml with 60–90% viability [2]. Additionally, cultured AFSCs double in a range of 36–48 h in serum free culture media and 99% of them display stem cell markers [3]. These data strongly suggests using term AFSCs clinically. We proposed that term AFSC samples processed and cryopreserved by the New York Blood Center, an approved clinical cell therapy facility, have similar characteristics to AFSC samples that have been previously processed and cryopreserved in the research laboratory. Moreover, these AFSC, after taken through a simulation where they would be clinically administered, are stable at room temperature and maintain their stem cell characteristics.
Materials and methods
Subjects
Healthy gravidas scheduled for cesarean birth for clinical indications without medical complications or urgency and not in labor were included. Excluded were patients with bleeding, complicated or abnormal pregnancy or abnormal fetus.
Ethical approval
The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance the tenets of the Helsinki Declaration, and has been approved by the authors’ Institutional Review Board or equivalent committee.
Sample collection
Informed consent was obtained prior to sample collection (NYU Grossman School of Medicine IRB study i15-01269, Lenox Hill Hospital Northwell Health IRB study 21–0,162). After laparotomy a small uterine incision was made at the proposed site for delivery and a sterile catheter inserted to collect the fluid into a sterile bag. After flow stopped the catheter was withdrawn, the cesarean completed and the collected fluid delivered to the cell therapy center for processing and cryostorage.
Simulating a clinical setting to test the stability of term AFSCs in normal saline
One sterile vial of 4 mL of cell therapy center processed term AFSCs stored in CryoStore™ at −80 °C was thawed at room temperature at approximately 21 °C for approximately 20 min. The cells were removed from the vial using an 18-gauge needle and 20 cc syringe with 11 mL sterile normal saline to a total volume of 15 mL. An aliquot of 100–200 µL was collected into an Eppendorf tube to assess cell viability and cell count. Viability and cell count were assessed using trypan blue staining, a hemocytometer, and light microscope. The sample was then allowed to sit for 1–3 h after the 20 min thaw. At the 1, 2, and 3 h mark the syringe was inverted five times and a 100–200 µL aliquot was taken and viability and cell count assessed.
Simulating a clinical setting to test the integrity of term AFSCs after passed through balloon catheter
In a clinical setting, the procedure would be to thaw the storage vial at room temperature and draw it up in a sterile fashion, diluted by normal saline for introduction to the patient by direct injection or through a catheter to the desired site. Therefore, we simulated clinical conditions using a 20 cc syringe and 18G hypodermic needle, and a balloon catheter used clinically for administering liquid to a tissue site in a patient.
Five mL NYBC sterile cells cryopreserved in CryoStore™ Multi-Chamber Freezing Chambers were taken from −80 °C and thawed at room temperature at approximately 20 °C. The mini chamber was thawed for approximately 20 min. The term AFSCs in the mini chamber were aspirated using closed sterile technique without exposure to air with an18 gauge needle and a 20 mL syringe. A 100 µL sample was collected into an Eppendorf tube. 10 mL of normal saline was used to dilute up the cells in the syringe and another 100 µL aliquot was taken using closed sterile technique and with time point 0 starting now. The sample, combined with the 10 mL normal saline wash, was then allowed to sit in the syringe for 3 h and at each hour time point, the syringe was inverted five times before a 100 µL aliquot was taken and viability and count assessed. The diluted sample in the syringe was then passed through an Olympus EndoTherapy balloon catheter and collected in a 50 mL conical tube. Another 100 µL aliquot was collected into an Eppendorf tube and viability and count assessed. The catheter was washed with 15 mL of normal saline and collected in a 50 mL conical tube and 100 µL was taken and viability and count assessed. Finally all the AFSC that passed through the balloon catheter were combined for culture. The suspension was centrifuged and the supernatant was aspirated and the pelleted cells were resuspended in cell culture medium [4] and a 100 µL aliquot was taken. Viability and cell count for each aliquot was assessed as described. The cells were transferred into cell culture at 37 °C at 5% CO2 and observed for cell growth.
Flow cytometry (fluorescence activated cell separation (FACS))
Cells were harvested and detached from a confluent plate and resuspended in an assay buffer with 5% fetal bovine serum (FBS) in 1X Dulbecco’s phosphate-buffered saline (1X DPBS). Cells were stained with 1:1,000 dilutions of antibodies to the surface markers in an assay buffer at 4 °C for at least 1 h and washed, centrifuged at 1,200 rpm for 5 min to pellet cells. The supernatant was decanted, the tubes taken to the Cytometry and Sorting Core facility at NYU Langone Health using a UVII Becton Dickinson Analyzer to run samples. Analysis of files via WinList program was performed to determine cell surface marker expression.
Spheroid production for differentiation assays
Plates of 0.8% of low melting agarose in alpha-MEM-GlutaMAX were prepared and filled the wells of tissue culture quality, round bottom 96-well plates with 100 uL of melted agarose. Term AFSC were seeded at a concentration of 10,000 cells in 100 uL to each coated well. The plate was incubated for two to three days for the three dimensional cell cultures to form [5].
Neural differentiation
Transferred 1 mL cell culture base medium into two wells of a 4-well chamber slide each and 1 mL of STEMDiff Neural Differentiation Medium into the remaining two wells. Transferred five spheroids into each well and incubated at 37 °C for 3 weeks with media changes every two to three days. Aspirated off media and washed wells with 2 mL 1X DPBS three times. Fixed spheroids with 3.7% buffered formalin at room temperature overnight. Washed with 2 mL 5% heat inactivated horse serum in 1X DPBS three times. Permeabilized spheroids/cells with 1% Triton X-100 5% heat inactivated horse serum in 1X DPBS overnight at room temperature. Washed with 5% heat inactivated horse serum in 1X DPBS at room temperature. Incubated 1:100 dilution AlexaFluor488-mouse-anti-human beta tubulin III and AlexaFluor647-mouse-anti-human Nestin in 5% heat inactivated horse serum in 1X DPBS at room temperature overnight. Washed with 2 mL 0.1% Tween-20 in 1X DPBS three times followed by three washes with 1X DPBS. Mounted slides with coverslips with VECTASHIELD Mounting Medium with DAPI and cured overnight. The cells were visualized under fluorescent microscopy with a Nikon Eclipse TE2000-E inverted microscope. The images were acquired with Nikon NIS-Elements Imaging Software BR 3.10. Images were analyzed and quantified using ImageJ 1.47v.
Chondrocyte differentiation
Transferred 1 mL cell culture base medium into two wells of a 4-well chamber slide each and 1 mL of differentiation medium into the remaining two wells. Transferred five spheroids into each well and incubated at 37 °C for 3 weeks with media changes and processing as above. Incubated 1:100 dilution rabbit-anti-human Aggrecan in 5% heat inactivated horse serum in DPBS at room temperature overnight. Washed with 2 mL 0.1% Tween-20 in DPBS three times followed by three washes with 1X DPBS. Incubated 1:200 dilution of the secondary antibody AlexaFluro594 goat anti-Rabbit IgG (H + L) in 5% inactivated horse serum in DPBS at room temperature overnight. Washed three times with 0.1% Tween 20 followed by three washes with 1X DPBS. Slides were prepared and analyzed as above.
Pancreatic differentiation
Transferred 1 mL cell culture base medium into two wells of a 4-well chamber slide each and 1 mL of differentiation medium into the remaining two wells and spheroids prepared as above. Incubated 1:100 dilution PE-mouse-anti human PDX1 and AlexaFluor488-mouse anti-human Insulin in 5% heat inactivated horse serum in 1X DPBS at room temperature overnight. Processing was described above for analysis.
Results
After thawing, the count and viability of the cell therapy center cryostored amniotic fluid cells was comparable to what was found using the standard laboratory methods (Table 1). In addition, when observed over a period of 3 h after thawing, the viability of the cells at room temperature remained constant (Figure 1). Simulating clinical conditions of thawing and passage through a sterile clinical delivery device, such as what is used in patients showed preservation of viability and count over a 3 h period after thawing and after passage through the delivery device (Table 2, Figure 2). Cell therapy center processed cells grew in culture in a manner comparable to the laboratory results (Table 3). Flow cytometry results revealed marker expression for stemness parallel to what was observed in the laboratory (Table 4). Differentiation of cell therapy center cryopreserved cells using the same process as performed in the laboratory demonstrated differentiation to mesoderm (Figure 3A, B), ectoderm (Figure 4), and endoderm (Figure 5A, B) lineages. The observed differences were attributed to heterogeneity of different samples and culture conditions.
Cell count and viability of room temperature thawed term AFSCs processed by the cell therapy center.
| Sample | Condition | Percentage viability |
|---|---|---|
| T20-14 | Thawed undiluted | 65% |
| Diluted – 0 h | 55% | |
| Diluted – 1 h | 48% | |
| Diluted – 2 h | 40% | |
| Diluted – 3 h | 50% | |
| T20-06 | Thawed undiluted | 40% |
| Diluted – 0 h | 57% | |
| Diluted – 1 h | 60% | |
| Diluted – 2 h | 72% | |
| Diluted – 3 h | 55% | |
| T20-18 | Thawed undiluted | 34% |
| Diluted – 0 h | 56% | |
| Diluted – 1 h | 43% | |
| Diluted – 2 h | 46% | |
| Diluted – 3 h | 55% |
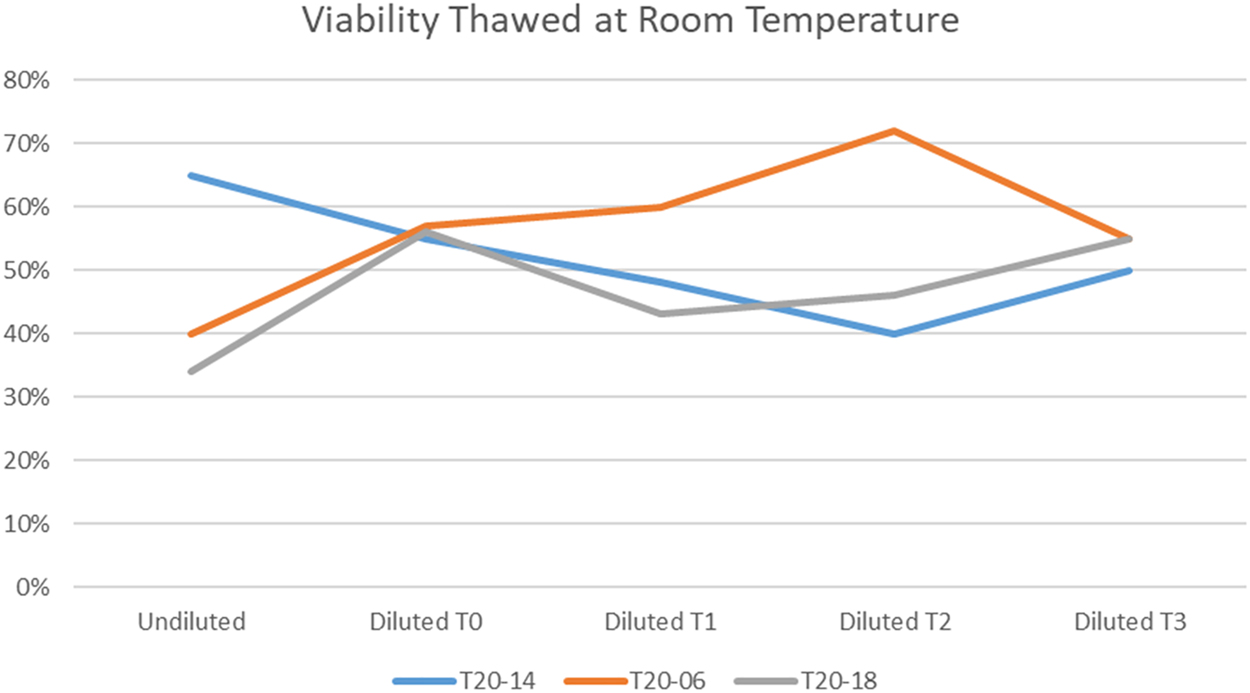
Cell therapy center processed AFSCs are relatively stable for 3 h after thawing at room temperature.
Cell therapy center processed term AFSCs viability before and after passage through balloon catheter.
| Sample | Test condition | Percent viability |
|---|---|---|
| T20-15 | Thawed undiluted | 46% |
| Diluted – 0 h | 42% | |
| Diluted – 1 h | 40% | |
| Diluted – 2 h | 43% | |
| Post catheter | 54% | |
| Resuspension in cell culture medium | 40% | |
| T20-02 | Thawed undiluted | 27% |
| Diluted – 0 h | 29% | |
| Diluted – 1 h | 24% | |
| Diluted – 2 h | 24% | |
| Post catheter | 25% | |
| Resuspension in cell culture medium | 20% | |
| T22-04 | Thawed undiluted | 75% |
| Diluted – 0 h | 74% | |
| Diluted – 1 h | 74% | |
| Diluted – 2 h | 74% | |
| Diluted – 3 h | 77% | |
| Post catheter | 79% | |
| Resuspension in cell culture medium | 74% | |
| T22-03 | Thawed undiluted | 67% |
| Diluted – 0 h | 68% | |
| Diluted – 1 h | 70% | |
| Diluted – 2 h | 70% | |
| Diluted – 3 h | 70% | |
| Post catheter | 70% | |
| Resuspension in cell culture medium | 68% | |
| T22-07 L | Thawed undiluted | 72% |
| Diluted – 0 h | 70% | |
| Diluted – 1 h | 68% | |
| Diluted – 2 h | 69% | |
| Diluted – 3 h | 69% | |
| Post catheter | 70% | |
| Resuspension in cell culture medium | 70% |
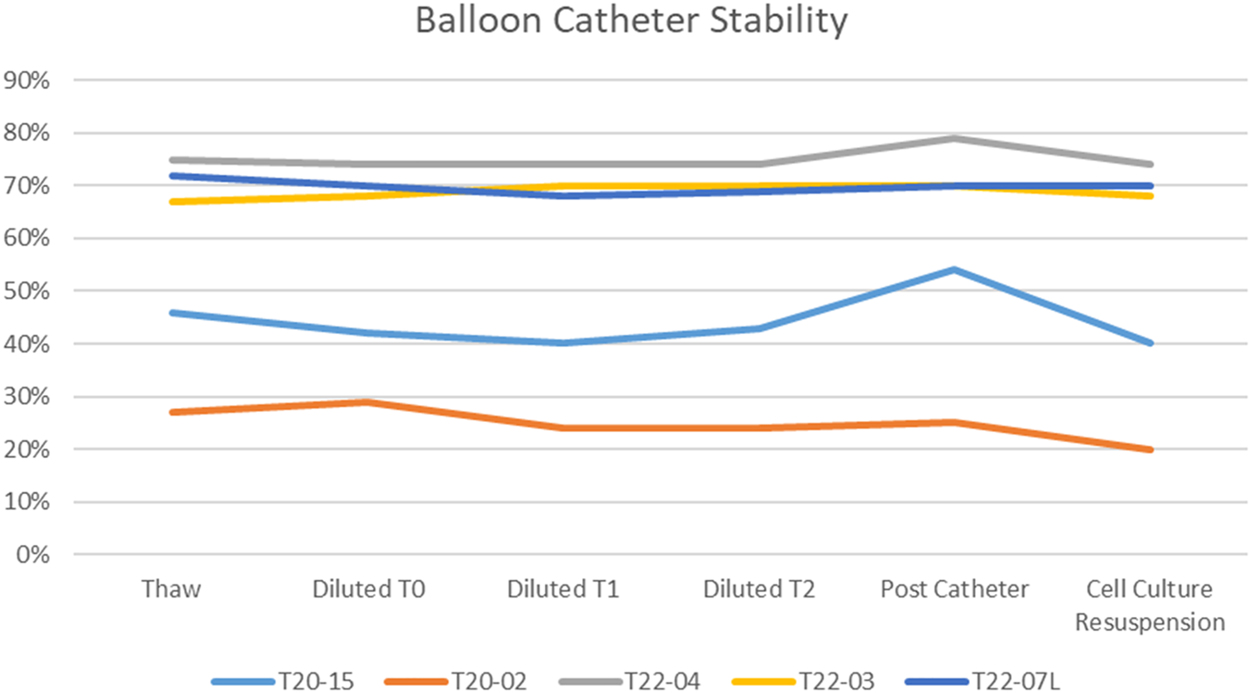
The viability of cell therapy processed AFSC thawed under a clinical simulation and passed through a clinically approved delivery device is remarkably stable.
Highest level of passage of cell therapy center processed term AFSC in cell culture.
| Sample | Highest level passaged |
|---|---|
| T20-02 | P14 |
| T21-01 | P12 |
| T22-03 | P18 |
| T22-07 L | P11 |
| T20-14 | P20 |
| T20-15 | P17 |
Flow cytometry for stem cell surface markers for term AFSC processed by cell therapy center.
| Sample | Passage | CD90 | SSEA4 | TRA-1-60 | CD133 | CD29 | CD44 | CD105 | CD73 |
|---|---|---|---|---|---|---|---|---|---|
| T20-02 | P4 | 99% | 68% | 8% | 0% | 97% | 98% | 0% | 100% |
| T21-01 | P1 | 99% | 18% | 1% | 0% | 99% | 97% | 0% | 100% |
| T22-03 | P3 | 98% | 0.1% | 1.3% | 0% | 28% | 100% | 7.6% | 100% |
| T20-20A | P3 | 99% | 34% | 1% | 1% | 99% | 98% | 0% | 100% |
| T20-20B | P2 | 93% | 24% | 6% | 0% | 88% | 81% | 0.25% | 88% |
| T21-06 | P4 | 99% | 23% | 0% | 2% | 99% | 98% | 0% | 100% |
-
All samples express a significant abundance of CD90, CD29, CD44, and CD73 surface markers. SSEA4 has varied abundance among the samples but is present in all samples. TRA-1-60, CD133, and CD105 have a significantly low presence in all samples.
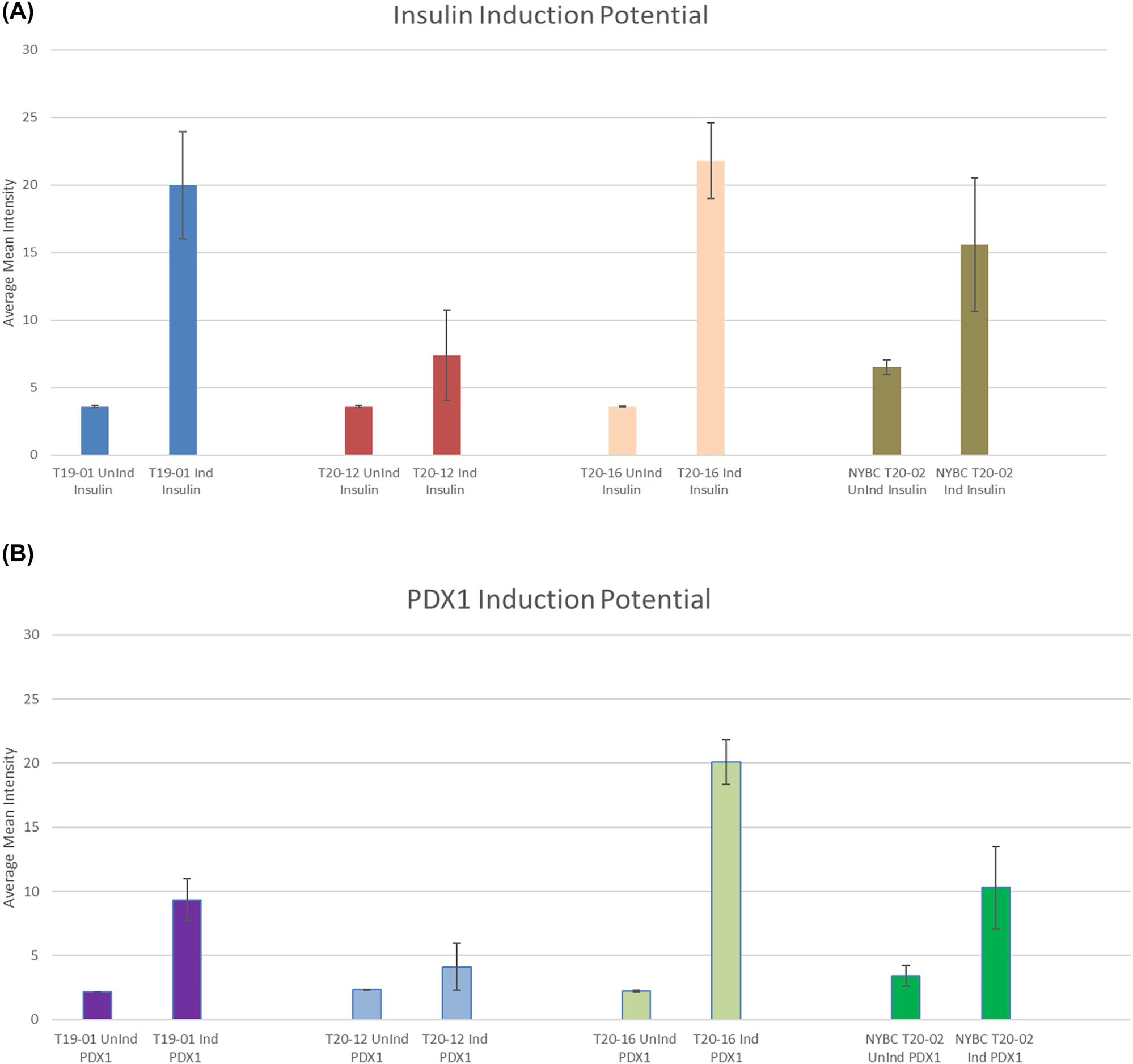
Pancreatic induction potential quantified by ImageJ. (A) Insulin induction potential. This graph displays that lab processed and NYBC processed term AFSC have the capabilities performing pancreatic induction using insulin as a marker. The ability of the cells to perform insulin induction makes it a promising clinical application. (B) PDX1 induction potential. This graph displays that term AFSC processed in the lab synthesize the promoter factor pancreatic and duodenal homeobox 1 (PDX1). The presence of PDX1 shows the ability for pancreatic development and beta-cell maturation.
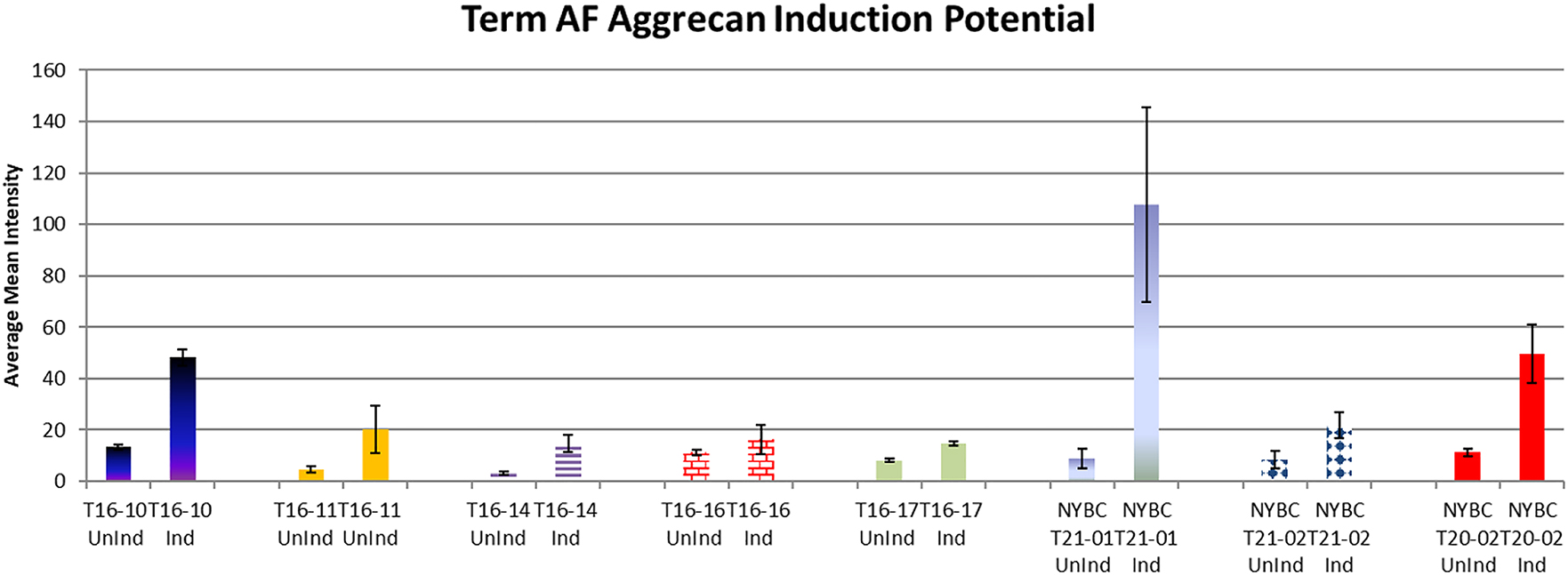
Chondrocyte induction potential quantified by ImageJ. In this graph NYBC and lab processed term AFSC showcase expression of aggrecan. Variable intensity of aggrecan levels exists between samples. However, aggrecan expression indicates the ability of NYBC term AFSCs to differentiate into chondrocytes.
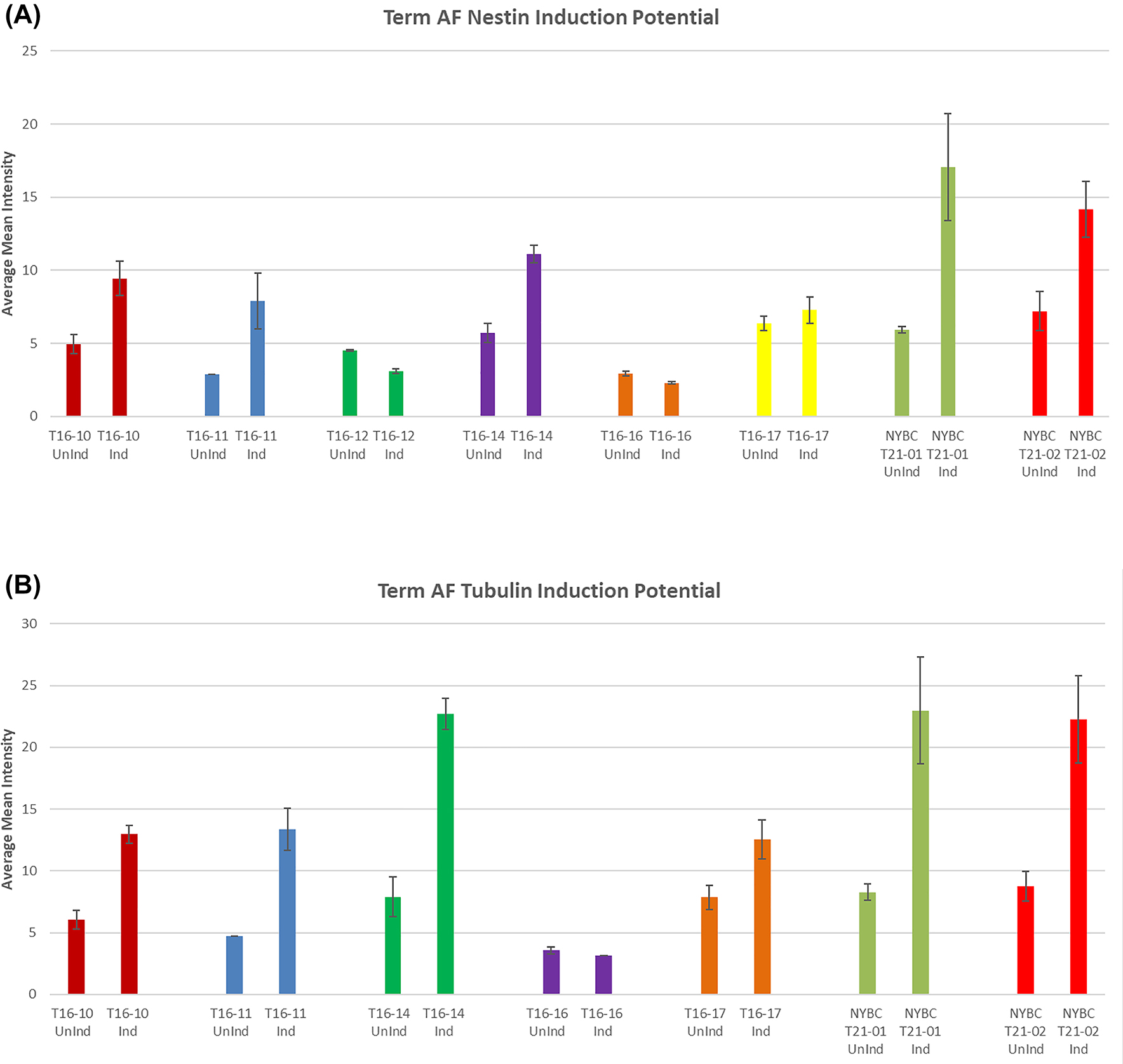
Neural induction potential quantified by ImageJ. (A) Term AF Nestin induction potential. Nestin expression indicates the ability of term AFSCs to differentiate into neural. In this graph NYBC processed and lab processed term AFSC showcase expression of Nestin. Variable intensity of Nestin levels exists between samples. Nestin is filament protein that are expressed mostly in nerve cells involved in the radial growth of axons. (B) Term AF Tubulin induction potential. This graph showcases the lab and NYBC processed term AFSC expression of varying levels of tubulin. NYBC samples on average show high levels of tubulin. Tubulin forms microtubule network and is essential for neurogenesis, axon guidance and maintenance.
Discussion
AFSC naturally demonstrate the full range of stem cell functions. They do not need viral or chemical agents to introduce the genes for pluripotency required to form induced pluripotent stem cells (IPSC). Unlike IPSC there is no alteration of the cell genome with an unpredictable potential, and AFSC do not form tumors [2]. Also they are available in large numbers immediately when obtained from term pregnancies. IPSC require a long process of harvesting cells from a tissue followed by multiple cultures to first develop and then grow sufficient numbers of cells for possible clinical applications.
The observations show the ability of the term AFSC to maintain stem cell characteristics and viability after cryopreservation by a clinical processing cell therapy center. Moreover, in a clinical setting that lacks equipment such as is used in a research laboratory, sterile preparation with an 18-gauge needle and 20 mL syringe, a standard clinical procedure for administering drugs, does not significantly affect the viability of term AFSC. Also, thawing cells frozen at −80 °C at room temperature does not significantly affect cell viability. The cells were able to grow in culture at a rate comparable to cells that were cryopreserved in the laboratory.
Each sample is stable for at least 3 h at room temperature when diluted with normal saline in a sterile 20 mL syringe. Viability and cell count are variable among samples due to inherent individual variation and to observer dependent variation when counting using a hemocytometer and trypan blue. Despite this the variation is within the expected margin of error for human reliant cell counting and viability [6, 7]. However, in a clinical setting, amniotic fluid cells that are cryopreserved by the cell therapy clinical center and diluted with normal saline for administration to a patient will maintain viability over the course of at least 3 h and the viability is stable after passing through a clinical Balloon Catheter.
The clinical cell therapy center cryopreserved term AFSC compared with laboratory processed cells maintain a cell count and grow in culture similarly, display the same cell surface markers and differentiate as well into all three germ layers–chondrocytes, neural precursors, and pancreatic cells. AFSC have the same pluripotency as IPSC and are readily available, abundant and do not alter the cells’ genome. They are an acceptable, perhaps preferable, alternative for potential clinical applications.
Conclusions
Term amniotic fluid stem cells are a promising source of therapeutic stem cells when prepared by a clinical cell therapy center. Additionally, in a clinical environment, after thawing AFSC in sterile normal saline in a 20 mL syringe maintain their viability and cell count for 3 h, allowing for delays in a busy clinical setting before administration to a patient.
The data supports potential clinical application of term amniotic fluid stem cells for regenerative medicine therapies.
Limitations
This study used a hemocytometer and trypan blue for manual cell count, and three to eight fields of visions were assessed. Publications support the use of at least three fields of vision when using this method of cell counting [6]. In comparison to other methods such as automated cell counting devices like the Vi-CELL® XR Cell Viability Analyzer (Beckman Coulter) and manual hemocytometer displayed no significant differences in cell counting capabilities [7]. However, using a hemocytometer requires a level of skill on the part of the user to be reliable [8]. Practical considerations such as transport delays after collection to the processing facility might affect the viability of term AFSC.
Acknowledgments
The authors would like to acknowledge Rona Weinberg, PhD of the New York Blood Center for her assistance in developing the clinical processing of the amniotic fluid cells.
-
Research funding: The Haughland Foundation provided the funds for this study. The foundation did not contribute to the design, performance, interpretation or writing the manuscript for this study.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: Authors state no conflict of interest.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Ethical approval: The research related to human use has complied with all the relevant national regulations, institutional policies, and in accordance with the tenets of the Helsinki Declaration, and has been approved by the authors’ Institutional Review Board, or equivalent committee (New York University Langone Health Institutional Review Board, study #i15-01269; and Lenox Hill Hospital Northwell Health Institutional Review Board study #21–0,162).
References
1. Dolin, CD, Chan, MK, Basch, RS, Young, BK. Human term amniotic fluid: a novel source of stem cells for regenerative medicine. Am J Obstet Gynecol 2018;219:308–9. Epub 2018 Jun 2. PMID: 29870738. https://doi.org/10.1016/j.ajog.2018.05.035.Search in Google Scholar PubMed
2. Dziadosz, M, Basch, RS, Young, BK. Human amniotic fluid: a source of stem cells for possible therapeutic use. Am J Obstet Gynecol 2016;214:321–7. Epub 2016 Jan 6. PMID: 26767797. https://doi.org/10.1016/j.ajog.2015.12.061.Search in Google Scholar PubMed
3. Young, BK, Chan, MK, Liu, L, Basch, RS. Amniotic fluid as a source of multipotent cells for clinical use. J Perinat Med 2016;44:333–7. PMID: 26115489. https://doi.org/10.1515/jpm-2015-0152.Search in Google Scholar PubMed
4. De Coppi, P, Bartsch, GJr, Siddiqui, MM, Xu, T, Santos, CC, Perin, L, et al.. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 2007;25:100–6. Epub 2007 Jan 7. PMID: 17206138. https://doi.org/10.1038/nbt1274.Search in Google Scholar PubMed
5. Chen, Z, Chan, MK, Strelchenko, N, Wang, F, Liu, L, Perle, MA, et al.. Heterogeneity of stem cells in human amniotic fluid. J Regen Med 2014;3:1. https://doi.org/10.4172/2325-9620.10001110.Search in Google Scholar
6. Marchenko, S, Flanagan, L. Counting human neural stem cells. J Vis Exp 2007;262. Epub 2007 Aug 22. PMID: 18989433; PMCID: PMC2565849. https://doi.org/10.3791/262.Search in Google Scholar PubMed PubMed Central
7. Cadena-Herrera, D, Esparza-De Lara, JE, Ramírez-Ibañez, ND, López-Morales, CA, Pérez, NO, Flores-Ortiz, LF, et al.. Validation of three viable-cell counting methods: manual, semi-automated, and automated. Biotechnol Rep (Amst) 2015;7:9–16. PMID: 28626709; PMCID: PMC5466062. https://doi.org/10.1016/j.btre.2015.04.004.Search in Google Scholar PubMed PubMed Central
8. Hsiung, F, McCollum, T, Hefner, E, Rubio, T. Comparisons of count reproducibility, accuracy, and time to get results between a hemocytometer and the TC20 automated cell counter. Bulletin 6003. Hercules, CA: Bio-Rad Laboratories, Inc; 2013:1–4 pp.Search in Google Scholar
© 2022 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorial
- Journal of Perinatal Medicine: Happy 50th anniversary
- Articles
- Fifty years of the Journal of Perinatal Medicine: an altmetric and bibliometric study
- Early origins of respiratory disease
- Oxygenation of the newborn. The impact of one molecule on newborn lives
- Emergency button cannula vs. umbilical catheter as neonatal emergency umbilical vein access – a randomized cross-over pilot study
- Covid and pregnancy in the United States – an update as of August 2022
- Facts and doubts on the beginning of human life – scientific, legal, philosophical and religious controversies
- Molecular subclasses of preeclampsia characterized by a longitudinal maternal proteomics study: distinct biomarkers, disease pathways and options for prevention
- Maternal telehealth: innovations and Hawaiʻi perspectives
- Prevention of risks of overweight and obesity in pregnant women
- Intraoperative ultrasound during repeat cesarean delivery facilitates sampling of uterine scar tissue
- The effect of abnormal placentation on maternal serum fetal fraction of cell-free DNA
- Prenatal predictors of adverse perinatal outcome in congenital cytomegalovirus infection: a retrospective multicenter study
- Diagnostic approach to fetal ventriculomegaly
- Clinical potential of human amniotic fluid stem cells
- Vaginal progesterone for the prevention of preterm birth: who can benefit and who cannot? Evidence-based recommendations for clinical use
- A second look at intrapartum fetal surveillance and future directions
- Computerized analysis of cardiotocograms in clinical practice and the SisPorto® system thirty-two years after: technological, physiopathological and clinical studies
- Acknowledgment
- Acknowledgment
Articles in the same Issue
- Frontmatter
- Editorial
- Journal of Perinatal Medicine: Happy 50th anniversary
- Articles
- Fifty years of the Journal of Perinatal Medicine: an altmetric and bibliometric study
- Early origins of respiratory disease
- Oxygenation of the newborn. The impact of one molecule on newborn lives
- Emergency button cannula vs. umbilical catheter as neonatal emergency umbilical vein access – a randomized cross-over pilot study
- Covid and pregnancy in the United States – an update as of August 2022
- Facts and doubts on the beginning of human life – scientific, legal, philosophical and religious controversies
- Molecular subclasses of preeclampsia characterized by a longitudinal maternal proteomics study: distinct biomarkers, disease pathways and options for prevention
- Maternal telehealth: innovations and Hawaiʻi perspectives
- Prevention of risks of overweight and obesity in pregnant women
- Intraoperative ultrasound during repeat cesarean delivery facilitates sampling of uterine scar tissue
- The effect of abnormal placentation on maternal serum fetal fraction of cell-free DNA
- Prenatal predictors of adverse perinatal outcome in congenital cytomegalovirus infection: a retrospective multicenter study
- Diagnostic approach to fetal ventriculomegaly
- Clinical potential of human amniotic fluid stem cells
- Vaginal progesterone for the prevention of preterm birth: who can benefit and who cannot? Evidence-based recommendations for clinical use
- A second look at intrapartum fetal surveillance and future directions
- Computerized analysis of cardiotocograms in clinical practice and the SisPorto® system thirty-two years after: technological, physiopathological and clinical studies
- Acknowledgment
- Acknowledgment

